Abstract
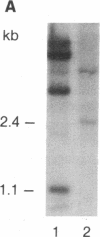
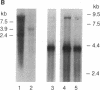
Retroviral transduction of cellular nucleic acid sequences requires illegitimate RNA or DNA recombination. To test a model that postulates transduction via efficient illegitimate recombination during reverse transcription of viral and cellular RNAs, we have measured the ability of Harvey sarcoma viruses (HaSVs) with artificial 3' termini to recover a retroviral 3' terminus from helper Moloney virus (MoV) by illegitimate and homologous recombination. For this purpose, mouse NIH 3T3 cells were transformed with Harvey proviruses and then superinfected with MoV. The proviruses lacked the 3' long terminal repeat and an untranscribed region of the 5' long terminal repeat to prevent virus regeneration from input provirus. Only 0-11 focus-forming units of HaSV were generated upon MoV superinfection of 3 x 10(6) cells transformed by Harvey proviruses with MoV-unrelated termini. This low frequency is consistent with illegitimate DNA recombination via random Moloney provirus integration 3' of the transforming viral ras gene in the 10(6)-kilobase mouse genome. When portions of murine viral envelope (env) genes were attached 3' of ras, 10(2)-10(5) focus-forming units of HaSV were generated, depending on the extent of homology with env of MoV. These recombinants all contained HaSV-specific sequences 5' and MoV-specific sequences 3' of the common env homology. They were probably generated by recombination during reverse transcription rather than by recombination among either input or secondary proviruses, since (i) the yield of recombinants was reduced by a factor of 10 when the env sequence was flanked by splice signals and (ii) HaSV RNAs without retroviral 3' termini would be inadequate templates for provirus synthesis. We conclude that there is no efficient illegitimate recombination in retroviruses. In view of known precedents of illegitimate DNA recombination, the structure of known viral onc genes, and our evidence for illegitimate DNA recombination via provirus integration, we favor the DNA model of transduction over the RNA model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Cancer genes: rare recombinants instead of activated oncogenes (a review). Proc Natl Acad Sci U S A. 1987 Apr;84(8):2117–2124. doi: 10.1073/pnas.84.8.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Zhou R. P., Goodrich D. Cancer genes by illegitimate recombination. Ann N Y Acad Sci. 1989;567:259–273. doi: 10.1111/j.1749-6632.1989.tb16477.x. [DOI] [PubMed] [Google Scholar]
- Fredrickson T. N., O'Neill R. R., Rutledge R. A., Theodore T. S., Martin M. A., Ruscetti S. K., Austin J. B., Hartley J. W. Biologic and molecular characterization of two newly isolated ras-containing murine leukemia viruses. J Virol. 1987 Jul;61(7):2109–2119. doi: 10.1128/jvi.61.7.2109-2119.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch J. W., Meier H. A short-term quantitative XC assay for murine leukemia virus. Virology. 1976 Jul 15;72(2):509–513. doi: 10.1016/0042-6822(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Tabin C. J., Wang J. Y., Weinberg R., Baltimore D. Transfection of fibroblasts by cloned Abelson murine leukemia virus DNA and recovery of transmissible virus by recombination with helper virus. J Virol. 1982 Jan;41(1):271–285. doi: 10.1128/jvi.41.1.271-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Structure of the provirus within NIH 3T3 cells transfected with Harvey sarcoma virus DNA. J Virol. 1981 Apr;38(1):125–135. doi: 10.1128/jvi.38.1.125-135.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral recombination during reverse transcription. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2052–2056. doi: 10.1073/pnas.87.6.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral transduction of oncogenic sequences involves viral DNA instead of RNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3733–3737. doi: 10.1073/pnas.85.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Mandahl N., Mitelman F. Genetic convergence and divergence in tumor progression. Cancer Res. 1988 Nov 1;48(21):5911–5916. [PubMed] [Google Scholar]
- Herman S. A., Coffin J. M. Efficient packaging of readthrough RNA in ALV: implications for oncogene transduction. Science. 1987 May 15;236(4803):845–848. doi: 10.1126/science.3033828. [DOI] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hay N., Bishop J. M. The role of RNA molecules in transduction of the proto-oncogene c-fps. Cell. 1986 Mar 28;44(6):935–940. doi: 10.1016/0092-8674(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Maisel J., Dina D., Duesberg P. Murine sarcoma viruses: the helper-independence reported for a Moloney variant is unconfirmed; distinct strains differ in the size of their RNAs. Virology. 1977 Jan;76(1):295–312. doi: 10.1016/0042-6822(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Lee W. H., Duesberg P. H. Generation of nondefective Rous sarcoma virus by asymmetric recombination between deletion mutants. J Virol. 1980 Nov;36(2):591–594. doi: 10.1128/jvi.36.2.591-594.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Kataoka K., Goto N., Fujiwara K. T., Kawai S. v-maf, a viral oncogene that encodes a "leucine zipper" motif. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7711–7715. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. R., Buckler C. E., Theodore T. S., Martin M. A., Repaske R. Envelope and long terminal repeat sequences of a cloned infectious NZB xenotropic murine leukemia virus. J Virol. 1985 Jan;53(1):100–106. doi: 10.1128/jvi.53.1.100-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Pfaff S. L., Zhou R. P., Young J. C., Hayflick J., Duesberg P. H. Defining the borders of the chicken proto-fps gene, a precursor of Fujinami sarcoma virus. Virology. 1985 Oct 30;146(2):307–314. doi: 10.1016/0042-6822(85)90014-5. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Hoffman J., Goff S. P., Baltimore D. Intramolecular integration within Moloney murine leukemia virus DNA. J Virol. 1981 Oct;40(1):164–172. doi: 10.1128/jvi.40.1.164-172.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Evolution of cancer genes as a mutation-driven process. Cancer Res. 1988 Apr 1;48(7):1697–1701. [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Zhou R. P., Duesberg P. H. Avian proto-myc genes promoted by defective or nondefective retroviruses are single-hit transforming genes in primary cells. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7721–7725. doi: 10.1073/pnas.86.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R. P., Kan N., Papas T., Duesberg P. Mutagenesis of avian carcinoma virus MH2: only one of two potential transforming genes (delta gag-myc) transforms fibroblasts. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6389–6393. doi: 10.1073/pnas.82.19.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]