Abstract
We report that a low-calorie diet can lessen the severity of neurochemical deficits and motor dysfunction in a primate model of Parkinson's disease. Adult male rhesus monkeys were maintained for 6 months on a reduced-calorie diet [30% caloric restriction (CR)] or an ad libitum control diet after which they were subjected to treatment with a neurotoxin to produce a hemiparkinson condition. After neurotoxin treatment, CR monkeys exhibited significantly higher levels of locomotor activity compared with control monkeys as well as higher levels of dopamine (DA) and DA metabolites in the striatal region. Increased survival of DA neurons in the substantia nigra and improved manual dexterity were noted but did not reach statistical significance. Levels of glial cell line-derived neurotrophic factor, which is known to promote the survival of DA neurons, were increased significantly in the caudate nucleus of CR monkeys, suggesting a role for glial cell line-derived neurotrophic factor in the anti-Parkinson's disease effect of the low-calorie diet.
Keywords: brain-derived neurotrophic factor, cell death, dopamine
Parkinson's disease (PD) results from the dysfunction and degeneration of dopamine (DA) neurons in the substantia nigra (SN) and DA axon terminals, resulting in progressive motor dysfunction (1). The risk of PD increases with advancing age, suggesting that cellular and molecular changes that occur in the brain during normal aging may promote the degeneration of DA neurons. In this regard, increased oxidative stress and impaired energy metabolism and protein turnover occur in DA neurons during normal aging, and these changes are greatly exacerbated in PD (2, 3). Although a small number of cases of PD result from inherited genetic mutations in one of three genes, α-synuclein, Parkin, or DJ-1 (4), most cases are sporadic with an unknown environmental cause(s). Nevertheless, studies of genetic and neurotoxin-based animal models of PD point to the involvement of oxidative stress and impaired energy metabolism and protein turnover in the pathogenesis of PD (3–6). There is currently no established intervention to prevent, or decrease the risk of, PD.
Most studies of humans have concluded that a decrease in the number of DA neurons in the SN occurs during normal aging, with PD being an acceleration of this process (7–9). However, some investigators have reported no age-related decline in the number of DA neurons (10). Studies in rhesus monkeys have been more consistent in reporting age-related decline in the number of tyrosine hydroxylase (TH)-positive SN neurons (11, 12). However, because some monkey studies have reported increased numbers of TH-positive nigral neurons after treatment with neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF), an age-related decline in expression of TH could be an alternative explanation to the results suggesting an age-related loss of nigral neurons (13).
In a wide range of laboratory animals, caloric restriction (CR) has been shown to prolong lifespan, decrease the incidence of several age-related diseases, improve stress responses, and maintain youthful function (14–16). CR can attenuate age-related decreases in DA signaling in rodents (2), and intermittent fasting can protect neurons against dysfunction and degeneration in rodent models of some neurodegenerative disorders (17, 18), but results from CR studies have not always been positive regarding neuroprotection (19). In the current study, we tested the hypothesis that CR would increase the resistance of DA neurons to dysfunction and degeneration in a nonhuman primate model of PD. Specifically, we assessed whether CR affects the vulnerability of nigro-striatal DA neurons to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). MPTP causes selective degeneration of DA neurons and PD-like neurochemical and histopathological alterations and motor dysfunction in humans (20) and monkeys (21–23) and therefore has been used extensively in rhesus monkeys to assess cellular and molecular mechanisms of PD and to evaluate potential treatments (24).
Methods
Animals. Thirteen adult male rhesus monkeys (Macaca mulatta) (9–17 years old) were singly housed and quasi-randomly divided into two diet groups, control diet (n = 6) and CR (30% restriction in calories, n = 7) with intention of matching initial body weights. Mean ± SEM body weights at the beginning of the study were 9.6 ± 0.5 kg for controls and 9.8 ± 0.8 kg for CR. The diets differed only in caloric content, but were otherwise identical in their nutrient composition. Details of the diet composition are described in ref. 25. Monkeys were maintained on the normal and low-calorie diets throughout the duration of the study. Changes in body weight of the monkeys in relation to major events in the protocol are shown in Fig. 1.
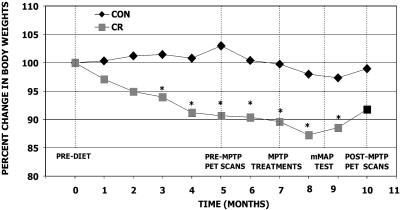
Fig. 1.
Experimental design and body weights of monkeys in each diet group during the course of the study. All monkeys were injected with MPTP after being on their respective diet for 6 months. PET scans were conducted 1–3 weeks before MPTP-injections (after being on the diet for 5 months) and 12–16 weeks after MPTP injection while monkeys were being maintained on their respective diet. Movement assessment panel testing to measure manual dexterity was conducted 7 weeks after MPTP injections. All monkeys were euthanized 16–18 weeks after MPTP injection. There was a significant decline in body weights of the CR group compared with the control diet group (CON) throughout the study (P < 0.05, repeated-measures ANOVA).
MPTP Treatment. After being on their respective diet for ≈6 months, all monkeys were injected with a single dose of the neurotoxin MPTP into the right carotid artery to induce hemiparkinsonism. Each monkey was administered 12 ml of a solution containing 0.2 mg/ml MPTP, for a total amount of 2.4 mg of MPTP per monkey. MPTP·HCl was purchased from Sigma-Aldrich and was dissolved in sterile saline on the day of the injection. Each monkey received the same dose regardless of body weight because the vast majority of the MPTP infused into the carotid artery enters the brain cells during the first pass through the cerebral vessels. Any residual MPTP is rapidly diluted in the peripheral circulation. Therefore, as a result of the MPTP infusion, the right side of the brain received the primary lesion, and the parkinsonian symptoms developed on the left side of the body. Thus, although the CR monkeys weighed less than the controls, they received an equal amount of MPTP.
Behavioral Assessments. At 5–6 and also 1–2 weeks before and then 6 weeks after MPTP (after the parkinsonian symptoms stabilized), all the monkeys were videotaped every other week for 2 h. The monkeys were moved from their home cages during morning hours to a separate quiet room containing a special holding cage made of clear acrylic plastic on all sides that permitted videotaping of their movements. Before and after MPTP treatment, they were allowed to acclimate to these cages for ≈1 h before a videotape session of up to 2 h. The videotapes were evaluated to assess parkinsonian symptoms [rigidity of the left affected side (upper and lower limbs), bradykinesia, posture, balance, and tremor] as described in refs. 26–28. Home-cage activity levels were quantified from videotapes described above by using the automated video-tracking system ethovision (Version 2.2, Noldus Information Technology, Asheville, NC). This automated method relies on determining the position of the animal in the cage at a rate of six samples per sec, and the resultant x-y coordinates are used to calculate the movement pattern during the observation period. For every sample, the cage is scanned and the position of the animal is determined by a subtraction method. To accomplish this procedure, a background scan of the empty cage is made. Then, the animal is introduced, and the cage is scanned at the sample rate. For each scan, the background-scan is subtracted, and the animal appears as the difference between the two images. The resulting x-y coordinates subsequently are related to actual distances in the cage by calibrating the software to the dimension of the cage. The video records were analyzed for distance traveled (cm) and movement speed (cm/sec). Each observation period was analyzed at a rate of six samples per second for 20 min. To assess the impact of MPTP on fine-motor movement, the monkeys were trained to use their left and right hands to retrieve treats from a monkey movement assessment panel. The time of treat retrieval (recorded from sensors attached to the panel) and also the ability of each monkey to retrieve treats from three levels of difficulty (platform, straight rod, and hook) were noted from the weekly testing procedure described in ref. 26. Monkeys received preliminary shaping on the task before their diet group assignments and then received formal training 6–10 weeks before MPTP treatment, and beginning 7 weeks afterward, they were tested every other week over a 10-week period.
Positron-Emission Tomography (PET). PET scans were performed 1–3 weeks before and 12–16 weeks after MPTP administration. Monkeys were anesthetized and placed into a GE Advance PET scanner (interslice interval of 4.25 mm and an in-plane full width at half maximum of ≈6.5 mm; General Electric) by using a head holder to acquire coronal slices. Two [O15]water injections of 10 mCi (1 Ci = 37 GBq) were followed by 60-sec scans. Subsequently, a 60-sec injection of ≈10 mCi of 6-[18F]fluoro-m-tyrosine ([18F]FMT) was followed by 2 h of dynamic scanning. Cerebellar regions of interest were then drawn on three adjacent slices of the averaged [O15]water scans and transferred onto the [18F]FMT scans to generate an FMT cerebellar input function. By using the cerebellar input function, we performed a pixel-by-pixel Patlak analysis (29) to create images of the irreversible uptake rate KI over the time interval of 60–120 sec. With the help of MRI scans obtained on each monkey, the KI for the entire left and right basal ganglia were obtained by manually drawing regions of interest on all slices containing basal ganglia.
Euthanization and Brain Tissue Collection. About 16–18 weeks after MPTP injections, all monkeys were euthanized, and brain tissue samples were collected for postmortem histochemical and neurochemical analyses. After receiving an initial dose of ketamine hydrochloride (20–25 mg/kg, i.m.), the animals were deeply anesthetized by using pentobarbital sodium (20 mg/kg, i.v.) and transcardially perfused with 6 liters of heparinized ice-cold physiological saline. This method is consistent with the recommendations of the American Veterinary Medical Association Panel on Euthanasia. The brains then were recovered and sectioned by using an ice-cold brain mold for histochemical and neurochemical analyses. According to procedures described in ref. 30, the brain tissue was blocked into sections of the SN (20-mm-thick block) and the striatal regions (4-mm-thick coronal tissue sections). The SN region was postfixed into 4% paraformaldehyde for histochemical staining. The remaining blocks of tissues from the striatum were used for neurochemical and neurotrophic factor measurements.
Tissue Staining. Details of these methods are described in ref. 30. The SN region of the brain was sectioned at an instrument setting of 40 μm by using a freezing microtome. Every 12th section was stained with the TH antibody (dilution 1:1,500, mouse monoclonal antibody, Chemicon) for stereological analysis of midbrain DA neurons. Sections were incubated in biotinylated goat anti-mouse IgG for 1 h followed by reaction in avidin–biotin–peroxidase complex for 1 h. A brown reaction was developed with 0.04% diaminobenzidine and 0.005% H2O2. Immunostaining controls included the omission of the primary antibody and replacement of the primary antibody with normal serum of the same species. After immunostaining, the sections were lightly counterstained with cresyl violet and then dehydrated in an alcohol series and coverslipped for microscopic analyses. Systematic-random sampling and TH histochemistry through the entire SN–ventral tegmental area (VTA) complex generated a series of 8–10 sections with a mean thickness of 17.4 μm after all tissue processing.
Stereology. The total number of TH-positive neurons was quantified in both the affected right and the contralateral left SN and VTA of each monkey by using systematic-random sampling and the optical fractionator method (30, 31). A single trained investigator blind to treatment group conducted the stereological analyses by using a computerized stereology system (stereologer, Systems Planning and Analysis, Alexandria, VA). The SN–VTA complex was outlined on each section at low magnification (×5), and the total number of TH-positive neurons was estimated by using the disector method at high magnification (×100, numerical aperture 1.4, oil immersion lens). With a guard volume of 2 μm to avoid sectioning artifacts (e.g., lost caps), TH-immunopositive cells were counted when they fell within a 12-μm-high disector or touched the top or side inclusion planes. This procedure was repeated at a total of 100–150 locations through all 8–10 sections containing SN–VTA, which led to an acceptably low coefficient of error (mean coefficient of error < 0.10).
Neurochemical Analyses. The striatal region was subdivided into the lateral, intermediate, and medial regions; multiple punches from the 4-mm-thick coronal tissue section were blocked; and fresh tissue was taken from the caudate nucleus (CN, n = 6 punches), putamen (n = 5 punches), and nucleus accumbens (n = 3 punches). These punches from each animal were used for the measurement of DA and DA metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) levels by HPLC with electrochemical detection as described in ref. 14.
Measurement of Neurotrophic Factors. Two additional punches from the CN were used for the measurement of the neurotrophic factors brain-derived neurotrophic factor (BDNF) and GDNF from both sides of the brain, which were determined by ELISA for both proteins, specifically the BDNF and GDNF Emax ImmunoAssay System (Promega) according to the manufacturer's protocol. A standard protein assay of each sample was conducted before performing the trophic factor assay to determine the total protein content within each sample. Then, the neurotrophic content determined from the assay kit was divided by the protein content of each sample to estimate the neurotrophic levels in picogram per milligram units.
Statistical Analysis. Changes in body weight over the course of the study were analyzed by using two-way (diet × time) repeated-measures ANOVA, followed by Fisher's post hoc test, by using statview software (SAS Institute, Cary, NC). Analyses of biochemical results involved two-way (diet × region) factorial ANOVA conducted separately by left and right hemispheres, followed by Fisher's post hoc comparisons. Differences in BDNF and GDNF levels were assessed by using two-way (diet × hemisphere) ANOVA, again with Fisher's post hoc comparisons. A similar analysis was used to assess the activity data. PET data and stereological counts of TH-positive neurons were analyzed by using two-tailed t tests to compare control and CR groups. Performance in the food retrieval task was assessed by using a χ2 analysis comparing the number of monkeys from each group reaching criteria on each component of the test. The significance level was set at P ≤ 0.05.
Results
As expected, a gradual loss in body weight (≈12%) occurred over a 6-month period in the CR group vs. the control diet group (Fig. 1). The difference in body weight between groups became significant 3 weeks after the initiation of CR and remained different throughout the remainder of the study, except for the last measurement. Four weeks after MPTP treatment, the body weight of the control group was 9.38 ± 0.9 kg (mean ± SEM), and for the CR group it was 8.6 ± 0.8 kg. Interestingly, after MPTP treatment, CR monkeys exhibited a gain in body weight even though they were being provided the same amount of food. This gain might have been due to the reduced activity after MPTP, but we saw no such gain in the control group. Thus, at 12 weeks after MPTP treatment, the body weights of the control and CR groups did not differ significantly, with 9.8 ± 0.8 kg for control and 9.2 ± 0.7 kg for CR monkeys.
After 5 months on the normal and low-calorie diets, the status of the nigro-striatal DA system was evaluated in each monkey by PET imaging of the basal ganglia by using the radioligand [18F]FMT, to determine presynaptic dopaminergic activity in vivo (32–34). Analyses of the PET images revealed no detectable differences between the two diet groups (data not shown).
After 6 months on their respective diets, all monkeys were administered MPTP, which was infused into the right carotid artery at a dose that induces degeneration of DA neurons in the right (but not the left) SN with consequent loss of DA terminals in the basal ganglia, resulting in motor dysfunction on the left side (hemiparkinsonism). Within 24 h of surgery, all monkeys exhibited motor symptoms of the hemiparkinson condition. They showed reduced general activity and bradykinesia. On the left affected side of the body, rigidity of both upper and lower limbs was observed, and most displayed an action tremor. Both groups of monkeys exhibited slight declines in body weight that recovered a few weeks after MPTP treatment (Fig. 1). CR monkeys were maintained on the same dietary level received before MPTP treatment.
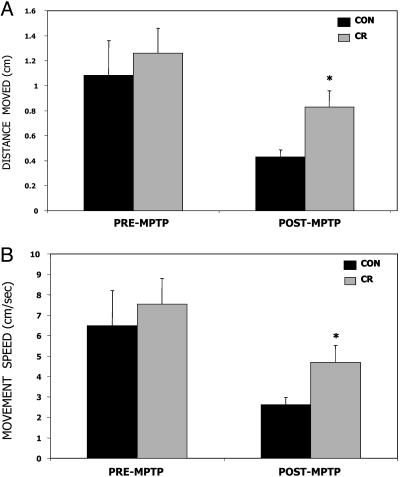
Before and after MPTP treatment, motor activity was evaluated by using image-analysis software to analyze the videotaped recordings. Before MPTP treatment (4–5 weeks), no significant group differences in these parameters were observed, although the mean movement distance and speed of movement were slightly elevated for the CR monkeys (Fig. 2). These measures of movement were significantly reduced in both groups of monkeys after MPTP treatment (6–8 weeks); however, measures of distance moved (Fig. 2 A) and speed of movement (Fig. 2B) were significantly greater (>2-fold) in the CR monkeys compared with those on the control diet. The type of movement observed was generalized around the test environment and did not involve stereotypic circling behavior.
Fig. 2.
Motor activity in monkeys maintained on CR compared with controls (CON) before and after exposure to the neurotoxin MPTP. Mean distance moved within the cage (A) and mean speed of movement (B) did not differ significantly between diet groups before MPTP treatment but were significantly higher in CR monkeys compared with CON monkeys after MPTP treatment. Values are the means and SEM. *, P < 0.05 compared with CON after MPTP, two-tailed, unpaired t test with Welch's correction.
To evaluate motor function before and after MPTP treatment, we used a monkey movement assessment panel that evaluates manual dexterity based on ability and time to retrieve a “lifesaver” treat by using the left (affected) hand from one of three locations: a platform, a straight rod, or a hook (26). The most complex task required a unique sequence of wrist and hand movements to maneuver a small round food reward over a rod curved like a hook before removing it from a small acrylic receptacle. Before the beginning of diet imposition and ≈6–10 weeks before MPTP treatments, all monkeys were trained to be proficient in using both limbs for all three tasks, and both diet groups were equivalent in their performance (data not shown). When the monkeys were evaluated 7–8 weeks after MPTP treatment, the results indicated that motor impairment was less severe in the monkeys in the CR group compared with the control diet group (the percentage of monkeys that were able to perform each task were as follows: platform: 33% control, 43% CR; rod: 33% control, 57% CR; and hook: 17% control, 57% CR), but these differences did not reach statistical significance.
PET analysis applying FMT imaging was repeated 3 months after MPTP. Results before MPTP treatment showed no significant differences between control and CR groups (results not shown). After MPTP treatment, the PET data revealed a clear effect of the MPTP lesion with marked decrease in the FMT signal in the affected side (see Fig. 5, which is published as supporting information on the PNAS web site). When the two diet groups were compared on estimates of presynaptic DA, higher activity in both the left and the right basal ganglia was observed in the CR monkeys compared with those on the control diet, but the values did not reach statistical significance.
To further evaluate the effects of diet and MPTP on the nigro-striatal system, we performed neurochemical and immunohistochemical analyses in selected brain regions of each monkey. Brain tissue was collected in 4-mm-thick sections from the monkeys 4.5 months after MPTP treatment. The tissue section containing the SN was used for immunocytochemical analysis of surviving cells. Tissue punches from designated regions of the striatum (medial, intermediate, and lateral) (Fig. 3A) were used for biochemical analyses (30).
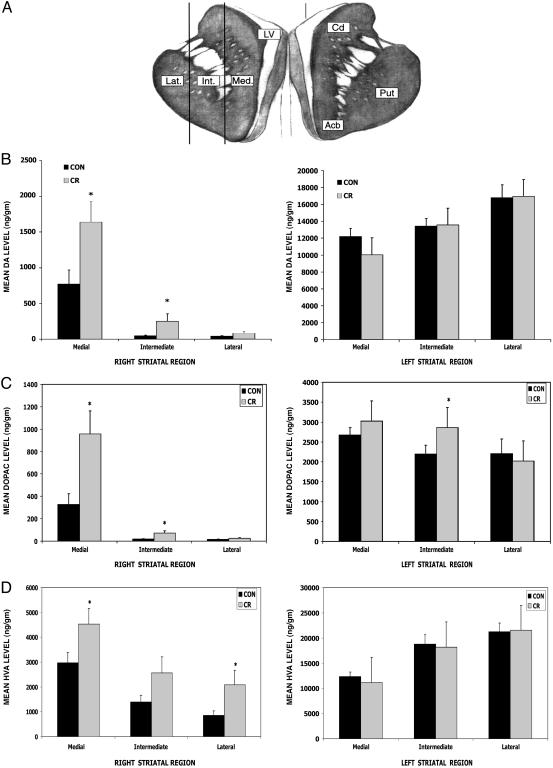
Fig. 3.
CR attenuates MPTP-induced depletion of DA and its metabolites in the striatum. (A) Concentrations of DA and its metabolites DOPAC and HVA were measured in tissue samples from three different regions of the striatum [lateral (Lat.), intermediate (Int.), and medial (Med.) regions] on both the left and right sides of the brain from MPTP-treated monkeys on the CR (n = 7) and control (n = 6) diets. Cd, caudate; Put, putamen; Acb, nucleus accumbens. (B–D) Measurements of levels of DA (B), DOPAC (C), and HVA (D) reveal that the CR monkeys had significantly higher DA levels in the medial (t = 2.5, df = 10; *, P = 0.016) and intermediate (t = 1.95, df = 6; *, P = 0.049) regions of the striatum compared with the control diet monkeys in the right striatum. (B) In the left (the side not receiving MPTP) striatum the DA levels were not significantly different between the CR and control diet groups (*, P > 0.05). (C) The CR group had significantly elevated DOPAC content in the intermediate region of the left striatum (t = 1.90, df = 10; *, P = 0.043). The DOPAC content was also significantly higher in both the medial (t = 2.76, df = 8; *, P = 0.012) and intermediate (t = 2.42, df = 6; *, P = 0.026) regions of the right striatum. (D) In the left striatum there was no significant diet effect on the HVA content (P > 0.05). In the right striatum, the HVA content was elevated significantly in both the medial (t = 2.06, df = 10; *, P = 0.033) and lateral regions (t = 2.02, df = 7; *, P = 0.042).
Concentrations of DA and the DA metabolites DOPAC and HVA were measured by HPLC-based methods. Levels of DA, DOPAC, and HVA were each significantly lower in the striatum on the right (MPTP injected) compared with the left side of the brain in every monkey (Fig. 3 B–D). In the left striatum, the only significant diet effect on levels of DA and its metabolites was in the intermediate regions where the DOPAC level was significantly greater in monkeys on CR compared with those on the control diet (data not shown). In the right striatum that received the primary lesion, we found that mean levels of DA, DOPAC, and HVA were significantly greater for monkeys in the CR group (Fig. 3 B–D). The diet effect on DA and DOPAC levels had a clear medial-to-lateral gradient with the greatest effects being observed in the punches from the medial and intermediate regions of the striatum. For HVA, levels detected in samples from CR monkeys were elevated compared with samples from control diet monkeys in all three regions with the differences being statistically significant in the medial and lateral regions. These data indicate that CR attenuates MPTP-induced depletion of DA, a result consistent with the generally higher level of motor function in the CR monkeys.
Dietary restriction (intermittent fasting) has been shown to increase the expression of BDNF in several different brain regions of rodents (35, 36). Moreover, the amount of BDNF available to DA neurons may be decreased in PD patients (37), and BDNF can protect DA neurons in animal and cell culture models of PD (38–40). We therefore quantified BDNF protein levels by ELISA analysis in tissue samples from the left and right CNs of each monkey. In the unaffected left CN, there was no significant difference in BDNF concentration between diet groups (Fig. 4A); however, in the affected right CN, BDNF concentrations were 40% higher in the CR group compared with the control diet group, although this difference did not reach statistical significance (P = 0.07).
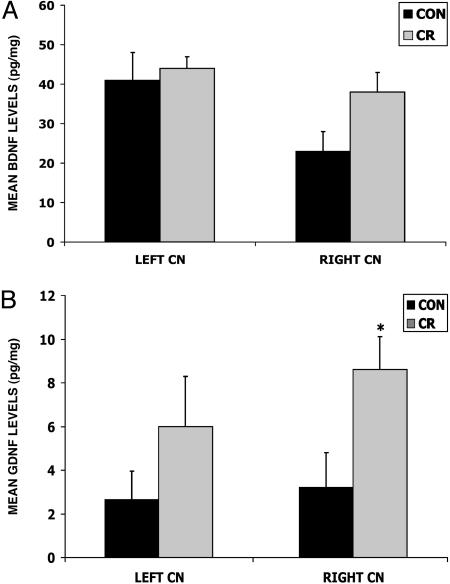
Fig. 4.
Levels of BDNF and GDNF are increased in the striatum of MPTP-treated monkeys that had been maintained on a CR diet. Levels of BDNF (A) and GDNF (B) were measured by ELISA analysis in tissue samples from the CN (18). Values are the mean and SEM. (A) The BDNF level was greater in the right CN of the CR monkeys, but the difference did not reach statistical significance (ANOVA, F1,8 = 4.2, P = 0.74). (B) The GDNF concentration was significantly greater in the right CN of the CR compared with the control diet monkeys (ANOVA, F1,8 = 5.5; *, P = 0.045).
GDNF has been demonstrated to have neuroprotective and neurorestorative actions in primate models of PD and is currently being administered to PD patients in clinical trials (41–45). The concentrations of GDNF in the right and left CN of the CR monkeys were nearly 3-fold higher than levels measured in the same region of monkeys on the control diet (Fig. 4B), with this difference statistically significant in the right affected side (P = 0.045). These findings suggest that a low-calorie diet can markedly increase the production of GDNF, an effect of the diet that may contribute to the improved outcome in this monkey model of PD.
To evaluate the extent of damage and death of DA neurons, we estimated the total number of TH-immunoreactive cells in tissue sections of both the left and right SN/VTA of each monkey by using methods of unbiased stereology. As expected, there was a large decrease (≈80%) in the number of TH-positive neurons in the right (compared with the left) SN/VTA of all monkeys. However, there were 15% more TH-positive cells in the right SN/VTA of monkeys in the CR group (36,521 ± 2,067) compared with those in the control diet group (31,672 ± 3814). The numbers of TH-positive neurons in the unaffected left side of the SN/VTA did not differ significantly between the diet groups (data not shown). Previous studies in rats have shown that dietary restriction can prevent the deaths of cortical neurons induced by a stroke (46) and of hippocampal neurons induced by the excitotoxin kainic acid (47). The greater numbers of TH-positive neurons in the right SN of CR monkeys is consistent with a neuroprotective effect of CR against MPTP-induced death of dopaminergic neurons. Preservation of these neurons likely contributes to the improved functional outcome in the hemiparkinson monkeys on the CR diet, compared with those on the control diet. Indeed, despite the large depletion of DA resulting from MPTP exposure, evident in the FMT PET images and in the measurements of DA in postmortem striatal tissues, levels of DA, DOPAC, and HVA were significantly greater in the striata of monkeys on the CR diet.
Discussion
The present findings suggest that the protective effect of reduced-calorie diets may result from up-regulation of GDNF and BDNF expression and consequent activation of neuroprotective signal transduction pathways in DA neurons. Environmental factors other than energy intake have been shown to up-regulate the expression of GDNF and BDNF and might, in this way, protect against PD. For example, physical exercise can reduce damage to DA neurons and improve motor function in a rat model of PD (48), and this effect is associated with an increase in GDNF levels in the striatum (49). Exercise also induces BDNF expression in several brain regions (50), and both BDNF and GDNF are induced in response to cognitive stimulation (51), in rodents. Studies of rodents suggest a pivotal role for BDNF signaling in the ability of dietary restriction to protect neurons against excitotoxicity (35) and to stimulate neurogenesis (36). Cell culture and in vivo studies have shown that GDNF and BDNF can protect neurons against excitotoxic, metabolic, and oxidative insults (38–40, 44, 45), suggesting mechanisms by which these neurotrophic factors may protect neurons against PD. Finally, CR also might protect DA neurons by mechanisms in addition to up-regulation of neurotrophic factors. For example, it has been shown that CR reduces levels of cellular oxidative stress (52) and also can up-regulate the expression of cytoprotective protein chaperones such as heat-shock protein-70 in rodents (53).
Data from epidemiological studies suggest that individuals with low-calorie, low-fat diets (54) and those who exercise more during their adult life (55) may be at reduced risk of PD. Our findings demonstrate the ability of a reduced-calorie diet to attenuate the cellular and biochemical alterations that result in dysfunction and degeneration of DA neurons in a monkey model of PD. Whereas preclinical and clinical trials of neurotrophic factors in PD involve direct infusion of the neurotrophic factor into the brain of the patient (42, 43), the ability of a CR diet to increase the amount of endogenous GDNF and possibly BDNF in the brains of monkeys suggests that it may be possible to ameliorate PD through dietary manipulations. The present findings suggest that long-term CR can protect the monkey DA motor system against environmental toxins associated with PD, suggesting that CR might reduce the risk of PD in humans. However, the impact of CR and other dietary manipulations on the course of PD in patients who are already symptomatic remains to be determined.
Supplementary Material
Acknowledgments
We acknowledge the contributions of the staff at the National Institutes of Health Animal Facility (Poolesville, MD), especially Alphie Ciser, Margaret Clark, Melisa Lorence, April Hobbs, and Dr. Doug Powell. We also thank Yi Ai for technical assistance.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BDNF, brain-derived neurotrophic factor; CN, caudate nucleus; CR, caloric restriction; DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; [18F]FMT, 6-[18F]fluoro-m-tyrosine; GDNF, glial cell line-derived neurotrophic factor; HVA, homovanillic acid; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD, Parkinson's disease; PET, positron-emission tomography; SN, substantia nigra; TH, tyrosine hydroxylase; VTA, ventral tegmental area.
See Commentary on page 17887.
References
- 1.Jellinger, K. A. (2002) J. Neural. Transm. 62, Suppl., 347–376. [DOI] [PubMed] [Google Scholar]
- 2.Roth, G. S. & Joseph, J. A. (1994) Ann. N.Y. Acad. Sci. 719, 129–135. [DOI] [PubMed] [Google Scholar]
- 3.Jenner, P. & Olanow, C. W. (1998) Ann. Neurol. 44, S72–S84. [DOI] [PubMed] [Google Scholar]
- 4.Dawson, T. M. & Dawson, V. L. (2003) Science 302, 819–822. [DOI] [PubMed] [Google Scholar]
- 5.Lotharius, J. & Brundin, P. (2002) Nat. Rev. Neurosci. 3, 932–942. [DOI] [PubMed] [Google Scholar]
- 6.Dauer, W. & Przedborski, S. (2003) Neuron 39, 889–909. [DOI] [PubMed] [Google Scholar]
- 7.Long, J. M., Mouton, P. R., Jucker, M. & Ingram, D. K. (1999) J. Gerontol. 54A, B407–B417. [DOI] [PubMed] [Google Scholar]
- 8.Cabello, C. R., Thune, J. J., Pakkenberg, H. & Pakkenberg, B. (2002) Neuropathol. Appl. Neurobiol. 28, 283–291. [DOI] [PubMed] [Google Scholar]
- 9.Ma, S. Y., Roytt, M., Collan, Y. & Rinne, J. O. (1999) Neuropathol. Appl. Neurobiol. 25, 394–399. [DOI] [PubMed] [Google Scholar]
- 10.Muthane, U., Yasha, T. C. & Shankar, S. K. (1998) Ann. Neurol. 43, 283–287. [DOI] [PubMed] [Google Scholar]
- 11.Emborg, M. E., Ma, S. Y., Mufson, E. J., Levey, A. I., Taylor, M. D., Brown, W. D., Holden, J. E. & Kordower, J. H. (1998) J. Comp. Neurol. 401, 253–265. [PubMed] [Google Scholar]
- 12.Peters, A., Rosene, D. L., Moss, M. B., Kemper, T. L., Abraham, C. R., Tigges, J. & Albert, M. S. (1996) J. Neuropathol. Exp. Neurol. 55, 861–874. [DOI] [PubMed] [Google Scholar]
- 13.Palfi, S., Leventhal, L., Chu, Y., Ma, S. Y., Emborg, M., Bakay, R., Deglon, N., Hantraye, P., Aebischer, P. & Kordower, J. H. (2002) J. Neurosci. 22, 4942–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weindruch, R. & Sohal, R. S. (1997) N. Engl. J. Med. 337, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanagat, J., Allison, D. B. & Weindruch, R. (1999) Toxicol. Sci. 52, S35–S40. [DOI] [PubMed] [Google Scholar]
- 16.Mattison, J. A., Lane, M. A., Roth, G. S. & Ingram, D. K. (2003) Exp. Gerontol. 38, 35–46. [DOI] [PubMed] [Google Scholar]
- 17.Duan, W., Zhang, Z., Gash, D. M. & Mattson, M. P. (1999) Ann. Neurol. 46, 587–597. [PubMed] [Google Scholar]
- 18.Duan, W., Jiang, H., Ware, M., Li, X. J. & Mattson, M. P. (2003) Proc. Natl. Acad. Sci. USA 100, 2911–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan, W. W., Richardson, A. G. & Nelson, J. F. (2003) J. Gerontol. A Biol. Sci. Med. Sci. 58, B394–B399. [DOI] [PubMed] [Google Scholar]
- 20.Langston, J. W., Forno, L. S., Tetrud, J., Reeves, A. G., Kaplan, J. A. & Karluk, D. (1999) Ann. Neurol. 46, 598–605. [DOI] [PubMed] [Google Scholar]
- 21.Guttman, M., Fibiger, H. C., Jakubovic, A. & Calne, D. B. (1990) J. Neurochem. 54, 1329–1334. [DOI] [PubMed] [Google Scholar]
- 22.Palombo, E., Porrino, L. J., Bankiewicz, K. S., Crane, A. M., Sokoloff, L. & Kopin, I. J. (1990) J. Neurosci. 10, 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moratalla, R., Quinn, B., DeLanney, L. E., Irwin, I., Langston, J. W. & Graybiel, A. M. (1992) Proc. Natl. Acad. Sci. USA 89, 3859–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerhardt, G. A., Cass, W., Huettle, P., Brock, S., Zhang, Z. & Gash, D. M. (1999) Brain Res. 817, 163–171. [DOI] [PubMed] [Google Scholar]
- 25.Ingram, D. K., Cutler, R. G., Weindruch, R., Renquist, D. M., Knapka, J. J., April, M., Belcher, C. T., Clark, M. A., Hatcherson, C. D., Marriott, B. M., et al. (1990) J. Gerontol. A Biol. Sci. Med. Sci. 45, B148–B163. [DOI] [PubMed] [Google Scholar]
- 26.Gash, D. M., Zhang, Z., Umberger, G., Mahood, K., Smith, M., Smith, C. & Gerhardt, G. A. (1999) J. Neurosci. Methods 89, 111–117. [DOI] [PubMed] [Google Scholar]
- 27.Ovadia, A., Zhang, Z. & Gash, D. M. (1995) Neurobiol. Aging 16, 931–937. [DOI] [PubMed] [Google Scholar]
- 28.Imbert, C., Bezard, E., Guiraud, S., Boraud, T. & Gross, C. E. (2000) J. Neurosci. Methods 96, 71–76. [DOI] [PubMed] [Google Scholar]
- 29.Patlak, C. S. & Laberg, R. G. (1985) J. Cereb. Blood Flow Metab. 5, 584–590. [DOI] [PubMed] [Google Scholar]
- 30.Grondin, R., Zhang, Z., Yi, A., Cass, W. A., Maswood, N., Andersen, A. H., Elsberry, D. D., Klein, M. C., Gerhardt, G. A. & Gash, D. M. (2002) Brain. 125, 2191–2201. [DOI] [PubMed] [Google Scholar]
- 31.Ai, Y., Markesbery, W., Zhang, Z., Grondin, R., Elseberry, D., Gerhardt, G. A. & Gash, D. M. (2003) J. Comp. Neurol. 461, 250–261. [DOI] [PubMed] [Google Scholar]
- 32.Jordan, S., Eberling, J. L., Bankiewicz, K. S., Rosenberg, D., Coxson, P. G., VanBrocklin, H. F., O'Neil, J. P., Emborg, M. E. & Jagust, W. J. (1997) Brain Res. 750, 264–276. [DOI] [PubMed] [Google Scholar]
- 33.Eberling, J. L., Pivirotto, P., Bringas, J. & Bankiewicz, K. S. (2000) (2000) Exp. Neurol. 165, 342–346. [DOI] [PubMed] [Google Scholar]
- 34.Doudet, D. J., Chan, G. L., Jivan, S., DeJesus, O. T., McGeer, E. G., English, C., Ruth, T. J. & Holden, J. E. (1999) J. Cereb. Blood Flow Metab. 19, 278–287. [DOI] [PubMed] [Google Scholar]
- 35.Duan, W., Guo, Z. & Mattson, M. P. (2001) J. Neurochem. 76, 619–626. [DOI] [PubMed] [Google Scholar]
- 36.Lee, J., Duan, W. & Mattson, M. P. (2002) J. Neurochem. 82, 1367–1375. [DOI] [PubMed] [Google Scholar]
- 37.Howells, D. W., Porritt, M. J., Wong, J. Y., Batchelor, P. E., Kalnins, R., Hughes, A. J. & Donnan, G. A. (2000) Exp. Neurol. 166, 127–135. [DOI] [PubMed] [Google Scholar]
- 38.Frim, D. M., Uhler, T. A., Galpern, W. R., Beal, M. F., Breakfield, X. O. & Isacson, O. (1994) Proc. Natl. Acad. Sci. USA 91, 5104–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukahara, T., Takeda, M., Shimohama, S., Ohara, O. & Hashimoto, N. (1995) Neurosurgery 37, 733–739. [DOI] [PubMed] [Google Scholar]
- 40.Cheng, B. & Mattson, M. P. (1994) Brain Res. 640, 56–67. [DOI] [PubMed] [Google Scholar]
- 41.Gash, D. M. Zhang, Z., Ovadia, A., Cass, W. A., Yi, A., Simmerman, L., Russell, D., Martin, D., Lapchak, P. A., Collins, F., et al. (1996) Nature 380, 252–255. [DOI] [PubMed] [Google Scholar]
- 42.Nutt, J. G., Burchiel, K. J., Comella, C. L., Jankovic, J., Lang, A. E., Laws, E. R., Jr., Lozano, A. M., Penn, R. D., Simpson, R. K., Jr., Stacy, M., et al. (2003) Neurology 60, 69–73. [DOI] [PubMed] [Google Scholar]
- 43.Gill, S. S., Patel, N. K., Hotton, G. R., O'Sullivan, K., McCarter, R., Bunnage, M., Brooks, D. J., Svendsen, C. N. & Heywood, P. (2003) Nat. Med. 9, 589–595. [DOI] [PubMed] [Google Scholar]
- 44.Gratacos, E., Perez-Navarro, E. Tolosa, E., Arenas, E. & Alberch, J. (2001) J. Neurochem. 78, 1287–1296. [DOI] [PubMed] [Google Scholar]
- 45.Hanbury, R., Ling, Z. D., Wuu, J. & Kordower, J. H. (2003) J. Comp. Neurol. 461, 307–316. [DOI] [PubMed] [Google Scholar]
- 46.Yu, Z. & Mattson, M. P. (1999) J. Neurosci. Res. 57, 830–839. [PubMed] [Google Scholar]
- 47.Bruce-Keller, A. J., Umberger, G., McFall, R., Mattson, M. P. (1999) Ann. Neurol. 45, 8–15. [PubMed] [Google Scholar]
- 48.Tillerson, J. L., Cohen, A. D., Philhower, J., Miller, G. W., Zigmond, M. J. & Schallert, T. (2001) J. Neurosci. 21, 4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen, A. D., Tillerson, J. L., Smith, A. D., Schallert, T. & Zigmond, M. J. (2003) J. Neurochem. 85, 299–305. [DOI] [PubMed] [Google Scholar]
- 50.Cotman, C. W. & Berchtold, N. C. (2002) Trends Neurosci. 25, 295–301. [DOI] [PubMed] [Google Scholar]
- 51.Young, D., Lawlor, P. A., Leone, P., Dragunow, M. & During, M. J. (1999) Nat. Med. 5, 448–453. [DOI] [PubMed] [Google Scholar]
- 52.Barja, G. (2002) Ageing Res. Rev. 1, 397–411. [DOI] [PubMed] [Google Scholar]
- 53.Guo, Z., Ersoz, A., Butterfield, D. A. & Mattson, M. P. (2000) J. Neurochem. 75, 314–320. [DOI] [PubMed] [Google Scholar]
- 54.Logroscino, G., Marder, K., Cote, L., Tang, M. X., Shea, S. & Mayeux, R. (1996) Ann. Neurol. 39, 89–94. [DOI] [PubMed] [Google Scholar]
- 55.Sasco, A. J., Paffenbarger, R. S., Jr., Gendre, I. & Wing, A. L. (1992) Arch. Neurol. 49, 360–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.