Abstract
The morphometric examination of autophagic bodies provides useful information about the mechanism and magnitude of macroautophagy, and yeast researchers frequently utilize various measurements of these structures as part of their quantification of the process. The utility of this approach, however, has led to the common misconception that autophagic bodies can be found in the mammalian lysosome, which is generally not correct.
Keywords: autophagic body, autophagy, lysosome, stress, vacuole
Yeast is a very useful model system that has certain advantages over other organisms for studying autophagy; however, the purpose of this article is not to extoll the virtues of yeast, per se, but rather to clear up a point of confusion. One of the morphological features of macroautophagy in yeast is the formation of autophagic bodies—the single-membrane vesicles that are generated upon fusion of an autophagosome with the vacuole. Autophagic bodies can accumulate in the vacuole lumen when their degradation is blocked by genetic (for example, the deletion of PEP4) or pharmacological (for example, treatment with the Prb1 inhibitor PMSF) means (Fig. 1). Because they accumulate in the vacuole, which is typically quite large, it is generally easier to identify and quantify autophagic bodies than autophagosomes. Furthermore, depending on the strain background and the fixation and staining protocol used for electron microscopy, the vacuole can appear as an essentially white, empty structure, further simplifying morphometric analyses. As a result, autophagic bodies can be measured with regard to size and number, providing useful information about the mechanism of autophagy following perturbation to the system, such as that resulting from a particular mutation. Autophagic bodies can also be monitored through fluorescence microscopy, allowing a less exact, but simpler estimation of autophagic flux. Unfortunately, the simple fact is that, as a general rule, autophagic bodies do not form in higher eukaryotes, other than plants, and some rare cell types in other species.†
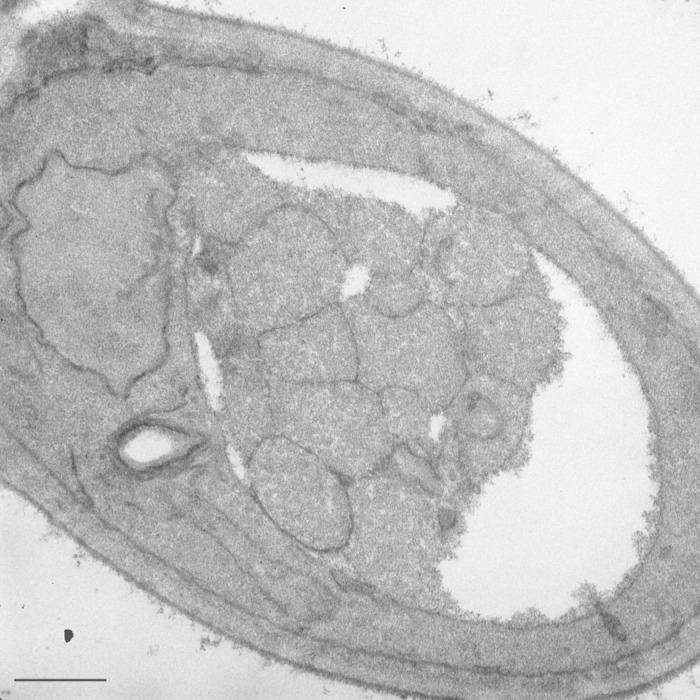
Figure 1. Autophagic bodies in the yeast vacuole. A yeast strain harboring a deletion of the PEP4 gene was grown in rich medium and shifted to nitrogen-starvation conditions for 4 h. Scale bar: 500 nm.
Unfortunately, we occasionally see references to autophagic bodies when people are discussing mammalian cell lysosomes, or the lysosomes of other eukaryotic systems, and in most cases this is not correct. Similarly, on several occasions we have noted schematic figures illustrating the presence of autophagic bodies within the lysosome. In most cases these errors have been corrected for papers published in Autophagy, but of course not all papers that concern autophagy are published in our journal; hence, the purpose of this article.
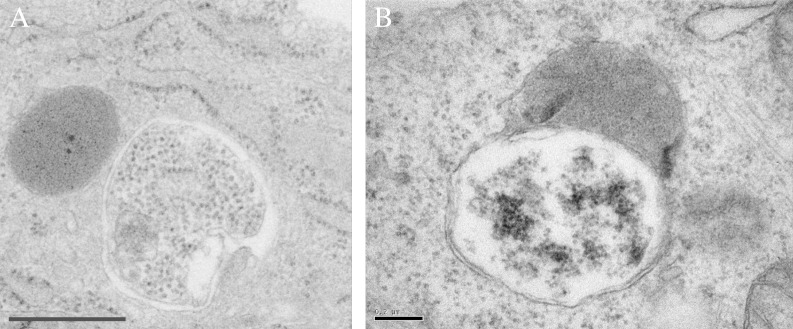
What it largely comes down to is that the yeast vacuole, is big. Actually, big is an understatement—this is a gigantic organelle. That is why researchers in the yeast field are speaking tongue-in-cheek when they refer to something as being “close to” or “in proximity of” the vacuole with respect to subcellular localization. After all, that is not a lot different than saying a particular human being is close to, or in proximity of, the earth as if that provided any useful information regarding that person’s whereabouts. The point is, almost everything in the yeast cell is close to the vacuole—it is just that big. An average Saccharomyces cerevisiae cell is approximately 5–10 µm in diameter, vacuoles are in the range of 2–3 µm, 1 - 3 and autophagic bodies are typically 0.4–0.8 µm. 4 Thus, an average vacuole can easily accommodate 20–30 autophagic bodies, and perhaps more than 100 (think 3-dimensionally), depending on their respective sizes (Fig. 1). In contrast, mammalian lysosomes are approximately 0.5 µm in diameter (really quite small by comparison to the vacuole), and mammalian autophagosomes are 0.5–2 µm. Thus, the average lysosome cannot accommodate even a single autophagic body (Fig. 3). This point is illustrated quite dramatically by the mixed media sculpture “Macroautophagy.” 5
Figure 3. Mammalian lysosomes are too small to accommodate autophagic bodies. Mouse embryonic fibroblasts showing an autophagosome next to (A) or fusing with (B) an electron dense lysosome. Scale bars: 600 nm (A) and 200 nm (B).
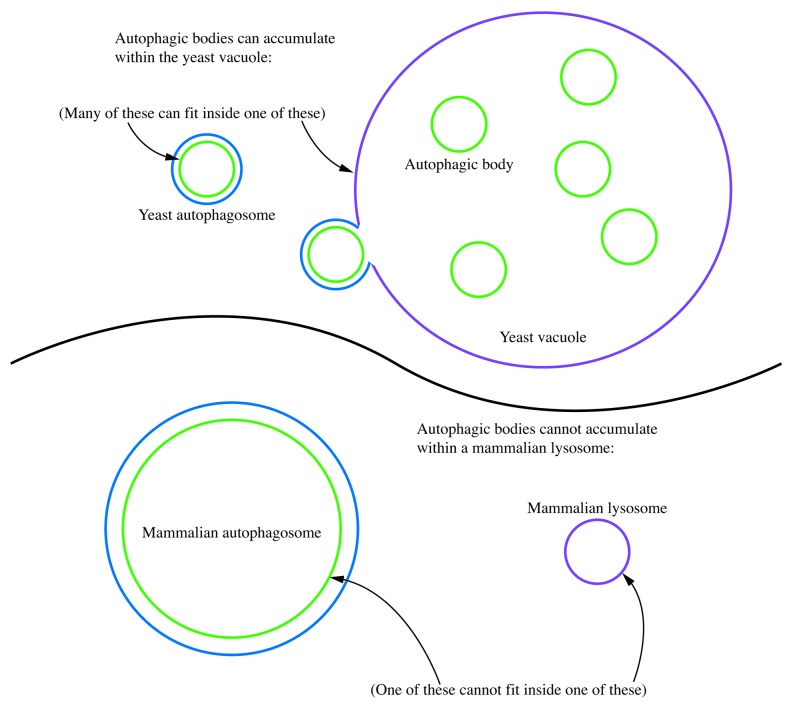
Thus, do not make the mistake of referring to autophagic bodies when describing the steps of macroautophagy in higher eukaryotes other than plants. Remember, as a general rule, lysosomes do not contain autophagic bodies (Fig. 4).
Figure 4. Autophagic bodies can accumulate within the yeast vacuole, but not the mammalian lysosome. In this schematic diagram, the relative sizes of yeast and mammalian autophagosomes are shown compared with the vacuole and lysosome. Even smaller mammalian autophagosomes are typically too large to allow the release of the inner vesicle into the lysosome lumen.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH grant GM053396 (to DJK) and Academy of Finland, Biocentrum Helsinki and Sigrid Juselius grants (to E-LE).
Footnote
†Structures that may be similar to autophagic bodies have been detected in mouse embryonic stem cells, and occasionally in mouse embryonic fibroblasts. In this case, however, these single-membrane compartments are probably generated by the fusion of autophagosomes with late endosomes, which are usually larger than lysosomes (Fig. 2).
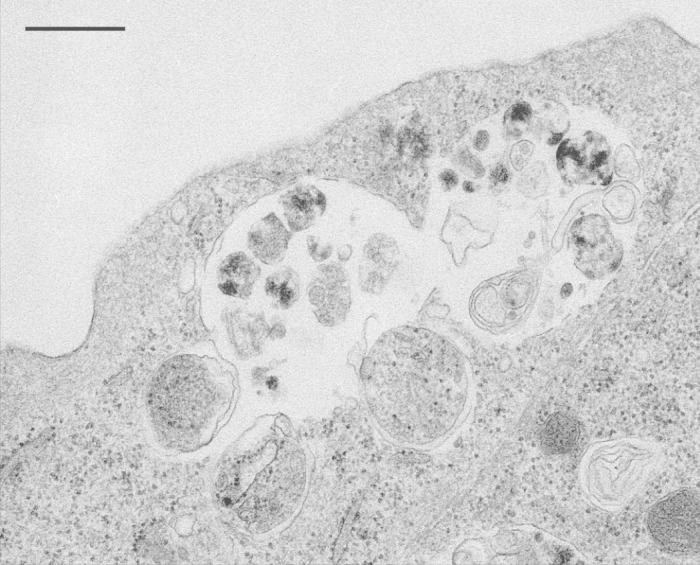
Figure 2. Single-membrane compartments in mouse embryonic fibroblasts. The “autophagic bodies” are likely generated by the fusion of an autophagosome with a late endosome/multivesicular body. This figure was reproduced from Figure 5B of Eskelinen, E-L. Meth Mol Biol, 2008; 445:11–28 with the permission of Springer.
References
- 1. Indge KJ. . The isolation and properties of the yeast cell vacuole. J Gen Microbiol 1968; 51:441 - 6; http://dx.doi.org/ 10.1099/00221287-51-3-441; PMID: 4968622 [DOI] [PubMed] [Google Scholar]
- 2. Matile P, Wiemken A. . The vacuole as the lysosome of the yeast cell. Arch Mikrobiol 1967; 56:148 - 55; http://dx.doi.org/ 10.1007/BF00408765; PMID: 4873367 [DOI] [PubMed] [Google Scholar]
- 3. Pratt P, Bryce J, Stewart G. . The yeast vacuole—a scanning electron microscopy study during high gravity wort fermentations. J Inst Brew 2007; 113:55 - 60; http://dx.doi.org/ 10.1002/j.2050-0416.2007.tb00256.x [DOI] [Google Scholar]
- 4. Eskelinen E-L, Reggiori F, Baba M, Kovács AL, Seglen PO. . Seeing is believing: the impact of electron microscopy on autophagy research. Autophagy 2011; 7:935 - 56; http://dx.doi.org/ 10.4161/auto.7.9.15760; PMID: 21566462 [DOI] [PubMed] [Google Scholar]
- 5. Dice JF, Klionsky DJ. . Artophagy: the art of autophagy--macroautophagy. Autophagy 2010; 6:320 - 1; http://dx.doi.org/ 10.4161/auto.6.3.11263; PMID: 20118653 [DOI] [PubMed] [Google Scholar]