Abstract
Located within 5′ untranslated region of HCV RNA is internal ribosome entry site (IRES) which directs cap-independent translation of viral polyprotein. Mutations in IRES sequence have been shown to cause changes in efficiency of protein translation in vitro in few instances. No study has been done to investigate association between frequency of nucleotide sequence variations in IRES region of HCV-3 RNA and response to pegylated interferon-α plus ribavirin therapy. Hence, this study was planned to analyze relationship between frequency of nucleotide sequence variations of HCV-3 IRES region and response to therapy. Twenty-seven HCV-3 patients were studied, of whom 19 responded to therapy and 8 did not. Alanine aminotransferase and aspartate aminotransferase levels were significantly lower in responders compared to non-responders. HCV RNA detection and genotyping was performed by nested-PCR and RFLP respectively. Viral load quantification in pre and post therapy samples was done by real time PCR. The viral load was significantly lower in the patients after treatment as compared to before treatment. HCV IRES region from pre-treatment sera of 27 HCV-3 infected patients was amplified by nested PCR and sequenced. Secondary structure of IRES region of HCV-3 was predicted using the M fold Web Server. Mutational analysis revealed hot spot of mutations in HCV-3 IRES region from 40–80 and 210–280 nucleotides. Though more mutations were found in non-responders as compared to responders, this difference was statistically insignificant. Therefore, in addition to IRES region of HCV-3, some other host and viral factors may contribute to therapy outcome.
Electronic supplementary material
The online version of this article (doi:10.1007/s13337-016-0335-7) contains supplementary material, which is available to authorized users.
Keywords: Hepatitis C virus genotype 3, Internal ribosome entry site, Nucleotide sequence variations, Pegylated interferon-alpha, Ribavirin
Introduction
Hepatitis C virus (HCV), a member of the family Flaviviridae, is the major causative agent of parenterally transmitted non-A, non-B hepatitis. HCV is an enveloped virus with a 9500-nucleotide-long single-stranded RNA genome of positive polarity encoding a single polyprotein, which is processed by the host cell and virus-encoded proteases into three major structural proteins and numerous non-structural proteins necessary for viral replication [14]. A comparison of genome and polyprotein sequences of HCV isolates has led to the identification of seven major genotypes, each with several subtypes [28]. Several studies have reported the presence of various HCV genotypes in India, and genotype 3 has been shown to be the predominant genotype in most parts of the country, followed by type 1 [9, 14, 19, 27].
The HCV genome contains a single open reading frame flanked by 5′ and 3′ untranslated regions (UTR) of 341 and about 230 nucleotides, respectively [4]. The 5′ UTR is uncapped and contains the internal ribosome entry site (IRES) which contains highly conserved secondary and tertiary structures that are necessary for proper binding and positioning of the viral RNA within the host cell’s protein translation machinery [4, 18]. The HCV IRES spans a region of ~340 nucleotides that encompasses most of the 5′ UTR of the viral mRNA and the first 24–40 nucleotides of the core-coding region. The critical role of 5′ UTR in initiation of poly-protein translation requires the highest degree of conservation of this region [5].
Although with the advent of second-generation direct-acting antiviral medications (DAAs) most of the other interferon-based regimens have been replaced, pegylated interferon-α2b with ribavirin being more cost-effective, is still being used in developing countries. Moreover, pegylated interferon along with second generation DAAs is still part of many treatment regimens [1]. Various studies have been done to study the relationship of genetic variability in 5′ UTR of hepatitis C virus 1, 2b/2c, and 4a with patients’ response to interferon-therapy [3, 4, 26]. However no such study has been reported on HCV-3. Though some studies have found decrease in translational efficiency of IRES region in patients suffering from HCV-3 responding to interferon therapy, while some other have found that interferon response is independent of IRES efficiency in HCV-3a, no study has been done on effect of mutations in IRES region in HCV-3 on response to interferon-treatment [23, 26, 31]. Hence, through the present study, we sought to investigate the relationship between nucleotide sequence variations in IRES region of HCV genotype 3 RNA and response to pegylated interferon-α and ribavirin therapy, in patients infected with HCV genotype 3.
Materials and methods
Place of study
The study was conducted in the virology laboratory of a 2000-bedded tertiary care teaching hospital in New Delhi.
Ethical approval and consent from study subjects
Ethical approval certificate for the study was obtained from the institutional ethical committee. Written informed consent from the study subjects were obtained in each case.
Inclusion/exclusion criteria
Patients above 18 years of age with established chronic liver disease (based on clinical features and elevated alanine aminotransferase for more than 6 months) who attended the medical outpatient department and wards of the hospital from January 2011 to December 2013 level were recruited in the study [10].
Patients positive for Hepatitis B surface antigen (HBsAg), Immunoglobulin M antibody to hepatitis B core antigen (HBcIgM), human immunodeficiency virus (HIV), hemochromatosis, any other cause of liver disease (e.g. α1 antitrypsin deficiency, Wilson’s disease, obesity-induced liver disease, drug-related liver disease), active seizures, ischemic cardiovascular disease within the last 6 months or having history of alcohol intake were excluded from the study.
Sampling and analysis
Five ml of blood sample was aseptically collected in plain vial from the study subjects. Serum was separated and aliquoted in different vials and stored at −70 °C until tested. Repeated freezing and thawing was avoided.
Screening for HBsAg was done using commercially available Enzyme-Linked Immunosorbent Assay (ELISA) Kit (Hepalisa, J. Mitra & Co., India) as per manufacturer instructions. Anti-HBcIgM antibody was tested using commercially available ELISA Kit (Bio-Rad, France) as per manufacturer instructions. Anti-HCV antibody was also tested using commercially available third generation ELISA Kit (Microlisa, J. Mitra and Co., India), comprising of Core, E1, E2, NS3, NS4 and NS5 antigens of HCV, as per manufacturer instructions. HBsAg or anti- HBcIgM antibody positive samples were excluded, while anti-HCV antibodies positive samples were further processed.
Detection of hepatitis C virus ribonucleic acid
HCV RNA was extracted from the serum sample using High Pure Viral RNA extraction kit (Roche Diagnostics, Mannheim, GmbH, Germany), according to manufacturers’ instructions. HCV RNA was extracted from 200 µL serum and eluted in 50 µL of elution buffer. Eluted RNA was stored at −80 °C until further processing.
Most of the commercial PCR amplification strategies for HCV detection are targeted against the 5′ UTR region since there is more than 90 % sequence identity among different HCV genotypes. The secondary and tertiary structures of this region are also mostly conserved. However, other than the 5′ UTR region, the core region is also targeted for PCR based detection of HCV, as a recent study has shown that sequence divergence of the core region is greater than the divergence of the 5′ UTR sequence. Hence, detection based on the sequence of the core region can reliably identify subtypes as well as major genotypes [13].
Reverse transcription polymerase chain reaction (RT-PCR) was carried out by modified method of Mellor et al. for the detection of hepatitis C virus RNA [22]. The RNA was denatured by heating at 70 °C for 3 min prior to RT-PCR, and reverse transcribed at 42 °C for 60 min, in a PCR tube containing 1X RT buffer, 10 mM deoxynucleotide triphosphates (dNTPs), 20 U RNase inhibitor, 50 U murine leukemia virus reverse transcriptase, and 20 pmol primer (core region P1: 5′ATGTACCCCATGAG/TA/GTCGGC 3′ anti-sense) to a final volume of 20 µL. The c-DNA product was denatured at 95 °C for 5 min, then cooled at 4 °C for 5 min and subjected to nested polymerase chain reaction (PCR) analysis.
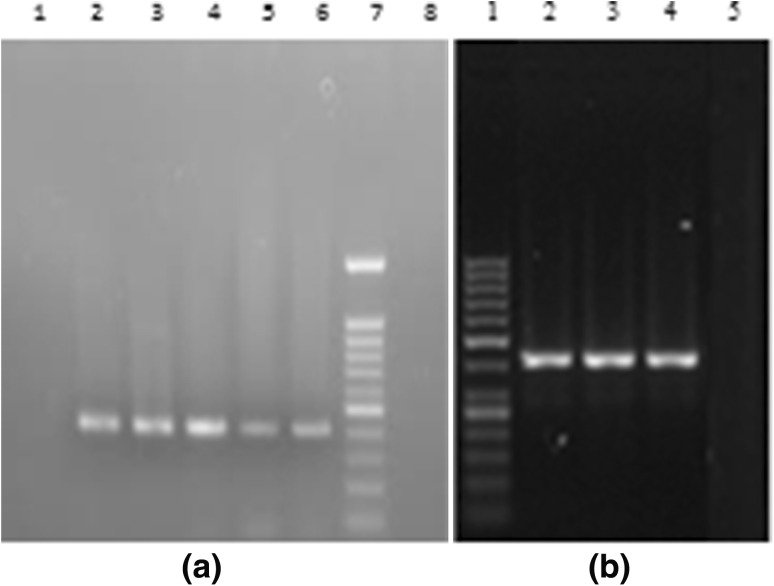
Nested-PCR was performed in the reaction mixture containing PCR buffer (10×), 2 mM MgCl2, 10 mM dNTPs, 20 pmol outer primers (sense P2:5′ACTGCCTGATAGGGTGC TTGCG AG 3′, anti-sense P1:5′ ATGTACCCCATGAG/TA/GTCGGC 3′) for 5′ NCR core region, 0.75 U Taq Deoxyribonucleic Acid (DNA) polymerase, in a total reaction volume of 25 µL. The amplified PCR product was subjected to second round of PCR using 20 pmol inner primers (sense P3:5′ACTGCCTGATAGGGTGCTTG CGAG3′, anti-sense P4:5′ ATGTACCCCATGAG/TA/GTCGGC 3′) for 5′ NCR core region, and 0.75 U Taq DNA polymerase, in a total reaction volume of 25 µL. The thermal cycling condition of nested PCR was as previously described by Verma and Chakravarti [30]. The amplified PCR product was electrophoresed in ethidium bromide stained 2 % Nusieve agarose gel (Sigma-Aldrich, USA) and was visualised under a ultraviolet (UV) transilluminator and gel documentation (Alpha Innotech, San Leandro, USA) unit for identifying desired 405 base pair (bp) fragment using molecular weight marker φX174/HaeIII digested product. Positive and negative controls were also included (Fig. 1).
Fig. 1.
a Agarose gel electrophoresis photograph of HCV reverse transcriptase PCR band of 405 base pairs. Lanes 1, 2, 3, 4 and 7 are the samples. Lane 5 is the DNA ladder of 100 base pairs. Lanes 6 and 8 are negative control and positive control respectively. b Amplification of internal ribosome entry site (IRES) region of HCV-3 isolated in the present study. Lane 1 is the molecular weight marker of 100 base pairs. Lanes 2 and 5 are positive control and negative control respectively. Lanes 3 and 4 are patients’ samples
HCV genotyping
HCV genotype was determined by restriction fragment length polymorphism (RFLP) analysis using the method of Chinchai et al. [8] /direct sequencing. In RFLP, the nested PCR product of RNA positive samples (20–30 µL) was digested with the three enzymes Accl, Mbol and BstN1 and incubated at 37 °C overnight in a specific endonuclease buffer. The digested product was loaded onto 3 % nusieve agarose gel and the restriction pattern was analysed using Gel-Doc System (Alpha Innotech, San Leandro, USA).
In direct sequencing the amplified PCR products were purified, using QIAquick Gel DNA Extraction Kit (Qiagen GmbH, Hilden) according to the manufacturer’s instructions. A 25–50 ng of the purified DNA products, was directly sequenced by the Sanger dideoxy method (Sanger 1977) (using DTCS starter kit (Beckman Coulter, U.S.A.), as per manufacturer instructions on Beckman coulter CEQ 8000 genetic analysis system (Fullerton, USA)/sent directly to Ocimum Bio solution Hyderabad. Sequence identity matrices and multi- sequence alignments from DNA databases were generated using Basic Local Alignment Search Tool (BLAST); available at National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/BLAST) and using Clustal W programme (http://align.genome.jp/).
Quantification of HCV-3 RNA in pre and post therapy samples
HCV- 3 RNA positive patients were subjected to quantification to determine the viral load. Quantitative HCV RT-PCR was performed using the Light Cycler TaqMan master mix kit (Roche Diagnostic GmbH, Mannheim, Germany) in Roche Light Cycler version 2.0 by the method of Martell et al. [21] using TaqMan chemistry. Viral load quantification in pre and post therapy samples was carried out by Real Time PCR. Each specimen was analyzed in duplicate and the mean value reported as the viremic level in the serum. The unit of the HCV-3 RNA quantification was copies/ml. A total of 7 standards of different copy numbers were included in HCV-3 RNA quantification assay. The range of standard used in quantitative analysis was 102–108 copies/mL. A known quantity of internal standard was included in each preparation of HCV-3 RNA. RT-PCR of a region in the 5′ UTR was performed as suggested by the manufacturer, i.e. at 95 °C for 20 s, which was followed by further 45 cycles at 95 °C for 10 s, at 58 °C for 15 s and at 72 °C for 10 s. Cooling was done at 40° C for 30 s.
Amplification of IRES region of HCV genome
Amplification of IRES region of HCV-3 RNA was done by RT-PCR, as described in details above. HCV IRES region from pre-treatment sera of HCV-3 infected patients was amplified by nested PCR and directly sequenced by Sanger’s method explained in the direct sequencing section. Nested-PCR was performed in the reaction mixture containing PCR buffer (10x), 2 mM MgCl2, 10 mM dNTPs, 20 pmol outer primers (sense IP1: 5′ ATGTACCCCATGAG/TA/GTCGGC 3′ and anti-sense IP2: 5′ CTG TGA GGA ACT ACT GTC TT 3′) for IRES region, 0.75U Taq DNA polymerase, in a total reaction volume of 25μl. The PCR product was subjected to 20 pmol inner primer (sense IP3: 5′ CTG TGA GGA ACT ACT GTC TT 3′ and anti-sense IP4: 5′ CAC/T GT A/G AGG GTA TCG ATG AC 3′) for IRES region with similar PCR conditions as described above. The reaction mixture for the nested PCR was subjected to 35 cycles of amplification at the following temperatures: initial denaturation at 94°C for 4 min, followed by annealing at 55°C for 60 s, elongation at 72°C for 60 s with final extension at 72°C for 10 min. Amplified PCR product was electrophoresed in ethidium bromide stained 2 % Nusieve agarose gel (Sigma-Aldrich, USA) and was visualised under a ultraviolet (UV) transilluminator and gel documentation (Alpha Innotech, San Leandro, USA) unit for identifying desired 720 bp fragment using 100 bp molecular weight marker φX174/HaeIII (MBI Fermentas, Lithunia) digested product. Positive and negative controls were also included (Fig. 1).
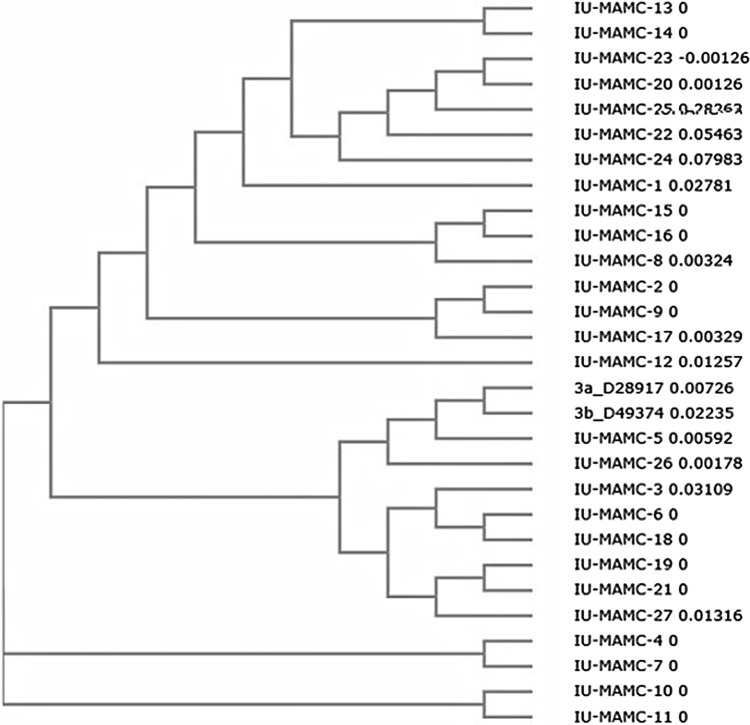
Fig. 2.
Phylogenetic tree demonstrating the genetic relationships between different responders (IU-1 to IU-19) and non-responders (IU-20 to IU-27) with reference sequences based on the nucleotide identity of the internal ribosome entry site (IRES) region (40–344 nucleotides). Accession no. of the sequences IU-1 to IU-27-KM281638, KM281639, KM281640, KM281641, KM281642, KM281643, KM281644, KM281645, KM281646, KM281647, KM281648, KM281649, KM281650, KM281651, KM281652, KM281653, KM281654, KM281655, KM281656, KM281657, KM281658, KM281659, KM281660, KM281661, KM281662, KM281663, KM281664. Accession no. of reference sequences—3a_D28917 and 3b_D49374
Direct sequencing of IRES region and analysis
The amplified PCR product was purified, using QIAquick Gel DNA Extraction Kit (Qiagen GmbH, Hilden) as per manufacturer instructions and it was directly sequenced by the Sanger’s dideoxy method, using DTCS starter kit (Beckman Coulter, USA), as per manufacturer instructions on Beckman coulter CEQ8000 genetic analysis system (Fullerton, USA). The sequences of responders and non-responders group were aligned using CLUSTAL W (version 2.1 multiple sequence alignment) to the reference sequences (Prototype) of the genotype from National Centre for Biotechnology Information (NCBI) Gene Bank. The most common nucleotide at each position of this study was taken as the consensus sequence. The factors associated with the mutations in each of the regions were studied, taking into account the median of the number of mutations in each region.
Alignment of IRES sequences were compared with the consensus sequence of HCV (http://www.ncbi.nlm.nih.gov/Genbank). The IRES sequences were aligned with the reported HCV genotype from National Center for Biotechnology Information (NCBI) Gene Bank using CLUSTAL W (version 2.1 multiple sequence alignment). The sequences in FASTA format were pasted in the submission form (available at http://www.ebi.ac.uk/cluatalw/), and the output obtained was represented by phylogenetic tree (Fig. 2). Secondary structure of IRES region of HCV genotype 3 was predicted using the M fold Web Server (mfold.rna.albany.edu). Sequences in FASTA format was given as input (Supplementry figure).
In addition to the molecular analysis, other parameters like biochemical and haemoglobin levels were obtained of the 27 HCV-3 RNA positive study subjects. The biochemical tests and viral load determination were repeated after 6 months of treatment.
Statistical analysis
Mann–Whitney and ANOVA tests were applied using Graph pad Prism 5 analysis to analyze the biochemical profile, haemoglobin levels and viral load of HCV-3 RNA positive samples. Comparisons between groups were made by the Chi-square or Fisher exact test for categorical variables and the Student t test for quantitative variables. The quantitative variables were expressed as means ± SD. A probability value (p value) <0.05 was considered statistically significant.
Results
Demographic features
Out of the 27 patients studied, 19 (70 %) responded to treatment (pegylated IFN-α and ribavarin) and 8(30 %) did not respond. Among the 19 responders, 12 were males and 7 were females (mean age—43.84 ± 10.19 years), while among the 8 responders, 5 were males and 3 were females (mean age—53.63 ± 8.86 years). The demography, biochemical profiles and number of mutations in IRES region of HCV RNA of the responders and non-responders are shown in Table 1.
Table 1.
Age, sex, haemoglobin levels, biochemical characteristics and number of mutations in HCV-3 RNA IRES region of the responders and non-responders
| Patients’ response to treatment/total patients | Age (years) | Sex (male/female) | Haemoglobin (g/dL) | Blood urea (mg%) | Alkaline phosphatase (KAU) | Total bilirubin (mg%) | Albumin (mg%) | AST (IU/L)a | ALT (IU/L)b | Number of mutations |
|---|---|---|---|---|---|---|---|---|---|---|
| Responders (19/27) | 43.84 ± 10.19 | 12/7 | 9.94 ± 2.87 | 33.0 ± 29.97 | 166.05 ± 87.36 | 3.43 ± 0.58 | 2.7 ± 0.80 | 77.25 ± 51.89 | 40.47 ± 20.16 | 4.95 ± 1.84 |
| Non-responders (8/27) | 53.63 ± 8.86 | 5/3 | 9.04 ± 1.92 | 21.63 ± 3.01 | 189 ± 78.43 | 3.82 ± 0.60 | 2.95 ± 0.97 | 87.68 ± 53.14 | 68.00 ± 25.81 | 5.75 ± 2.49 |
| p value* | 0.06 | 0.65 | 0.42 | 0.30 | 0.52 | 0.34 | 0.51 | 0.038* | 0.006* | 0.36 |
Normal values of the biochemical indices and haemoglobin levels
Haemoglobin—≥11 g/dL. Blood urea—20–40 mg/dL. Total bilirubin—<1.2 mg%. Aspartate aminotransferase—<40 IU/L. Alanine aminotransferase—<35 IU/L. Alkaline phosphatase—80–260 KAU. Albumin—3.5–5 mg%
* p value <0.05 is taken as statistically significant
aAspartate aminotransferase
bAlanine aminotransferase
Biochemical profiles
Out of the 7 biochemical parameters studied in the responders and non-responders, two parameters alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were significantly lower in responders as compared to non-responders (Table 1). Thus, these parameters can act as the indicators of response to pegylated IFN-α plus ribavirin therapy.
Mutational analysis
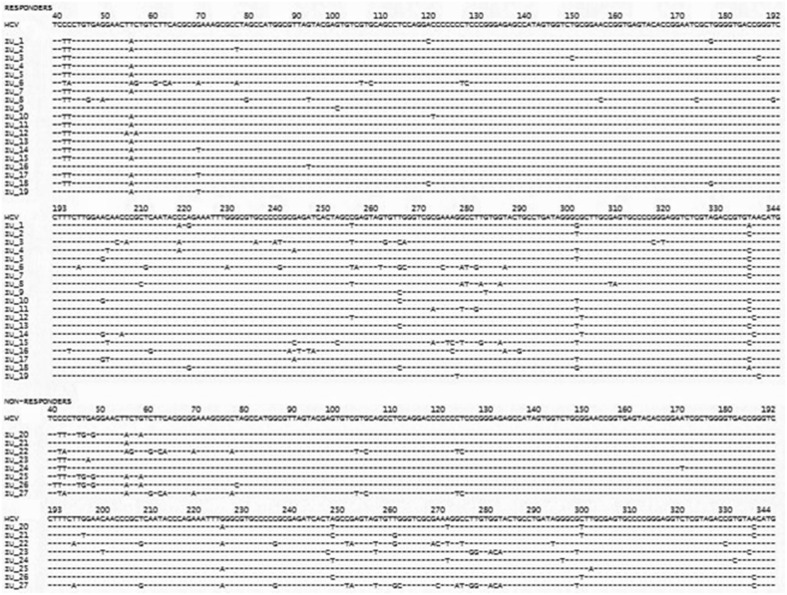
The no. of nucleotide variations within the entire internal ribosome entry site (40–344 nucleotides) region was compared in responders and non-responders. Mutational analysis revealed hot spot of mutations in internal ribosome entry site (IRES) region from 40–80 and 210–280 nucleotides, which was not significantly associated with response to therapy, though the number of mutations in non-responders was more than in responders (Table 1). Among the responders, nucleotide variations were most commonly found at positions 42–43, 303 and 338, while among the non- responders nucleotide variations were commonest at positions 42–43, 46–60, 250–284, 300 and 340 (Fig. 3).
Fig. 3.
Alignment of internal ribosome entry site (IRES-nucleotides 40–344) sequence of the 27 HCV-3 isolates. The sequences were compared with the consensus sequence of HCV (http://www.ncbi.nlm.nih.gov/Genbank). Nucleotide variations were found in both responders (19) and non-responders (8), however the number of mutations was more in non-responders than responders. The short lines indicate identity with the consensus HCV sequence. Hot spot of mutations were found in nucleotides 40–80 and 210–280
Biochemical profile and viral loads of HCV-3 RNA positive patients before and after treatment
The baseline biochemical profile and viral loads of the HCV-3 RNA positive patients were noted before starting treatment, and the same were noted again at completion of the treatment. The biochemical profiles and viral load of patients before and after treatment is shown in Table 2. The biochemical parameters, except alkaline phosphatase, were significantly deranged in pre-therapy as compared to post-therapy patients (Table 2). The viral load was also significantly lower in the patients after treatment as compared to before treatment (Table 2).
Table 2.
Pre- therapy and post-therapy biochemical profiles and viral load of HCV-3 RNA positive study subjects
| AST (IU/L)a | ALT (IU/L)b | ALP (KAU)c | Total bilirubin (mg%) | Albumin (mg%) | Viral load (copies/mL) | |
|---|---|---|---|---|---|---|
| Pre-therapy | 87.31 ± 77.13 | 91.39 ± 89.22 | 251.14 ± 174.13 | 1.53 ± 2.92 | 3.9 ± 0.76 | 370,438 ± 28,613 |
| Post-therapy | 38.48 ± 22.76 | 34.79 ± 17.01 | 78.89 ± 18.71 | 0.57 ± 0.26 | 4.00 ± 0.37 | 238,971 ± 146,604 |
| p value* | 0.003* | 0.001* | 0.06 | 0.02* | 0.003* | 0.03* |
Normal values of the biochemical indices
Aspartate aminotransferase—<40 IU/L. Alanine aminotransferase—<35 IU/L. Alkaline phosphatase—80–260 KAU. Total bilirubin—<1.2 mg%. Albumin—3.5–5 mg%
* p value <0.05 is taken as statistically significant
aAspartate aminotransferase
bAlanine aminotransferase
cAlkaline phosphatase
Phylogenetic tree and secondary structure prediction of IRES region
The 27 sequences of the NS5A region (Nucleotides 40–344) from all of the patients studied were used to make phylogenetic tree (Fig. 2). No clustering was observed among the sequences according to the treatment response. Secondary structure prediction of IRES region of HCV genotypes 3 was performed in both responders and non-responders (Fig. 3). The alignment of nucleotide sequences of IRES region was compared with the reference sequence of HCV genotype 3. The nucleotide variations were identified from 42–52 and 242–282 nucleotides. None of these nucleotide variations seemed to contribute to any gross modification of the secondary structure of IRES region of HCV genotype 3 when compared with the reference secondary structure of IRES region of HCV genotype 3.
Discussion
HCV infection is considered a significant risk factor for development of hepatocellular carcinoma (HCC) [7, 17]. Nucleotide substitutions in the viral genome result in its diversity into quasispecies, subtypes and distinct genotypes. The various genotypes differ in their infectivity and immune response due to these nucleotide variations [17].
Both natural and recombinant interferon- α (IFN-α) have immunomodulatory and anti-proliferative activities, thereby interfering with HCV replication. Interferons are natural cellular proteins with a number of immune functions including induction of the antiviral state, secretion of cytokines, enrolment of immune cells, and induction of cell differentiation. IFN-α can normalize liver function tests (LFT) results and improve hepatic inflammation by interfering with viral replication in patients with HCV infection [17]. In treating infection with Peg-IFN-α2b and ribavirin, knowledge of a patient’s viral genotype is very important as it determines the duration of the therapy [2].
Initiation of translation of hepatitis C viral RNA occurs internally and is mediated by IRES, which is a segment of about 330 nucleotides located at the 5′ end region [31]. IRES initiates the translation of a very long protein containing about 3000 amino acids. This large pre-protein has major role in viral replication and assembly [25]. While this is the most conserved part of the genome, this region also acquires nucleotide substitutions which are often covariant [26]. In spite of thermodynamic stability of RNA structure, mutations affecting binding affinity of factors may be associated with either more flexibility or rigidity, which in turn regulates efficiency of translation initiation [4].
Combination therapy with IFN-α and ribavirin in chronic hepatitis C exhibits the best responses against HCV genotype 3. Still, it has been found to be ineffective in 20–30 % of patients infected with this genotype [31]. The reason for the absence of response is not fully understood yet. Studies have shown that HCV IRES is sensitive to interferon treatment [11, 16]. Yasmeen et al. [31] have found correlation between IRES activity in vitro and response to treatment in HCV-3 patients. Panigrahi et al. [23] demonstrated that the synergistic antiviral action of IFN-α and ribavirin combination treatment is at the point of inhibition of HCV IRES mediated translation through prevention of polyribosome formation using two different mechanisms involving PKR (double-stranded RNA-dependent protein kinase) activation and depletion of intracellular guanosine pool through inhibition of inosine-5′-monophosphate dehydrogenase. However, the detailed mechanism of how IFN-α and ribavirin combination treatment leads to efficient translation arrest of HCV IRES mRNA has not yet been understood completely.
Studies have been done on hepatitis genotypes other than genotype 3 on the relation between frequency of mutations in IRES region and efficacy of IFN therapy. Awady et al. [4] have reported that in case of HCV-4a, the influence of mutations inside domain III of IRES on the response of HCV patients to combination therapy depends primarily on the position and not on the frequency, of these mutations within IRES domain III. In a study by Jubin et al. [15], no association was seen between sequence variation of IRES element of HCV-1b and response to IFN treatment. Saiz et al. [26] showed that response to IFN therapy and the activity of IRES elements of HCV genotypes 1b, 2a/2c and 3a present in sera from treated patients are independent events. The present study is the first study to be conducted aiming at finding the relationship between frequency of mutations in IRES region and response to therapy in patients suffering from HCV-3 infection. In this study, though frequency of mutations in patients not responding to treatment was more than those responding to treatment, no significant association was found between the frequency of mutations and patients’ response to treatment. Therefore difference in treatment outcome among patients with HCV genotype 3 infection can’t be explained only by the sequence variability seen in the IRES. Some other host and viral factors may be responsible for the therapy outcome.
There are four subgenomic regions of HCV other than IRES in which distinct mutations have been observed in response to IFN therapy. The regions include E2-PePHD (PKR/eIF2α phosphorylation homology domain); NS5A-ISDR (IFN sensitivity-determining region), PKRBD (PKR binding domain), and V3 (Variable region 3). A PKR/eIF2α phosphorylation homology domain (PePHD) within the E2 protein has been found to interact with PKR and inhibit PKR in vitro, which suggests a possible mechanism for HCV to elude the antiviral effects of IFN therapy [25, 29]. Genetic heterogeneity of a specific domain of NS5A region, called the IFN sensitivity-determining region (ISDR), is closely related to the response in Japanese patients with HCV genotype 1b, so that patients with at least 4 mutations within ISDR achieve a sustained virologic response (SVR) to IFN-α monotherapy. Mutations within the PKRBD of HCV genotype 1 are associated with a long-term sustained response to IFN-α and IFN-α/ribavirin therapy [6, 20, 25]. Some clinical studies have projected that the number of amino acid variations within variable region V3 may be associated with the treatment outcome [12, 24, 25].
To the best of our knowledge, this is the first study performed aiming to investigate the association between frequency of mutations in IRES region and response to pegylated IFN-α plus ribavirin treatment in HCV genotype 3 patients. We found that though the frequency of mutations in IRES region was more in non-responders compared to responders, the association between mutation-frequency and therapy outcome was statistically insignificant. Therefore we conclude that difference in treatment outcome among patients with HCV-3 infection can’t be explained only by the sequence variability seen in the IRES region and it may be affected by some other viral and host factors. However, this issue deserves further investigation, including tests of other viral markers and whole-genome screening. Precise analysis of the factors influencing therapy response will give us the opportunity to better understand the interferon resistance phenomena and will also help us to identify specific targets for the development of novel antiviral strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Secondary structure of internal ribosome entry site (IRES) region of responders, non-responders and HCV-3 reference sequence. Supplementary material 1 (TIFF 139 kb)
Acknowledgments
We thankfully acknowledge the financial assistance provided to us by University Grant Commission and Indian Council of Medical Research for conducting this research work.
References
- 1.Aghemo A, Back D, Dusheiko G, Forns X, Puoti M, Sarrazin C. EASL recommendations on treatment of hepatitis C. J Hepatol. 2015;63:199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Alghamdi AS, Sanai FM, Ismail M, Alghamdi H, Alswat K, Alqutub A, Altraif I, Hemant Shah H, Alfaleh FZ. SASLT practice guidelines: management of hepatitis C virus infection. Saudi J Gastroenterol. 2012;18(Suppl):S1–S32. doi: 10.4103/1319-3767.101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araújo FMG, Sonoda IV, Rodrigues NB, Teixeira R, Redondo RAF, Oliveira GC. Genetic variability in the 5′UTR and NS5A regions of hepatitis C virus RNA isolated from non-responding and responding patients with chronic HCV genotype 1 infection. Mem Inst Oswaldo Cruz. 2008;103(6):611–614. doi: 10.1590/S0074-02762008000600018. [DOI] [PubMed] [Google Scholar]
- 4.Awady MKE, Azzazy HM, Fahmy AM, Shawky SM, Badreldin NG, Yossef SS, Omran MH, Zekri ARN, Goueli SA. Positional effect of mutations in 5′UTR of hepatitis C virus 4a on patients’ response to therapy. World J Gastroenterol. 2009;15(12):1480–1486. doi: 10.3748/wjg.15.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barría MI, González A, Vera-Otarola J, León U, Vollrath V, Marsac D, Monasterio O, Pérez-Acle T, Soza A, López-Lastra M. Analysis of natural variants of the hepatitis C virus internal ribosome entry site reveals that primary sequence plays a key role in cap-independent translation. Nucleic Acids Res. 2009;37(3):957–971. doi: 10.1093/nar/gkn1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg T, Marques AM, Höhne M. Mutations in the E2-PePHD and NS5A region of hepatitis C virus type 1 and the dynamics of hepatitis C viremia decline during interferon alfa treatment. Hepatology. 2000;32:1386–1395. doi: 10.1053/jhep.2000.20527. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarti A, Anzar A, Malik S. A study of changing trends of prevalence and genotypic distribution of hepatitis C virus among high risk groups in north India. Indian J Med Microbiol. 2013;31(4):354–359. doi: 10.4103/0255-0857.118877. [DOI] [PubMed] [Google Scholar]
- 8.Chinchai T, Labout J, Noppornpanth S, Theamboonlers A, Haagmans BL, Osterhaus ADME, Poovorawan Y. Comparative study of different methods to genotype hepatitis C virus type 6 variants. J Virol Methods. 2003;109:195–201. doi: 10.1016/S0166-0934(03)00071-5. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury A, Santra A, Chaudhuri S, Dhali GK, Chaudhuri S, Maity SG, Naik TN, Bhattacharya SK, Mazumder DN. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology. 2003;37:802–809. doi: 10.1053/jhep.2003.50157. [DOI] [PubMed] [Google Scholar]
- 10.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. doi: 10.1002/hep.1840190629. [DOI] [PubMed] [Google Scholar]
- 11.Dhar D, Roy S, Das S. Translational control of the interferon regulatory factor 2 mRNA by IRES element. Nucleic Acids Res. 2007;35(16):5409–5421. doi: 10.1093/nar/gkm524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duverlie G, Khorsi H, Castelain S, Jaillon O, Izopet J, Lunel F, Eb F, Penin F, Wychowski C. Sequence analysis of the NS5A protein of European hepatitis C virus 1b isolates and relation to interferon sensitivity. J Gen Virol. 1998;79:1373–1381. doi: 10.1099/0022-1317-79-6-1373. [DOI] [PubMed] [Google Scholar]
- 13.Firdaus R, Saha K, Biswas A, Sadhukhan PC. Current molecular methods for the detection of hepatitis C virus in high risk group population: a systematic review. World J Virol. 2015;4(1):25–32. doi: 10.5501/wjv.v4.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta R, Subramani M, Khaja MN, Madhavi C, Roy S, Habibullah CM, Das S. Analysis of mutations within the 5′ untranslated region, interferon sensitivity region, and PePHD region as a function of response to interferon-therapy in hepatitis C virus-infected patients in India. J Clin Microbiol. 2006;44(3):709–715. doi: 10.1128/JCM.44.3.709-715.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jubin R, Vantuno NE, Kieft JS, Murray MG, Doudna JA, Lau JYN, Baroudy BM. Hepatitis C virus internal ribosome entry site (IRES) stem loop IIId contains a phylogenetically conserved GGG triplet essential for translation and IRES folding. J Virol. 2000;74(22):10430–10437. doi: 10.1128/JVI.74.22.10430-10437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato J, Kato N, Moriyama M, Goto T, Taniguchi H, Shiratori Y, Omata M. Interferons specifically suppress the translation from the internal ribosome entry site of hepatitis C virus through a double-stranded RNA-activated protein kinase–independent pathway. J Infect Dis. 2002;186:155–163. doi: 10.1086/341467. [DOI] [PubMed] [Google Scholar]
- 17.Khaliq S, Latief N, Jahan S. Role of different regions of the hepatitis C virus genome in the therapeutic response to interferon-based treatment. Arch Virol. 2014;159(1):1–15. doi: 10.1007/s00705-013-1780-x. [DOI] [PubMed] [Google Scholar]
- 18.Kieft JS, Zhou K, Grech A, Jubin R, Doudna JA. Crystal structure of an RNA tertiary domain essential to HCV IRES-mediated translation initiation. Nat Struct Biol. 2002;9:370–374. doi: 10.1038/nsb781. [DOI] [PubMed] [Google Scholar]
- 19.Lole KS, Jha JA, Shrotri SP, Tandon BN, Prasad VG, Arankalle VA. Comparison of hepatitis C virus genotyping by 5′noncoding region- and core-based reverse transcriptase PCR assay with sequencing and use of the assay for determining subtype distribution in India. J Clin Microbiol. 2003;41:5240–5244. doi: 10.1128/JCM.41.11.5240-5244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macquillan G, Niu CX, Speers D, Englihs S, Garas G, Harnett GB, Reed WD, Allan JE, Jeffrey GP. Does sequencing the PKRBD of hepatitis C virus NS5A predict therapeutic response to combination therapy in an Australian population? J Gastroenterol Hepatol. 2004;19:551–557. doi: 10.1111/j.1440-1746.2003.03319.x. [DOI] [PubMed] [Google Scholar]
- 21.Martell M, Gomez J, Esteban JI, Sauleda S, Quer J, Cabot B, Esteban R, Guardia J. High-throughput real-time reverse transcription-PCR quantitation of hepatitis C virus RNA. J Clin Microbiol. 1999;37:327–332. doi: 10.1128/jcm.37.2.327-332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellor J, Holmes EC, Jarvis LM, Yap PL, Simmonds P. Investigation of the pattern of hepatitis C virus sequence diversity in different geographical regions: implications for virus classification. The International HCV Collaborative Study group. J Gen Virol. 1995;76:2493–2507. doi: 10.1099/0022-1317-76-10-2493. [DOI] [PubMed] [Google Scholar]
- 23.Panigrahi R, Hazari S, Chandra S, Chandra PK, Datta S, Kurt R, Cameron CE, Huang Z, Zhang H, Garry RF, Balart LA, Dash S. Interferon and ribavirin combination treatment synergistically inhibit HCV internal ribosome entry site mediated translation at the level of polyribosome formation. PLoS ONE. 2013;8(8):e72791. doi: 10.1371/journal.pone.0072791. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Puig-Basagoiti F, Forns X, Furcic I, Ampurdanes S, Gimenez-Barcons M, Franco S. Dynamics of hepatitis C virus NS5A quasispecies during interferon and ribavirin therapy in responder and non-responder patients with genotype 1b chronic hepatitis C. J Gen Virol. 2005;86:1067–1075. doi: 10.1099/vir.0.80526-0. [DOI] [PubMed] [Google Scholar]
- 25.Raza A, Zafeer M, Arshad BG, Shakeel SN. Mutational analysis of various sub-genomic regions of HCV and their role in interferon therapy response. Int J Sci Emerg Technol. 2012;4(3):159–166. [Google Scholar]
- 26.Sáiz JC, de Quinto SL, Ibarrola N, López-Labrador FX, Sánchez-Tapias JM, Rodés J, Martínez-Salas E. Internal initiation of translation efficiency in different hepatitis C genotypes isolated from interferon treated patients. Arch Virol. 1999;144:215–229. doi: 10.1007/s007050050499. [DOI] [PubMed] [Google Scholar]
- 27.Singh S, Malhotra V, Sarin SK. Distribution of hepatitis C virus genotypes in patients with chronic hepatitis C infection in India. Indian J Med Res. 2004;119:145–148. [PubMed] [Google Scholar]
- 28.Smith DB, Bukh J, Kuiken C, Muerhoff S, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 30.Verma V, Chakravarti A. Comparision of 5′ noncoding-core region with 5′ noncoding region of HCV by RT PCR: importance and clinical application. Curr Microbiol. 2008;57:206–211. doi: 10.1007/s00284-008-9175-z. [DOI] [PubMed] [Google Scholar]
- 31.Yasmeen A, Hamid S, Granath FN, Lindström H, Elliott RM, Siddiqui AA, Persson MA. Correlation between translation efficiency and outcome of combination therapy in chronic hepatitis C genotype 3. J Viral Hepat. 2006;13(2):87–95. doi: 10.1111/j.1365-2893.2005.00660.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Secondary structure of internal ribosome entry site (IRES) region of responders, non-responders and HCV-3 reference sequence. Supplementary material 1 (TIFF 139 kb)