Abstract
Worldwide, nearly 1.7 billion people per year contract diarrheal infectious diseases (DID) and almost 760 000 of infections are fatal. DID are a major problem in developing countries where poor sanitation prevails and food and water may become contaminated by fecal shedding. Diarrhea is caused by pathogens such as bacteria, protozoans and viruses. Important diarrheal pathogens are Vibrio cholerae, Shigella spp. and rotavirus, which can be prevented with vaccines for several years. The focus of this review is on currently available vaccines against these three pathogens, and on development of new vaccines. Currently, various types of vaccines based on traditional (killed, live attenuated, toxoid or conjugate vaccines) and reverse vaccinology (DNA/mRNA, vector, recombinant subunit, plant vaccines) are in development or already available. Development of new vaccines demands high levels of knowledge, experience, budget, and time, yet promising new vaccines often fail in preclinical and clinical studies. Efficacy of vaccination also depends on the route of delivery, and mucosal immunization in particular is of special interest for preventing DID. Furthermore, adjuvants, delivery systems and other vaccine components are essential for an adequate immune response. These aspects will be discussed in relation to the improvement of existing and development of new vaccines against DID.
Keywords: diarrheal diseases, human pathogen, oral vaccine, recombinant vaccine, Vibrio cholerae, Shigella spp. Campylobacter spp., rotavirus
Introduction
Infections of the gastrointestinal tract triggered by bacterial, parasitic and viral pathogens rank among the most frequent infectious diseases. Every year, 2 million people die of diarrheal diseases, 760 000 of these are children under 5 y of age.1,2 Diarrhea is defined as the passage of loose or watery stools occurring three or more times in a 24-h period. Three types of diarrhea are differentiated: (1) acute diarrhea, including Cholera, (2) acute bloody diarrhea, also called dysentery characterized by damage to the intestinal mucosa, and (3) the persistent diarrhea with disease duration of 14 or more days.2 Most of the pathogens causing diarrhea share a similar way of transmission, i.e., the fecal-oral transmission. However, the dose of the infectious agent and the specific process causing diarrheal symptoms varies widely between various pathogens.3
Water and sodium are necessary for life and if diarrhea leads to insufficient absorption of water and nutrients, patients will dehydrate and death occurs after losing 10% of body fluids.4 One of the main reasons for the prevalence of diarrheal infections is a shortage of potable water and sewage facilities. Therefore, infectious diarrhea is the leading causes of morbidity and mortality in less developed countries. Adding to the burden of morbidity and mortality, there are under-recognized long-term effects of frequent early childhood diarrheal, such as impaired absorption of nutrients, especially during the first two years of life in a phase of brain and synapse development. Along with the deficits in nutrients, significant developmental defects were observed in patients with childhood diarrhea. These include a permanent reduction in growth (up to 8 cm by the age of 7), a loss of 10 IQ points and lack of a year of school education by the age of nine.5,6 Despite of improving life conditions due to control of potable water and better sanitary facilities in developed areas, there are also problems with bacterial, parasitic and viral pathogens causing diarrhea. This disease mainly occurs in connection with hospitalization and the administering of antibiotics. The risk of infections can be reduced by preventions policies. However, only vaccines provide protection against disease outbreak and can guarantee long-term immunization.
The first vaccine against cowpox developed by Edward Jenner at the end of the 18th century together with new developed methods, that allow the attenuation and inactivation of microorganisms,7,8 laid the foundation for the scientific era of vaccinology.9 Today vaccines are the most cost efficient strategy to lower the burden of infectious diseases all over the world. To generate new vaccine candidates, it is required to know the virulence strategies of the bacterial, parasitic and viral pathogens and the specific immune mechanisms of the host. We will briefly describe important diarrheal pathogens with clinical presentation, main virulence factors and therapeutic approaches.
Important Pathogens Causing Diarrhea
There are numerous pathogens from different life forms such as bacteria, parasites and viruses that cause diarrheal infectious diseases (DID) in humans. The main focus of this article is on Vibrio cholerae, Shigella spp. and R rotavirus for which vaccines are available or in development (see further below). A comprehensive description of pathogens associated with DID can be found in the Supplemental Material and Table S1.
Vibrio cholerae
The Gram-negative curved rod Vibrio cholerae is responsible for 100,000 – 120,000 deaths annually (World Health Organization, WHO July 2012). The main virulence factor is Cholera toxin (CT), a secreted AB-toxin,that triggers hyper-secretion of electrolytes by manipulation in second messenger levels in enterocytes (Fig. 1). The serotypes responsible for the disease defined clinically and epidemiologically as Cholera are V. cholerae O1 and O139.10,11 The currently prevailing 7th pandemic is caused by V. cholerae serotype O1. Despite of efforts to fight the disease by oral rehydration and in certain cases also antibiotic therapy, Cholera forms a major public health problem, especially in regions with low hygienic standards, such as developing countries and conflict areas.12
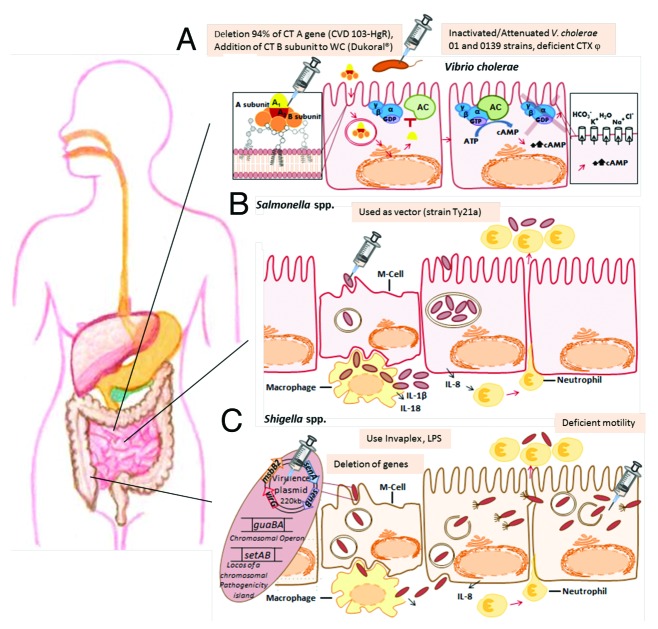
Figure 1. The gastrointestinal tract, interactions between pathogens and host and approaches to vaccine design. (A) Vibrio cholerae secrets an enterotoxin, which is an AB Toxin and comprises a single catalytic A subunit and a pentameric B subunit for specific binding to host cells. The receptor for CT is the glycolipid ganglioside GM1. Following internalization by receptor-mediated endocytosis, transport to the Golgi and the ER, the A1 subunit is finally transferred to the cytoplasm. The A1 fragment is a NAD-dependent ADP ribosyltransferase and activates the G protein Gsα (GTP-bound), thereby continually stimulating adenylate cyclase (AC) produce cAMP. The high cAMP level enables the protein kinase A which induces a dramatic electrolyte transport, which is typically for diarrhea. Possible vaccine approaches (indicated by syringes) are the cholera toxin (CT), different virulence genes and use of inactivated/attenuated strains.(B) Salmonella spp. translocates effector proteins by a T3SS, encoded by Salmonella Pathogenicity Island 1, inducing pro-inflammatory responses and uptake of the pathogen via macropinocytosis. Uptake also occurs via M-cells or phagocytes. After phagocytosis by macrophages apoptosis is triggered, thereby triggering inflammation reactions with recruitment of neutrophils. Internalized Salmonella survive and replicate within the ‘Salmonella-containing vacuole’. Salmonella enterica Serovar Typhi Ty21 represents a promising live vector for presentation of foreign antigens from unrelated bacterial, viral and parasitic pathogens. (C) Shigella spp. infects the epithelium from the intestinal lumen of the terminal small intestine and colon through M-cells. After phagocytosis, the bacteria are able to escape from the macrophage by triggering apoptosis. By remodeling the host cell actin cytoskeleton and forming large membrane protrusions, invasion occurs similar to S. enterica. Within host cells, Shigella is motile in the cytoplasm by a mechanism involving the formation of actin tails, also leading to infection into neighboring host cells. Spreading from cell to cell within intestinal tissue is accompanied by emission of bloody mucopurulent stools. Strains deficient in intracellular motility, in enterotoxin and further virulence genes present good vaccine candidates. Furthermore, cross-linked O-antigen polysaccharides of the relevant Shigella serotypes to a carrier protein is also a vaccine design approach.
Shigella spp.
Further members of the Enterobacteriaceae family and major cause of diarrhea and dysentery are Shigella spp., which are closely related to Escherichia coli. Shigella spp. is transmitted through person-to-person contact and ingested by contaminated water or food. Only a small infective dose (10–100 bacteria)13 of one of the four Shigella species is enough to trigger epidemic mucosal ulceration and bloody diarrhea (see Fig. 1C). Despite of the self-limiting infection, the dysentery especially causes death of children in developing countries. Two virulence mechanisms are crucial for the pathogenesis, which are cell invasion and formation of the Shiga toxin. For invasion, the bacteria attach to M cells of the terminal ileum and of the colon. Via transcytosis, they reach macrophages in the subserosa and are taken up by phagocytosis. After inducing apoptosis in the phagocytes, released Shigella may invade epithelial cells via their basolateral membranes. Within enterocytes, Shigella escapes into the cytosol and triggers F-actin formation at one cell pole, resulting in actin-mediated intracellular motility and intercellular spread.14
Rotavirus
The most common cause of diarrhea in young children is an infection with rotavirus.15 It is a non-enveloped virus and stable against environmental influences.16 Rotavirus infects intestinal epithelial cells, within it produces an enterotoxin called NSP4, which interacts with calcium-activated chloride channels.17 Furthermore, it reduces the effectiveness of digestive enzymes, leading to nutritional deficiencies.18 The disease is self-limiting in otherwise healthy humans and therapy is based on rehydration.16 Worldwide, the number of deaths of young children associated with rotavirus was determined as 453 000 in 2008.19 To prevent and reduce rotavirus infections, in 2009 the WHO proposed to include rotavirus vaccination into the immunization plan.20
Benefits and Limitations of Currently Available Vaccines Against Diarrheal Infections
Cholera immunization
Cholera is caused by two of more than 200 V. cholerae serogroups, i.e., O1 and O139. Furthermore, V. cholerae O1 can be classified into the classical or the predominant El-Tor biotypes. Although, prevention measures like the procurement of drinking water and improved sanitary facilities are important in areas with epidemic diseases, also vaccines against the pathogens are necessary. It could be demonstrated that only hygiene measures are not enough to prevent Cholera in Africa.21 In over 100 y of Cholera vaccine development, mainly the oral type of vaccine prevailed (see below).
Parenteral Cholera vaccines
Parenteral vaccines available during the 1990s consisted of phenol-killed whole cells of V. cholerae O1, which were about 50% protective for a short duration. Because of the short period duration of protection and painful local inflammatory reactions, the injectable vaccine has never been recommended for use.22 One parenteral Cholera vaccine, currently available in the United States, shows similar results and has no positive effect on controlling epidemic Cholera outbreaks.23 Because of the low protective efficacy, the high reactogenicity of this vaccine type and the knowledge that oral administration of antigens results in mucosal immune response in the 1980s began the developing of oral Cholera vaccines.
Oral Cholera vaccines (OCV)
Two types of oral Cholera vaccines are currently available (Table 1): (1) Dukoral® (WC-rBS) a monovalent vaccine based on formalin and heat-killed whole-cells (WC) of V. cholerae O1 plus recombinant Cholera toxin B subunit (rBS), (2) Shanchol® and mORCVAX®, both identical bivalent vaccines based on serogroups O1 and O139, but formulated by different manufacturers. These oral Cholera vaccines received good results in controlling epidemic diseases24 and trace all back to the early oral Cholera vaccine research of the 1980s.25
Table 1. Licensed oral vaccines against V. cholerae O1 (modified according to Ref6,102,103)* Produced by VABIOTECH, Vietnam and only available in Vietnam.
| Vaccine | No. of doses | Booster dose | Active component(s) | Licensed product name (manufacturer) | Duration of protection | Age range for vaccination |
|---|---|---|---|---|---|---|
| iInactivated whole Vibrio combination + CTX B subunit | 2, given 7–42 d apart (3 for children aged 2–5 y) | Every 2 y (every 6 mo for children aged 2–5 y) | Mix of inactivated V. cholerae O1 of classical and El Tor biotypes and Inaba and Ogawa serotypes plus CT B subunit | Dukoral® (SBL) | 2 y (6 mo for children aged 2–5 y) | ³ 2 y |
| Inactivated whole Vibrio combination | 2, given 14 d apart | Every 2 y (likely to be extended to every 3 y) | Killed whole cells only (O1 classical and El Tor biotypes plus O139) | Shanchol ® / mOrcVAX ®* | 3 y | Shanchol ³ 1 y; mORCVAX ³ 2 y |
| CVD 103-HgR recombinant live vaccine | 1 | Unknown | Recombinant classical, Inaba strain with deletion of 94% of the gene encoding the CT A, + Hg2+ resistance gene (mer) introduced into the hemolysin A locus on the chromosome | Orochol ®, Mutachol® (Berna Biotech)** | 6 mo (established only in North American volunteers) | ³ 2 y |
Note: *Produced by VABIOTECH, Vietnam and only available in Vietnam; ** Production discontinued; re-initiation requires modification of manufacturing facility
Dukoral® has the advantage that the B subunit governs protection against ETEC. Shanchol® and mORCVAX® also have the advantages over Dukoral® that (1) no buffer (mostly clean water) is needed, (2) less storage space is required and (3) doses are less expensive.26 The third cholera vaccine CVD 103-HgR, an oral live-attenuated vaccine (Table 1), ceased production in 2004 due to failure in a large field trail performed in Indonesia.27 Further attenuated oral vaccines are still in different development phases and not yet licensed (Table 2). Many of these were created by removal of the CTXφ prophage, an inactivation of hemagglutinin/protease activity by sequence insertion of the Clostridium thermocellum endoglucanase A and modification of the recA region.
Table 2. New generation and unlicensed vaccines against V. cholerae and Shigella spp. (modified according Ref.103).
| Vaccine | Immunization route | No. of doses | Status | Cause of attenuation | Relevant immune response(s) | Developer, references | ||
|---|---|---|---|---|---|---|---|---|
| V. cholerae O1 attenuated vaccines | ||||||||
| Peru 15 or CholeraGarde® recombinant live vaccine | Oral | 1 (107-109 CFU) | Phase IIb | Core deletion of CT Modification of recA |
Intestinal secretory IgA and serum IgG specific for LPS and other surface antigens: serum vibriocidal antibodies | Avant Immuno-therapeutics104 | ||
| V. cholerae 638 | Oral | 1 (109 CFU) | Phase I/II | Deletion of CTXφ prophage from VC strain C7258 El Tor Ogawa Inactivation of hemagglutinin/protease activity by sequence insertion of the Clostridium thermocellum endoglucanase A |
Intestinal secretory IgA and serum IgG specific for LPS and other surface antigens: serum vibriocidal antibodies | Finlay Institute, Cuba105 | ||
| VA1.3 and VA1.4 | Oral | 1 (5*109 CFU) (VA1.3) |
Phase I/II | Non-toxigenic V. cholerae + insertion of the ctxB, VA1.3 + AmpR |
Anti-CT-antibodies serum vibriocidal antibodies |
Three different laboratories | ||
| IEM 108 | Oral | 1 (109 CFU) in rabbits | Pre-clinical | deficient in CTXφ (IEM101) +introduced ctxB gene, + rstR gene |
Anti-CT IgG and vibriocidal antibodies | China CDC106 | ||
| V. cholerae O139 attenuated vaccines | ||||||||
| TLP01 | 1 (109 CFU) in rabbits | Pre-clinical | CRC266 0139 strain with CTXφ, mshA deletion, inativation of hemagglutinin/ protease activity by sequence insertion of the Clostridium thermocellum endoglucanase A | Intestinal secretory IgA and serum IgG specific for LPS, IgM, vibriocidal antibodies | Finlay Institute, Cuba107 | |||
| VCUSM2 | Oral | 1 (109 CFU) in rabbits | Pre-clinical | VC strain C7258 El Tor Ogawa with hemA deletion causes an ALA auxotrophy | Intestinal secretory IgAs and serum IgGs specific for LPS and anti-CT, vibriocidal antibodies | Universiti Sains Malaysia108 | ||
| CVD 112 | 107 CFU | Phase I | VC 0139 with ctxA, zot, ace, cep deletion | vibriocidal antibodies, anti-CT IgG | University of Maryland, US109 | |||
| Attenuated Shigella spp. vaccines | ||||||||
| S. sonnei strain WRSS1 | Oral | 2 (104 CFU) | Phase II | Deletion of the plasmid-encoded virG (icsA) protein → προτειν ϕ τηε πλασμιδ−ενχοδεδ γ110 | Intestinal secretory IgA and serum IgG specific for O-antigen and virulence plasmid proteins | Walter Reed Army Institute of Research111 | ||
| S. sonnei strain WRSs2, WRSs3 | Oral | 2? | Pre-clinical | Deletion of the plasmid-encoded virG (icsA), senA, senB, msbB2 |
Unknown | 112 - 114 | ||
| S. flexneri 2a strain CVD 1208S | Oral | 2 | Phase I | Deletion of guaBA, setAB, and senA110 | Intestinal secretory IgA and serum IgG specific for O-antigen and virulence plasmid proteins | Center for Vaccine Development115 | ||
| S. flexneri 2a strain SC602 | Oral | 1–2 (104 CFU) | Phase IIb | Deletion of the plasmid-encoded virG (icsA) protein →προτειν οϕ τηε πλασμιδ−ενχοδεδ υ110 | Intestinal secretory IgA and serum IgG specific for O antigen and virulence plasmid proteins | Pasteur Institute116 | ||
| S. dysenteriae 1 strain SC599 | Oral | 2 | Phase II | Loss of invasion (icsA, iron chelation (ent, fep) and Shiga toxin A subunit (stxA) | Intestinal secretory IgA and serum IgG specific for O-antigen and virulence plasmid proteins | Pasteur Institute32 | ||
| Inactivated and other Shigella spp. vaccines | ||||||||
| Parenteral Shigella glycoconjugates | Intra-muscular | 2 | Phase III | O polysaccharide covalently linked to carrier protein | Serum IgG specific O-antigen | NICHD33 | ||
| Shigella invasion complex (Invaplex) | Nasal | 3 | Phase I | Invaplex is composed of the invasion plasmid antigen (Ipa), proteins IpaB and IpaC in a native complex with Shigella LPS |
Intestinal secretory IgA and serum IgG specific for O-antigen and virulence plasmid proteins | Walter Reed Army Institute of Research117 | ||
| Proteosomes to which S. sonnei or S. flexneri 2a LPS is adsorbed | Nasal | 2 | Phase II | outer membrane protein vesicles of Group B meningitides | Intestinal secretory IgA and serum IgG specific for O antigen | ID BiomedicalA118 | ||
| Inactivated S. sonnei | Oral | 3–5 | Phase I | By formalin inactivation | Intestinal secretory IgA and serum IgG specific for O antigen | Emergent Biosolutions119 | ||
| Salmonella vaccine vector Ty21a | Oral | 3 | Pre-clinical | live Salmonella Typhi vaccine vector expressing S. sonnei or S. dysenteriae antigens | Intestinal secretory IgA and serum IgG specific for O antigen | Aridis120 | ||
Shigella vaccine development
The immunity observed after infection with Shigella spp. is species-specific and each species divides into various serotypes and subtypes.28 After infection, an IgM immune response is triggered against LPS O-antigen of the serotype.29,30 The serotype specificity and wide range of Shigella serotypes are reasons for the need of multivalent vaccines.
All vaccines against shigellosis developed so far are unlicensed, but some are currently undergoing clinical testing phases (see Table 2). Target populations for Shigella vaccine are infants and young children in developing countries and travelers from industrialized to developing countries.31
The unlicensed vaccines for shigellosis follow different approaches and are in various phases of trials. Two of the vaccine strategies demonstrated their efficacy by positive result in controlled large scale field trials.32 First vaccine strategy is the creation of parenteral conjugate vaccines in which O-antigen polysaccharides of the relevant Shigella serotypes are covalently linked to carrier proteins. The National Institute of Child Health and Human Development (NICHD) is investigating this approach,33 because it has been suggested by several studies that immunity against shigellosis is conferred by anti O-antigen serum IgG34 (see Table 2). This studies comprise several field studies, which yield following results: (1) A single dose of S. sonnei conjugate conferred 74% protection efficacy against S. sonnei gastroenteritis,33 and (2) S. sonnei and S. flexneri 2a conjugate vaccine had no protection efficacy in younger than 3 y of age but S. sonnei showed a 71% protective efficacy in 3–4 y-old children.35
The second strategy is creation of live oral vaccines based on attenuated strains of Shigella. The greatest benefits of this strategy are the needle-free delivery route, an easier way to manufacture than for other potential vaccines and good tolerability. Due to the fact that this approach uses living Shigella, the right degree of attenuation is a critical parameter. Over-attenuation will lead to insufficient immunogenicity, whereas moderate attenuation causes excessive reactogenicity.36 For this, the invasion plasmid-encoded protein antigens (Ipas) are most qualified for reducing virulence.37 The three distinct enterotoxins produced by Shigella strains can also be used for attenuation.38 The most currently developed vaccines are the new generation, live attenuated vaccines, as shown in Table 2. The new approaches are based on the use of proteosomes, orally administered inactivated S. sonnei, a Shigella invasion complex (Invaplex), or a core-linked LPS expression of S. dysenteriae serotype 1 O-antigen in a living Salmonella Typhi vaccine vector.6 So far, these Shigella vaccine strategies have no documented evidence of protection in humans.
Rotavirus immunization
The first rotavirus vaccine RotaShield was licensed in 1998, but was removed from marked in 1999 after intussusceptions were detected in immunized young children.39 Since 2006, two new licensed rotavirus vaccines Rotarix® and RotaTeq® are available and drastically reduced worldwide infection rates.40,41 Rotarix® is based on a monovalent, live, attenuated human G1P[8] (glycoprotein VP7 defines the G serotypes, protease-sensitive protein VP4 defines P serotypes) rotavirus strain (G1 serotype, P[8] genotype), the most common worldwide, but also prevents infections by serotypes G3, G4 and G9.42,43 This attenuated strain was found in a child at Cincinnati Children’s Hospital.44 After isolation, Vero cells (derived from monkey kidneys) were used for vaccine production.45 The immunization is about 100% for the proposed serotypes, but decreases when infected with other serotypes.43 However, the mechanism of protection is still unclear.43
In contrast, RotaTeq® consist of genotypes G1, G2, G3, G4 and P1A(8) from human and bovine isolates.46 Four of the five genotypes used are from human and each synthesizes one of the proteins of the outer capsid (G1, G2, G3 or G4) and a bovine adhesion protein (serotype P7). The fifth genotype P1A(8), express a bovine outer capsid protein (G6) and the human adhesion protein P1A (genotype P[8], serotype P1A[8]).46 They are also multiplied in Vero cells and after vaccination, immunization is nearly 100%.46 Similar to Rotarix®, the mechanism of immunization is not completely understood, but the serum anti-rotavirus IgA titers were 3-fold higher in volunteers with RotaTeq® treatment than those with placebo.46
The vaccine is administered orally to infants.47 First dose is given between the 6 and 12 wk after birth (1 mL Rotarix, 2 mL Rotateq) and the latest vaccination before 24 wk (min. four weeks between both doses, Rotarix) or 32 wk (min. four weeks between all three doses, Rotateq).43,46,47 In the first weeks after vaccination there is a small risk of an invagination (1–2 of 100 000 vaccinated infants), but negligible comparable to a rotavirus infection.42,47 Other side effects can be bloody stool, severe stomach pain or bilious emesis.47
Since 2009, rotavirus vaccination is part of the immunization program.20 However, both named vaccines are very expensive for developing countries, therefore non-profit organizations like the Global Alliance for Vaccines and Immunization (GAVI) offer them for a much lower price to reduce the infection rate in problematic regions.48 In the meantime, Indian scientists developed a new rotavirus vaccine called Rotavac that is much cheaper (1 $ per dose).49 It is an oral, live attenuated vaccine applied in 3 doses (6, 10, 14 wk after birth), which passed clinical phase III.49 The vaccine is based on a G9P[11] strain called 116E, originally isolated from a child in Delhi, with a VP4 similar to various bovine RVs.50-53 There is also a second candidate based on live attenuated rotavirus strain called RV3, a G3P[6], which contains human RV-like VP7 and VP4 proteins and was isolated from neonates.50
However, there is a newer approach reported from Wen et al. in 2012 based on truncated recombinant VP8* (ΔVP8*) proteins of human rotavirus strain Wa P[8], DS-1 P[4] or 1076 P[6], which was expressed in E. coli in high yield.54 Three doses of 10–20µg of ΔVP8* vaccine (without adjuvants) were injected in guinea pigs and showed high amount of homotypic and heterotypic neutralizing antibodies.54 Further, mice were treated with clinical relevant dosage of DS-1 P[4]ΔVP8* vaccine and produced increased level of VP8*-specific serum IgG antibodies.54 This new development has the advantages of cost-effectiveness, lack of reversions, and based on the experience that rotavirus G types are often detected in parallel with P[8], P[4] and P[6] ΔVP8* proteins, excluded serotypes could be covered.54
Problems and Limitations for Creating New Vaccines
In the previous parts, benefits and limitations of available vaccines against certain diarrheal infections were discussed. Next, problems and limitations during the research process of developing new vaccines will be described.
General obstacles in vaccination
Vaccinations against Cholera, shigellosis and other diarrheal diseases underlie limitations and barriers, such as general technical and logistical problems. Some vaccines require administration of further doses that are difficult for mobile populations. The use of oral vaccines, which sometimes required purified water for administration, the cost-effectiveness of the vaccines, strain variations from region to region, hygiene behavior and sanitation are additional wide ranged problems.
Moreover, logistical settings rely on stable infrastructure and human resources, both are scarce commodity in crisis areas, which are predestined for Cholera or other infection outbreaks. One further barrier is the transport of the bulky and large amount of vials, under respect of the cold chain principles. The public and political response in general and during times of crisis also plays a role in vaccination21.
Barriers for vaccine development against parasites
Beside general problems discussed earlier in vaccine development, especially parasitic vaccine research and production is difficult. First, the parasitic (eukaryotic) genome size differs from prokaryotic bacteria and viruses which makes it hard to identify possible antigen targets. Further, suitable in vitro systems are missing and related expression vectors such as E. coli could be problematic due to a different codon usage which leads to a misfolded protein.- If there is an existing organism for expression, the expression rate has to be high enough to guaranty sales turnover. In addition, protozoans e.g., Entamoeba histolytica, Giardia lamblia and Cryptosporidium have complex lifecycles with different stages in which they form various virulence factors (see Suppl. Material). Often it is impossible to culture and analyze all stages of a lifecycle and virulence mechanisms. Accordingly, these parasites may cause chronic diseases. And last, investigations require long observation time and appropriate animal models are thus problematic (further animal model limitations are discussed below). However, potential targets and approaches exist in parasitic vaccine development (see Table S1 and Table 3).
Table 3. Types of vaccines.
| Name | Features | Advantage | Disadvantage | Example, Reference |
|---|---|---|---|---|
| Live, attenuated vaccine | Use of live attenuated bacteria and viruses, often isolated from patients or cell cultures | • booster effect (antibody production) • easy to create virus vaccines • mucosal immunization (IgA) |
• reverse mutation • high side effects • must be cultivable • not suitable forimmuno-compro-mised and pregnant people • difficult to create bacteria vaccines (high level of knowledge) • refrigeration |
Mumps, measles, rubella virus74,121,122 diarrhea caused by rotavirus (see rotavirus immunization) tests with S. Typhimurium ssaV mutants in mice123 ETEC mutants tested in phase II124,125 |
| Inactive/ killed pathogen vaccine | Consist of killed microorganisms such as bacteria and viruses inactivated with chemicals (formaldehyde), radiation, antibiotics or heat | • suitable for immune-com-promised and pregnant people • no back mutation |
• low booster effect (immunization up to 2 y) • repeated dosing • must be cultivable |
Dukoral and Shanchol against Cholera (oral, see Cholera immunization)26,63,74 |
|
Subunit vaccine/ recombinant subunit vaccine |
Use of an antigen or epitope, produced and isolated from microorganism | • use mixture of antigens • low side effects |
• genetic information required • low booster effect |
Hemagglutinin and neuraminidase of the influenza virus74,126,127 |
| Toxoid vaccine | Use of inactivated toxins from pathogenic bacteria, can be combined with killed pathogens | • booster effect (serum antibody IgA, IgG) • combination of many toxins |
• only toxin producing bacteria • the toxin has to be known |
Cholera, diphtheria, tetanus, tests with Clostridium difficile74,128,129 |
| Conjugate vaccine | Combination of the capsule lipopolysaccharide (LPS) and a toxin or an antigen subunit, because LPS often causes a weak immune response | • booster effect (T-cell dependent immunization) | • only capsulated bacteria | Diarrhea caused by Campylobacter jejuni, Hemophilus influenzae “type b” (Hib)74,130,131 Pneumococcal conjugate vaccine (PCV) (Prevnar, Synflorix and Prevnar 13) N. meningitides Group C polysaccharide-tetanus toxoid (NeisVac-C) |
| DNA vaccine | The DNA contains the information of the antigen and is injected with a needle, some cells take it up and integrate it, after a time they synthesize the antigen and present it on the surface | • stimulate innate and adaptive immune response (in mice) • general side effects of the pathogen will be avoided • cheap and easy to create • no cold chain • stable DNA |
• the antigen and its sequence has to be known • integration of DNA can be a risk • uptake effectiveness? • low booster effect (in human) • gold particles are expensive |
Cancer, tests in mice with CpP2-DNA of Cryptosporidium parvum74,132,133 |
| mRNA vaccine | See “DNA vaccine” | • see “DNA vaccine” • no risk of genome integration |
• see “DNA vaccine” • instable mRNA |
Prostate cancer58,74 |
| Recombinant vector vaccine | Similar to “DNA vaccine,” they use an attenuated virus or bacteria as a vector with the antigen information | • similar to a real infection - > better immune response • humoral and cellular response (in mice) • cheap and easy to create |
• back mutation? • suitable for immunosuppressed people? • integration of DNA can be a risk? • low booster effect (in human)? |
Vectors for HIV proteins,74,134 tests in mice with Cryptosporidium parvum Cp15, profilin, and apyrase expressed in Salmonella Typhi vector135 |
| Plant vaccine | Use of genetically modified plants (with viral and bacterial vectors) to produce antigens. Isolation of the antigen from plant or consummation of the plant | • booster effect? • no cold chain • long shelf life • cheap production |
• correct translation of the antigen information? • enough amount of antigen? • stability/delivery system? |
Tests in mice with rice expressing Cholera and rotavirus antigens79,136,137 |
Antigen variation of gastrointestinal pathogens
Many pathogens causing diarrhea have developed strategies for avoiding recognition by the host immune system and thereby improve their long-term survival. One of these strategies is antigen variation. This method describes the change of antigenic molecules in infectious organisms such as bacterial, fungal and parasitic in response to the immune system of the host. This can be achieved with phase variation, epigenetic modifications, or DNA recombination. Phase variation is characterized by individual particular gene on/off switch. The switch can be made through slipped-strand mispairing at the level of transcription and translation or through only translational regulated mechanisms such as early dissociation of ribosomes and mRNA instability.55 The majority of bacterial phase-variable molecules are surface structures involved in virulence, such as fimbriae (S. Typhimurium, Proteus mirabilis, E. coli), or flagella (S. Typhimurium, Campylobacter coli). Furthermore, multiples serotypes result from variation of LPS such as the O-antigen in Shigella spp56 or the lipooligosaccharide (LOS) of Campylobacter jejuni.57 The O-antigen modification in Shigella flexneri is transmitted by serovar-specific temperate bacteriophages.58
Antigen variation in pathogens may call the generation of multivalent vaccines, and recent examples are the pneumococcal polyvalent polysaccharide vaccines representing 23, or pneumococcal conjugate vaccines (PCV) representing 10 or 13 of the 83 capsule serotypes, respectively.59 Production of polyvalent vaccines is cost intensive, since various antigens have to be produced and combined in amounts sufficiently high to trigger immunity against the important serotypes of the pathogen. The cost-effectiveness of new pneumococcal conjugate vaccines is under debate (example in ref. 60). Given a higher degree of serotype variation, as for example in non-typhoidal Salmonella enterica, generation of polyvalent vaccines may become technical difficult and expensive. Vaccination against a subset of serotypes also imposes selective pressure on pathogen. This may give rise to the infections by serotypes not represented by the polyvalent vaccine, and the need for reformulation of polyvalent vaccines.
Lack of suitable animal models
For Shigella vaccine development a major obstacle is the lack of an animal model that sufficiently resembles pathogenesis of human bacillary dysentery.36 Various animals have been tested for developing disease models. For example, newborn mice were used, but fail in evaluation of protective immunity because of the short timeframe in which the vaccine has to be administrated before the mice is infected.61 One further recently developed animal model is the guinea pig colitis model. After infection with wild-type Shigella strains, an acute inflammation of the colon was observed, mimicking human shigellosis.62 This limitation also applies to a number of other pathogens associated with DID.
Limitations of killed pathogen and subunit vaccines
A general problem of killed pathogen vaccines is the low duration of protection (< 2 y) and the need for repeated vaccine administration after this time.63 This fact is problematic in regions where medical facilities are far away from the clients. The route of administration may partially compensate the lower duration of protection and mucosal immunization appears most efficient.63
Killed pathogen vaccines require the propagation of bacteria, viruses or parasites. Subunit vaccines consist of certain antigenic constituents of pathogens, which can be toxins, capsules or cell envelope components such as LPS. These molecules have a better booster effect comparable to killed pathogen vaccines, for instance the Cholera toxoid vaccine showed a higher immunization than the killed pathogen alone.64 Additionally, these fragments can be used as mono- or polyvalent vaccine or can be combined with inactivated vaccines. However, subunit vaccines are limited to toxin or capsule-producing pathogens. Furthermore, to identify the right toxin or antigen that causes a disease and effective in immunization is time-consuming and requires the proper expression vector and the right pathogen cultivation, a high concentration and an adequate purification. All of these vaccines are based on cultivatable microorganisms, but only a subset of disease-causing bacteria and viruses are so. A practical limitation of many subunits vaccines is the requirement for refrigerated transport chains.
Benefits and limitations of mucosal vaccination
Most microorganisms associated with DID enter the body orally via ingestion of food or water uptake, and the mucosal surface is one of the first body barriers against various pathogens (see Fig. 3). In order to overcome this barrier, or to persist in it, the pathogens need the necessary durability to survive. The ability to survive in this environment leads to initiation and transmission of diseases. To prevent DID at this stage, a local induced immune reaction through mucosal vaccination is of benefit. The mechanism of mucosal protection and reaction in humans against pathogens is described in the section “Mucosal immunization”. For inducing an effective mucosal immune response, a vaccine should be directly delivered to the place of mucosal immune activation. Typical immunization routes for mucosal vaccination are nasal, oral, rectal and vaginal. Effective mucosal vaccines are available for rotavirus and V. cholerae. In particular, the nasal immunization route for mucosal vaccine generates the highest amount of systemic antibodies in human trials and mouse models.65,66 Several advantages of nasal and oral vaccines are known, especially compared with parenteral vaccines, because both mucosal and systematic immune reactions are activated. However, one advantage of the common intramuscularly/subcutaneously administration is the known quantity of vaccine that actually enters the body, plus the measurable amount of antibodies generated and lymphoid cells in the blood.67 Nevertheless, the mucosal immunization enables rapid mass immunization and the needle- and syringe-free administration, which can preserve from infection by HIV, hepatitis B or C.
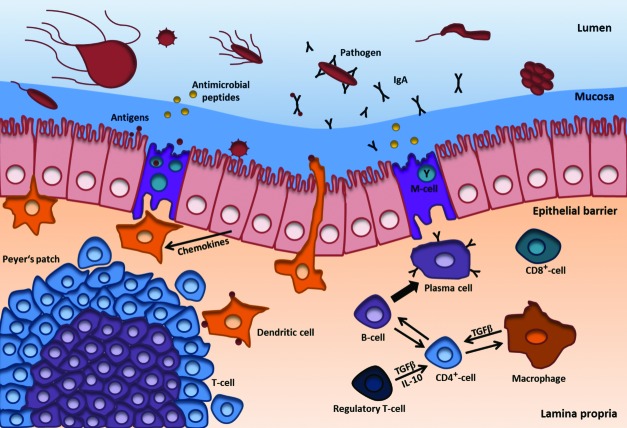
Figure 3. Mucosal immune responses. The gastrointestinal tract (GI) is covered by a mucus layer covering a monolayer of epithelial cells and M-cells that are connected by tight junctions (not shown). Inside the GI pathogens come in contact with the mucus, natural barrier not only against pathogens, but also against the gastric acid in the stomach. Mucus also protects against drying of the nasal mucosa and serves as an adhesion surface for the intestinal flora. Components of the mucus are mucin glycoprotein chains, antimicrobial peptides such as lysozyme, histatine and cystatine, defensins and specific secretory IgA. The mucus is produced from subjacent monolayer of enterocytes; ultimately they form the protective barrier. A further layer is the lamina propria with its gut-associated lymphoid tissues (GALT) such as Peyer’s patches and isolated lymphoid follicles. It also contains a high amount of immune cells such as CD4+- and CD8+-T-cells, B-lymphocytes and plasma cells, dendritic cells and macrophages. For long-term immunization IgA producing plasma cells are very important. M-cells transport antigens via transcytosis to antigen presenting cells such as dendritic cells. These and macrophages interact with various types of T-cells (such as CD4+- and CD8+-cells) in the Peyer’s patches, lamina propria and other lymphatic tissues through their receptors and various signal molecules. After T-cell activation, they also interact with B-cells, which then move to the target side and change into IgA producing plasma cells. IgA is secreted as mono- or dimer and binds to pathogens (e.g., viruses, bacteria and parasites) and antigens. Thus, the adaptive immune response does not proceed in an uncontrolled manner there are also regulatory T-cells. However, there are other defense mechanisms for instance antimicrobial peptides, defensins, digestive enzymes, the complement system and the mucin glycoprotein chains (not shown). Modified according to Macdonald and Monteleone et al. 2005.138
What are challenges in creating new mucosal vaccines? One uncertainty is the administration of the right amount of vaccine. Because of the mucosal host defense mechanisms, such as the attack through proteases, nucleases and the risk for dilution by bulk flow, a high dose is are required.68 Inducing tolerance by low intake of antigens and the host’s own flora are further barriers for designing new vaccines. The lower immunogenicity of oral vaccines in developing nations compared with industrialized countries could be shown in some studies,69-72 underlies probably unknown host factors73 and is also to be considered for development. Application of multimeric and/or particulate vaccines mimicking mucosal adherent pathogens or adjuvants (see below) could increase and compensate the low immune response.67
New Strategies for Vaccine Development
This section describes new strategies, the assets and drawbacks of vaccine candidates. A variety of new vaccines have been designed deploying distinct mechanisms to activate the innate and adaptive immune system.
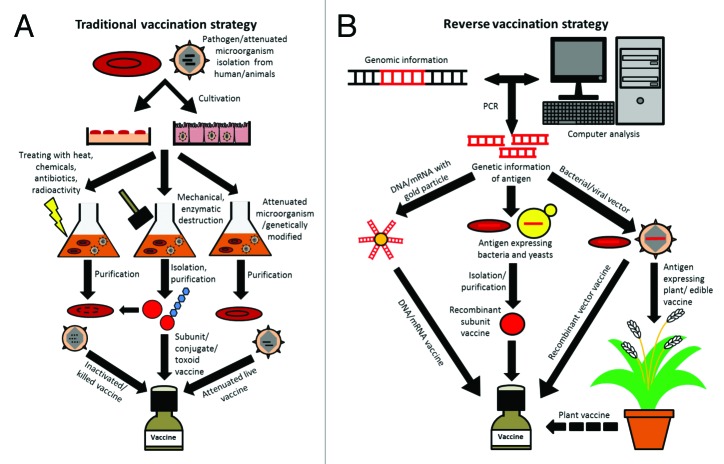
The most known are killed/inactivated pathogen vaccines (V. cholerae), live attenuated vaccines and subunit vaccines (V. cholerae) such as conjugate and toxoid vaccines,74 which all belong in this review to the traditional methods and are based on Pasteur’s principles of isolation, inactivation, injection or additional purification of antigens and vaccination (see Fig. 2A). Further, bacterial subunit vaccines are commonly synthesized in the bacterium. In contrast, viral vaccines such as Rotarix® and Rotateq® against rotavirus are produced in Vero cells, other opportunities can be embryos or tissues, due to a lack of virus metabolism. However, the traditional way to produce new vaccines is lengthy, depends on the cultivation of pathogens and often failed the target, due to insufficient or absent or overreacting immune response.
Figure 2. Traditional vaccine production and reverse genetics strategies. (A) Simplified representation of the individual steps of the traditional vaccine production by isolation of the pathogen over the cultivation, processing, up to the final vaccine. With this approach, different vaccines such as inactivated and attenuated live vaccines or subunit vaccines and special types like conjugate and toxoid vaccines can be produced and combined. Dashed line means destroyed DNA or RNA. (B) Simplified scheme of steps for reverse vaccine manufacturing. Different from traditional methods, reverse vaccinology starts with the analysis of the pathogen genome and epitope libraries searching for possible antigens used for immunization. After cloning/synthesis of the candidate sequence, it is transformed into vectors, antigen-expressing microorganisms or conjugated to gold particles. Vectors then can be used as vaccine or for plant modification. Antigen-expressing plants may be used as edible vaccines or are further processed to plant vaccines. Recombinant subunit vaccines are derived from antigen-expressing microorganisms. DNA- or mRNA-conjugated gold particles are also used as DNA or mRNA vaccines.
A newer approach is the reverse vaccinology; with this strategy a vaccine against N. meningitides has already been found. Based on the genome of an isolated pathogen, genes can be analyzed and via databases and bioinformatics calculations, potential antigen candidates such as surface proteins are identified (see Fig. 2B). These target genes/proteins are synthesized recombinant in bacteria (e.g., E. coli) and injected in mice or other animal models.75 Further, screening of immune sera of various tested antigen candidates can reveal their function as surface proteins and bactericidal effects.75 With the last few candidates further tests and development are performed, which lead to a potential vaccine. By this method, mono- and polyvalent vaccines, DNA (e.g., first attempts with C. parvum), mRNA, recombinant vectors (e.g., first attempts with S. Typhimurium) and genetically modified plants (e.g., first attempts with rice expressing Cholera toxin or rotavirus antigen) can be generated (see Fig. 2B). All of these strategies have advantages and disadvantages as specified in Table 3.
Use of attenuated live vaccines or recombinant vaccines
Unlike inactivated vaccines, live attenuated vaccines have a high potential of long-term immunization, as exemplified by comparing rotavirus vaccines (live attenuated) with Cholera vaccines (recombinant, toxoid). However, live vaccines harbor risks such as back mutations and adverse side effects. Cultivation is required and application to immuno-compromized individuals may be critical. For live attenuated vaccine generation, bacteria and viruses can be isolated from patients without symptoms of disease and are cultivated. Another method is the use of genetically modified microorganisms. The deletion of virulent genes leads to attenuation, but requires a detailed knowledge about the bacteria or virus. In contrast, the transformation of antigen encoding genes into non-pathogenic bacteria or yeast cells results in recombinant proteins, which can be isolated and processed to recombinant vaccines. Important requirements for the production of such vaccines are a save and rapidly growing microorganism with low nutrition claims and correct post-translational modifications. Recombinant vaccines are significantly more secure than live attenuated, have lower side effects, are accessible to all and can be used as mono- or polyvalent vaccines. An example of a recombinant vaccine is the inactivated Cholera vaccine with its recombinant toxin B subunit,26,76 also approaches with Shigella are based on recombinant protein synthesis (see Fig. 1C and Table 2).
Improving mucosal immunization
Another approach to improve vaccines is mucosal immunization, with various advantages such as easy administration even by untrained personnel, less waste by the avoidance of syringes and needles, less pain in application and no risk of infection by contaminated needles. Mucosal vaccine delivery also induces mucosal immunity by stimulation of IgA production (see Fig. 3) and, in some cases, a systemic immunity via IgG production.77 Nonetheless, this type of immunization has also some disadvantages such as a complex composition of adjuvants and delivery systems, which are required to protect the antigen against gastric acid and digestive enzymes. These ingredients and the vaccine itself can cause allergies and other side effects, such as RotaShield against rotavirus.
Administration in form of aerosols is a possible type of mucosal immunization. An example for nasal vaccination is FluMist for influenza vaccination, but there are also orally administered vaccines available for Cholera and rotavirus infections.47,76,78 Other approaches are based on genetically manipulated, edible plants, which expresses the antigen for immunization.79
Improved adjuvants and delivery systems
Additional factors to significantly improve effects of vaccines are adjuvants and delivery systems. Most of the available vaccines are combined with adjuvants, substances that boost the immune response and promote long-term immunity.80 In addition to the booster function, positive effects of adjuvants are reduction of the amount of antigen and increase in absorption.81 Only few adjuvants for human vaccines are licensed, one of the most commonly used is aluminum salt-based adjuvants (alum). Alum stimulate the immune and complement system, but little knowledge about the mechanism is available.82,83 Alum is part of many formulations such as tetanus, diphtheria or hepatitis B vaccines.84,85 Allergic reactions to alum-containing vaccines were observed.86 A further adjuvant is squalene-oil-in-water suspension, as used in Freund’s adjuvants.87 First use was in an influenza vaccine (LUAD, Chiron) and also triggers an immune response.88 Further adjuvants which are used in combating diarrhea are based on non-toxic Cholera toxin B subunit (CTB) from V. cholerae and heat-labile enterotoxin B subunit (LTB) from E. coli, which acts as pentamer and are essential for cell binding.89 CTB has already been used for Cholera vaccine Dukoral®. Both CTB and LTB are well characterized toxin subunits which showed increased mucosal immune response in combination with coupled antigens and are therefore well suitable adjuvants.90 Both bind together with their antigens to the GM1 ganglioside receptor on host cells such as antigen presenting cells and promote immune reaction.90
Beside adjuvants, delivery systems such as liposomes and virosomes are also important for immunization. They protect the vaccine against degradation through gastric acid and proteases and deliver it to the target side. In case of influenza virus, it consists of the influenza virus envelope with its host cell binding and merging features, but without the genetic material.91 Vaccine formulations may require preservatives such as thimerosal, a mercury-containing compound.92 Other components can be amino acids and polysorbate for stabilization, formaldehyde and antibiotics for preventing bacterial growth and phenol and phenoxyethanol as preservatives. However, all these ingredients are often debated points.
How to reliably test vaccine performance?
Before a new vaccine enters the market, many obstacles and regulatory stages must be overcome. The whole process (research and development, preclinical and clinical studies, licensure) takes several years or decades and cost many millions of dollars. Often new vaccine candidates fail in the preclinical phase, because they could not guarantee safety and efficacy. After researchers found and developed a candidate vaccine, preclinical studies with cell cultures, tissues and animals begin. For example, the live, attenuated rotavirus vaccines were cultured and tested in monkey kidney cells MA-104.93,94 However, the main focus is still on animal experiments. Depending on the vaccine and route of administration, different animal models are used. Frequently, experiments are performed in rats, mice, dogs and primates. Parameters that determine the attempt are the species, gender, age and group size.95 Also important elements are the weight and food intake, as it might indicate side effects.
In order to verify vaccine efficacy, more parameters have to be monitored, i.e., (1) antibody titer (ELISA), (2) the dose that leads to an immunization, (3) types of immune cells responding, and (4) adverse effects (toxicity studies). In addition, possible influences of the vaccine on central and immune organs need to be investigated.95 If the vaccine contains a new adjuvants, genotoxicity, carcinogenicity and pharmacokinetic studies have to be performed, in order to determine potential effects on the organism and to verify the effectiveness of the vaccine.95 Interestingly, Cholera vaccines are available on the market, although there was no suitable animal model for analysis of immunization.96
Future Perspectives
Despite various new approaches in developing vaccines, important in preventing DID are also improving hygiene measures and strengthening the immune system of the population at risk. This includes hand washing and disinfection, awareness campaign and, most important, non-contaminated water. Various approaches for water disinfection were implemented, such as filtration though Sari tissues (48% reduction of Cholera infections),97 the Lifestraw family of filters with a pore-size as small as 20 nm,98 and solar disinfection (SODIS) in PET bottles.99 Another possibility to reduce the infection rate is household water chlorination, thus fecal-based contamination could be reduced.100 Furthermore, hygiene education plays the major role. In addition to the hygiene, enhancing the immune system via breast milk feeding (contains maternal antibodies and nutrients) and zinc supplementation (important for enzyme synthesis)101 is also of interest. Although these methods reduce the frequency of infections, but do not necessarily provide protection by immunization.
There is still a lack of vaccines against diarrheal pathogens, especially against parasites. Here, economic constrains are an issue, since parasites are problematic in developing countries, but not in industrialized nations. Vaccines may be too expensive for endemic regions and not profitable for business investment. As a consequence, research will be stopped.
Research and development lasts for decades until the vaccine is on the market. During this time, the antigen composition of the target pathogen may chance, and adjustment of formulations may be required. Furthermore, often trained personnel for vaccination and correct storage places are missing. Lack the long-term immunity after vaccine may explain the need for repeated administration, again rising costs for vaccination campaigns.
Therefore, faster, more effective and more cost-effective vaccine development processes need to be developed. One area which deals with the development of new vaccines is reverse vaccinology. Based on genomic informations and computer analyses (epitope library), possible antigens can be found, modified for most effective immunization and produced recombinant (see Fig. 2B). Promising approaches are DNA-, mRNA-, plant-based and recombinant vector vaccines (see Table 3). Also, in order to enhance the immune response and reduce the administration rate, adjuvants and delivery systems (virosomes, liposomes, etc.) are used. However, in this area is a lot of potential for further vaccine improvement.
Attempts for an easier and safer delivery of vaccines are aerosols/nasal sprays, oral vaccines, special skin patches, powders and liquid jets, thus would reduce needles and trained personnel. Finally, for reducing costs, such as for packaging and refrigeration, plant-based vaccines could be an option, for example in form of rice, which is long lasting and does not need to be cooled and would act simultaneously immunogenic.
These are possible approaches with high potential for the optimization of vaccines and vaccine availability. However, this still requires further research and development.
Supplementary Material
Disclosure of Potential Conflicts of Interest
The Author states he has no conflict of interest
References
- 1.WHO. World Health Report 2006: working together for health. Geneva: World Health Organization, 2006. [Google Scholar]
- 2.WHO. WHO fact sheet N°330. Diarrhoeal disease. WHO, 2013. [Google Scholar]
- 3.Diarrhoea WHO. why children are still dying and what can be done. Geneva: World Health Organization, 2009. [Google Scholar]
- 4.RehydrationProject. Why is diarrhoea dangerous?, 2013.
- 5.Steiner TS, Samie A, Guerrant RL. Infectious diarrhea: new pathogens and new challenges in developed and developing areas. Clin Infect Dis. 2006;43:408–10. doi: 10.1086/505874. [DOI] [PubMed] [Google Scholar]
- 6.Petri WA, Jr., Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–90. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasteur L. De l'atténuation du virus du choléra des poules. CR Acad Sci Paris 1880. C. R. T. 1880;91:673–80. [Google Scholar]
- 8.Salmon D, Smith T. On a new method of producing immunity from contagious diseases. Proc Biol Soc Wash. 1886;3:4. [Google Scholar]
- 9.Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, Ahmed R, Bhan MK, Plotkin SA. Accelerating next-generation vaccine development for global disease prevention. Science. 2013;340:1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamang MD, Sharma N, Makaju RK, Sarma AN, Koju R, Nepali N, Mishra SK. An outbreak of El Tor cholera in Kavre district, Nepal. Kathmandu Univ Med J (KUMJ) 2005;3:138–42. [KUMJ] [PubMed] [Google Scholar]
- 11.López-Gigosos RM, Plaza E, Díez-Díaz RM, Calvo MJ. Vaccination strategies to combat an infectious globe: oral cholera vaccines. J Glob Infect Dis. 2011;3:56–62. doi: 10.4103/0974-777X.77297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam C, Octavia S, Reeves PR, Lan R. Multi-locus variable number tandem repeat analysis of 7th pandemic Vibrio cholerae. BMC Microbiol. 2012;12:82. doi: 10.1186/1471-2180-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine MM. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134:614–27. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. 2008;21:134–56. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holloway G, Coulson BS. Innate cellular responses to rotavirus infection. J Gen Virol. 2013;94:1151–60. doi: 10.1099/vir.0.051276-0. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Immunization and Respiratory Diseases (NCIRD) DoVD. Rotavirus Clinical Information. 2011.
- 17.Ko EA, Jin BJ, Namkung W, Ma T, Thiagarajah JR, Verkman AS. Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut. 2013 doi: 10.1136/gutjnl-2013-305663. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorrot M, Vasseur M. [Physiopathology of Rotavirus diarrhea] Arch Pediatr. 2007;14(Suppl 3):S145–51. doi: 10.1016/S0929-693X(07)80018-2. [DOI] [PubMed] [Google Scholar]
- 19.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee PI, Chen PY, Huang YC, Lee CY, Lu CY, Chang MH, Lin YZ, Chiu NC, Ni YH, Chen CM, et al. Recommendations for rotavirus vaccine. Pediatr Neonatol. 2013;54:355–9. doi: 10.1016/j.pedneo.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 21.von Seidlein L, Jiddawi M, Grais RF, Luquero F, Lucas M, Deen J. The value of and challenges for cholera vaccines in Africa. J Infect Dis. 2013;208(Suppl 1):S8–14. doi: 10.1093/infdis/jit194. [DOI] [PubMed] [Google Scholar]
- 22.Mosley WH, Aziz KM, Mizanur Rahman AS, Alauddin Chowdhury AK, Ahmed A, Fahimuddin M. Report of the 1966-67 cholera vaccine trial in rural East Pakistan. Bull World Health Organ. 1972;47:229–38. [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan ET, Calderwood SB. Cholera vaccines. Clin Infect Dis. 2000;31:561–5. doi: 10.1086/313951. [DOI] [PubMed] [Google Scholar]
- 24.Lucas ME, Deen JL, von Seidlein L, Wang XY, Ampuero J, Puri M, Ali M, Ansaruzzaman M, Amos J, Macuamule A, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005;352:757–67. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- 25.Clemens JD, Harris JR, Sack DA, Chakraborty J, Ahmed F, Stanton BF, Khan MU, Kay BA, Huda N, Khan MR, et al. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J Infect Dis. 1988;158:60–9. doi: 10.1093/infdis/158.1.60. [DOI] [PubMed] [Google Scholar]
- 26.WHO Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–28. [PubMed] [Google Scholar]
- 27.Richie EE, Punjabi NH, Sidharta YY, Peetosutan KK, Sukandar MM, Wasserman SS, Lesmana MM, Wangsasaputra FF, Pandam SS, Levine MM, et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000;18:2399–410. doi: 10.1016/S0264-410X(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 28.Strockbine NAMA. In: Brenner DJ, Krieg NR, Staley TE, editors. Bergey's mannual of systematic bacteriology. New York: Springer, 2005. [Google Scholar]
- 29.Niyogi SK. Shigellosis. J Microbiol. 2005;43:133–43. [PubMed] [Google Scholar]
- 30.Lindberg AA, Kärnell A, Weintraub A. The lipopolysaccharide of Shigella bacteria as a virulence factor. Rev Infect Dis. 1991;13(Suppl 4):S279–84. doi: 10.1093/clinids/13.Supplement_4.S279. [DOI] [PubMed] [Google Scholar]
- 31.Hyams KC, Bourgeois AL, Merrell BR, Rozmajzl P, Escamilla J, Thornton SA, Wasserman GM, Burke A, Echeverria P, Green KY, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325:1423–8. doi: 10.1056/NEJM199111143252006. [DOI] [PubMed] [Google Scholar]
- 32.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–53. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen D, Ashkenazi S, Green MS, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–9. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 34.Robbins JB, Chu C, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis. 1992;15:346–61. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 35.Passwell JH, Ashkenzi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, et al. Israeli Shigella Study Group Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine. 2010;28:2231–5. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kweon MN. Shigellosis: the current status of vaccine development. Curr Opin Infect Dis. 2008;21:313–8. doi: 10.1097/QCO.0b013e3282f88b92. [DOI] [PubMed] [Google Scholar]
- 37.Cam PD, Pál T, Lindberg AA. Immune response against lipopolysaccharide and invasion plasmid-coded antigens of shigellae in Vietnamese and Swedish dysenteric patients. J Clin Microbiol. 1993;31:454–7. doi: 10.1128/jcm.31.2.454-457.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotloff KL, Noriega FR, Samandari T, Sztein MB, Losonsky GA, Nataro JP, Picking WD, Barry EM, Levine MM. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect Immun. 2000;68:1034–9. doi: 10.1128/IAI.68.3.1034-1039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: the role of age at the time of vaccination. J Infect Dis. 2005;192(Suppl 1):S36–43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- 40.Esona MD, Mijatovic-Rustempasic S, Yen C, Parashar UD, Gentsch JR, Bowen MD, LaRussa P. Detection of PCV-2 DNA in stool samples from infants vaccinated with RotaTeq. Hum Vaccin Immunother. 2014;10:25-32. doi: 10.4161/hv.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemming M, Vesikari T. Genetic diversity of G1P[8] rotavirus VP7 and VP8* antigens in Finland over a 20-year period: No evidence for selection pressure by universal mass vaccination with RotaTeq® vaccine. Infect Genet Evol. 2013;19:51–8. doi: 10.1016/j.meegid.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Nakagomi O, Iturriza-Gomara M, Nakagomi T, Cunliffe NA. Incorporation of a rotavirus vaccine into the national immunisation schedule in the United Kingdom: a review. Expert Opin Biol Ther. 2013;13:1613–21. doi: 10.1517/14712598.2013.840285. [DOI] [PubMed] [Google Scholar]
- 43.GlaxoSmithKline. WHO Package insert Rotarix (liquid formulation). 2009.
- 44.FDA USFada. Vaccines, Blood & Biologics - Background on Rotavirus Vaccines. 2010. [Google Scholar]
- 45.FDA USFada. Vaccines, Blood & Biologics - Background on Viral Vaccine Development. 2010. [Google Scholar]
- 46.Merck&CoInc. Package insert RotaTeq. 2008:11.
- 47.Koch J, Wiese-Posselt M, Remschmidt C, Wichmann O, Bertelsmann H, Garbe E, Hengel H, Meerpohl JJ, Mas Marques A, Oppermann H, et al. Background paper to the recommendation for routine rotavirus vaccination of infants in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:957–84. doi: 10.1007/s00103-013-1777-3. [DOI] [PubMed] [Google Scholar]
- 48.Atherly DE, Lewis KD, Tate J, Parashar UD, Rheingans RD. Projected health and economic impact of rotavirus vaccination in GAVI-eligible countries: 2011-2030. Vaccine. 2012;30(Suppl 1):A7–14. doi: 10.1016/j.vaccine.2011.12.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koshy JP. The making of Rotavac Live mint 2013.
- 50.Rippinger CM, Patton JT, McDonald SM. Complete genome sequence analysis of candidate human rotavirus vaccine strains RV3 and 116E. Virology. 2010;405:201–13. doi: 10.1016/j.virol.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gentsch JR, Das BK, Jiang B, Bhan MK, Glass RI. Similarity of the VP4 protein of human rotavirus strain 116E to that of the bovine B223 strain. Virology. 1993;194:424–30. doi: 10.1006/viro.1993.1280. [DOI] [PubMed] [Google Scholar]
- 52.Das BK, Gentsch JR, Hoshino Y, Ishida S, Nakagomi O, Bhan MK, Kumar R, Glass RI. Characterization of the G serotype and genogroup of New Delhi newborn rotavirus strain 116E. Virology. 1993;197:99–107. doi: 10.1006/viro.1993.1570. [DOI] [PubMed] [Google Scholar]
- 53.Das BK, Gentsch JR, Cicirello HG, Woods PA, Gupta A, Ramachandran M, Kumar R, Bhan MK, Glass RI. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32:1820–2. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen X, Cao D, Jones RW, Li J, Szu S, Hoshino Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine. 2012;30:6121–6. doi: 10.1016/j.vaccine.2012.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deitsch KW, Lukehart SA, Stringer JR. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol. 2009;7:493–503. doi: 10.1038/nrmicro2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Q, Lan R, Wang Y, Wang J, Wang Y, Li P, Du P, Xu J. Isolation and genomic characterization of SfI, a serotype-converting bacteriophage of Shigella flexneri. BMC Microbiol. 2013;13:39. doi: 10.1186/1471-2180-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Woude MW, Bäumler AJ. Phase and antigenic variation in bacteria. Clin Microbiol Rev. 2004;17:581–611. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Sun Y, Jia T, Zhang R, Zhang K, Wang L. Messenger RNA vaccine based on recombinant MS2 virus-like particles against prostate cancer. Int J Cancer. 2014;134:1683–94. doi: 10.1002/ijc.28482. [DOI] [PubMed] [Google Scholar]
- 59.Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med 2013; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farkouh RA, Klok RM, Postma MJ, Roberts CS, Strutton DR. Cost-effectiveness models of pneumococcal conjugate vaccines: variability and impact of modeling assumptions. Expert Rev Vaccines. 2012;11:1235–47. doi: 10.1586/erv.12.99. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez MI, Thuizat A, Pedron T, Neutra M, Phalipon A, Sansonetti PJ. A newborn mouse model for the study of intestinal pathogenesis of shigellosis. Cell Microbiol. 2003;5:481–91. doi: 10.1046/j.1462-5822.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 62.Shim DH, Suzuki T, Chang SY, Park SM, Sansonetti PJ, Sasakawa C, Kweon MN. New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J Immunol. 2007;178:2476–82. doi: 10.4049/jimmunol.178.4.2476. [DOI] [PubMed] [Google Scholar]
- 63.Amanna IJ, Slifka MK. Wanted, dead or alive: new viral vaccines. Antiviral Res. 2009;84:119–30. doi: 10.1016/j.antiviral.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamorsky KT, Kouokam JC, Bennett LJ, Baldauf KJ, Kajiura H, Fujiyama K, Matoba N. Rapid and scalable plant-based production of a cholera toxin B subunit variant to aid in mass vaccination against cholera outbreaks. PLoS Negl Trop Dis. 2013;7:e2046. doi: 10.1371/journal.pntd.0002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, Neutra MR. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–74. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 66.Staats HF, Montgomery SP, Palker TJ. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for the induction of systemic and mucosal anti-HIV antibody responses. AIDS Res Hum Retroviruses. 1997;13:945–52. doi: 10.1089/aid.1997.13.945. [DOI] [PubMed] [Google Scholar]
- 67.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 68.Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng. 2012;14:17–46. doi: 10.1146/annurev-bioeng-071811-150054. [DOI] [PubMed] [Google Scholar]
- 69.John TJ, Jayabal P. Oral polio vaccination of children in the tropics. I. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol. 1972;96:263–9. doi: 10.1093/oxfordjournals.aje.a121457. [DOI] [PubMed] [Google Scholar]
- 70.Vesikari T, Isolauri E, D’Hondt E, Delem A, André FE, Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984;1:977–81. doi: 10.1016/S0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- 71.Hanlon P, Hanlon L, Marsh V, Byass P, Shenton F, Hassan-King M, Jobe O, Sillah H, Hayes R, M’Boge BH, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;1:1342–5. doi: 10.1016/S0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- 72.Rill RL, Shaw BR, Van Holde KE. Isolation and characterization of chromatin subunits. Methods Cell Biol. 1978;18:69–103. doi: 10.1016/S0091-679X(08)60134-X. [DOI] [PubMed] [Google Scholar]
- 73.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–39. doi: 10.1093/clinids/13.5.926. [review] [DOI] [PubMed] [Google Scholar]
- 74.NIAID NIoAaID. Health & Research Topics - Vaccines - Types of Vaccines 2012
- 75.Pizza M, Scarlato V, Masignani V, Giuliani MM, Aricò B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–20. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 76.Leung DT, Rahman MA, Mohasin M, Patel SM, Aktar A, Khanam F, Uddin T, Riyadh MA, Saha A, Alam MM, et al. Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin Vaccine Immunol. 2012;19:690–8. doi: 10.1128/CVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen-Jarolim E. Gastrointestinaltrakt: Mukosale Pathophysiologie und Immunologie. Springer, 2006. [Google Scholar]
- 78.MedImmune. FluMist® Quadrivalent (package insert). 2013:25.
- 79.Tokuhara D, Álvarez B, Mejima M, Hiroiwa T, Takahashi Y, Kurokawa S, Kuroda M, Oyama M, Kozuka-Hata H, Nochi T, et al. Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J Clin Invest. 2013;123:3829–38. doi: 10.1172/JCI70266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mastelic B, Garçon N, Del Giudice G, Golding H, Gruber M, Neels P, Fritzell B. Predictive markers of safety and immunogenicity of adjuvanted vaccines. Biologicals. 2013;41:458–68. doi: 10.1016/j.biologicals.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 81.EMEA TEMA. Guideline on adjuvants in vaccines for human use. 2005:18.
- 82.Ohlsson L, Exley C, Darabi A, Sandén E, Siesjö P, Eriksson H. Aluminium based adjuvants and their effects on mitochondria and lysosomes of phagocytosing cells. J Inorg Biochem. 2013;128:229–36. doi: 10.1016/j.jinorgbio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 83.Güven E, Duus K, Laursen I, Højrup P, Houen G. Aluminum hydroxide adjuvant differentially activates the three complement pathways with major involvement of the alternative pathway. PLoS One. 2013;8:e74445. doi: 10.1371/journal.pone.0074445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahboubi A, Fazeli MR, Dinarvand R, Samadi N, Sharifzadeh M, Ilka H, Azadi S, Soleimanian R, Kalkouei H, Hajikhanmirzaei R, et al. Comparison of the adjuvanticity of aluminum salts and their combination in hepatitis B recombinant protein vaccine in assessed mice. Iran J Immunol. 2008;5:163–70. doi: 10.22034/iji.2008.17162. [DOI] [PubMed] [Google Scholar]
- 85.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–93. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171–7. doi: 10.1007/s00431-012-1841-2. [DOI] [PubMed] [Google Scholar]
- 87.Ott G, Rashakrishnan R, Fang J-H, Hora M. The adjuvant MF59: a 10-year perspective. Methods Mol Med. 2000;42:211–28. [Google Scholar]
- 88.WHO. Global Vaccine Safety - Squalene-based adjuvants in vaccines. 2006.
- 89.Basset C, Thiam F, Martino CD, Holton J, Clements JD, Kohli E. Cholera-like enterotoxins and Regulatory T cells. Toxins (Basel) 2010;2:1774–95. doi: 10.3390/toxins2071774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanchez J, Holmgren J. Cholera toxin - a foe & a friend. Indian J Med Res. 2011;133:153–63. [PMC free article] [PubMed] [Google Scholar]
- 91.Huckriede A, Bungener L, Stegmann T, Daemen T, Medema J, Palache AM, Wilschut J. The virosome concept for influenza vaccines. Vaccine. 2005;23(Suppl 1):S26–38. doi: 10.1016/j.vaccine.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 92.Sharpe MA, Livingston AD, Baskin DS. Thimerosal-Derived Ethylmercury Is a Mitochondrial Toxin in Human Astrocytes: Possible Role of Fenton Chemistry in the Oxidation and Breakage of mtDNA. J Toxicol. 2012;2012:373678. doi: 10.1155/2012/373678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Afzal MA, Christian PD, Bentley MN, Zhou TQW. WHO Workshop in Training Performance of Rotavirus Vaccine Potency Testing. 2007:8. [Google Scholar]
- 94.Londrigan SL, Hewish MJ, Thomson MJ, Sanders GM, Mustafa H, Coulson BS. Growth of rotaviruses in continuous human and monkey cell lines that vary in their expression of integrins. J Gen Virol. 2000;81:2203–13. doi: 10.1099/0022-1317-81-9-2203. [DOI] [PubMed] [Google Scholar]
- 95.Griffiths E, Gruber M, Masset D, Verdier F, Wood D, Knezevic I. WHO Guidelines on nonclinical evaluation of vaccines. 2003:27.
- 96.Bishop AL, Camilli A. Vibrio cholerae: lessons for mucosal vaccine design. Expert Rev Vaccines. 2011;10:79–94. doi: 10.1586/erv.10.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huq A, Yunus M, Sohel SS, Bhuiya A, Emch M, Luby SP, Russek-Cohen E, Nair GB, Sack RB, Colwell RR. Simple sari cloth filtration of water is sustainable and continues to protect villagers from cholera in Matlab, Bangladesh. MBio. 2010;1:1. doi: 10.1128/mBio.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boisson S, Kiyombo M, Sthreshley L, Tumba S, Makambo J, Clasen T. Field assessment of a novel household-based water filtration device: a randomised, placebo-controlled trial in the Democratic Republic of Congo. PLoS One. 2010;5:e12613. doi: 10.1371/journal.pone.0012613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.SODIS. SODIS Method. 2009.
- 100.Mengistie B, Berhane Y, Worku A. Household water chlorination reduces incidence of diarrhea among under-five children in rural Ethiopia: a cluster randomized controlled trial. PLoS One. 2013;8:e77887. doi: 10.1371/journal.pone.0077887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182–90. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 102.Clemens J, Shin S, Sur D, Nair GB, Holmgren J. New-generation vaccines against cholera. Nat Rev Gastroenterol Hepatol. 2011;8:701–10. doi: 10.1038/nrgastro.2011.174. [DOI] [PubMed] [Google Scholar]
- 103.Pastor M, Pedraz JL, Esquisabel A. The state-of-the-art of approved and under-development cholera vaccines. Vaccine. 2013;31:4069–78. doi: 10.1016/j.vaccine.2013.06.096. [DOI] [PubMed] [Google Scholar]
- 104.Qadri F, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, Saha A, Al Tarique A, Seidlein LV, Park E, et al. PXV Study Group Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine. 2007;25:231–8. doi: 10.1016/j.vaccine.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 105.García L, Jidy MD, García H, Rodríguez BL, Fernández R, Año G, Cedré B, Valmaseda T, Suzarte E, Ramírez M, et al. The vaccine candidate Vibrio cholerae 638 is protective against cholera in healthy volunteers. Infect Immun. 2005;73:3018–24. doi: 10.1128/IAI.73.5.3018-3024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang W, Wang S, Yu F, Zhang L, Qi G, Liu Y, Gao S, Kan B. Construction and evaluation of a safe, live, oral Vibrio cholerae vaccine candidate, IEM108. Infect Immun. 2003;71:5498–504. doi: 10.1128/IAI.71.10.5498-5504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ledón T, Ferrán B, Pérez C, Suzarte E, Vichi J, Marrero K, Oliva R, Fando R. TLP01, an mshA mutant of Vibrio cholerae O139 as vaccine candidate against cholera. Microbes Infect. 2012;14:968–78. doi: 10.1016/j.micinf.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 108.Ravichandran M, Ali SA, Rashid NH, Kurunathan S, Yean CY, Ting LC, Bakar AS, Lalitha P, Zainuddin ZF. Construction and evaluation of a O139 Vibrio cholerae vaccine candidate based on a hemA gene mutation. Vaccine. 2006;24:3750–61. doi: 10.1016/j.vaccine.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 109.Tacket CO, Losonsky G, Nataro JP, Comstock L, Michalski J, Edelman R, Kaper JB, Levine MM. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–6. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 110.Barnoy S, Jeong KI, Helm RF, Suvarnapunya AE, Ranallo RT, Tzipori S, Venkatesan MM. Characterization of WRSs2 and WRSs3, new second-generation virG(icsA)-based Shigella sonnei vaccine candidates with the potential for reduced reactogenicity. Vaccine. 2010;28:1642–54. doi: 10.1016/j.vaccine.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Orr N, Katz DE, Atsmon J, Radu P, Yavzori M, Halperin T, Sela T, Kayouf R, Klein Z, Ambar R, et al. Community-based safety, immunogenicity, and transmissibility study of the Shigella sonnei WRSS1 vaccine in Israeli volunteers. Infect Immun. 2005;73:8027–32. doi: 10.1128/IAI.73.12.8027-8032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kotloff KL, Taylor DN, Sztein MB, Wasserman SS, Losonsky GA, Nataro JP, Venkatesan M, Hartman A, Picking WD, Katz DE, et al. Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect Immun. 2002;70:2016–21. doi: 10.1128/IAI.70.4.2016-2021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bedford L, Fonseka S, Boren T, Ranallo RT, Suvarnapunya AE, Lee JE, Barnoy S, Venkatesan MM. Further characterization of Shigella sonnei live vaccine candidates WRSs2 and WRSs3-plasmid composition, invasion assays and Sereny reactions. Gut Microbes. 2011;2:244–51. doi: 10.4161/gmic.2.4.17042. [DOI] [PubMed] [Google Scholar]
- 114.Barnoy S, Baqar S, Kaminski RW, Collins T, Nemelka K, Hale TL, Ranallo RT, Venkatesan MM. Shigella sonnei vaccine candidates WRSs2 and WRSs3 are as immunogenic as WRSS1, a clinically tested vaccine candidate, in a primate model of infection. Vaccine. 2011;29:6371–8. doi: 10.1016/j.vaccine.2011.04.115. [DOI] [PubMed] [Google Scholar]
- 115.Kotloff KL, Simon JK, Pasetti MF, Sztein MB, Wooden SL, Livio S, Nataro JP, Blackwelder WC, Barry EM, Picking W, et al. Safety and immunogenicity of CVD 1208S, a live, oral DeltaguaBA Deltasen Deltaset Shigella flexneri 2a vaccine grown on animal-free media. Hum Vaccin. 2007;3:268–75. doi: 10.4161/hv.4746. [DOI] [PubMed] [Google Scholar]
- 116.Katz DE, Coster TS, Wolf MK, Trespalacios FC, Cohen D, Robins G, Hartman AB, Venkatesan MM, Taylor DN, Hale TL. Two studies evaluating the safety and immunogenicity of a live, attenuated Shigella flexneri 2a vaccine (SC602) and excretion of vaccine organisms in North American volunteers. Infect Immun. 2004;72:923–30. doi: 10.1128/IAI.72.2.923-930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oaks EV, Turbyfill KR. Development and evaluation of a Shigella flexneri 2a and S. sonnei bivalent invasin complex (Invaplex) vaccine. Vaccine. 2006;24:2290–301. doi: 10.1016/j.vaccine.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 118.Fries LF, Montemarano AD, Mallett CP, Taylor DN, Hale TL, Lowell GH. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect Immun. 2001;69:4545–53. doi: 10.1128/IAI.69.7.4545-4553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McKenzie R, Walker RI, Nabors GS, Van De Verg LL, Carpenter C, Gomes G, Forbes E, Tian JH, Yang HH, Pace JL, et al. Safety and immunogenicity of an oral, inactivated, whole-cell vaccine for Shigella sonnei: preclinical studies and a Phase I trial. Vaccine. 2006;24:3735–45. doi: 10.1016/j.vaccine.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 120.Xu Q, Cisar JO, Osorio M, Wai TT, Kopecko DJ, Xu de Q Core-linked LPS expression of Shigella dysenteriae serotype 1 O-antigen in live Salmonella Typhi vaccine vector Ty21a: preclinical evidence of immunogenicity and protection. Vaccine. 2007;25:6167–75. doi: 10.1016/j.vaccine.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 121.Weibel RE, Buynak EB, McLean AA, Hilleman MR. Persistence of antibody after administration of monovalent and combined live attenuated measles, mumps, and rubella virus vaccines. Pediatrics. 1978;61:5–11. [PubMed] [Google Scholar]
- 122.FDA USFada. Vaccines, Blood & Biologics - Complete List of Vaccines Licensed for Immunization and Distribution in the US. 2013. [Google Scholar]
- 123.Periaswamy B, Maier L, Vishwakarma V, Slack E, Kremer M, Andrews-Polymenis HL, McClelland M, Grant AJ, Suar M, Hardt WD. Live attenuated S. Typhimurium vaccine with improved safety in immuno-compromised mice. PLoS One. 2012;7:e45433. doi: 10.1371/journal.pone.0045433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Daley A, Randall R, Darsley M, Choudhry N, Thomas N, Sanderson IR, Croft NM, Kelly P. Genetically modified enterotoxigenic Escherichia coli vaccines induce mucosal immune responses without inflammation. Gut. 2007;56:1550–6. doi: 10.1136/gut.2006.112805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turner AK, Stephens JC, Beavis JC, Greenwood J, Gewert C, Randall R, Freeman D, Darsley MJ. Generation and characterization of a live attenuated enterotoxigenic Escherichia coli combination vaccine expressing six colonization factors and heat-labile toxin subunit B. Clin Vaccine Immunol. 2011;18:2128–35. doi: 10.1128/CVI.05345-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Salvador A, Igartua M, Hernández RM, Pedraz JL. An overview on the field of micro- and nanotechnologies for synthetic Peptide-based vaccines. J Drug Deliv. 2011;2011:181646. doi: 10.1155/2011/181646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bramwell VW, Eyles JE, Oya Alpar H. Particulate delivery systems for biodefense subunit vaccines. Adv Drug Deliv Rev. 2005;57:1247–65. doi: 10.1016/j.addr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 128.Donald RG, Flint M, Kalyan N, Johnson E, Witko SE, Kotash C, Zhao P, Megati S, Yurgelonis I, Lee PK, et al. A novel approach to generate a recombinant toxoid vaccine against Clostridium difficile. Microbiology. 2013;159:1254–66. doi: 10.1099/mic.0.066712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kotloff KL, Wasserman SS, Losonsky GA, Thomas W, Jr., Nichols R, Edelman R, Bridwell M, Monath TP. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect Immun. 2001;69:988–95. doi: 10.1128/IAI.69.2.988-995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol. 2012;2:7. doi: 10.3389/fcimb.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lesinski GB, Westerink MA. Novel vaccine strategies to T-independent antigens. J Microbiol Methods. 2001;47:135–49. doi: 10.1016/S0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 132.Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012;11:189–209. doi: 10.1586/erv.11.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Benitez A, Priest JW, Ehigiator HN, McNair N, Mead JR. Evaluation of DNA encoding acidic ribosomal protein P2 of Cryptosporidium parvum as a potential vaccine candidate for cryptosporidiosis. Vaccine. 2011;29:9239–45. doi: 10.1016/j.vaccine.2011.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Virnik K, Ni Y, Berkower I. Live attenuated rubella viral vectors stably express HIV and SIV vaccine antigens while reaching high titers. Vaccine. 2012;30:5453–8. doi: 10.1016/j.vaccine.2012.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manque PA, Tenjo F, Woehlbier U, Lara AM, Serrano MG, Xu P, Alves JM, Smeltz RB, Conrad DH, Buck GA. Identification and immunological characterization of three potential vaccinogens against Cryptosporidium species. Clin Vaccine Immunol. 2011;18:1796–802. doi: 10.1128/CVI.05197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jain A, Saini V, Kohli DV. Edible transgenic plant vaccines for different diseases. Curr Pharm Biotechnol. 2013;14:594–614. doi: 10.2174/138920101131400225. [DOI] [PubMed] [Google Scholar]
- 137.Tokuhara D, Yuki Y, Nochi T, Kodama T, Mejima M, Kurokawa S, Takahashi Y, Nanno M, Nakanishi U, Takaiwa F, et al. Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc Natl Acad Sci U S A. 2010;107:8794–9. doi: 10.1073/pnas.0914121107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.