Abstract
Introduction
The C677T functional variant in the methylene-tetrahydrofolate reductase (MTHFR) gene leads to reduced enzymatic activity and elevated blood levels of homocysteine. Hyperhomocysteinemia has been linked with higher rates of cardiovascular diseases, cognitive decline, and late-life depression.
Methods and Materials
Here, 3D magnetic resonance imaging data was analyzed from 738 individuals (age: 75.5 ± 6.8 years; 438 men/300 women) including 173 Alzheimer's patients, 359 subjects with mild cognitive impairment, and 206 healthy older adults, scanned as part of the Alzheimer's Disease Neuroimaging Initiative (ADNI).
Results
We found that this variant associates with localized brain atrophy, after controlling for age, sex, and dementia status, in brain regions implicated in both intellectual and emotional functioning, notably the medial orbitofrontal cortices. The medial orbitofrontal cortex is involved in the cognitive modulation of emotional processes, and localized atrophy in this region was previously linked with both cognitive impairment and depressive symptoms. Here, we report that increased plasma homocysteine mediates the association between MTHFR genotype and lower medial orbitofrontal volumes, and that these volumes mediate the association between cognitive decline and depressed mood in this elderly cohort. We additionally show that vitamin B12 deficiency interacts with the C677T variant in the etiology of hyperhomocysteinemia.
Conclusion
This study sheds light on important relationships between vascular risk factors, age-related cognitive decline, and late-life depression, and represents a significant advance in our understanding of clinically relevant associations relating to MTHFR genotype.
Keywords: MTHFR, homocysteine, brain atrophy, age-related cognitive decline, late-life depression, MRI
INTRODUCTION
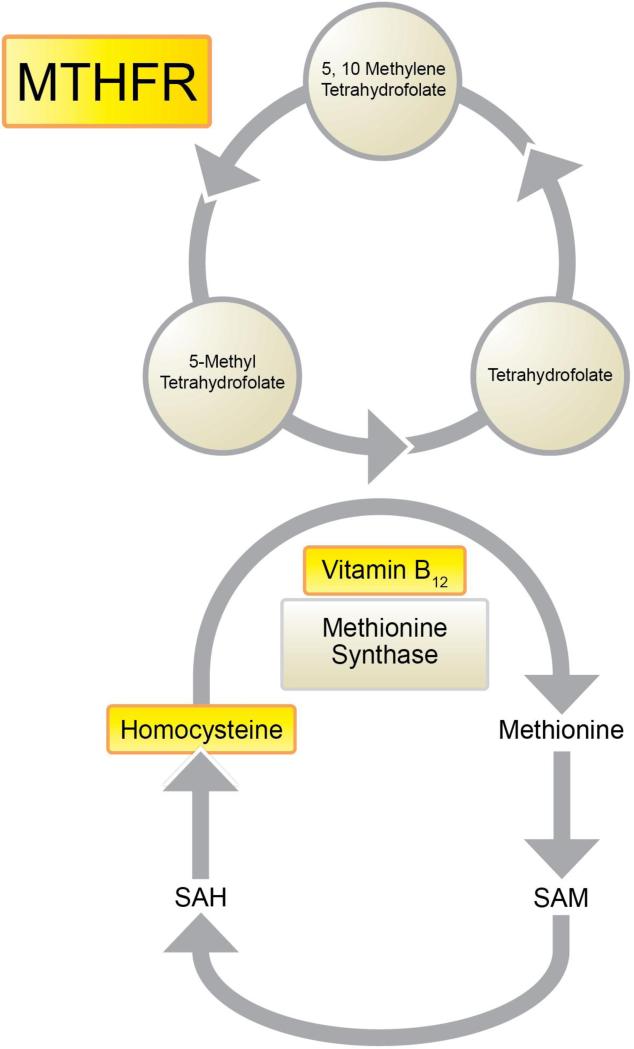
Hyperhomocysteinemia, a metabolic anomaly involving elevated levels of the amino acid homocysteine in the blood, is associated with higher rates of numerous age-related disorders, such as cardiovascular diseases (CVDs) (1, 2) including vascular dementia (3, 4); cognitive decline (5-9); and depressed mood (10-12). Elevated plasma homocysteine levels may stem from the use of certain therapeutic drugs, elevated alcohol ingestion, intestinal malabsorption, or from impaired metabolism due to genetic alterations in metabolic enzymes, including methyltetrahydrofolate reductase (MTHFR), most commonly when combined with insufficient dietary intake of B vitamins. Notably, homocysteine is recycled to methionine using vitamin B12 as a cofactor, and MTHFR – the rate-limiting enzyme in the methyl cycle – catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a cosubstrate for homocysteine remethylation by methionine synthase. These relationships are illustrated in Figure 1.
Figure 1.
Simplified illustration of the one-carbon cycle
We recently reported that older adults with higher homocysteine levels had more pronounced regional brain atrophy (13) and thinner cortical gray matter (14) on MRI. We also found that the C677T variant in MTHFR was associated with smaller regional brain volumes in two independent elderly cohorts with mild cognitive impairment (MCI) (15). Increased susceptibility for CVDs (16), which are strongly linked to both cognitive decline and depressive symptoms in old age (17), are also associated with the C677T variant.
Here, we first determined if our previously reported associations between the T “risk” allele and more pronounced brain atrophy extended beyond individuals with MCI to both dementia patients and healthy older adults. We further sought to model some of the mechanisms underlying relationships between brain integrity, clinical outcomes, and the genetic and environmental modulators of homocysteine metabolism. To this end, we first examined whether the effects of this MTHFR polymorphism on medial orbitofrontal volumes were mediated by its impact on plasma homocysteine. We also determined whether vitamin B12 deficiency influenced the strength of the relationship between this variant and homocysteine levels. We then found that lower cognitive performance and reduced medial orbitofrontal volumes were significant predictors of depressed mood and tested the hypothesis that compromised integrity in this brain region involved in the cognitive control of emotion may partially explain the relationship between cognitive impairment and depressive symptoms.
METHODS AND MATERIALS
Subjects
We analyzed a large sample of elderly individuals from the Alzheimer's Disease Neuroimaging Initiative (ADNI). The study was conducted according to the Good Clinical Practice guidelines, the Declaration of Helsinki, and U.S. 21 CFR Part 50 (Protection of Human Subjects), and Part 56 (Institutional Review Boards). Written informed consent was obtained from all participants before protocol-specific procedures were performed. All ADNI data are publicly available (at http://adni.loni.usc.edu). To avoid the known effects of population stratification on genetic analysis (18), we only included non-Hispanic Caucasian subjects identified by self-report and confirmed by multi-dimensional scaling (MDS) analysis (19). The ADNI cohort included multiple diagnostic groups: patients with Alzheimer's disease (“AD”), subjects with MCI, and healthy elderly (cognitively normal, “CON”) participants. All subjects were administered the Mini Mental Status Examination (MMSE (20)) and the 15-item version of the Geriatric Depression Scale (GDS-15 (21)). Our final analysis comprised 738 individuals (average age ± s.d. = 75.53 ± 6.78 years; 438 men/300 women) including 173 AD, 359 MCI, and 206 healthy older adults. All participants received pre-mortem clinical diagnoses, as described in detail in ADNI's General Procedures Manual: http://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf). Table 1 illustrates demographic, cognitive, and mood data for all participants, stratified by genotype and sub-stratified by diagnostic groups.
Table 1.
Demographic data by diagnostic and C677T genotype groups (N=738)
| CC | CT | TT | Total | Group Comparisons | ||
|---|---|---|---|---|---|---|
| CON | N | 84 (37 F) | 96 (45 F) | 26 (12 F) | 206 (94 F) | p = 0.929 |
| Age | 75.95 (± 4.75) [76] | 75.98 (± 5.00) [75] | 77.42 (± 5.59) [77] | 76.15 (± 4.98) [76] | p = 0.380 | |
| MMSE | 29.25 (± 0.94) [30] | 29.08 (± 0.99) [29] | 29.08 (± 0.85) [29] | 29.15 (± 0.95) [29] | p = 0.464 | |
| GDS-15 | 1.06 (± 1.25) [1] | 0.68 (± 1.14) [0] | 0.92 (± 0.89) [1] | 0.86 (± 1.17) [0] | p = 0.089 | |
| MCI | N | 149 (57 F) | 157 (54 F) | 53 (17 F) | 359 (128 F) | p = 0.656 |
| Age | 75.27 (± 6.88) [76] | 75.02 (± 7.50) [76] | 75.15 (± 7.45) [74] | 75.14 (± 7.22) [76] | p = 0.957 | |
| MMSE | 26.96 (± 1.74) [27] | 27.06 (± 1.83) [27] | 27.55 (± 1.75) [28] | 27.09 (± 1.78) [27] | p = 0.115 | |
| GDS-15 | 1.58 (± 1.29) [1] | 1.59 (± 1.42) [1] | 1.28 (± 1.28) [1] | 1.54 (± 1.35) [1] | p = 0.330 | |
| AD | N | 81 (40 F) | 67 (25 F) | 25 (13 F) | 173 (78 F) | p = 0.256 |
| Age | 75.04 (± 7.69) [76] | 75.91 (± 7.30) [77] | 76.48 (± 8.34) [77] | 75.58 (± 7.61) [77] | p = 0.644 | |
| MMSE | 23.33 (± 1.88) [23] | 23.45 (± 2.22) [24] | 23.24 (± 1.81) [23] | 23.36 (± 2.00) [23] | p = 0.892 | |
| GDS-15 | 1.64 (± 1.42) [1] | 1.58 (± 1.35) [1] | 1.84 (± 1.55) [1] | 1.65 (± 1.40) [1] | p = 0.737 | |
| Total | N | 314 (134 F) | 320 (124 F) | 104 (42 F) | 738 (300 F) | p = 0.602 |
| Age | 75.39 (± 6.61) [76] | 75.50 (± 6.80) [76] | 76.04 (± 7.26) [77] | 75.53 (± 6.78) [76] | p = 0.697 | |
| MMSE | 26.64 (± 2.70) [27] | 26.91 (± 2.63) [27] | 26.89 (± 2.67) [28] | 26.79 (± 2.66) [27] | p = 0.399 | |
| GDS-15 | 1.50 (± 1.33) [1] | 1.31 (± 1.39) [1] | 1.33 (± 1.30) [1] | 1.38 (± 1.35) [1] | p = 0.382 |
N indicates sample sizes, followed by the number of women in parentheses. Average age, MMSE, and GDS-15 scores are followed by their standard deviations in parentheses. Median values are indicated in brackets. p-values for Pearson chi-squared tests (for sex) or one-way ANOVAs (for age, MMSE, and GDS-15 scores) are reported in the last column. CON=healthy controls, MCI=mild cognitive impairment, AD=Alzheimer's disease
Genotyping and Allele Frequency
The ADNI sample was genotyped using the Illumina 610-Quad BeadChip (San Diego, CA, USA). The only polymorphism examined in this study was the C677T functional variant in the methylene-tetrahydrofolate reductase (MTHFR) gene, at the rs1801133 locus. Allele frequency was computed from genotype frequency. The distributions of allele frequencies by diagnostic groups were evaluated by χ2 tests with a 0.05 significance level, using 3 × 2 and 2 × 2 contingency tables in SPSS 23.0.
Neuroimaging
Whole-Brain Analyses: Tensor Based Morphometry (TBM)
The C677T polymorphism was analyzed for association with regional brain volumes in ADNI participants, as detailed below. Subjects were scanned with a standardized MRI protocol developed for this cohort (22, 23). Briefly, high-resolution structural brain MRI scans were acquired at 58 sites across North America, using 1.5 Tesla MRI scanners. A sagittal 3D MP-RAGE sequence was used, optimized for consistency across sites (23) (TR/TE = 2400/1000 ms; flip angle = 8°; FOV = 24 cm; final reconstructed voxel resolution = 0.9375 × 0.9375 × 1.2 mm3). Image corrections were applied using a processing pipeline at the Mayo Clinic, consisting of: 1) a procedure termed GradWarp to correct geometric distortion due to gradient non-linearity (24), 2) a “B1-correction”, to adjust for image intensity inhomogeneity due to B1 non-uniformity using calibration scans (23), 3) “N3” bias field correction, for reducing residual intensity inhomogeneity (25), and 4) geometrical scaling, according to a phantom scan acquired for each subject (23), to adjust for scanner- and session-specific calibration errors. To adjust for global differences in brain positioning and scale, all subjects’ scans were linearly registered to the stereotaxic space defined by the International Consortium for Brain Mapping (ICBM-53) (26), using a 9-parameter (9P) transformation (three translations, three rotations, three scales) (27). We used standard trilinear interpolation and resampled the resulting aligned scans to have 1mm isotropic voxels.
We then created a minimal deformation target (MDT), which serves as an unbiased average template image for automated image registration, and to reduce statistical bias. The MDT was created from the MRI scans of 40 randomly selected healthy elderly subjects, as detailed elsewhere (28, 29). To quantify 3D patterns of volumetric tissue variations, all individual T1-weighted images were non-linearly aligned to the template with an inverse-consistent 3D elastic warping technique using a mutual information cost function (30). Consequently, for each subject, a separate Jacobian matrix field was derived from the gradients of the deformation field that aligned that individual brain to the MDT template. The determinant of the local Jacobian matrix was derived from the forward deformation field to characterize local volume differences. Color-coded Jacobian determinants were used to illustrate regions of volume expansion, i.e. those with det J(r) > 1, or contraction, i.e., det J(r) < 1 (31-34) relative to the group template. As all images were registered to the same study-specific template, these Jacobian maps shared common anatomical coordinates, defined by the normal template. Individual Jacobian maps were retained for further statistical analyses.
To model effects of the C677T functional variant in MTHFR on local brain volumes, we used univariate linear regression to associate the number of minor T alleles (0,1, or 2) with the Jacobian values (describing the amount of brain tissue deficit or excess relative to the standard template) at each voxel in the brain, after controlling for age, sex, and diagnosis. To minimize Type I errors, we used a searchlight method for false discovery rate (FDR) correction (35), which controls the false discovery rate in all reported statistical maps. We implemented this method to correct the maps of statistical associations between the image phenotype (morphometry) and genotype at the rs1801133 locus (number of T alleles). All maps shown were thresholded at the appropriate corrected p-value, after performing searchlight FDR (q=0.05), to show only regions of significance that passed the multiple comparisons correction.
Region of Interest (ROI) Analyses: Medial Orbitofrontal Volumes
Five MRI core analysis laboratories have provided feature extraction and numeric summaries from the high quality ADNI MRI data, which are publicly available in the ADNI data archive (http://adni.loni.usc.edu). One of these core analysis laboratories, the Center for Imaging of Neurodegenerative Diseases at UCSF (co-investigator: Norbert Schuff), has provided volumetric segmentation using the FreeSurfer image analyses suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Version 4.3 is used for ADNI's cross-sectional data. The input for ADNI FreeSurfer is a T1 weighted image in NiFTI format, which has been preprocessed (gradient warping, scaling, B1 correction, and N3 inhomogeneity correction) by the Mayo Clinic preprocessing stream as describe above.
Briefly, Freesurfer processing includes motion correction, removal of non-brain tissue using a hybrid watershed/surface deformation procedure (36), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter (37, 38), intensity normalization (25), tessellation of the gray matter/white matter boundary, automated topology correction (39, 40), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (41-43), followed by rigorous QC procedures that allow for the exclusion of failed segmentations, due to poor image quality, registration issues, or processing errors. We downloaded the numeric summaries for “medial orbital frontal” volumes, and retained the data pertaining to subjects with frontal segmentations that satisfied all QC requirements. We obtained the volumes of the medial orbitofrontal cortices (in mm3) for 640 of our participants.
Blood-based markers
In the ADNI public database, plasma levels of homocysteine were available for 732 of our participants, 634 of whom also had usable medial orbitofrontal volumes. The database also contained plasma vitamin B12 concentrations for 680 of our subjects (including 675 with available homocysteine levels and 587 with both homocysteine and medial orbitofrontal volume data). Homocysteine and vitamin B12 levels (in pg/mL) were extracted from blood samples collected via standard venipuncture protocols. Vitamin B12 deficiency was defined as plasma levels < 250 pg/mL.
Statistical analyses
To ensure consistency, we used an additive model of minor T allele effects – the number of T allele carried by each participant was coded as 0, 1, or 2 – in all analyses aimed at testing association between the C677T variant and another variable. However, since medial orbitofrontal volumes seemed to be affected in a recessive manner, we additionally ran every regression model using a recessive model of minor allele effects (i.e., comparing C allele carriers with T homozygotes). These analyses produced similar results, which are presented in the Supplemental Information section (Tables S2-S4).
We used general linear models to examine the predictors of medial orbitofrontal volumes, plasma homocysteine concentrations, and GDS-15 scores. Shapiro-Wilk tests showed that medial orbitofrontal volumes were normally distributed (p=0.601), but homocysteine levels (p<0.001) and GDS-15 scores (p<0.001) were not. We therefore used standardized scores in all regression models including plasma homocysteine or mood scores as the dependent variable. Age and sex were included as covariates in all analyses. We also controlled for diagnosis, except when MMSE was used as a factor in the model, since MMSE scores are one of the major diagnostic criteria for dementia (as described in ADNI's General Procedures Manual: http://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf). These statistical analyses were conducted in SPSS 23.0.
Simple mediation analyses were conducted using Andrew Hayes's PROCESS Procedure (v2.15) for SPSS (http://www.processmacro.org/download.html). We obtained path coefficients (a, b, c, and c’) representing the linear regression coefficients for each path in the mediation model. We standardized all variables to facilitate the interpretation of path coefficients, now bounded by −1 and 1 across all measures. The a-path represents the association between the predictor and mediator variables. The b-path denotes the relationship between the mediator and outcome variables, while also controlling for the predictor variable. The c’-path (also called “direct effect”) and the c-path (also known as “total effect”) represent the associations between the predictor and outcome variables including and excluding the mediator variable, respectively. If the difference between c and c’ is statistically significant, then there is a significant mediation effect. It was previously shown that a*b = c-c’; therefore, we tested the significance of a*b (also known as “indirect effect”) using bootstrapped confidence intervals (44). Bootstrapping creates thousands of simulated datasets using resampling with replacement and runs the analysis once in each simulated sample (45). 95% of the generated statistics fall between two values, and if that confidence interval (CI) for a*b does not include 0, then a significant (p<0.05) mediation has occurred. Percent mediation [PM] is a measure of effect size interpreted as the percent of the total effect (c) accounted for by the indirect effect (a*b); that is, PM = (a*b)/c (44).
RESULTS
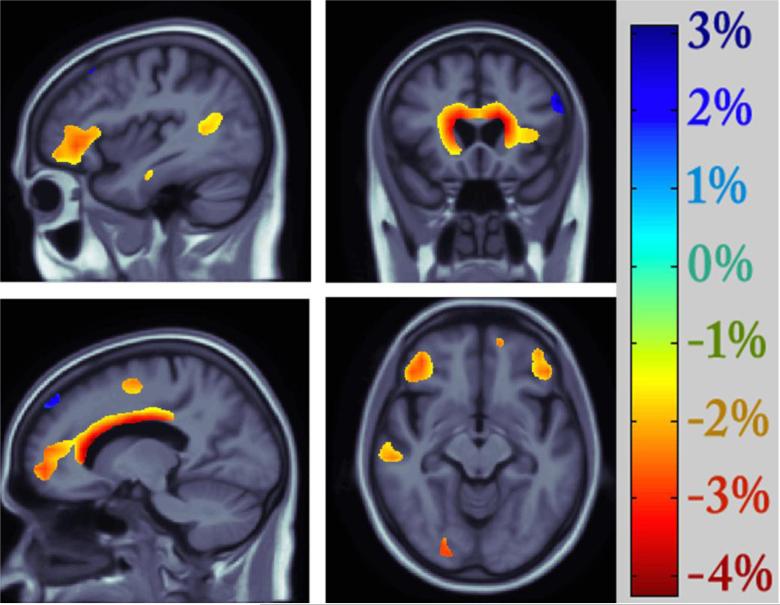
Consistent with the prevailing view that the MTHFR C677T polymorphism may not be a risk factor for Alzheimer's disease (AD), but appears to be associated with various types of age-related disorders (46), the distributions of genotype (p=0.592) and allele frequency (p=0.667) for rs1801133 did not significantly differ across the 3 diagnostic groups in the ADNI cohort (Table 2). Nonetheless, this functional variant in MTHFR predicted differences in regional brain volumes in our large elderly sample, after controlling for age, sex, and diagnosis. As depicted in Figure 2, smaller volumes in the frontal (including the bilateral cingulate gyri, middle frontal gyri, as well as lateral and medial orbitofrontal cortices), parietal (notably the inferior parietal lobule), and temporal lobes (especially the superior temporal gyrus), as well as in the thalamus, were statistically related to carrying the minor T allele at the rs1801133 locus. Strong genotype group differences were also observed in periventricular regions. Each copy of the “risk” allele was associated with a 2-4 % reduction in local brain volumes(Figure 2).
Table 2.
Genotype and allele frequency by diagnostic groups (N=738)
| CON | MCI | AD | Pearson Chi-Square Test | ||
|---|---|---|---|---|---|
| Total | N=738 | 206 | 359 | 173 | |
| Genotype Frequency | CC | 84 (41%) | 149 (41%) | 81 (47%) | |
| CT | 96 (46%) | 157 (44%) | 67 (39%) | p = 0.592 | |
| TT | 26 (13%) | 53 (15%) | 25 (14%) | ||
| Allele Frequency | C | 264 (64%) | 455 (63%) | 229 (66%) | |
| T | 148 (36%) | 263 (37%) | 117 (34%) | p = 0.667 |
CON=healthy controls, MCI=mild cognitive impairment, AD=Alzheimer's disease
Figure 2. Effects of the C677T variant on regional brain volumes (N=738).
Negative beta values (warm colors) show regions where each risk T allele was associated with a 2-4 % volume deficit, as shown on the color bar. Tests for associations are adjusted for age, sex, and diagnosis; maps are corrected for multiple comparisons with the searchlight false discovery rate method at q=0.05. Images are in radiological convention (left side of the brain shown on the right).
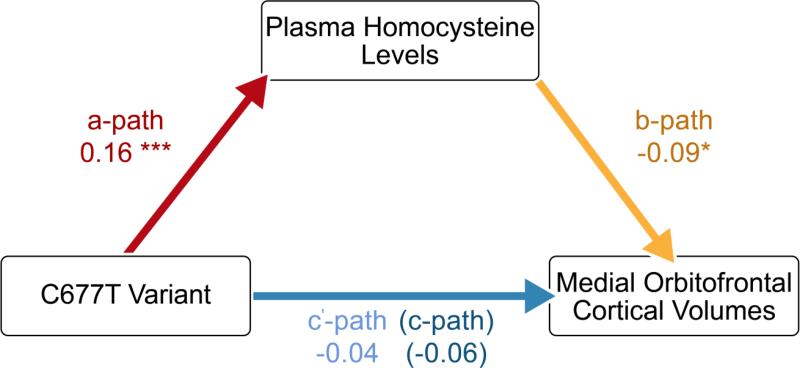
Among the regions showing a significant association with MTHFR genotype in our whole-brain analyses, the medial orbitofrontal cortex was particularly noteworthy because of its involvement, not just in mood states and intellectual functioning, but specifically in the cognitive modulation of emotional processes. This brain area was therefore selected as the region of interest in our investigations of the relationships between brain integrity, clinical outcomes, and homocysteine metabolism. Our ROI analyses, which used Freesurfer volumes in a subset of the same elderly sample (Table S1), confirmed that the C677T variant was associated with reduced medial orbitofrontal volumes (p=0.033, F-ratio=3.428) after controlling for age, sex, and diagnosis (Table S2). As expected, the C677T variant was also associated with significant elevations in plasma homocysteine levels (p<0.001, F-ratio=10.375) after controlling for age, sex, and dementia status (Table S2). Mediation analyses further revealed a significant indirect effect of the number of T alleles on medial orbitofrontal volumes through plasma homocysteine levels, a*b=−0.015, CI [−0.039, −0.003]. The mediator accounted for about one quarter of the total effect, PM=0.253 (Figure 3).
Figure 3. Mediation of the association between genotype and medial orbitofrontal volumes by plasma homocysteine levels (N=634).
Path coefficients for simple mediation analysis using the PROCESS Procedure for SPSS. The a-path represents the association between genotype and plasma homocysteine levels. The b-path denotes the relationship between plasma homocysteine levels and medial orbitofrontal volumes, while also controlling for genotype. The c’-path and the c-path represent the associations between genotype and medial orbitofrontal volumes with and without plasma homocysteine levels included as a mediator, respectively. *p<0.05, **p<0.01, ***p<0.001.
We then tested the hypothesis that the relationship between genotype and plasma homocysteine concentrations may differ between individuals with inadequate circulatory levels of vitamin B12 (i.e., who are deficient in a major cofactor used by methionine synthase to re-methylate homocysteine into methionine) and non-deficient subjects, by introducing vitamin B12 deficiency status as a covariate in addition to age, sex, and diagnosis in the regression models. The C677T variant showed even stronger associations with elevations in plasma homocysteine levels (p<0.001, F-ratio=12.143), vitamin B12 deficiency was an independent predictor of homocysteine concentrations (p=0.021, F-ratio=5.348), and the genotype by deficiency status interaction term was significant (p=0.011, F-ratio=4.529), supporting our hypothesis (Table S3). Within-group analyses confirmed that, in vitamin B12 deficient individuals, carrying the allele conferring reduced enzymatic activity was more strongly associated with increased plasma homocysteine concentrations (p=0.008, F-ratio=5.122) than in non-deficient subjects (p=0.055, F-ratio=2.911), after controlling for age, sex, and dementia status (Table S3).
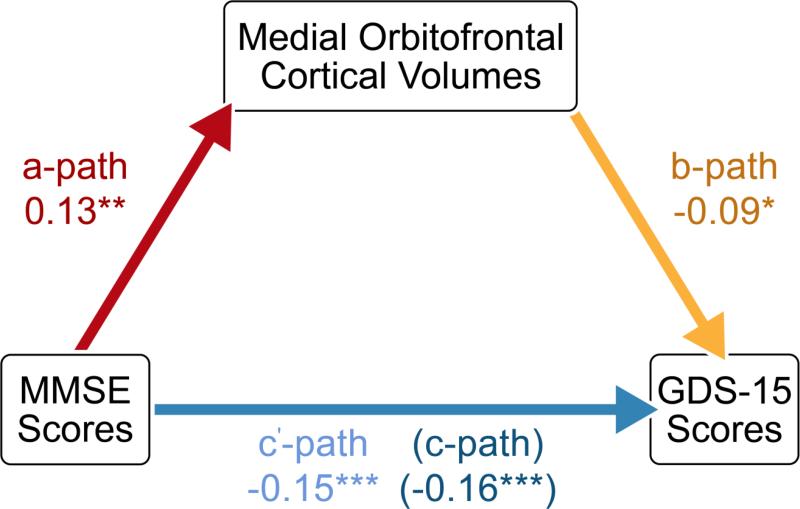
We subsequently examined predictors of GDS-15 scores, across the whole sample. We found no significant main effects of the C677T variant (p=0.256, F-ratio=1.368), homocysteine levels (p=0.823, F-ratio=0.050), or vitamin B12 deficiency status (p=0.961, F-ratio=0.002) on mood scores. However, cognitive decline, assessed with the MMSE (p<0.001, F-ratio=12.808), and medial orbitofrontal atrophy (p=0.005, F-ratio=7.840) were associated with greater depressive symptoms (i.e., higher GDS-15 scores), after controlling for age and sex (Table S4). Further analyses revealed a significant mediation of MMSE-GDS associations by medial orbitofrontal volumes, a*b=−0.012, CI [−0.027, −0.003]. The mediator accounted for about 8% of the total effect, PM=0.075 (Figure 4). These results are summarized in Figure S1.
Figure 4. Mediation of the association between MMSE performance and GDS-15 scores by medial orbitofrontal volumes (N=640).
Path coefficients for simple mediation analysis using the PROCESS Procedure for SPSS. The a-path represents the association between MMSE scores and medial orbitofrontal volumes. The b-path denotes the relationship between medial orbitofrontal volumes and GDS scores, while also controlling for MMSE performance. The c’-path and the c-path represent the associations between MMSE and GDS scores with and without medial orbital cortical volumes included as a mediator, respectively. *p<0.05, **p<0.01, ***p<0.001.
DISCUSSION
The C677T functional variant in MTHFR is a risk factor for hyperhomocysteinemia, and has been associated with higher rates of various age-related disorders (46). This study expands on our earlier report of a link between the C677T variant and regional brain atrophy in two independent elderly cohorts with mild cognitive impairment (15), by providing evidence for these associations across the spectrum of normal cognitive aging, MCI, and AD. For the first time in this report, we address the mechanisms through which genetic variation in MTHFR alters brain integrity, and affects mood-cognition associations in the elderly. These novel findings bring together prior work reporting that both the C677T variant (15) and plasma homocysteine levels (13) are significant predictors of reduced regional brain volumes in older adults. These results also suggest the importance of adequate vitamin B12 intake, especially in carriers of the thermolabile variant, consistent with a prior report highlighting the significance of this vitamin in relation to homocysteine-induced regional brain atrophy (47).
Whole-brain TBM analyses revealed associations between the C677T variant and reduced volumes in several brain regions involved in intellectual functioning (e.g., the middle frontal gyrus and inferior parietal lobule), and in the regulation of emotional and cognitive aspects of goal-directed behavior (e.g., the superior temporal gyrus, cingulate gyrus, and orbitofrontal cortex). In particular, the medial orbitofrontal cortex is a functionally complex structure with extensive projections to and from primary sensory cortices, different prefrontal regions and other association areas, limbic structures, and the medial temporal lobe. It is implicated in high-level aspects of cognition and mediates important aspects of emotional behavior. Notably, converging evidence from multiple lines of study suggests its involvement in the cognitive modulation of the affective and reward value of stimuli and emotion-related states (48).
Medial orbitofrontal atrophy occurs early in the course of dementia (49, 50) and structural abnormalities in this region have been associated with depressive symptoms in both middle-aged (51) and geriatric subjects (52-54). In fact, the largest ever and most recent worldwide meta-analysis of cortical thickness reductions in depressed patients relative to controls reported the largest effect sizes in medial orbitofrontal cortices (55). Cognitive decline in the elderly is frequently accompanied by depressed mood (56, 57), and neurodegeneration appears to play an important role in the pathogenesis of depression associated with cognitive complaints (58). Here, we examined predictors of mood scores and found that the only two variables significantly associated with depressive symptoms were medial orbitofrontal atrophy and cognitive impairment. We also uncovered a significant mediation of these mood-cognition associations by medial orbitofrontal volumes, thereby integrating findings from multiple prior studies.
Our TBM analyses also showed significant associations between the C677T variant and reduced volumes in periventricular regions. A decrease in the size of periventricular structures allows the ventricles to expand; thus, this finding appears consistent with prior reports of an association between plasma homocysteine and ventricular enlargement in older adults (59, 60). However, this result should be interpreted with caution. Movement artifacts are sometimes more evident at tissue interfaces where changes in signal intensity are most pronounced – such as along the brain-CSF border – and head motion during MRI acquisition can also tend to reduce gray matter volume and thickness estimates (61). Moreover, participant motion is increased in elderly and clinical populations (62) and most image processing methods combined with the exclusion of low quality scans based on visual inspection are not always sufficient to fully account for motion as a confounding variable (61). Therefore, despite the rigorous motion correction and QC procedures implemented in this study, we cannot exclude the possibility that the periventricular volume differences we observed may be due in part to greater head motion.
Carriers of the T allele have higher plasma homocysteine concentrations, as evidenced by multiple genome-wide association studies (63-67). Homocysteine is prothrombotic and proatherogenic, resulting in damage to vessel walls. It is also toxic to neurons via multiple mechanisms, including inflammation and pro-oxidation, direct DNA damage, and glutamate excitotoxicity (68). Elevated homocysteine levels have been associated with brain atrophy in the elderly, which may be due to the cerebrovascular as well as the direct neurotoxic effects of this amino acid. Notably, we showed that higher plasma homocysteine levels predicted regional brain volume deficits in older adults (13), and the present study suggests that our previously reported associations between the C677T variant and more pronounced brain atrophy in MCI (15) also apply to dementia patients and healthy older adults. Here, we additionally show that the effect of this polymorphism on medial orbitofrontal volumes is mediated by increased plasma homocysteine levels, which further elucidates a possible causal pathway between MTHFR genotype and brain tissue loss in the elderly.
Our results also suggest that vitamin B12 deficiency interacts with the C677T variant in the etiology of hyperhomocysteinemia and associated disorders. While it may be possible to offset the pathogenic effects of certain variants via dietary supplementation, our findings imply that the same interventions may be ineffective in individuals who do not carry these variants. This provides a potential explanation for the discrepancies reported in studies evaluating the efficacy of supplementation with B vitamins (69). The metabolism of homocysteine is complex; thus, stratifying participants by genetic and physiological risk factors for hyperhomocysteinemia – or a combined risk index based on genetic, imaging, and peripheral blood markers – may allow for more sensitive and focused future clinical trials of degenerative brain diseases and age-related disorders. Furthermore, as high intake of B vitamins can have detrimental effects in some individuals (70), this report also highlights the importance of personalized medicine in determining appropriate levels of B vitamins intake, and underscores the need for novel approaches to reducing plasma homocysteine concentrations (e.g., by enhancing the conversion of this amino acid to cysteine in the liver or by supporting its urinary excretion (71)).
Since the Alzheimer's Disease Neuroimaging Initiative was established in 2004, over 500 studies of the ADNI dataset have been published, and have resulted in numerous major accomplishments (72). Notably, following the identification of novel genetic risk factors for age-related disorders, many studies have focused on associations between these risk variants and brain measures. These include our prior reports of the effects of Alzheimer's risk variants in APOE and CLU on ventricular expansion rate (73) and obesity-related polymorphism in FTO on regional brain atrophy (74). An important insight from this line of study was the understanding that genetic risk factors affect the trajectory of brain aging even in cognitively normal individuals. Another area of focus in research using ADNI data has been the elucidation of relationships between blood metabolites and various imaging, genetic, and clinical correlates. Examples include our prior studies of associations between plasma leptin and brain volumes (75), and between serum cholesterol, a cholesterol-related gene, and white matter microstructure (76). This line of study has led to a better understanding of the link between different biomarkers associated with aging and neurodegenerative disorders.
The present report goes one step further and proposes a mechanistic model of the relationships among three clusters of information - homocysteine metabolism (MTHFR genotype, vitamin B12 deficiency, and plasma homocysteine concentrations), regional brain volumes, and clinical symptoms (MMSE and GDS-15 scores). We found that the association between the C677T variant and reduced volumes of medial orbitofrontal cortices was mediated by increased plasma homocysteine levels and that the link between cognitive impairment and depressive symptoms was mediated by decreased medial orbitofrontal volumes, suggesting that this functional variant may affect the relationship between cognitive decline and depressed mood in older adults, perhaps through its effect on regional atrophy in brain regions involved in the cognitive modulation of emotional processes.
Future studies will be needed to provide a validation of these models in different elderly cohorts. It will also be important for future investigations to address how this genetic variant interacts with other polymorphisms (especially variants that also confer a predisposition to hyperhomocysteinemia, such as those in the ZNF366 and PTPRD genes (64)) and other environmental factors (including vitamin deficiencies as well as alcohol consumption and therapeutic drug use) to affect the trajectory of brain aging, and, indirectly intellectual and emotional functioning in older adults. Nonetheless, by modeling some of the mechanisms through which the C677T variant affects regional brain volumes, and how these changes relate to cognitive impairment and depressive symptoms across the spectrum of healthy aging, MCI, and AD; this study represents an important advance in our understanding of clinically relevant associations relating to this widely studied polymorphism in MTHFR.
Supplementary Material
ACKNOWLEDGEMENTS
F.F.R. was supported, in part, by a Turken Research Award from the Sam and Ida Turken Charitable Foundation, and by a training grant from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (T32NS048004). This work was additionally supported by National Institute of Health grants (R01 MH097268, R01 AG040060) to P.M.T. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Cattaneo M. Hyperhomocysteinemia, atherosclerosis and thrombosis. Thrombosis and haemostasis. 1999;81:165–176. [PubMed] [Google Scholar]
- 2.Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. Biofactors. 2009;35:120–129. doi: 10.1002/biof.17. [DOI] [PubMed] [Google Scholar]
- 3.McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke; a journal of cerebral circulation. 2002;33:2351–2356. doi: 10.1161/01.str.0000032550.90046.38. [DOI] [PubMed] [Google Scholar]
- 4.Hainsworth AH, Yeo NE, Weekman EM, Wilcock DM. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochimica et biophysica acta. 2016;1862:1008–1017. doi: 10.1016/j.bbadis.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budge MM, de Jager C, Hogervorst E, Smith AD, Oxford Project To Investigate M, Ageing Total plasma homocysteine, age, systolic blood pressure, and cognitive performance in older people. Journal of the American Geriatrics Society. 2002;50:2014–2018. doi: 10.1046/j.1532-5415.2002.50614.x. [DOI] [PubMed] [Google Scholar]
- 6.Riggs KM, Spiro A, 3rd, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. The American journal of clinical nutrition. 1996;63:306–314. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann M, Gottfries CG, Regland B. Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dementia and geriatric cognitive disorders. 1999;10:12–20. doi: 10.1159/000017092. [DOI] [PubMed] [Google Scholar]
- 8.Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ. Homocysteine, B vitamin status, and cognitive function in the elderly. The American journal of clinical nutrition. 2002;75:908–913. doi: 10.1093/ajcn/75.5.908. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, Ilango K, Singh PK, Karmakar D, Singh GP, Kumari R, et al. Age dependent levels of plasma homocysteine and cognitive performance. Behav Brain Res. 2015;283:139–144. doi: 10.1016/j.bbr.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. The British journal of psychiatry : the journal of mental science. 2008;192:268–274. doi: 10.1192/bjp.bp.107.039511. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia P, Singh N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundam Clin Pharmacol. 2015 doi: 10.1111/fcp.12145. [DOI] [PubMed] [Google Scholar]
- 12.Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008;65:1286–1294. doi: 10.1001/archpsyc.65.11.1286. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan P, Hua X, Toga AW, Jack CR, Jr., Weiner MW, Thompson PM. Homocysteine effects on brain volumes mapped in 732 elderly individuals. Neuroreport. 2011;22:391–395. doi: 10.1097/WNR.0b013e328346bf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsen SK, Rajagopalan P, Joshi SH, Toga AW, Thompson PM, Alzheimer's Disease Neuroimaging I. Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer's Disease Neuroimaging Initiative. Neurobiology of aging. 2015;36(Suppl 1):S203–210. doi: 10.1016/j.neurobiolaging.2014.01.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopalan P, Jahanshad N, Stein JL, Hua X, Madsen SK, Kohannim O, et al. Common folate gene variant, MTHFR C677T, is associated with brain structure in two independent cohorts of people with mild cognitive impairment. NeuroImage: Clinical. 2012;1:179–187. doi: 10.1016/j.nicl.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheweita SA, Baghdadi H, Allam AR. Role of genetic changes in the progression of cardiovascular diseases. International journal of biomedical science : IJBS. 2011;7:238–248. [PMC free article] [PubMed] [Google Scholar]
- 17.da Costa AL, Santos Varela J, Mazetti O, Restelatto L, F. CA, Godinho C, et al. Comparison of the Mini Mental State Examination and depressive symptoms between high cardiovascular risk and healthy community elderly groups. Dementia & Neuropsychologia. 2008;2:294–299. doi: 10.1590/S1980-57642009DN20400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 19.Stein JL, Hua X, Morra JH, Lee S, Hibar DP, Ho AJ, et al. Genome-wide analysis reveals novel genes influencing temporal lobe structure with relevance to neurodegeneration in Alzheimer's disease. Neuroimage. 2010;51:542–554. doi: 10.1016/j.neuroimage.2010.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. International journal of geriatric psychiatry. 1999;14:858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Leow AD, Klunder AD, Jack CR, Jr., Toga AW, Dale AM, Bernstein MA, et al. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. Neuroimage. 2006;31:627–640. doi: 10.1016/j.neuroimage.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 25.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 26.Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 28.Hua X, Leow AD, Lee S, Klunder AD, Toga AW, Lepore N, et al. 3D characterization of brain atrophy in Alzheimer's disease and mild cognitive impairment using tensor-based morphometry. Neuroimage. 2008;41:19–34. doi: 10.1016/j.neuroimage.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, et al. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer's disease: an MRI study of 676 AD, MCI, and normal subjects. Neuroimage. 2008;43:458–469. doi: 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leow A, Huang SC, Geng A, Becker J, Davis S, Toga A, et al. Inverse consistent mapping in 3D deformable image registration: its construction and statistical properties. Inf Process Med Imaging. 2005;19:493–503. doi: 10.1007/11505730_41. [DOI] [PubMed] [Google Scholar]
- 31.Freeborough PA, Fox NC. Modeling brain deformations in Alzheimer disease by fluid registration of serial 3D MR images. J Comput Assist Tomogr. 1998;22:838–843. doi: 10.1097/00004728-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 33.Chung MK, Worsley KJ, Paus T, Cherif C, Collins DL, Giedd JN, et al. A unified statistical approach to deformation-based morphometry. Neuroimage. 2001;14:595–606. doi: 10.1006/nimg.2001.0862. [DOI] [PubMed] [Google Scholar]
- 34.Riddle WR, Li R, Fitzpatrick JM, DonLevy SC, Dawant BM, Price RR. Characterizing changes in MR images with color-coded Jacobians. Magn Reson Imaging. 2004;22:769–777. doi: 10.1016/j.mri.2004.01.078. [DOI] [PubMed] [Google Scholar]
- 35.Langers DR, Jansen JF, Backes WH. Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. Neuroimage. 2007;38:43–56. doi: 10.1016/j.neuroimage.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 36.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 40.Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 41.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 42.Dale AM, Sereno MI. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 43.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press; 2013. [Google Scholar]
- 45.Hesterberg T, Monaghan S, Moore DS, Clipson A, Epstein R. Bootstrap Methods and Permutation Tests. W. H. Freeman; New York: 2003. [Google Scholar]
- 46.Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet. 2015;58:1–10. doi: 10.1016/j.ejmg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, et al. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9523–9528. doi: 10.1073/pnas.1301816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Progress in neurobiology. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Prestia A, Baglieri A, Pievani M, Bonetti M, Rasser PE, Thompson PM, et al. The in vivo topography of cortical changes in healthy aging and prodromal Alzheimer's disease. Suppl Clin Neurophysiol. 2013;62:67–80. doi: 10.1016/b978-0-7020-5307-8.00004-1. [DOI] [PubMed] [Google Scholar]
- 50.Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, et al. Brain atrophy in Alzheimer's Disease and aging. Ageing research reviews. 2016 doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biological psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 52.Lee SH, Payne ME, Steffens DC, McQuoid DR, Lai TJ, Provenzale JM, et al. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biological psychiatry. 2003;54:529–533. doi: 10.1016/s0006-3223(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 53.Taylor WD, Macfall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, et al. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychol Med. 2007;37:1763–1773. doi: 10.1017/S0033291707000128. [DOI] [PubMed] [Google Scholar]
- 54.Ribeiz SR, Duran F, Oliveira MC, Bezerra D, Castro CC, Steffens DC, et al. Structural brain changes as biomarkers and outcome predictors in patients with late-life depression: a cross-sectional and prospective study. PloS one. 2013;8:e80049. doi: 10.1371/journal.pone.0080049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular psychiatry. 2016 doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Blazer DG. Depression and cognition in the elderly. Annu Rev Clin Psychol. 2015;11:331–360. doi: 10.1146/annurev-clinpsy-032814-112828. [DOI] [PubMed] [Google Scholar]
- 57.Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Annals of the New York Academy of Sciences. 2015;1345:36–46. doi: 10.1111/nyas.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HK, Nunes PV, Oliveira KC, Young LT, Lafer B. Neuropathological relationship between major depression and dementia: A hypothetical model and review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;67:51–57. doi: 10.1016/j.pnpbp.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Jochemsen HM, Kloppenborg RP, de Groot LC, Kampman E, Mali WP, van der Graaf Y, et al. Homocysteine, progression of ventricular enlargement, and cognitive decline: the Second Manifestations of ARTerial disease-Magnetic Resonance study. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013;9:302–309. doi: 10.1016/j.jalz.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Feng L, Isaac V, Sim S, Ng TP, Krishnan KR, Chee MW. Associations between elevated homocysteine, cognitive impairment, and reduced white matter volume in healthy old adults. Am J Geriatr Psychiatry. 2013;21:164–172. doi: 10.1016/j.jagp.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJ, Fischl B. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 2015;107:107–115. doi: 10.1016/j.neuroimage.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pardoe HR, Kucharsky Hiess R, Kuzniecky R. Motion and morphometry in clinical and nonclinical populations. Neuroimage. 2016;135:177–185. doi: 10.1016/j.neuroimage.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Hazra A, Kraft P, Lazarus R, Chen C, Chanock SJ, Jacques P, et al. Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum Mol Genet. 2009;18:4677–4687. doi: 10.1093/hmg/ddp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malarstig A, Buil A, Souto JC, Clarke R, Blanco-Vaca F, Fontcuberta J, et al. Identification of ZNF366 and PTPRD as novel determinants of plasma homocysteine in a family-based genome-wide association study. Blood. 2009;114:1417–1422. doi: 10.1182/blood-2009-04-215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pare G, Chasman DI, Parker AN, Zee RR, Malarstig A, Seedorf U, et al. Novel associations of CPS1, MUT, NOX4, and DPEP1 with plasma homocysteine in a healthy population: a genome-wide evaluation of 13 974 participants in the Women's Genome Health Study. Circ Cardiovasc Genet. 2009;2:142–150. doi: 10.1161/CIRCGENETICS.108.829804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka T, Scheet P, Giusti B, Bandinelli S, Piras MG, Usala G, et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. 2009;84:477–482. doi: 10.1016/j.ajhg.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lange LA, Croteau-Chonka DC, Marvelle AF, Qin L, Gaulton KJ, Kuzawa CW, et al. Genome-wide association study of homocysteine levels in Filipinos provides evidence for CPS1 in women and a stronger MTHFR effect in young adults. Hum Mol Genet. 2010;19:2050–2058. doi: 10.1093/hmg/ddq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sachdev PS. Homocysteine and brain atrophy. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1152–1161. doi: 10.1016/j.pnpbp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 69.Morris MS. The role of B vitamins in preventing and treating cognitive impairment and decline. Advances in nutrition. 2012;3:801–812. doi: 10.3945/an.112.002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shorter KR, Felder MR, Vrana PB. Consequences of dietary methyl donor supplements: Is more always better? Progress in biophysics and molecular biology. 2015 doi: 10.1016/j.pbiomolbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Loscalzo J. Homocysteine trials--clear outcomes for complex reasons. The New England journal of medicine. 2006;354:1629–1632. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- 72.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, et al. 2014 Update of the Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11:e1–120. doi: 10.1016/j.jalz.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roussotte FF, Gutman BA, Madsen SK, Colby JB, Thompson PM, Alzheimer's Disease Neuroimaging I. Combined effects of Alzheimer risk variants in the CLU and ApoE genes on ventricular expansion patterns in the elderly. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:6537–6545. doi: 10.1523/JNEUROSCI.5236-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, et al. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8404–8409. doi: 10.1073/pnas.0910878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajagopalan P, Toga AW, Jack CR, Weiner MW, Thompson PM, Alzheimer's Disease Neuroimaging I. Fat-mass-related hormone, plasma leptin, predicts brain volumes in the elderly. Neuroreport. 2013;24:58–62. doi: 10.1097/WNR.0b013e32835c5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warstadt NM, Dennis EL, Jahanshad N, Kohannim O, Nir TM, McMahon KL, et al. Serum cholesterol and variant in cholesterol-related gene CETP predict white matter microstructure. Neurobiology of aging. 2014;35:2504–2513. doi: 10.1016/j.neurobiolaging.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.