For decades, bone marrow (BM) has been the preferred source of hematopoietic stem and progenitor cells (HSPCs) for transplants following myeloablative conditioning. At present, mobilized peripheral blood stem cells are commonly used for transplantation, particularly in the autologous setting.1
Hematopoietic stem cells (HSCs) are maintained in their niche by binding to adhesion molecules and diverse strategies are used to promote their egress from BM to peripheral blood (PB).2 Granulocyte-colony stimulating factor (G-CSF) represents the gold standard agent to mobilize HSPCs for transplantation. Nevertheless, other compounds have recently been tested.3
One of the most successful mobilizing agents is plerixafor,4 a bicyclam molecule that selectively and reversibly antagonizes the binding of stromal cell derived factor-1 (SDF-1) on the surface of BM stromal cells, to chemokine CXC-receptor-4 (CXCR4) on HSPCs, with their subsequent mobilization in the blood. Clinical trials demonstrated that plerixafor alone safely and rapidly mobilizes HSCs in healthy donors, β-thalassemia patients and patients affected by malignancies.3,5,6
Characterization studies of non-human primates and human samples of plerixafor-mobilized cells in comparison to cells mobilized by G-CSF alone or in combination with plerixafor showed a different expression profile, cell composition and engrafting potential in xenotransplant models.7–11
However, these studies did not solve whether plerixafor, G-CSF, or their combination mobilizes different primitive HSC populations.
In order to define the content of HSPCs mobilized by plerixafor, CD34+ cells were isolated from leukapheresis (Plx PB), steady-state BM and BM following plerixafor administration (Plx BM) of thalassemic patients enrolled in a phase II trial of mobilization.
We performed a detailed immunophenotype analysis of primitive HSPCs by using the “gold standard” cell surface markers.12
The analysis revealed an increased frequency of long-term HSCs (LT-HSCs) and a decrease in intermediate HSCs (INT-HSCs) in Plx PB versus BM. Moreover, multipotent progenitor (MPP) frequency was lower in Plx PB samples as compared to BM and Plx BM, indicating that they are not predominantly mobilized by plerixafor (Figure 1A,B).
Figure 1.
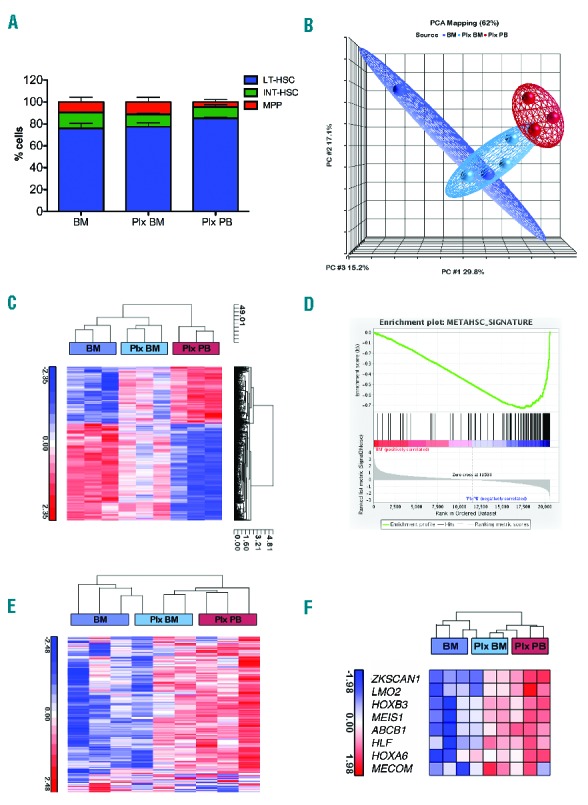
Cell surface marker and gene expression analysis of primitive HSPCs in paired sources derived from thalassemic patients. A. Distribution of LT-HSCs, INT-HSCs and MPPs in different sources derived from thalassemic patients: BM (n=2), Plx BM (n=3) and Plx PB (n=3). The expression of CD38, CD90, CD45RA and CD49f cell surface markers is considered the “gold standard” for predicting primitive HSCs.12 Using these markers three main subpopulations of CD34+ cells were identified: HSCs (CD34+ CD38−/low CD90+/− CD45RA−CD49f+); LT-HSCs (CD34+ CD38−/low CD90+ CD45RA− CD49f+); INT-HSC (CD34+ CD38−/low CD90− CD45RA- CD49f+); MPPs (CD34+ CD38−/low CD90- CD45RA− CD49f−). The indicated subsets are reported as relative fractions of the more primitive hematopoietic populations (gated on CD34+ CD38−/low CD90+/− CD45RA− cells). Data are represented as mean±SEM. B. Population distance analysis of microarray data presented in three principal components (PCs1–3). Each point represents a single array. Stem cell sources are color-coded. C. Hierarchical cluster analysis to assess relative distance of the transcriptome of each stem cell source. The branched tree is based on differentially expressed genes in plerixafor-mobilized CD34+ cells versus BM (FDR<0.05). In this image, the normalized expression levels of genes are presented according to a colored gradient from the highest (red) to lowest (blue, see colored scale). D. GSEA plot enrichment of METAHSC_SIGNATURE in Plx PB (n=3) vs. BM (n=3) expression profile (P<0.01). E. Our data were compared with previously generated gene sets METAHSC_SIGNATURE (Online Supplementary Figure S3), derived from bone marrow stem and progenitor population. Plx PB gene expression positively correlates with primitive gene sets. F. Heat maps of selected genes involved in HSC regulation are more highly expressed in Plx PB than in other samples. PCA: principal component analysis; BM: bone marrow; PB: peripheral blood; HSC: hematopoietic stem cell; LT-HSC: long-term HSC; INT-HSC: intermediate HSC; MPP: multipotent progenitor.
To identify the molecular mechanisms underlying the higher quality of plerixafor-mobilized HSPCs, gene expression profiling was performed on CD34+ cell samples isolated from the same individuals (n=3). Principal component analysis (PCA) revealed a segregation of Plx PB, BM and Plx BM in three discrete clusters on the basis of the cell source (Figure 1C). Plx BM samples were positioned between Plx PB and BM, suggesting that the transcriptional profile begins to change by plerixafor administration and progressed in HSPCs collected by leukapheresis.
Comparing Plx PB to BM samples, we identified 829 differentially expressed probe sets (Online Supplementary Table S1). 37% of the probe sets was significantly upregulated in plerixafor-mobilized CD34+ cells, whereas the expression of 523 probe sets was greater in BM (FDR < 0.05). Cluster analysis showed that Plx BM clustered with BM samples indicating that these cells share many similarities (Figure 1D). Most of the probe sets upregulated in Plx PB were enriched in cellular and metabolic processes, while most of the downregulated probe sets were involved in cell cycle processes13 (Online Supplementary Figure S1A–D), including MIK67, cyclins, CDC6, CHEK1, BRCA1, BRCA2, kinases and transcription factors related to cell proliferation (Online Supplementary Figure S2A and Online Supplementary Table S2). Another characteristic of Plx PB is the underexpression of DNA repair genes associated to the S-phase of cell cycle, such as RAD51, FEN1 and TOP2A (Online Supplementary Table S2), suggesting that Plx PB is enriched in HSCs, characterized by a slow division rate since quiescent. By using this gene list (PROGENITORS_PROGRAM), gene set enrichment analysis (GSEA) revealed that BM samples were enriched in markers of proliferation (P<0.01, Online Supplementary Figure S2B).
Performing a meta-analysis on gene expression data from human purified hematopoietic subpopulations, we identified 174 genes upregulated in HSCs and multipotent progenitors (MPPs) (METAHSC_SIGNATURE) (Online Supplementary Figure S2). GSEA analysis revealed that Plx PB samples were enriched in METAHSC_SIGNATURE (P<0.01, Figure 1D), with a gene expression profile positively correlated with primitive gene sets (Figure 1E).
We selected genes (Online Supplementary Table S3) for transcription factors playing a key role in HSC regulation (HSC expression program):13 MECOM, MEIS1, HOXB3, ZKSCAN1 and HLF.14 These genes were found significantly upregulated in Plx PB compared to BM and Plx BM cells (Figure 1F). This program is already active in cells primed by plerixafor (Plx BM), indicating an early change in subpopulation composition (Figure 1F). This finding might be due to a direct transcriptional activation by the drug on resident cells, or to the contribution of circulating plerixafor CD34+ cells in the BM harvest, although these hypotheses are difficult to test.
In order to proceed to comparative studies including other clinically relevant HSC sources, available mostly from healthy donors (HD), we performed immunophenotype and gene expression profile analysis of BM-derived CD34+ cells from thalassemic patients (THAL) and HD. No differences were observed in LT-HSC frequencies (Online Supplementary Figure S4) and in gene expression profile (Online Supplementary Table S4), thus excluding a disease-related contribution to specific properties, at least in the CD34+ bulk population. These results were confirmed by analysis on pediatric samples (manuscript in preparation).
Extending immunophenotype analysis to other stem cells sources (Online Supplementary Figure S5A), a higher percentage of LT-HSCs was observed in Plx PB cells compared to G-CSF-mobilized cells (G-CSF PB), which are indeed preferentially enriched in MPPs and INT-HSCs (Figure 2A,B). Interestingly, the combined use of G-CSF and plerixafor (G+Plx PB) mobilizes a LT-HSCs population similar to that found in G-CSF PB, but with a lower frequency of INT-HSCs and MPPs (Figure 2A,B).
Figure 2.
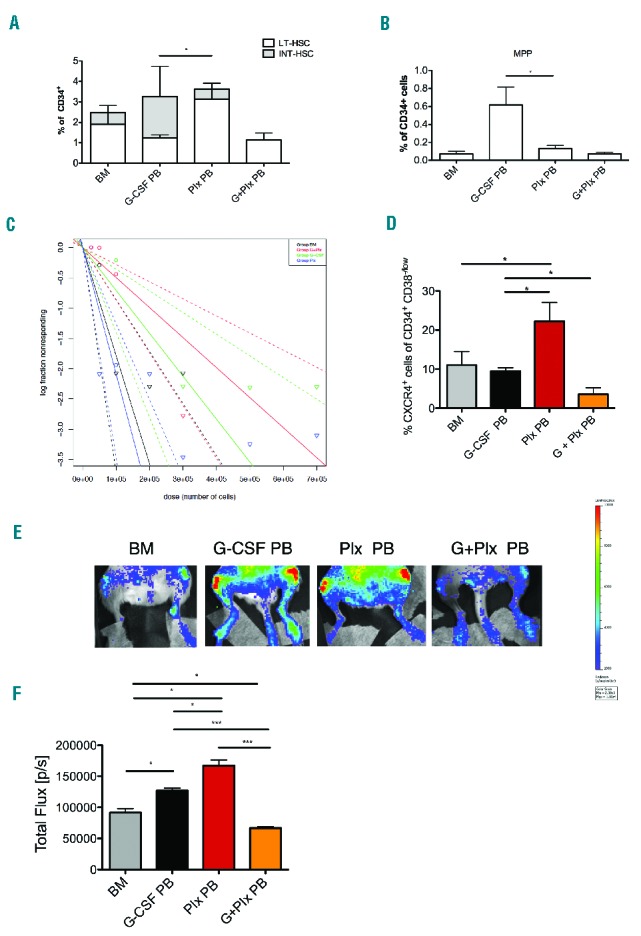
Hierarchy of HSC sources. A. Analysis of the frequencies of primitive hematopoietic stem cells LT-HSC, INT-HSC and MPP (B) in BM (n=9), G-CSF (n=3), Plx (n=3) and G+Plx (n=2) CD34+ cells. Data are represented as mean±SEM. C. Stem cell frequency: a single-hit Poisson model was used to calculate the SRC frequency at limiting dilution for cells derived from Plx PB, G-CSF PB, G+Plx PB and from BM. Mice (n=123) were considered engrafted if the frequency of human cells was ≥1%CD45+. ELDA software was used to calculate the SRC frequency. The calculated SRC frequency is 1/47875 in plerixafor-mobilized CD34+ cells, 1 SRC in 141203 G-CSF-mobilized CD34+ cells and 1 SRC in 1/201803 G+Plx PB. The frequency in BM is 1 SRC in 56020 CD34+ cells. D. Percentage of CXCR4+ in CD34+ CD38−/low was analyzed in all samples. It was statistically significantly higher in plerixafor-mobilized cells compared to all samples (*P<0.05). Data are represented as mean±SEM. E. Sorted CD34+ CD38−/low cells were transduced with a LVPGK/Luc and transplanted in NSG mice. Four different cohorts of mice (n=3/each group) were generated by transplantation of BM (CD34+ cells from a pool of 5 donors), G-CSF PB (pool of 3 donors), Plx PB (pool of 3 donors) and G+Plx PB (pool of 2 donors). 24hrs after injection into the mouse circulation, a BLI signal emanating from the area corresponding to the femurs was detected. F. Histogram represents total Flux [p/s] emanating from the area corresponding to both femurs. Mice that received the CD34+CD38−/low cells isolated from Plx PB exhibited higher signals than those that received the CD34+CD38−/low cells isolated from other sources. (***P<0.001; *P<0.05). Data are represented as mean±SEM. BM: bone marrow; PB: peripheral blood; HSC: hematopoietic stem cell; LT-HSC; long-term HSC; INT-HSC: intermediate HSC; MPP: multipotent progenitor.
Since SDF1-CXCR4 signaling is essential to maintain the HSC pool, and plerixafor is able to interfere with this axis, we reasoned that the cells might be enriched with functionally primitive HSCs. Therefore, we performed a limiting dilution assay in NOD/ShiLtSz-scid/IL2Rγnull (NSG) mice to calculate the frequency of primitive severe combined immunodeficiency (SCID) repopulating cells (SRCs).
Sub-lethally irradiated mice were transplanted with escalating doses of CD34+ cells from Plx PB, G-CSF PB, G+Plx PB and BM. Limiting dilution assay showed that the SRC frequencies of Plx PB, G-CSF PB, G+Plx PB and BM were 1/47875, 1/141203, 1/201803 and 1/56020 CD34+ cells, respectively (Figure 2C, Online Supplementary Table S5) with statistically significant differences between Plx PB versus G+Plx PB and Plx PB versus G-CSF PB (Figure 2C, Online Supplementary Table S6). These data are in accordance with previous results.7,10
Since the signaling provided by the interaction of SDF1 with CXCR4 also plays an essential role in the migration of transplanted cells toward the BM niche, likewise in the xenotransplantation model,15 we evaluated the CXCR4 expression and the in vivo homing capacity of BM, G-CSF PB, Plx PB and G+Plx PB. Flow cytometry results revealed that CXCR4 expression was lower in G-CSF PB CD34+ cells (Online Supplementary Figure S5B), whereas the analysis restricted to the primitive compartment of CD34+CD38−/low cells, showed a lower percentage of CXCR4+ cells in the G+Plx PB source (Figure 2D). These data suggest that the reduced reconstitution activity of both G-CSF- and G+Plx-mobilized cells observed in SRCs assay could be the result of a lower frequency of CXCR4+ primitive cells.
To address this issue, CD34+CD38−/low cells from CD34+ samples were examined for their in vivo homing potential by non-invasive bioluminescent imaging (BLI). After 24hrs, mice injected with Plx PB cells exhibited a higher signal than those with cells from BM, G-CSF PB and G+Plx PB (Figure 2E), despite comparable transduction efficiencies (Online Supplementary Figure S5C). The most relevant differences were between Plx PB versus G+ Plx PB (P<0.001) and Plx PB versus G-CSF PB (P<0.05) groups (Figure 2F). Thus, we could ascribe the reduced number of SRCs mobilized by G-CSF and G+Plx to a lower migrating and long-term repopulating capacity compared to plerixafor-mobilized cells.
When the transcriptional relationships among different mobilized PB CD34+ samples (Plx PB, G-CSF PB and G+Plx PB) were analyzed, we observed that METAHSC_SIGNATURE and HSC expression program are preferentially expressed in Plx PB samples compared to the others (Figure 3A,B), whereas the PROGENITOR_PROGRAM is particularly active in G+Plx PB cells (Figure 3C). The differences in gene expression profile reflect the cell composition, both in terms of primitive immature (Figure 2) and more mature progenitors (Figure 3D).
Figure 3.
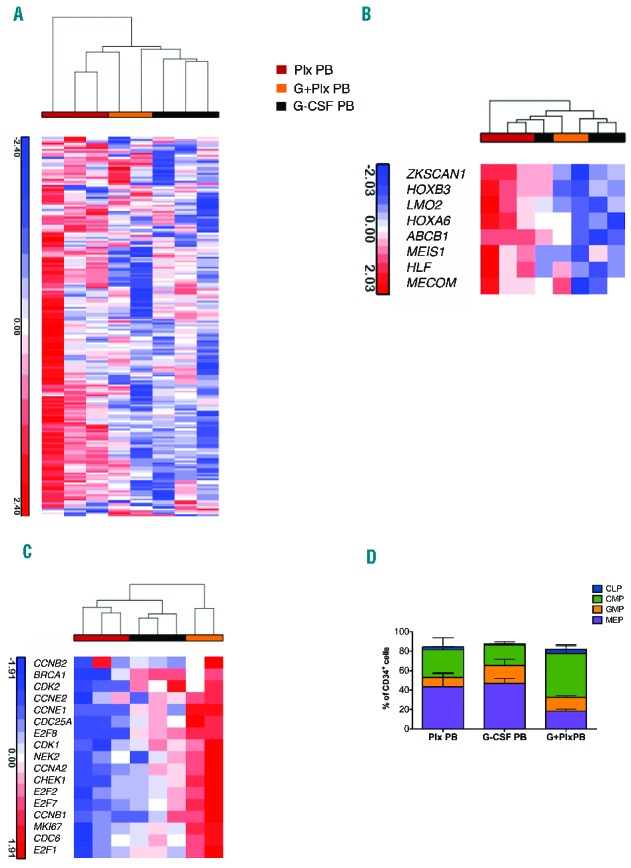
‘Stemness’ signature in mobilized CD34+ cell sources. A. Hierarchical cluster analysis to assess relative distance of the transcriptome of each stem cell source. Data from mobilized stem cell sources were compared with previously generated gene sets derived from bone marrow stem and progenitor population (Online Supplemenary Figure S3). Plx PB gene expression was positively correlated with primitive gene sets. B. Heat maps of selected genes involved in HSC regulation are more highly expressed in Plx PB than in other stem cell sources. C. Heat map of regulators of cell cycle and DNA repair, showing an enrichment in the progenitor associated program in G+Plx PB expression profile. In these images, the normalized expression levels of genes are presented according to a colored gradient from the highest (red) to lowest (blue, see colored scale). D. Immunophenotypic analysis of progenitor composition in G-CSF PB, Plx PB and G+Plx PB sources. G-CSF PB (n=2); Plx PB (n=3); G+Plx PB (n=2): CMP, common myeloid progenitor (CD34+ CD38+ CD45RA− CD135+ CD10− CD7−), GMP, granulocyte-macrophage progenitor (CD34+ CD38+ CD45RA+ CD135+ CD10− CD7−), MEP, megakaryocyte-erythrocyte progenitor (CD34+ CD38+ CD45RA− CD135− CD10− CD7−), and CLP, common lymphoid progenitor (CD34+ CD38+ CD45RA+ CD10+ CD7−). The CLP, CMP, GMP and MEP subsets are reported as percentages of CD34+ cells, according to the expression of CD34, CD38, CD45RA, CD135, CD10 and CD7 surface markers. Statistically significant differences were observed by comparing the three mobilized sources with the higher CMP and the lower MEP content in G+Plx PB samples, as compared to G-CSF PB and Plx PB, respectively (P<0.05). Data are represented as mean±SEM. PB: peripheral blood.
In summary, we show that HSCs from different sources are endowed with specific properties. Plerixafor-mobilized cells possess the highest ability to home to hematopoietic niches and engraft in immunodeficient mice. The higher content of CXCR4+ and CD49f+ cells correlates with this feature, whereas the reduced number of SRCs and homing capacity of G-CSF- and G+Plx-mobilized cells could be ascribed to lower expression of CXCR4 due to a direct effect of G-CSF.7,11 Global gene expression profiling highlights the superior in vivo reconstitution activity of plerixafor-mobilized cells. We hypothesized that the “stemness” signature of cells dislodged from their niche by plerixafor is attenuated by the combined use with G-CSF, which emphasizes the gene expression profile induced by G-CSF treatment. Since the number of analyzed samples is limited, in addition, further studies on purified subpopulations will define if the combined use of the two drugs affects the self-renewal of LT-HSCs. These findings suggest the use of more primitive HSCs when target cell numbers for transplantation is limited, or when disease related characteristics dictate caution in the choice of G-CSF as a mobilizing agent. Regarding the population mobilized by both G-CSF and plerixafor, the reduction of SRCs is counterbalanced by the superior harvest of mobilized cells, since the single addition of plerixafor synergizes with multiple doses of G-CSF to mobilize greater numbers of CD34+/kg than that obtained by a single agent.5
Among the different hypotheses on the effect of combined mobilizing agents, on the basis of our results, we hypothesized that plerixafor mobilizes cells previously partially disengaged from the BM niche and/or expanded by G-CSF. Further studies will contribute to dissecting the molecular basis underlying the direct and indirect actions of single and combined treatment on the HSC compartment in humans.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Korbling M, Freireich EJ. Twenty-five years of peripheral blood stem cell transplantation. Blood. 2011;117(24):6411–6416. [DOI] [PubMed] [Google Scholar]
- 2.Lapid K, Glait-Santar C, Gur-Cohen S, Canaani J, Kollet O, Lapidot T. Egress and mobilization of hematopoietic stem and progenitor cells: a dynamic multi-facet process. StemBook; Cambridge (MA), 2008. [PubMed] [Google Scholar]
- 3.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26(1):34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2(7):581–587. [DOI] [PubMed] [Google Scholar]
- 5.Yannaki E, Karponi G, Zervou F, et al. Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with beta-thalassemia major. Hum Gene Ther. 2013;24(10):852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. [DOI] [PubMed] [Google Scholar]
- 7.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donahue RE, Jin P, Bonifacino AC, et al. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009;114(12):2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruehauf S, Seeger T, Maier P, et al. The CXCR4 antagonist AMD3100 releases a subset of G-CSF-primed peripheral blood progenitor cells with specific gene expression characteristics. Exp Hematol. 2006;34(8):1052–1059. [DOI] [PubMed] [Google Scholar]
- 10.Hess DA, Bonde J, Craft TP, et al. Human progenitor cells rapidly mobilized by AMD3100 repopulate NOD/SCID mice with increased frequency in comparison to cells from the same donor mobilized by granulocyte colony stimulating factor. Biol Blood Marrow Transplant. 2007;13(4):398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larochelle A, Krouse A, Metzger M, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107(9):3772–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–221. [DOI] [PubMed] [Google Scholar]
- 13.Akashi K, He X, Chen J, et al. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood. 2003;101(2):383–389. [DOI] [PubMed] [Google Scholar]
- 14.Gazit R, Garrison BS, Rao TN, et al. Transcriptome analysis identifies regulators of hematopoietic stem and progenitor cells. Stem Cell Reports. 2013;1(3):266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capitano ML, Hangoc G, Cooper S, Broxmeyer HE. Mild heat treatment primes human CD34(+) cord blood cells for migration toward SDF-1alpha and enhances engraftment in an NSG mouse model. Stem Cells. 2015;33(6):1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


