Abstract
The current gold standard for sperm preservation is storage at cryogenic temperatures. Dry preservation is an attractive alternative, eliminating the need for ultralow temperatures, reducing storage maintenance costs, and providing logistical flexibility for shipping. Many seeds and anhydrobiotic organisms are able to survive extended periods in a dry state through the accumulation of intracellular sugars and other osmolytes and are capable of returning to normal physiology postrehydration. Using techniques inspired by nature's adaptations, attempts have been made to dehydrate and dry preserve spermatozoa from a variety of species. Most of the anhydrous preservation research performed to date has focused on mouse spermatozoa, with only a small number of studies in nonrodent mammalian species. There is a significant difference between sperm function in rodent and nonrodent mammalian species with respect to centrosomal inheritance. Studies focused on reproductive technologies have demonstrated that in nonrodent species, the centrosome must be preserved to maintain sperm function as the spermatozoon centrosome contributes the dominant nucleating seed, consisting of the proximal centriole surrounded by pericentriolar components, onto which the oocyte's centrosomal material is assembled. Preservation techniques used for mouse sperm may therefore not necessarily be applicable to nonrodent spermatozoa. The range of technologies used to dehydrate sperm and the effect of processing and storage conditions on fertilization and embryogenesis using dried sperm are reviewed in the context of reproductive physiology and cellular morphology in different species.
Keywords: : spermatozoa, ambient preservation, dehydration, biostabilization, biobanking
Introduction
The history of sperm cryopreservation began in the 1780s with the first artificial insemination of a canine by Lazzaro Spallanzani, resulting in the birth of three pups.1 Others successfully repeated this work in many species, including humans, through the early 1900s. However, it was not until 1939 with the founding of the American Society of Animal Production (ASAP) that research into semen preservation began. Several different medium formulations were investigated for their effect on post-thaw motility.1 Egg yolk was most notably used as it was observed that the lipids in egg yolk reduced the sensitivity of sperm cells to cold temperatures.1 With the discovery of glycerol as a cryoprotectant, the field of sperm cell cryopreservation advanced considerably, with many successes reported for a range of mammalian species.2 Polge et al.3 were the first to attempt to preserve fowl spermatozoa using glycerol, followed by Sherman4 with bull spermatozoa. The egg yolk–glycerol method is still used as a standard extender for semen cryopreservation today to buffer and protect the sperm cells from cold and osmotic shock, preserving their fertilization ability.
Cryopreservation routinely involves exposing cells to a series of nonphysiological conditions, including immersion in a cryoprotective agent, cooling to a subzero temperature, storage in liquid nitrogen vapor, then thawing and cryoprotectant removal before a return to physiological conditions. The challenge during freezing is not the cell's endurance of ultralow temperatures (−80°C to −196°C), but rather the intermediate temperature zone (−15°C to −60°C) where damaging ice crystals can form.5 Cryoinjury (cell membrane disruption, cytoplasmic fragmentation, DNA damage) can occur during slow cooling as a result of the increasing concentration of solutes in the unfrozen fraction that develops with progressive extracellular ice formation (solution effects) and during fast cooling due to lethal intracellular ice formation.6–9 During cryopreservation, adding a permeating cryoprotectant (e.g., glycerol, dimethyl sulfoxide, 1,2-ethanediol, or 1,2-propanediol) and/or a nonpermeating cryoprotectant (e.g., sucrose, trehalose) can minimize solution effect injury and intracellular ice formation.5,10 With these material advances, cryopreservation has become the gold standard for long-term preservation of many species.
Rapid progress in the field of genetic engineering has led to a steady increase in the number of valuable transgenic and rare genetic models that need to be preserved.11 However, the requirement for liquid nitrogen to maintain cryogenic storage temperatures in specialized containers poses several logistical problems centered on handling, storage, and shipping difficulties, especially for low resource settings where liquid nitrogen and/or dry ice are not readily available. Dry preservation methods have been explored in response to the need for more efficient storage and transport approaches for gametes and to support the fields of reproductive biology, species conservation, and biobanking of research strains of animals.8,11–13 A more effective and efficient stabilization method for nucleated cells would transform these areas of medicine and research. Dehydration and storage of biological samples at nonfreezing temperatures would eliminate the high cost of liquid nitrogen dewars for storage as well as the constant expense of regenerating liquid nitrogen to maintain cryogenic temperatures. Storage in the dehydrated state would also reduce transport volumes and the complexity of shipping.
Inspiration for dry preservation as a strategy for storage of gametes can be found in observations of seeds and anhydrobiotic organisms (e.g., tardigrades, artemia, rotifers). Anhydrobiotic organisms are able to survive the damaging effects of desiccation, with up to 90% water loss, by intracellular synthesis of protective agents such as trehalose.13,14 Mammalian sperm cells lack this in vivo production of trehalose and are desiccation sensitive, therefore trehalose must be loaded intracellularly using biotechnological methods or other engineered strategies.15–19
Advances in reproductive technologies have made possible the fertilization of an oocyte by an immotile, but genetically competent, sperm cell.20 This not only has allowed advances in reproductive science, but it also suggests that there is a minimal set of cellular components that must be adequately preserved for proper fertilization and subsequent embryo development. Understanding the damage that occurs to these critical components during dry preservation is key to determining the safeguards necessary for effective and efficient dry preservation processing and storage. For sperm cells, these considerations are species dependent. This article will review the basics of the science behind dry preservation, the range of technologies used to dehydrate spermatozoa, and the effect of processing and storage conditions on fertilization and embryogenesis using dried spermatozoa. These technological advances will be reviewed in the context of comparative cellular anatomy.
The Science and Technology of Dry Preservation
Learning from nature
Nature has taught us that the concept of dry preservation is possible as seeds and many anhydrobiotic organisms are able to survive extended periods in the dry state.13 Currently, many proteins, bacteria, pharmaceutical drugs, and foods are successfully preserved in the dry state.21,22 Mammalian cells are, by nature, desiccation sensitive and cannot be stabilized in the dry state without the use of biotechnological interventions. The in vivo accumulation of specific solutes in response to stress allows anhydrobiotic organisms to survive an extreme loss of cellular water, up to 90%, and desiccation for extended periods of time with a return to fully functional states with minimal cellular loss.13 Trehalose, a natural sugar comprising two glucose molecules joined by a 1-1 glycosidic bond, is found at particularly high concentrations in many desiccation-tolerant organisms.13 This molecule promotes the formation of amorphous glassy systems, inhibits crystallization, and interacts with biological structures to stabilize them during drying.
Buitink et al., using electron paramagnetic resonance spectroscopy in seeds and pollen, showed that molecular mobility decreases with lowering moisture content during desiccation and then increases when the water content becomes very low.23 Maintaining negligible molecular mobility is critical for long-term storage, and it seems as though there is a threshold, both upper and lower, of moisture content that must be obtained during drying and then maintained during storage to sufficiently stabilize critical cellular components.23 However, motion within glasses involves a variety of molecular dynamics, ranging from atomic vibrations, motion of cages, and secondary or β-relaxation, including Johari−Goldstein (JG) relaxation, to the fully cooperative α-relaxation associated with the transition into a glassy state.24–26 For example, loss of enzymatic activity of proteins embedded in sugar glasses has been shown to not directly correlate with α-relaxation, but instead has been demonstrated to be a function of the fast high-frequency (THz) dynamics or fast β-relaxation of the solvent matrix.25 The relationship between secondary relaxation phenomena and preservation outcome has not been well studied in more complex cellular systems.
Trehalose is thought to provide protection through several mechanisms, including the nonexclusive and complementary theories of vitrification, preferential exclusion, and water replacement.27–33 The vitrification theory proposes that trehalose forms a glassy matrix around the biomolecules that comprise the cell membrane, physically shielding them from stresses.30 Trehalose vitrifies at low water contents and is characterized by an extremely high glass transition temperature (Tg).34,35 Below the Tg, in the viscous state, the viscosity is so high that there is negligible molecular mobility and metabolic function. Trehalose also has a highly stable glycosidic bond, preventing the browning reactions to which other sugars are susceptible.36
In the presence of water, trehalose is capable of transforming to the crystalline dihydrate, allowing it to sequester water, avoiding plasticization in the remaining uncrystallized portion, thus maintaining a high Tg. It should be noted that if preservation of the entire sample is critical, even partial crystallization can cause degradation and be undesirable, requiring samples to be maintained below 44% relative humidity (RH) or otherwise formulated with additional components that suppress crystallization.37
The preferential exclusion theory suggests that there is no direct interaction between trehalose and biomolecules, but that trehalose sequesters the water molecules surrounding the biomolecules, decreasing the hydration radius and increasing compactness and stability during drying.27,32 The water replacement theory asserts that trehalose substitutes for the water molecules bound to and surrounding the biomolecules, maintaining their native structure and therefore maintaining their function.30,32,33 Trehalose has been shown to hydrogen bond with head groups of phospholipid bilayers under dehydration conditions, depressing the gel-to-liquid crystalline phase transition (Tm), which can prevent damaging phase changes during rehydration.29,32
Drying technology
Numerous processing technologies have been explored to prepare cells into a dry state, including lyophilization (or freeze-drying),38–40 convective drying,10 spin drying,41 and microwave-assisted drying.12,42,43 Lyophilization has been historically used in the food and pharmaceutical industries to preserve perishable materials and render them more convenient for transport. In this approach, the material is frozen and then the environmental pressure is reduced to allow frozen water in the material to sublimate directly from the solid phase to the gas phase.
Convective drying involves drying in an environment (usually nitrogen gas) that has a lower water vapor pressure compared with the sample so that drying occurs due to differences in the water vapor pressure between the sample and the environment.10 Convective drying is also used for foods and pharmaceuticals, but freeze-drying is the most common process for drying protein pharmaceuticals, biological standards, and preserving specimens in biological banks.44 However, convective drying has some drawbacks, including long processing time, complex vapor pressures at the surface of concentrated solutes, and the inability to control final moisture content.45
Forced convection, using a gas flow over the sample, can increase drying rates, but such rates are often nonuniform, making them undesirable for biological samples.40 Microwave-assisted or heat-assisted convective drying can circumvent some of these limitations, enabling faster drying times and more controlled and uniform final moisture contents.43
Bacterial cells46 and platelets39 have been successfully lyophilized and survive dry storage. Most recently, microwave-assisted drying has been used to successfully preserve the germinal vesicle in oocytes.43 However, nucleated cells pose additional difficulties with their high level of intracellular complexity, making survival more challenging.8 Cells have membranes, organelles such as mitochondria and nuclei, proteins, and morphologies that need to be maintained to retain proper function. Success with dry preservation of mammalian cells has been somewhat elusive.8,12
Techniques for loading trehalose intracellularly
Previous work has suggested that the presence of the sugar on both sides of the plasma membrane is required for trehalose to be maximally effective as a protectant.15,17–19,47,48 Mammalian cells lack the enzymatic pathways to produce trehalose and therefore trehalose loading is a common first step in processing cells for dehydration. Several methods have been explored to accomplish this, including ATP poration,19 genetically engineered α-hemolysin poration,17 transfection,49 and fluid-phase endocytosis.42,50 In the specific case of spermatozoa, introduction of trehalose has been accomplished through poration of cellular membranes with α-hemolysin, which is added exogenously to create pores in the plasma membranes, allowing trehalose to be loaded into the cytosol through concentration gradients.16
With these advances in loading trehalose, significant progress has been made toward the goal of dry preservation. However, despite the efficient introduction of protective solutes into the cell, the ability to maintain cellular viability is hampered by the low viabilities at low water contents. Regardless of the loading technique, reports of survival are diminished at water contents below 0.2–0.5 g H2O/g DW.8,51 A low level of residual water is required and must be maintained throughout the storage period to achieve viability postrehydration.
Dry Preservation of Spermatozoa
Reproductive technologies influence preservation approaches
The advent of intracytoplasmic sperm injection (ICSI), or the manual injection of a single spermatozoon into an oocyte for in vitro fertilization, has allowed an immotile spermatozoon to fertilize an oocyte, with the first successful fertilization in 1998 showing that an immotile spermatozoon can retain genetic integrity.20 With the use of reproductive technologies, neither the plasma membrane nor motility of spermatozoa is an aspect of the sperm cell that must be preserved to maintain function. ICSI bypasses the need for sperm motility to bind to cells of the female reproductive tract, and the need for spermatozoa to undergo hyperactivation, zona binding, and the acrosome reaction.42–54
Sperm cells remain viable and capable of fertilization through ICSI based on the fact that sperm nuclear integrity is maintained for proper chromatin decondensation, and in some species, centrosomal integrity is maintained for proper sperm aster formation in the zygote.54 It is important to understand the damage that can occur to the critical components of the sperm cell during drying that would render them incapable of performing their necessary tasks for proper embryo development to determine the safeguards and interventions necessary to further develop this technique.
Sperm morphology and function
The mature sperm cell is a terminally differentiated cell, ∼60 μm long and 5 μm wide and completely enveloped by the plasma membrane, a dynamic system resulting from many changes during spermatogenesis in the seminiferous tubules.1,55–57 First of all, the nuclear chromatin is highly compacted during spermatogenesis through the replacement of histones with protamines. During spermiogenesis, cellular volume is then reduced, including the loss of most organelles and cytoplasm, increasing the cell's aerodynamic properties, followed by epididymal maturation where the spermatozoon surface is modified by integration of proteins, glycoproteins, and lipids such as phosphatidylcholine that are significant in the induction of progressive motility, capacitation, and the acrosome reaction.55
The structure of the mammalian sperm cell is broken down into three distinct regions, the head containing the nucleus, the midpiece, and the tail. Sperm motility depends on ATP production by the mitochondria, located in the midpiece, which must produce ample energy in the form of ATP to power the flagellar motion of the tail region to propel the spermatozoa to the ova. Once at the site of fertilization, the roles of the sperm head come into play. The sperm head comprises mainly an acrosome, a large secretory vesicle that overlies the apical region of the sperm head, and a densely packed region of chromatin safeguarding the sperm genetic material.56 Once the spermatozoon head has bound to and penetrated the plasma membrane of the oocyte, several events involving the DNA within the sperm head must take place, including decondensation of the sperm nucleus, exchange of protamines for histones, development of the male and female pronuclei, and pronuclear migration to the center of the oocyte.57
Simultaneous to chromatin decondensation, sperm aster formation must start to take place. In nonrodent mammalian species, the sperm centrosome contributes the dominant nucleating seed consisting of the proximal centriole surrounded by pericentriolar components onto which the oocyte's centrosomal material is assembled.58 With the exception of mitotic spindle, the oocyte lacks any centriolar structure until penetration by the spermatozoon. During oogenesis, the egg centrosome is reduced and inactivated since there should be only one functional centrosome to ensure normal development. During spermiogenesis, there is a partial reduction of the male centrosome, during which the proximal centriole is retained intact in the sperm neck, while the distal centriole progressively degenerates and is partially reduced, eventually merging with the sperm axoneme in the midpiece and tail.2,52,58
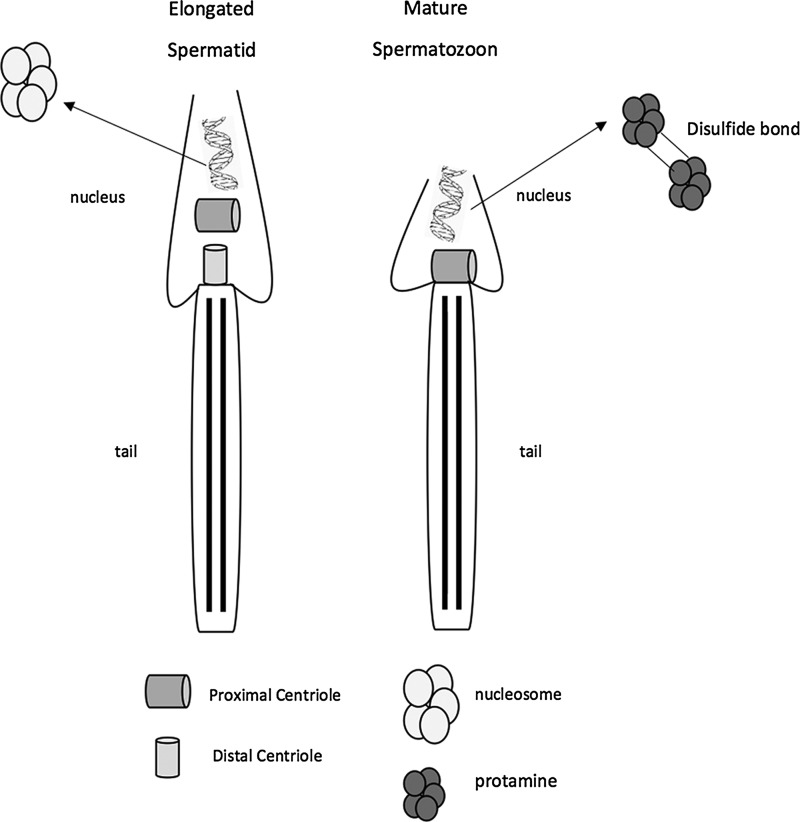
The sperm centrosome, displaying the pinwheel structure of nine triplet microtubules surrounded by pericentriolar components, is primarily responsible for nucleating and organizing the sperm aster, which pushes the sperm head toward the oocyte center and guides migration of the female pronucleus for union with the male pronucleus, completing the fertilization process.53,58,59 It has been shown that poor patterns of aster formation can contribute to delayed first cell division and decreased developmental rates, demonstrating that sperm centrosome maturity, obtained during epididymal maturation, contributes to fertilizing ability and embryo quality.60 Unlike rodent mammalian species that inherit the whole centrosome from the oocyte, nonrodent mammalian species partly inherit the centrosome from the sperm cell (Fig. 1).
FIG. 1.
Illustration of DNA supercompaction and centrosomal reduction during spermatogenesis. Supercompaction of the nuclear DNA is achieved through the replacement of lysine-rich histones with smaller more basic arginine and cysteine-rich protamines. Protamines further compact the DNA through the formation of disulfide bonds during maturation through the epididymis. In nonrodent mammalian species, there is partial reduction of the male centrosome. The distal centriole is reduced during spermatogenesis, resulting in the presence of only the proximal centriole in the mature spermatozoon.
Molecular Damage in Spermatozoa and Its Contribution to Preservation Induced Injury
DNA injury
ICSI can overcome membrane damage and loss of motility, but cannot overcome DNA damage, one of the most important components of the reproductive outcome in the spermatozoa. It has been clinically established that sperm DNA damage is positively correlated with lower fertilization outcomes and embryo development in both natural and technology-assisted reproduction.61–63 The two characteristics that differentiate sperm cells from somatic cells are protamination and DNA repair mechanisms.64 The dense compaction of sperm DNA provides protection to the DNA from a wide range of assailants that might impart fragmentation. The normal and necessary processes of meiotic crossing over and the replacement of histones with protamines to supercoil the sperm DNA impart levels of temporary DNA fragmentation to the cells, which if unfixed, evolve into DNA fragmentation of mature spermatozoa.64,65 These levels of damage are typically repaired during epididymal transit and before ejaculation, but unrepaired damage leads to permanent DNA fragmentation.65 DNA repair in spermatozoa is terminated as transcription and translation stop postspermatogenesis, therefore sperm cells have no means of repairing DNA damage that occurs during migration through the epididymis and postejaculation.60,64,66,67
It has been shown that oocytes and early embryos can help repair sperm DNA damage, so the effect of sperm damage on the developing embryo depends on both sperm chromatin damage and the ability of the oocyte to repair some of this damage.68 Fertilization of an oocyte by a spermatozoon damaged by extensive double-stranded DNA fragmentation can be incompatible with complete fertilization and embryo development. Activation checkpoints during embryogenesis slow down cell cycle progression until DNA damage is resolved, and if it remains unrepaired, cellular senescence and apoptosis are initiated.64
Studies in some mammalian species (rodent, nonrodent, and human) found that sperm exposure to high oxidant environments causes high levels of DNA damage. This damage did not necessarily minimize the ability of the spermatozoon to fertilize an oocyte, but showed a detrimental effect on embryo development.68–72 When this DNA damage in the sperm cell is not completely repaired by the oocyte and resulting zygote, and embryo progression continues, the damage gets passed on to the resulting embryo, transitioning any pathology associated with the unrepaired damage to the offspring. Most oxidative damage research has focused on plasma membrane and acrosome damage, and not nuclear DNA integrity; however, we know that fragmentation of genomic DNA is a hallmark of apoptosis, the most common form of eukaryotic cellular death.73 Some work specific to cats has examined antioxidant capacity to ameliorate ROS DNA damage generated during cryopreservation, and it was found that the addition of 5 mM cysteine to the resuspension medium increased DNA integrity at 6 hours post-thaw.74
Mitochondrial DNA is also present in the sperm cell and should be noted as a source of additional ROS generation and site of damage in the cell. The likelihood of damage to the sperm mitochondrial DNA during the life span of the sperm cell is so great that they are ubiquitinated and destroyed in the oocyte after fertilization to avoid any possibility of contributing to the mitochondrial content of the embryo.68 Uniparental mitochondrial inheritance is common to all eukaryotic species, but it should be noted that in humans, the incidence of injury was found to be greater in mitochondrial DNA than in nuclear DNA, proposing that mitochondrial damage may be recognized earlier and may be indicative of later DNA damage.73
Centrosomal damage
In nonrodent mammalian species, the sperm centrosome is the second critical component of the sperm cell necessary for function and fertilization. Defects in proper centrosomal function have been documented to have serious consequences on fertilization and embryo development.60,75 The mature MII oocyte supplies the molecular machinery that separates oocyte chromosomes postfertilization, allowing the extrusion of half of the chromosomes into the second polar body and retention of the other half to form the female pronucleus.75 The spermatozoon contributes the centriole–centrosome complex, which has been reduced during spermatogenesis, but has retained the functional proximal centriole required for sperm aster formation, for zygote nuclear formation, and for the bipolar mitotic apparatus that forms after centriolar duplication during the pronuclear stage, as well as critical centrosomal proteins (tubulin) required for the recruitment of additional centrosomal proteins (tubulin) from the oocyte.75
The sperm centriolar complex consists of a proximal centriole surrounded by centrosomal proteins, tubulin, pericentrin, and centrin, and a distal centriole that is degraded postfertilization. Research is ongoing concerning the complexity, functionality, and pathologies associated with the centrosome and surrounding centrosomal components. It has been reported that insufficient tubulin or centrin proteins in the sperm centriolar complex result in abnormal sperm aster formation and decreased fertilization.60,75,76 In the domestic cat, decreases in the size of the sperm aster formed postfertilization have been correlated with delayed first cleavage divisions, slower developmental rates, and reduced morula and blastocyst formations.60 These poor developmental patterns can be reversed by replacing the centrosome, at the time of ICSI fertilization, with a more mature one, indicating that centrosomal maturation and function are essential to proper development of the embryo.60
Sperm developmental stage and its relationship to reproductive outcome
There is a level of DNA fragmentation damage that is initiated in the sperm cell during the necessary processes of chromatin repackaging during spermatogenesis and these levels of damage are typically repaired during epididymal transit and before ejaculation, and as discussed previously, unrepaired damage leads to permanent DNA fragmentation in the resulting spermatozoa.65 For species conservation purposes and also for some procedures in humans, spermatozoa removed from the testes can be an alternative to ejaculated spermatozoa.
To achieve the best reproductive outcome, several aspects must be considered, including which spermatozoa have the least amount of DNA damage, which have the highest embryo growth potential, and which set of spermatozoa are more easily available, especially when considering wild or endangered species. It has been shown in cats that testicular spermatozoa injected into in vitro matured cat oocytes have proper, but lower, embryo growth potential (11%) than that of ejaculated sperm (21%). This shows that domestic cat testicular sperm are capable of supporting proper embryo development and that even though there are complex mechanisms involving epididymal maturation that are beneficial for conventional fertilization, these processes are not absolutely necessary to produce blastocyst embryos in the cat model.60
Studies assessing cat chromatin stability and resistance of the DNA to acidic denaturation have been evaluated in different regions of the epididymis—caput/head receiving spermatozoa from the testis through the efferent ducts, moving to the corpus/body, then to the cauda/tail. These data showed that as spermatozoa progressed from the caput to the cauda regions, the percentage of sperm heads with persistent histones through aniline blue staining decreased from 31.8% to 7.8%, indicating a further compaction of the chromatin through protamine replacement, increasing stability and decreasing exposure to damage. These data also showed that chromatin stability in the form of dsDNA through acridine orange staining increased significantly from the caput (51.1%) to the cauda (86.5%) regions of the epididymis.77 These data suggest that epididymal spermatozoa collected from the caudal epididymis have the best embryo growth potential outside of ejaculated spermatozoa.
Dry preservation of rodent spermatozoa
Most of the sperm dry preservation research conducted to date has been centered on mouse spermatozoa, with only a small number of studies in nonrodent mammalian species, as summarized in Table 1. Bhowmick et al. reported fetal mouse production using forced convective drying of mouse spermatozoa to an end moisture content of less than 5% with nitrogen gas at ambient temperatures.10 The resulting mean moisture contents achieved were variable at 3.6% ± 1.1% (rapid), 1.5% ± 0.6% (moderate), and 1.2% ± 0.3% (slow), with the sample spread the highest for rapid drying, indicating less controlled drying. Despite the variability, the success was highest for rapidly dried samples, yielding 13% fetal production at day 15 of gestation compared with 40% in control experiments that used fresh spermatozoa. In this study, it was reported that the final moisture content (5%–7%) did not affect blastocyst formation as much as the drying rate.10 Samples were not stored for longer than 24 hours.
Table 1.
Fertilization Outcomes with Dried Sperm Organized by Species, Drying Method, and Storage Conditions, Including Temperature and Duration of Storage
| Species | Method | Tstorage (°C) | Duration | % Blastocyst | Pregnancy outcome | Reference no. |
|---|---|---|---|---|---|---|
| Mouse | CD | 4 | 18–24 Hours | 64 | 11 Fetuses on day 15 from 85 embryos | 10 |
| 4 | 1 Month | 63 | 3 Pups from 40 embryos (7.5%) | 78 | ||
| 4 | 1 Year | 30.4 | 2 Pups from 38 embryos (5%) | 81 | ||
| 22 | 3 Months | 12.2 | 2 Pups from 59 embryos (3%) | |||
| 4 | 2 Years | 41.4 | 82 | |||
| 22 | 2 Years | 3 Pups from 51 embryos (6%) | ||||
| FD | 4 | 9 Pups from 32 embryos (21%) | 85 | |||
| 25 | 16 Pups from 86 embryos (19%) | |||||
| 4 | 6 Months | 13 | 40 | |||
| 4 | 6 Months | 50 | 79 | |||
| 4 | 5 Months | 9 Pups from 43 embryos (21%) | 80 | |||
| 4 | 1 Year | 22 Fetuses on day 15 from 60 embryos | 83 | |||
| 4 | 1.5 Years | 15 Pups from 72 embryos (21%) | ||||
| 4 | 3 Years | 17 Pups from 92 embryos (47%) | 84 | |||
| Rat | FD | 4 | 1 Year | 7 Pups from 102 embryos (7%) | 86 | |
| 4 | 1 Year | 3 Pups from 19 embryos (16%) | 88 | |||
| 4 | 5 Years | 10 Pups from 92 embryos (11%) | 89 | |||
| Cat | FD | 4 | <72 Hours | 27.9 | 90 | |
| Rabbit | 4 | 12–24 Months | 24 | 1 Dead kit from 230 embryos (0.4%) | 91 | |
| Horse | 4 | 3.5 Months | 5 Pregnancies from 7 embryos, 2 foals from 3 gestations | 92 | ||
| Bull | 4 | 1–3 Months | 29.6 | 93 | ||
| CD + heat | 4 | 1 Month | 14 | 94 | ||
| 25 | 7–10 Days | 5 | ||||
| Pig | FD | 4 | 1 Month | 10.7 | 95 | |
| Monkey | 25 | 1–2 Months | 25 | 96 |
CD, convective drying; FD, freeze-drying.
This group later reported on the function of mouse sperm cells convectively dried with nitrogen gas, with and without exposure to trehalose, using α-hemolysin to create transmembrane channels in the sperm plasma membrane.78 Their findings showed that the percentage of blastocysts produced following ICSI was significantly higher at all drying times than those without trehalose, indicating that drying in the presence of trehalose helped sperm cells to retain their developmental potential. They also observed that dried spermatozoa stored at 4°C produced more blastocysts at all storage times than those stored at 22°C, but the percentage of eggs forming blastocysts decreased with the storage time of sperm at both temperatures. The temperature-dependent degradation with time results suggest that the matrix may be slowing, but not suspending, molecular motion, pointing to opportunities for further composition and process improvements. These blastocyst percentages were within the range of those reported for lyophilized mouse spermatozoon samples stored for 6 months at 4°C.40,79
Implantation successes from trehalose-loaded convectively dried spermatozoa, stored for 1 and 3 months at 4°C, were 81% and 48% with live-born outcomes of 26% and 5%, respectively.78 For comparison, the live-born outcome obtained with lyophilized samples stored in a different medium (without trehalose) for 5 months at 4°C was reported at 9%.80 Using similar methodology and compositions, Li et al. investigated sperm storage lengths of 1 week to 5 months at 4°C, −20°C, and −80°C.16 Fertilization outcomes were not significantly different for any combination of storage length or temperature. Blastocyst formation was also not significantly different at any storage temperature for a storage length of 1 week or 1 month.
For a sperm storage period of 3 months, the percentage of oocytes that formed blastocysts decreased as storage temperature increased (74.4%, 54.3%, 35.1%).16 Storage of sperm for 5 months at 4°C before ICSI yielded a significant drop in blastocyst formation (10.2%), whereas results obtained after storage of spermatozoa at −20°C and −80°C for 5 months were not significantly different than 3-month storage outcomes, suggesting that at subzero temperatures, the molecular mobility of samples was reduced to the extent that long-term storage might be possible. Regression analysis indicated significant deterioration at 4°C, slightly less at −20°C, and no degeneration of sperm fetal production ability at −80°C.16
These findings showed that developmental potential (protection of genetic material and proteins required for oocyte activation and embryonic development) of dehydrated mouse spermatozoa could be maintained at some level, and that drying time, storage temperature, and length of storage play a large role in the level of developmental potential that can be preserved. Further storage advances were made by optimizing the drying media used during convective drying and by precise control over moisture content during storage.
Liu et al. reported blastocyst formation outcomes of 30.4% for sperm samples convectively dried in 3-O-methyl-d-glucose media and stored in a 12% RH environment for 12 months at 4°C and 12.2% for samples stored at 22°C.81 In a separate study, they were able to demonstrate ambient storage of convectively dried spermatozoa for up to 2 years, with a live birth outcome of 5.9%.82 These percentages are lower than those observed for lyophilized samples stored at 4°C for 1.5–3 years,83,84 but they are the highest outcomes observed thus far for storage at temperatures above 20°C. Wakayama et al. reported a birth outcome of 18.6% with lyophilized mouse spermatozoa that were stored for 1 month at 25°C, but these studies indicated that samples were progressively degrading during storage.85 Taken together, these studies suggest that successes with convective drying methods are approaching those of the more mature lyophilization approach and may be superior for room temperature storage conditions.
Successes have also been reported for lyophilized rat spermatozoa, but live births have not been achieved for storage temperatures above 4°C. Hochi et al. reported a live birth outcome of 7% for dried spermatozoa from Crjlj:Wistar rats that had been stored for 12 months at 4°C, but no pups were generated from equivalent samples stored at 25°C.86 High levels of chromosomal abnormalities were reported in the samples stored at ambient conditions,86 even though the pH of the media used (8.0) was consistent with the level that was shown to limit chromosomal damage in mice.87 Kaneko et al. were also able to generate pups at a level of 16% with equivalently stored lyophilized spermatozoa (1 year, 4°C) from Wistar rats, using optimized postinjection culture media.88 They later extended this storage time at 4°C to 5 years, with a 11% live birth outcome, indicating that degradation during storage at this temperature was minimal.89 Although these outcomes were lower than those achieved in mice, the difference could not be attributed to DNA damage. The authors suggested that further optimization of ICSI and embryo culture methods might improve outcomes.89
Dry preservation of nonrodent spermatozoa
There is a significant difference between sperm function in rodent and nonrodent mammalian species with respect to centrosomal inheritance, with rodent mammalian species inheriting the centrosome from the oocyte and nonrodent mammalian species inheriting the centrosome from the spermatozoa. Most of the sperm dry preservation work that has been conducted in nonrodent mammalian species has utilized freeze-drying, with examples in cat,90 rabbit,91 horse,92 bull,93,94 pig,95 and primate.96 Studies on lyophilized cat spermatozoa have only been taken out to the 8-day/blastocyst stage, with storage limited to 72 hours. When oocytes were activated with ethanol to override deficiencies in the ability of freeze-dried spermatozoa to induce the required intracellular calcium spikes, a blastocyst outcome of nearly 28% was achieved compared with 54.6% in controls.90 Freeze-dried cat spermatozoa displayed evidence of surface damage, broken membranes, and decapitation. Morphological damage of this nature has also been observed in freeze-dried mouse spermatozoa,85 but this did not limit the ability to produce pups, possibly due to the fact that the paternal centrosome is not critical for fertilization in this species. Kaneko recently demonstrated that freeze-dried Jaguar spermatozoa injected into mouse oocytes could form pronuclei, demonstrating promise for extending this approach to endangered cat species.97 Further studies to investigate DNA damage during drying as well as a determination of pregnancy outcomes with dried cat spermatozoa are warranted.
Studies in rabbit are also sparse, but Liu et al. were able to achieve the production of one full-term, but dead, kit from lyophilized sperm of 230 embryos that were transferred.91 Only spermatozoa that survived lyophilization with the tails intact were used, ensuring that the proximal centrosome was also injected. Many rehydrated spermatozoa presented with missing tails and the axenome in the neck and mid-piece regions exposed. Activation of the oocytes was also necessary, suggesting that lyophilization may have caused damage to sperm-born oocyte-activating factor or other critical features. The damaged spermatozoon plasma membrane was also observed to persist longer after spermatozoon injection, which the authors suggested may be due to chemical changes that occurred during processing.91 Blastocyst formation levels approached that of controls, highlighting the need to understand damage to factors that are responsible for continued development past the blastocyst stage. Given the low success rate of ICSI with fresh spermatozoa (<4%), efforts to expand this approach might need to focus first on optimizing ICSI procedures.
In larger animals, most studies have focused on early fertilization events and have not progressed to an evaluation of pregnancy outcomes, with the one exception being the horse. Choi et al. obtained blastocyst levels of 6% using horse semen that was lyophilized in the presence of sperm cytoplasmic extract, compared with 28% in controls, with five of seven embryo transfers yielding a pregnancy.92 The first live birth using freeze-dried semen from a nonlaboratory animal was also reported, with two of three pregnancies resulting in live births of healthy foals.92 The parentage testing of foals indicated that one originated from the intended lyophilized spermatozoa and the other from the sperm extract.
Keskintepe et al. obtained blastocyst outcomes of 29.6% when using lyophilized bovine spermatozoa, but optimized oocyte activation protocols were necessary to overcome damage to the spermatozoa.93 The use of heat to dry bovine spermatozoa has also been attempted, with fertilization and blastocyst development observed to varying degrees based on drying temperature, storage length, and storage time.94 Lee et al. reported blastocyst outcomes approaching 15% when using bovine spermatozoa dried for 8 hours at 50°C and stored for 7–10 days at 4°C.94 Storage of sperm beyond 1 month at 4°C resulted in a large decrease in blastocyst formation. Later work by Hara et al. achieved a cleavage outcome of 36%, but with a much reduced blastocyst outcome of 1%.95 This study investigated the effect of freeze-drying on DNA fragmentation, sperm aster formation, and the microtubule network. Results were comparable between fresh and freeze-dried spermatozoa that were used for ICSI. The microtubule-organizing center was more compromised in ICSI-fertilized oocytes in general compared with in vitro fertilized eggs.98 Strategies to overcome deficiencies in microtubule assembly were recently reviewed by Hochi, which may improve ICSI outcomes with both frozen and freeze-dried bovine spermatozoa.99 Modest fertilization success with lyophilized spermatozoa has been reported in pigs. A blastocyst outcome of 10.7% was achieved for lyophilized spermatozoa that had been stored at 4°C for 1 month, but samples stored at 25°C did not progress beyond the morula stage.97
A limited study in primates has shown that early reproductive potential is preserved in freeze-dried monkey spermatozoa stored for 1–2 months in the dark at room temperature, with a blastocyst outcome of 25% achieved with both freeze-dried spermatozoa and fresh controls.96 Trehalose media were shown to be essential for preserving the proximal centriole. Samples dried in trehalose media also presented with normal microtubule asters, acrosomal contents were retained, and some mitochondrial function was preserved. Despite the small sample sizes, this study demonstrated the importance of trehalose drying media for preventing damage to the sperm head, thus preserving the centrosomal function necessary for oocyte fertilization and development. In contrast to other studies, oocyte activation with chemicals was not necessary for samples preserved in trehalose. To the authors' best knowledge, fertilization of eggs with freeze-dried human spermatozoa has not been attempted, but recent studies show that DNA and chromosomal integrity are preserved during freeze-drying.100,101
Conclusions and Next Steps for the Field
With the use of reproductive technologies such as sperm injection, neither the plasma membrane nor motility of the spermatozoa must be preserved to maintain function. DNA integrity, on the other hand, must be maintained as delivering an intact set of chromosomes to the oocyte is the sole purpose of a mature sperm cell. For nonrodent mammalian species, the mature sperm cell also provides the centrosome to the oocyte, the structure of which must also be maintained for proper sperm function. Understanding the damage to these critical components during the dry preservation process is an important aspect of developing a functional dry preservation protocol.
Studies in both rodent and nonrodent species have demonstrated that with an appropriate choice of media, DNA integrity can be conserved during freeze-drying.89,95,100–102 Structural damage to the sperm head can result in a loss of fertilization ability, some of which can be recovered by utilizing exogenous chemicals to induce calcium signaling.90,91,93 Optimization of freeze-drying media may reduce this type of injury.96 There are also many underexplored options in terms of the drying modality and many processing considerations that can be optimized, including establishing the optimal moisture content to minimize degradation during storage and developing storage systems to maintain these levels.103,104 Optimization of drying and rehydration media and postinjection culture conditions can also lead to increased success rates.78,81,87,88,96
Initial attempts to optimize drying media have mainly focused on sugars, with successes reported with Trehalose and 3-O-methyl-d-glucose, but there are many other natural protectants that can be evaluated in future studies, including the late embryogenic abundant proteins and many other compatible osmolytes that occur naturally in desiccation-tolerant organisms.13,105 Successful methodologies developed for rodent species can be used as a starting point for nonrodent sperm models, but as the limited studies have shown, differences between species exist not just in the response to freeze-drying but also the amenability to ICSI.91,98 Although considerable work is still needed, early results show promise that dry preservation methodologies can serve as a more economical method for biobanking of spermatozoa from a range of species.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Walters EM, Benson JD, Woods EJ, Critser JK. The history of sperm cryopreservation. In: Pacey AA, Tomlinson MJ. (eds). Sperm Banking: Theory and Practice. New York: Cambridge University Press; 2009: 1–17 [Google Scholar]
- 2.Sathananthan A, Ratnasooriya W, de Silva P, Menzes J. Characterization of human gamete centrosomes for assisted reproduction. Ital J Anat Embryol 2001;106:61–73 [PubMed] [Google Scholar]
- 3.Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949;164:666. [DOI] [PubMed] [Google Scholar]
- 4.Sherman JK. Freezing and freeze-drying of human spermatozoa. Fertil Steril 1957;5:357–371 [DOI] [PubMed] [Google Scholar]
- 5.Mazur P. Equilibrium, quasi-equilibrium, and nonequilibrium freezing of mammalian embryos. Cell Biophys 1990;17:53–92 [DOI] [PubMed] [Google Scholar]
- 6.Sherman JK, Liu KC. Ultrastructure before freezing, while frozen, and after thawing in assessing cryoinjury of mouse epididymal spermatozoa. Cryobiology 1982;19:503–510 [DOI] [PubMed] [Google Scholar]
- 7.Gao D, Critser JK. Mechanisms of cryoinjury in living cells. Inst Anim Lab Res J 2000;41:187–196 [DOI] [PubMed] [Google Scholar]
- 8.Oliver A. Dry state preservation of nucleated cells: Progress and challenges. Biopreserv Biobank 2012;10:376–385 [DOI] [PubMed] [Google Scholar]
- 9.Di Santo M, Tarozzi N, Nadalini M, Borini A. Human sperm cryopreservation: Update on techniques, effect on DNA integrity, and implications for ART. Adv Urol 2012;13:837–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storey BT, Noiles EE, Thompson KA. Comparison of glycerol, other polyols, trehalose, and raffinose to provide a defined cryoprotectant medium for mouse sperm cryopreservation. Cryobiology 1998;37:46–58 [DOI] [PubMed] [Google Scholar]
- 11.Bhowmick S, Zhu L, McGinnis L, Lawitts J. Desiccation tolerance of spermatozoa dried at ambient temperature: Production of fetal mice. Biol Reprod 2003;68:1779–1786 [DOI] [PubMed] [Google Scholar]
- 12.Cellemme S, Van Vorst M, Paramore E, Elliott GD. Advancing microwave technology for dehydration processing of biologics. Biopreserv Biobank 2013;5:278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowe JH, Crowe LM. Preservation of mammalian cells–learning nature's tricks. Nat Biotechnol 2000;18:145–146 [DOI] [PubMed] [Google Scholar]
- 14.Clegg JS. Desiccation tolerance in encysted embryos of the animal extremophile, Artemia. Integr Comp Biol 2005;45:715–724 [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Acker JP, Eroglu A, Cheley S, Bayley H. Beneficial effects of intracellular trehalose on the membrane integrity of dried mammalian cells. Cryobiology 2001;43:168–181 [DOI] [PubMed] [Google Scholar]
- 16.Li MW, Biggers JD, Elmoazzen HY, Toner M, McGinnis L, Lloyd KC. Long-term storage of mouse spermatozoa after evaporative drying. Reproduction 2007;33:919–929 [DOI] [PubMed] [Google Scholar]
- 17.Eroglu A, Russo MJ, Bieganski R, et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol 2000;18:163–167 [DOI] [PubMed] [Google Scholar]
- 18.Eroglu A, Toner M, Toth TL. Beneficial effects of microinjected trehalose on the cryosurvival of human oocytes. Fertil Steril 2002;77:152–158 [DOI] [PubMed] [Google Scholar]
- 19.Elliott G, Liu XH, Cusick JL, Menze M, Vincent J. Trehalose uptake through P2X7 purinergic channels provides dehydration protection. Cryobiology 2006;52:114–127 [DOI] [PubMed] [Google Scholar]
- 20.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:8–17 [DOI] [PubMed] [Google Scholar]
- 21.Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm 2000;203:1–60 [DOI] [PubMed] [Google Scholar]
- 22.Sramek M, Schweiggert RM, van Kampen A, Carle R, Kohlus R. Preparation of high-grade powders from tomato paste using a vacuum foam drying method. J Food Sci 2015;80:E1756–E1762 [DOI] [PubMed] [Google Scholar]
- 23.Buitink J, Claesssens N, Hemminga MA, Hoekstra F. Influences of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant Physiol 1998;118:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu L, Zhang C, Yue Y, Bian X. A new threshold of uncovering the nature of glass transition: The slow β relaxation in glassy states. Chin Sci Bull 2010;55:457–472 [Google Scholar]
- 25.Cicerone MT, Douglas JF. β-Relaxation governs protein stability in sugar-glass matrices. Soft Matter 2012; 8:2983–2991 [Google Scholar]
- 26.Weng L, Elliott GD. Local minimum in fragility for trehalose/glycerol mixtures: Implications for biopharmaceutical stabilization. J Phys Chem B 2015; 119:6820–6827 [DOI] [PubMed] [Google Scholar]
- 27.Jain N, Roy I. Effects of trehalose on protein structure. Protein Sci 2009;18:24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hengherr S, Heyer AG, Kohler HR, Schill RO. Trehalose and anhydrobiosis in tardigrades–evidence for divergence in response to dehydration. FEBS J 2007;275:281–288 [DOI] [PubMed] [Google Scholar]
- 29.Crowe JH. Trehalose as a “chemical chaperone”: Fact and Fantasy. In Csermely P, Vígh L. (eds). Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks. New York: Springer Science and Business Media; 2007: 143–158 [DOI] [PubMed] [Google Scholar]
- 30.Crowe JH, Carpenter JF, Crowe LM. The role of vitrification in anhydrobiosis. Annu Rev Physiol 1998;60:73–103 [DOI] [PubMed] [Google Scholar]
- 31.Timasheff SN. The control of protein stability and association by weak interactions with water: How do solvents affect these processes? Annu Rev Biophys Biomol Struct 1992;22:67–97 [DOI] [PubMed] [Google Scholar]
- 32.Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984;223:701–703 [DOI] [PubMed] [Google Scholar]
- 33.Clegg JS. The physical properties and metabolic status of Artemia cysts at low water contents: The ‘water replacement hypothesis’. In Leopold CA. (ed). Membranes, Metabolism, and Dry Organisms. London: Comstock Publishing Association; 1986:169–187 [Google Scholar]
- 34.Green JL, Angell CA. Phase relations and vitrification in saccharide-water solutions and the trehalose anomaly. J Phys Chem 1989;93:2880–2882 [Google Scholar]
- 35.Chen T, Fowler A, Toner M. Literature review: Supplemented phase diagram of the trehalose-water binary mixture. Cryobiology 2000;40:277–282 [DOI] [PubMed] [Google Scholar]
- 36.Elbein AD, Pan YT, Pastuszak I, Carroll D. Review: New insights on trehalose: A multifunctional molecule. Glycobiology 2003;13:17R–27R [DOI] [PubMed] [Google Scholar]
- 37.Iglesias HA, Chirife J, Buera MP. Adsorption isotherm of amorphous trehalose. J Sci Food Agric 1997;75:183–186 [Google Scholar]
- 38.Wolkers WF, Walker NJ, Tablin F, Crowe JH. Human platelets loaded with trehalose survive freeze-drying. Cryobiology 2001;42:79–87 [DOI] [PubMed] [Google Scholar]
- 39.Bode AP, Fischer TH. Lyophilization of platelets: Fifty years in the making. Artif Cells Blood Substit Immobil Biotechnol 2007;35:125–133 [DOI] [PubMed] [Google Scholar]
- 40.Kawase Y, Araya H, Kamada N, Jishage K, Suzuki H. Possibility of long term preservation of freeze dried mouse spermatozoa. Biol Reprod 2005;72:568–573 [DOI] [PubMed] [Google Scholar]
- 41.Chakraborty N, Chang A, Elmoazzen H, Menze MA, Hand SC, Toner M. A spin-drying technique for lyophilization of mammalian cells. Ann Biomed Eng 2011;39:1582–1591 [DOI] [PubMed] [Google Scholar]
- 42.Chakraborty N, Biswas D, Parker W, Moyer P, Elliott GD. A role for microwave processing in the dry preservation of mammalian cells. Biotechnol Bioeng 2008;100:782–796 [DOI] [PubMed] [Google Scholar]
- 43.Elliott G, Lee PC, Paramore E, Van Vorst M, Comizzoli P. Resilience of oocyte germinal vesicles to microwave-assisted drying in the domestic cat model. Biopreserv Biobank 2015;13:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Paoli P. From sample collection to epidemiology, diagnosis and research. FEMS Microbiol Rev 2005;29:897–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanias T, Acker JP. Mammalian cell desiccation: Facing the challenges. Cell Preserv Technol 2006;4:253–277 [Google Scholar]
- 46.Miyamoto-Shinohara Y, Sukenobe J, Imaizumi TC, Nakahara T. Survival of freeze dried bacteria. J Gen Appl Microbiol 2008;54:9–24 [DOI] [PubMed] [Google Scholar]
- 47.Acker J, Fowler A, Lauman B, Cheley S, Toner M. Survival of desiccated mammalian cells: Beneficial effects of isotonic media. Cell Preserv Technol 2002;1:129–138 [Google Scholar]
- 48.Crowe JH, Crowe LM, Oliver AE, Tsvetkove N. The trehalose myth revisited: Introduction into a symposium on stabilization of cells in the dry state. Cryobiology 2001;43:89–105 [DOI] [PubMed] [Google Scholar]
- 49.Guo N, Puhlev I, Brown DR, Mansbridge J, Levine F. Trehalose expression confers desiccation tolerance on human cells. Nat Biotech 2000;18:168–171 [DOI] [PubMed] [Google Scholar]
- 50.Oliver AE, Jamil K, Crowe JH, Tablin F. Loading human mesenchymal stem cells with trehalose by fluid-phase endocytosis. Cell Preserv Technol 2004;2:35–49 [Google Scholar]
- 51.Weng L, Ziaei S, Elliott GD. Effects of water on structure and dynamics of trehalose glasses at low water contents and its relationship to preservation outcomes. Sci Rep 2016;6:28795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palermo G, Colombero LT, Rosenwak Z. The human sperm centrosome is responsible for normal syngamy an early embryonic development. Rev Reprod 1997;2:19–27 [DOI] [PubMed] [Google Scholar]
- 53.Desai N, Abdel-Hafez F, Sabanegh E. Paternal effect on genomic activation, clinical pregnancy and love birth rates after ICSI with cryopreserved epididymal versus testicular spermatozoa. Reprod Biol Endocrinol 2009;7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martins C, Bao SN, Ddoe MN, Correa GA, Rumpf R. Effects of freeze-drying on cytology, ultrastructure, DNA fragmentation, and fertilizing ability of bovine sperm. Theriogenology 2007;67:1307–1315 [DOI] [PubMed] [Google Scholar]
- 55.Abraham-Perkir J, Chantler E, Uggerhoj E, Fedder J. Response of mid-piece vesicle on human sperm to osmotic stress. Hum Reprod 2002;17:375–382 [DOI] [PubMed] [Google Scholar]
- 56.Wassarman P. A profile of fertilization in mammals. Nat Cell Biol 2001;3:59–64 [DOI] [PubMed] [Google Scholar]
- 57.Plachot M. Fertilization. Hum Reprod 2000;15:19–58 [DOI] [PubMed] [Google Scholar]
- 58.Schatten H, Sun QY. The role of centrosomes in mammalian fertilization and its significance for ICSI. Mol Hum Reprod 2009;15:531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moomjy M, Colombero LT, Veek LL, Rosenwaks Z, Palermo GD. Sperm integrity is critical for normal mitotic division and early embryonic development. Mol Hum Reprod 1999;5:836–844 [DOI] [PubMed] [Google Scholar]
- 60.Comizzoli P, Wildt DE, Pukazhenthi BS. Poor centrosomal function of cat testicular spermatozoa impairs embryonic development in vitro after intracytoplasmic sperm injection. Biol Reprod 2006;75:252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 1980; 210:1131–1133 [DOI] [PubMed] [Google Scholar]
- 62.Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproduction techniques. Hum Reprod 2000;15:1717–1722 [DOI] [PubMed] [Google Scholar]
- 63.Fernandez-Gonzalez R, Moreira PN. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod 2008;78:761–772 [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Marin C, Gosalvez J, Toy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci 2012;13:14026–14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mengual L, Ballesca JL, Ascaso C, Oliva R. Marked differences in protamine content and P1/P2 ration in sperm cells from percoll fractions between patients and controls. J Androl 2003;24:438–447 [DOI] [PubMed] [Google Scholar]
- 66.Ramalho-Santos J, Amaral A, Sousa AP, et al. Probing the structure and function of mammalian sperm using optical and fluoresence microscopy. In: Medez-Vils A, Diaz J. (eds). Modern Research and Educational Topics in Microscopy. Badajoz, Spain: Formatex; 2007: 394–402 [Google Scholar]
- 67.Twiggs JP, Irvine DS, Aitken RJ. Oxidative damage to DNA in human spermatozoa does not preclude pronuclear formation at intracytoplasmic sperm injection. Hum Reprod 1998;13:1864–1871 [DOI] [PubMed] [Google Scholar]
- 68.Aitken J, Krausz C. Oxidative stress, DNA damage, and the Y chromosome. Reproduction 2001;122:497–506 [DOI] [PubMed] [Google Scholar]
- 69.O WS, Chen H, Chow PH. Male genital tract antioxidant enzymes—Their ability to preserve sperm DNA integrity. Mol Cell Endocrinol 2006;250:80–83 [DOI] [PubMed] [Google Scholar]
- 70.Aitken RJ, Gordon E, Harkiss D, Twigg JP. Relative impact of oxidative stress on the functional competence and genomic integrity of the human spermatozoa. Biol Reprod 1998;59:1037–1046 [DOI] [PubMed] [Google Scholar]
- 71.Morris I, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod 2002;17:990–998 [DOI] [PubMed] [Google Scholar]
- 72.Chen H, Cheung MP, Chow PH, Cheung AL. Protection of sperm DNA against oxidative stress in vivo by accessory sex gland secretions in male hamsters. Reproduction 2002;124:491–499 [DOI] [PubMed] [Google Scholar]
- 73.Donnelly E, O'Connell M, McClure N, Lewis S. Differences in nuclear DNA fragmentation and mitochondrial integrity of semen and prepared human spermatozoa. Hum Reprod 2000;15:1552–1561 [DOI] [PubMed] [Google Scholar]
- 74.Thuwanut P, Chatdarong K, Techakumphu M, Axner E. The effect of antioxidants on motility, viability, acrosome integrity, and DNA integrity of frozen-thawed epididymal cat spermatozoa. Theriogenology 2008;70:233–240 [DOI] [PubMed] [Google Scholar]
- 75.Schatten H, Sun QY. New Insights into the role of centrosomes in mammalian fertilization and implications for ART. Reprod Rev 2011;142:793–801 [DOI] [PubMed] [Google Scholar]
- 76.Hinduja I, Baliga NB, Zaveri K. Correlation of human sperm centrosomal proteins with fertility. J Hum Reprod Sci 2010;3:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hingst O, Blottner S, Franz C. Chromatin condensation in cat spermatozoa during epididymal transit as studied by analine blue and acridine orange staining. Andrologia 1995;27:275–279 [DOI] [PubMed] [Google Scholar]
- 78.McGinnis L, Zhu L, Lawitts J, Biggers J. Mouse sperm desiccated and stored in trehalose medium without freezing. Biol Reprod 2005;73:627–633 [DOI] [PubMed] [Google Scholar]
- 79.Kawase Y, Hani T, Kamada N, Jishage K, Suzuki H. Effect of pressure at primary drying of freeze-drying mouse sperm reproduction ability and preservation potential. Reproduction 2007;133:841–846 [DOI] [PubMed] [Google Scholar]
- 80.Kaneko T, Nakagata N. Relation between storage temperature and fertilizing ability of freeze dried mouse sperm. Comp Med 2005;55:140–144 [PubMed] [Google Scholar]
- 81.Liu J, Lee G, Lawitts J, Toner M, Biggers J. Preservation of mouse sperm by convective drying and storing in 3-O-methyl-D-glucose. PLoS One 2012;7:e29924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Lee G, Lawitts J, Toner M, Biggers J. Live pups from evaporatively dried mouse sperm stored at ambient temperature for up to 2 years. PLoS One 2014;10:e1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ward M, Kaneko T, Kusakabe H, Biggers J, Whittingham D, Yanagimachi R. Long- term preservation of mouse spermatozoa after freeze-drying and freezing without cryoprotection. Biol Reprod 2003;69:2100–2108 [DOI] [PubMed] [Google Scholar]
- 84.Kaneko T, Serikawa T. Long-term preservation of freeze-dried mouse spermatozoa. Cryobiology 2012;64:211–214 [DOI] [PubMed] [Google Scholar]
- 85.Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol 1998;6:639–641 [DOI] [PubMed] [Google Scholar]
- 86.Hochi S, Watanabe K, Kato K, Hirabayashi M. Live rats resulting from injection of oocytes with spermatozoa freeze-dried and stored for one year. Mol Reprod Dev 2007;75:890–894 [DOI] [PubMed] [Google Scholar]
- 87.Kaneko T, Wittingham DGW, Yanagimachi R. Effect of pH Value of freeze-drying solution on the chromosome integrity and developmental ability of mouse spermatozoa. Biol Reprod 2003;68:136–139 [DOI] [PubMed] [Google Scholar]
- 88.Kaneko T, Kimura S, Nagata N. Importance of primary culture conditions for the development of rat ICSI embryos and long-term preservation of freeze-dried sperm. Cryobiology 2009;58:293–297 [DOI] [PubMed] [Google Scholar]
- 89.Kaneko T, Serikawa T. Successful long-term preservation of rat sperm by freeze-drying. PLoS One 2012;7:e35043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ringleb J, Waurich R, Wibbelt G, Streich WJ, Jewgenow K. Prolonged storage of epididymal spermatozoa does not affect their capacity to fertilize in vitro-matured domestic cat (Felis catus) oocytes when using ICSI. Reprod Fert Dev 2011;23:818–825 [DOI] [PubMed] [Google Scholar]
- 91.Liu J-L, Kusakabe H, Chang C-C, et al. Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol Reprod 2004;70:1776–1781 [DOI] [PubMed] [Google Scholar]
- 92.Choi YH, Varner DD, Love CC, Hartment DL. Production of live foal via intracytoplasmic injection of lyophilized sperm and sperm extract in the horse. Reproduction 2011;142:529–538 [DOI] [PubMed] [Google Scholar]
- 93.Keskintepe L, Pacholczyk G, Machinicka A, et al. Bovine blastocyst development from oocytes injected with freeze-dried spermatozoa. Biol Reprod 2002;67:409–415 [DOI] [PubMed] [Google Scholar]
- 94.Lee K, Niwa K. Fertilization and development in vitro of bovine oocytes following intracytoplasmic injection of heat dried sperm heads. Biol Reprod 2006;74:146–152 [DOI] [PubMed] [Google Scholar]
- 95.Kwon I, Park K, Niwa K. Activation, pronuclear formation, and development in vitro of pig oocytes following intracytoplasmic injection of freeze-dried spermatozoa. Biol Reprod 2004;71:1430–1436 [DOI] [PubMed] [Google Scholar]
- 96.Sanchez-Partida L, Simerly C, Ramalho-Santos J. Freeze-dried primate sperm retains early reproductive potential after intracytoplasmic sperm injection. Fertil Steril 2008;3:742–745 [DOI] [PubMed] [Google Scholar]
- 97.Kaneko T, Ito H, Sakamoto H, Onuma M, Inoue-Murayama M. Sperm preservation by freeze-drying for the conservation of wild animals. PLoS One 2014;9:e113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hara H, Abdalla H, Morita H, Kuwayama M, Hirabayashi M, Hochi S. Procedure for bovine ICSI, not sperm freeze-drying, impairs the function of the microtubule-organizing center. J Reprod Dev 2011;57:428–432 [DOI] [PubMed] [Google Scholar]
- 99.Hochi S. Microtubule assembly crucial to bovine embryonic development in assisted reproductive technologies. Anim Sci J 2016;87:1076–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gianaroli L, Magli MC, Stanghellini I, et al. DNA integrity is maintained after freeze-drying of human spermatazoa. Fertil Steril 2012;97:1067–1073 [DOI] [PubMed] [Google Scholar]
- 101.Kusakabe H, Yanagimachi R, Kamiguchi Y. Mouse and human spermatazoa can be freeze-dried without damaging their chromosomes. Hum Reprod 2008;23:233–239 [DOI] [PubMed] [Google Scholar]
- 102.Olaciregui M, Gil L. Freeze-dried spermatozoa: A future tool? Reprod Dom Anim 2016;51:1–7 [DOI] [PubMed] [Google Scholar]
- 103.Loi P, Iusa D, Czernik M, Zacchini F, Ptak G. Towards storage of cells and gametes in dry form. Trends Biotechnol 2013;31:688–695 [DOI] [PubMed] [Google Scholar]
- 104.Gil L, Olaciregu M, Luno V, Malo C, Gonzalez N, Martinez F. Current status of freeze-drying technology to preserve domestic animals sperm. Reprod Dom Anim 2014;49:72–81 [DOI] [PubMed] [Google Scholar]
- 105.Yamaguchi A, Tanaka S, Yamaguchi S, et al. Two novel heat-soluble protein families abundantly expressed in an anhydrobiotic tardigrade. PLoS One 2012;7:e44209. [DOI] [PMC free article] [PubMed] [Google Scholar]