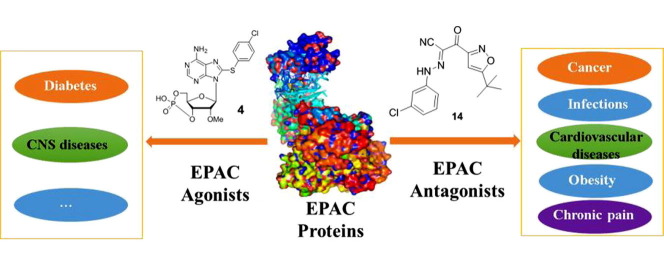
Graphical abstract
Keywords: EPAC, cAMP, Agonist, Antagonist, Modulator
Abstract
Exchange proteins directly activated by cAMP (EPACs) are critical cAMP-dependent signaling pathway mediators. The discovery of EPAC proteins has significantly facilitated understanding on cAMP-dependent signaling pathway and efforts along this line open new avenues for developing novel therapeutics for cancer, diabetes, heart failure, inflammation, infections, neurological disorders and other human diseases. Over the past decade, important progress has been made in the identification of EPAC agonists, antagonists and their biological and pharmacological applications. In this review, we briefly summarize recently reported novel functions of EPACs and the discovery of their small molecule modulators. The challenges and future perspectives are also discussed.
Cyclic adenosine monophosphate (cAMP), also known as 3′,5′-cyclic adenosine monophosphate, is converted from adenosine triphosphate (ATP) by adenylate cyclases (ACs). It is a prototypic second messenger that plays crucial roles in cellular responses to various stimulations, and mediates signaling pathways related to many human diseases including cancer, cardiac and urinary dysfunction, diabetes, immunological diseases and nerve disease.1, 2, 3, 4 Protein kinase A (PKA) and cyclic nucleotide-regulated ion channels were the first discovered cAMP mediators and originally considered as the only ones. In 1998, two independent groups reported another cAMP mediator named exchange proteins directly activated by cAMP (EPACs).5, 6 EPAC is a family of cAMP-binding proteins with guanine nucleotide exchange factors (GEF) activity that directly activate Ras-like small GTPases (Rap1 and Rap2).5, 6 The discovery of EPAC proteins has significantly facilitated the understanding of cAMP-dependent signaling pathway and opens new avenues for developing novel therapeutics targeting cancer, diabetes, heart failure, inflammation, infectious diseases, neurological disorders and other human conditions.1, 7, 8, 9, 10
Structures and functions of EPAC family proteins: To date, two members of the EPAC family proteins have been identified, known as EPAC1 (coded by Rapgf3 gene, in human) and EPAC2 (coded by Rapgf4 gene, in human).5, 6 EPAC1 (cAMP-GEF-I) is an about 100 kDa molecular weight multi-domain protein that is highly expressed in developing and mature human tissues. The multi-domain protein EPAC2 has three isoforms (EPAC2A, EPAC2B and EPAC2C) with about 115 kDa of molecular weight and is enriched in nervous system and endocrine tissues. EPAC1 and EPAC2 proteins have considerable similarity in the structure and sequence (68% similarity in human).11 Both EPAC1 and EPAC2 consist of two regions, the N-terminal regulatory region and the C-terminal catalytic region. The regulatory region of EPAC protein includes a disheveled, Egl-10, pleckstrin (DEP) domain and cyclic nucleotide binding domain (CNBD). The C-terminal catalytic regions of EPAC1 and EPAC2 are composed of three basic domains named as cell division cycle 25 homology GEF domain (CDC25-HD), Ras association (RA) domain, and Ras exchange motif (REM) domain.12, 13
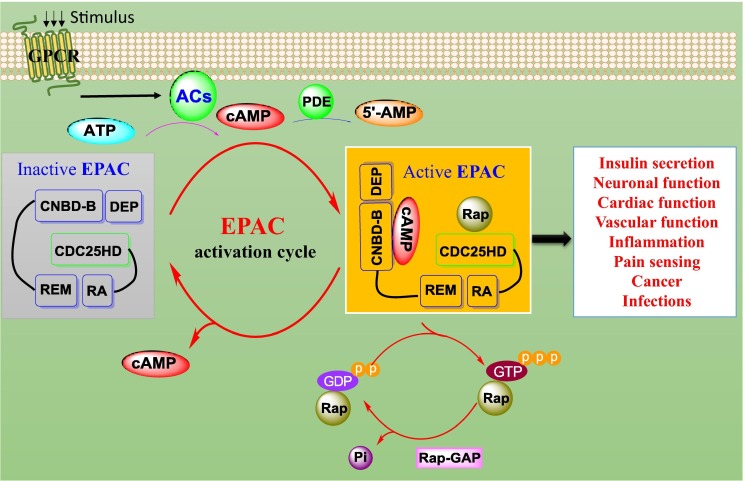
In the absence of cAMP, the activity of EPAC is auto-inhibited. The N-terminal regulatory region and the C-terminal catalytic region of EPAC are held together through intramolecular interactions, thereby preventing Rap binding to the CDC25-HD of EPAC and keeping EPAC inactive (Fig. 1 ).14 When cell is stimulated by extracellular signals, ACs are activated through various ligands which bind to G-protein-coupled receptors (GPCRs) and promote the conversion of ATP into cAMP.15 The binding of cAMP to CNBD allows the regulatory region to rotate about 90° sideways and leaves enough space for Rap binding to CDC25-HD.15 Consequently, active EPAC catalyzes the exchange of guanosine diphosphate (GDP) to guanosine triphosphate (GTP) and controls Rap-mediated biological functions (Fig. 1). The EPAC signaling pathway plays a critical role in various biological responses including insulin secretion, neuronal function, cardiovascular function, vascular function, inflammation, cancer, pain, and infections.1, 7, 8, 9, 10
Fig. 1.
Postulated mechanisms of EPAC activation and associated biological functions. Under the G-protein-coupled receptor (GPCR) stimulation, adenylate cyclases (ACs) convert adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). The excessive cAMP can be degraded into 5′-AMP by phosphodiesterases (PDEs). The binding of cAMP to inactive EPAC leads to the activation of EPAC, which facilitates the exchange of guanosine diphosphate (GDP) to guanosine triphosphate (GTP) and controls Rap-mediated biological functions. Meanwhile, Rap-GTPase-activating proteins (Rap-Gap) facilitate the intrinsic GTPase activity of Rap to breakdown GTP into GDP and phosphorus inorganic (Pi).
The EPAC signaling pathway is involved in insulin secretion from pancreatic β cells. EPAC2 promotes glucose-stimulated insulin secretion (GSIS) by regulation of intracellular Ca2+ concentration.16, 17, 18 To date, three pathways have been revealed for EPAC2-mediated insulin secretion. First, EPAC2/Rap can activate phospholipase Cε (PLCε), protein kinase C (PKC), ryanodine receptor (RyR) and sarco/endoplasmic reticulum Ca2+-ATPase (SERCA).19, 20 Second, EPAC2 can directly interact with sulfonylurea receptor 1 (SUR1), leading to ATP-sensitive potassium channel (KATP) closure in response to the increase in the ATP/ADP ratio, thus regulating the intracellular Ca2+ level.21 Third, interaction of EPAC2 with Rim2, Munc 13-1 and Piccolo potentiates rapid Ca2+-dependent exocytosis.22, 23 According to a recent study, EPAC1 may also play an important role in GSIS.24 The EPAC1 knockout mouse model showed the decreased expression of glucose transporter Glut2 and transcription factor PDX1. Collectively, these studies suggest that EPAC represents a potential therapeutic target for diabetes and obesity.
The interaction of EPAC2 with Rim1 has an important role in regulating neurotransmitter release.25 In addition, a recent EPAC2 knockout mice model study provides in vivo evidence that EPAC2 promotes transmitter release by maintaining the readily releasable pool (RRP) at mossy fiber (MF) synapses in the hippocampus.26 Growing evidence demonstrates that EPAC participates in neurite growth and neuronal differentiation.27, 28 In PC12 and NS-1 cells, EPAC2 is necessary for mediating growth arrest and neurite extension during neuronal differentiation through the mitogen activated protein kinase (MAPK) pathways including p38 and extracellular signal-regulated kinase (ERK).29
Studies based on EPAC1 and EPAC2 knockout mouse model have revealed that EPAC proteins exert significant physiological roles in learning, memory and social interactions in brain.30 Furthermore, EPAC2-deficent mice show reduced dendritic spine motility and density in cortical neurons, and display defects in social interactions and ultrasonic vocalizations.31 Thus, targeting EPAC signaling pathways may present a novel strategy for the treatment of CNS diseases.
In the heart, EPAC can enhance cardiac contractility by regulating intracellular Ca2+ concentration through PLCε, PKC, RyR and Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling pathways.32, 33 In the hypertrophic heart, EPAC (mainly EPAC1) is found to be overexpressed.34 It suggests that EPAC may play an important role in cardiac hypertrophy.35 Activation of EPAC can prevent H2O2-induced production of reactive oxygen radical and inhibit the activation of caspase-3 and apoptosis in cardiomyocytes.3 Recently, it was reported that the activation of β1-adrenergic receptors (β1-AR) could lead to EPAC2-dependent sarcoplasmic reticulum (SR) Ca2+ leak and arrhythmia through phosphorylation of RyR2 by CaMKIIδ or PKA.36 Of note, the distributions between EPAC1 and EPAC2 in mice myocytes are significantly different. EPAC1 is limited in nuclear signaling while EPAC2 is found to gather around the T tubules, indicating that EPAC2 is involved in the arrhythmogenic SR Ca2+ leak.37 EPAC also plays a critical role in the development of cardiac fibrosis.38 The important involvement of EPAC in cardiovascular functions offers a new direction for the discovery of new treatment of cardiovascular diseases.
The roles of EPAC in vascular functions involve the regulation of smooth muscle cells migration and proliferation, vascular tone, endothelial barrier (EB) function and inflammation.39 In smooth muscle cells, EPAC enhances smooth muscle cells migration in the process of vascular remodeling and neointimal formation in response to femoral artery mechanical injuring.40 Further evidence supports that EPAC enhances smooth muscle cell migration through inducing integrin β1 activation41 and regulating extracellular matrix components scretion.42 Besides, EPAC can induce vasorelaxation or vasoconstriction through distinct regulation of intracellular Ca2+ concentration and KATP.39, 43, 44 In vivo studies reveal that knockout of the EPAC1 gene can attenuate neointima formation by inhibition of smooth muscle cells migration, in the meantime, Ca2+ concentration and cofilin-mediated lamellipodia formation are decreased.45 Most recently, Cheng and co-workers found that EPAC1 involved in neointima formation through PI3K/AKT signaling pathway and mitochondrial fission in response to vascular injury in the mouse carotid artery ligation model.46 Therefore, EPAC is a promise therapeutic target for vascular diseases. In addition, EPAC exhibits anti-inflammatory activity against interleukin 6 (IL-6) receptor signaling through up-regulating the expression of suppressor of cytokine signaling 3 (SOCS3) in VECs.47 Both in vitro and in vivo studies revealed that inhibition of EPAC led to the expression vascular cell adhesion molecule 1 (VCAM-1) decrease and mediated the effect of Phosphodiesterase 4 (PDE4) activity. These findings indicate that EPAC-PDE4 signaling pathway may provide a novel means for the treatment of vascular inflammation inclusive of atherosclerosis and in-stent restenosis.48 Moreover, recent hyperalgesia priming and EPAC knockout mouse model studies uncovered a key role of EPAC1 in chronic inflammatory pain under the control of EPAC1 phosphorylation mediated by GPCR kinase 2 (GRK2).49, 50 It is worth to mention that EPAC is also associated with airway diseases via modulating airway smooth muscle cell functions.51, 52, 53 Taken together, EPAC is an emerging therapeutic target for the drug discovery of cardiovascular diseases, inflammation and airway diseases.
More and more studies are focused on the function role of EPAC1 in cancer proliferation, apoptosis and migration. Recently, it was reported that the EPAC-ERK and AKT signaling pathways promote the B cell antigen receptor (BCR) mediated growth arrest and apoptosis in the B lymphoma cell line WEHI-231.54 In human glioblastoma cell lines A172 and U87MG, EPAC1-Rap1 and PKA pathways mediate cell death and cell cycle arrest in the process of rolipram regulation of glioblastoma cell density.55 While in the prostate cancer cells, EPAC1 can increase cell proliferation and survival involved in EPAC/Rap1/B-Raf, Ras/MAPK, and PI3K-mTOR signaling pathways.56, 57 EPAC is thus crucial in regulating cell migration and proliferation. Numerous papers support that EPAC1 promote various cancer cells (including cervical,58 fibrosarcoma,59 melanoma,60 ovarian,61 pancreatic62 and prostate63) migration and metastasis. The cAMP-mediated immune functions are well documented, but the breakthrough work on implication of EPAC in anti-cancer immunotherapy has not been reported until recently. EPAC1 was identified to play an essential role in the regulation of T-cell-mediated immunosuppression.64 Interestingly, results from a recent study suggest that EPAC signaling pathway may up-regulate the aerobic glycolysis, and promote oncogenesis.65 All these studies demonstrate EPAC1 is an emerging target for cancer therapy.
The cAMP signaling controls a wide range of processes in pathogenic bacteria, fungi and protozoa, and cAMP is a critical mediator of microbial pathogens infectious virulence gene expression.66 It is well known that the functions of cAMP are mainly regulated by EPAC and PKA.67 Thus, it is not surprising that EPAC has been shown to play a critical role in bacterial infection. For example, according to recent genetic manipulation and pharmacological approaches in vivo, knockout EPAC1 gene or inhibition of EPAC1 activity can prevent bacterial adhesion and invasion during fatal rickettsioses.68 These results offer a new avenue for fighting with bacterial infection and display a novel mode of action for EPAC1 and host-pathogen interactions.
Small molecules targeting EPAC proteins: Given the essential role of EPAC in various biological functions and human diseases, tremendous efforts have been devoted to discover small molecule EPAC modulators as pharmacological probes and potential drug candidates for the treatment of human diseases. Based on the different chemical structures, currently reported EPAC modulators can be divided into two major categories: cAMP analogues and non-cyclic nucleotide small molecules. Naturally, the cAMP analogues of an EPAC modulator subclass often exhibit EPAC agonist activity, while most of non-cyclic nucleotide ligands tend to act as EPAC antagonists or EPAC inhibitors.
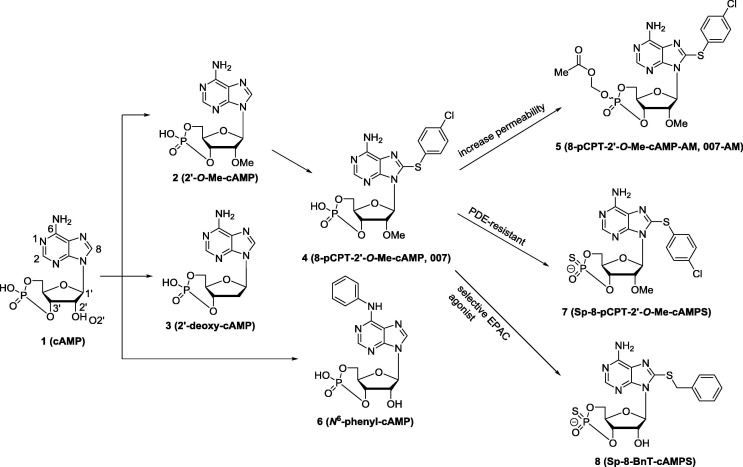
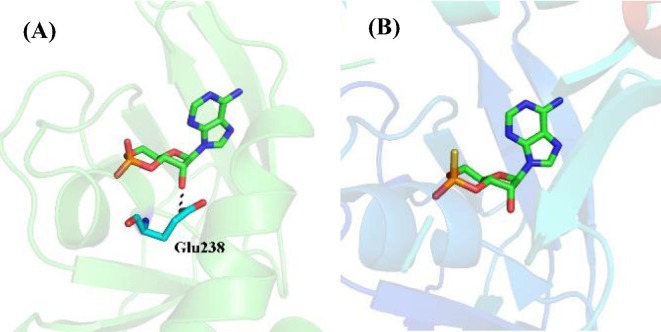
cAMP analogues as EPAC modulators: Earlier effort on discovery and development of EPAC modulators was mainly focused on design and synthesis of the analogues of compound 1 (cAMP, Fig. 2 ), but none of these analogues exhibited selectivity between EPAC and PKA, limiting their usage for further studies.69 In order to investigate the different biological functions of EPAC and PKA, developing EPAC specific modulators was imperative. A breakthrough on EPAC selective modulators was made by Bos and co-workers27, 70 Through the comparison of the binding sites of compound 1 with EPAC and PKA cAMP domains, they found that the 2′-OH group of the compound 1 could form hydrogen bond with highly conserved glutamate residue (Glu238 in human PKA protein) in the PKA cAMP domain, which is absent in the EPAC cAMP binding site (Fig. 3 ). Interaction between the 2′-OH group and conserved glutamate residue is essential for cAMP binding to the PKA cAMP domain and modification of the 2′-OH group could lead to selective EAPC modulators. Based on these findings, a series of 2′-O-alkyl-modified analogues of compound 1, for example, compound 2 (2′-O-Me-cAMP, Fig. 2), were designed, and they exhibited about 10- to 100-fold EPAC/PKA selectivity.27 It is worth to mention that replacing the 2′-OH group by hydrogen led to compound 3 (2′-deoxy-cAMP, Fig. 2).71 Compound 3 showed some EPAC/PKA selectivity, but with a dramatic loss of its EPAC activation activity compared to compound 1.
Fig. 2.
The chemical structures of cAMP analogues as EPAC modulators.
Fig. 3.
(A) The co-crystal structure of cAMP (1) with PKA CNBD (PDB code: 1RGS). (B) The co-crystal structure of cAMP (1) with EPAC2 CNBD (PDB code: 3CF6).
Further modifications on the 8-position of compound 2 led to compound 4 (8-pCPT-2′-O-Me-cAMP, a.k.a. 007, Fig. 2),70 which is a potent (half-maximal activation of EPAC1 at 2.2 µM) and selective (about 100-fold EPAC/PKA selectivity) EPAC agonist. Since the discovery of compound 4, it has been widely used as a powerful tool for elucidating the EPAC functions.72, 73, 74, 75 In rat models, injection of compound 4 can provoke mechanical hyperalgesia through the mechanism of activating the ε isoform of PKC.72 When 5-HT at the threshold and subthreshold concentrations, compound 4 remarkably increased the frequency of Ca2+ oscillations.73 Human peripheral blood lymphocytes (PBL) cultures, treated with compound 4, at a 100 µM level, decreased approximately 40% HIV-1 viral replication.74 According to the most recent studies, compound 4 recapitulated the cocaine-induced increase in GluA2-lacking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) in dopamine neurons of the ventral tegmental area (VTA), indicating its therapeutic potential for drug additions.75Based on its aforementioned advantages, compound 4 has been widely employed as a useful chemical probe for EPAC function studies, but its biological application is limited due to its poor plasma membrane penetrating capability. Esterification of the negatively charged singly bonded oxygen of the compound 4 phosphate group led to compound 5 (8-pCPT-2′-O-Me-cAMP-AM, a.k.a. 007-AM, Fig. 2), which contains a mixture of the equatorial and the axial isomers.76 Compound 5 works as a prodrug of 4, and can be hydrolyzed to release 4 quickly after crossing the plasma membrane. Compound 5 displays an effect of over 100-fold more potent than compound 4 without affecting its EPAC/PKA selectivity, and it has greatly facilitated the studies of EPAC signaling pathways.1, 12 However, it is a good substrate for phosphodiesterases (PDE) and also acts as a PDE inhibitor.10
On the other hand, introducing a phenyl group to compound 1 at the N-6 position led to compound 6 (N 6-phenyl-cAMP, Fig. 2).27 Biological studies suggest that compound 6 acts as a full PKA agonist instead of EPAC agonist. This finding indicates that modification on the N-6 position of compound 1 may lead to PKA/EPAC selective agonists.
Recently, several other analogues of compound 1 as EPAC modulators were reported. For example, compound 7 (Fig. 2), a highly specific EPAC activator, has better lipophilicity and membrane permeability than compound 1.77 Nevertheless, it still suffers from PDE inhibition side effects. Rehmann and co-workers developed a series of compound 1 analogues as EPAC2-selective agonists through a structure-guided approach. The represented compound 8 exhibits over 100-fold EPAC2 active potency toward EPAC1.78 Although cAMP analogues (e.g. compounds 4 and 5) have been widely used as the powerful chemical probes for the study of EPAC functions, more attention should be paid to their selectivity against other targets beyond PKA and EPAC. According to recent studies, most cAMP analogues (e.g. compounds 4 and 5) have multiple cellular targets, thereby leading to cross-target activities and off-target effects.79, 80, 81, 82, 83
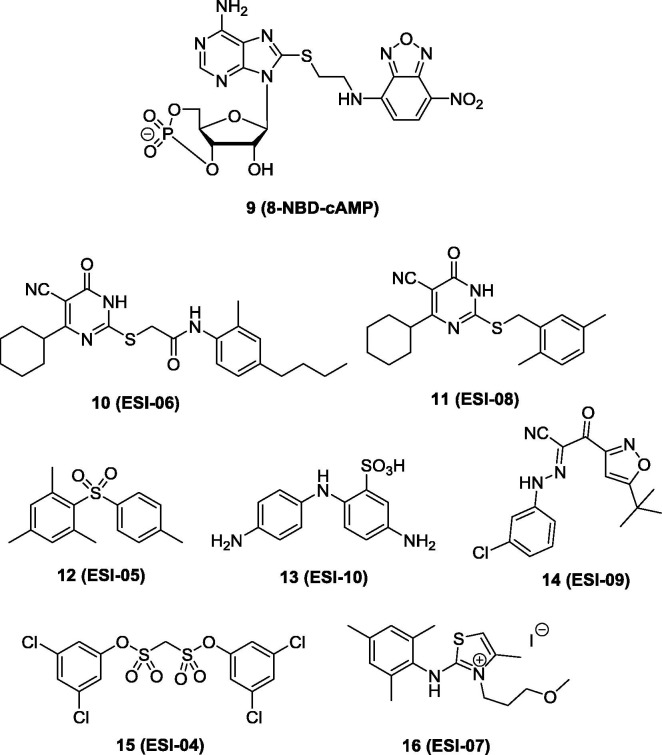
Non-cyclic nucleotide small molecules as EPAC modulators: A series of non-cyclic nucleotide EPAC selective inhibitors were reported by Cheng and colleagues.84, 85 They established a sensitive and robust fluorescence-based high throughput (HTS) assay for screening EPAC specific inhibitors that compete with compound 9 (Fig. 4 ).84 Compound 9 is suitable for screening EPAC specific inhibitors because it can induce more than 100-fold fluorescent change when binding to purified full-length EPAC2, and can be reversed by addition of excessive cAMP. Three compounds from the NCI diversity set library were identified with IC50 values range from 1.7 to 7.9 µM in competing with compound 9 in binding EPAC2.84 Furthermore, they expanded their screening to the Maybridge Hitfinder compound library with about 14,400 compounds.85 Seven compounds (compounds 10 to 16, Fig. 4) were discovered to completely inhibit EPAC2 GEF activities at 25 µM in the presence of a same concentration of cAMP.
Fig. 4.
The chemical structure of fluorescent probe compound 9 and HTS hits 10 to 16 as EPAC- inhibitors.
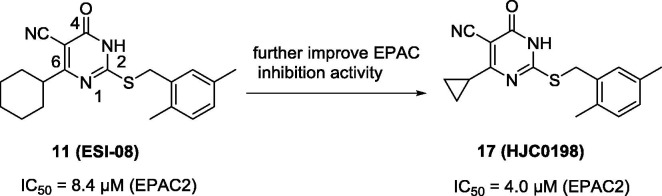
To develop novel EPAC inhibitors, Zhou and co-workers86 optimized the HTS hit 11 as the chemical lead. After modifications of the substituents on the phenyl ring or C6-position of compound 11, compound 17 (Fig. 5 ) was identified to be the more potent compound in this series with an IC50 value of 4.0 µM (EPAC2). Docking studies revealed that the size and position of substituents played a role in EPAC inhibitory activity. At the concentration of 25 µM, compound 17 selectively blocked cAMP-induced EPAC activation and did not inhibit cAMP-mediated PKA activation. In HEK293/EPAC1 and HEK293/EPAC2 cell lines, compound 17 completely blocked EPAC1- and EPAC2-mediated AKT phosphorylation at the concentration of 10 µM.
Fig. 5.
The chemical structure of EPAC inhibitor 17.
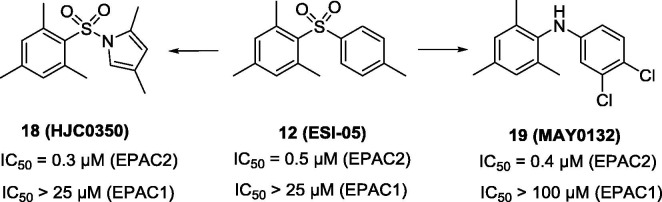
Starting from HTS hit 12, Zhou and co-workers conducted structural modifications to afford compound 18 (Fig. 6 ),87 showing an IC50 of 0.3 µM for competitive binding to EPAC2 in the presence of compound 9. Compound 18 has no inhibitory EPAC1-mediated Rap1-GDP exchange activity or altering cAMP-induced type I and II PKA holoenzymes activation at the concentration of 25 µM. This result suggests that compound 18 is an EPAC2-specific inhibitor. Moreover, this conclusion was also confirmed by live cell imaging studies using EPAC1, EPAC2 or PKA FRET sensor. Replacing the para-toluenesulfonyl motif in compound 12 with a 4-cholor-3-(trifluoromethyl)aniline group led to compound 19 (Fig. 6).88 Compound 19 acts as an excellent EPAC2 specific inhibitor with an IC50 value of 0.4 µM (competitively binding to EPAC2 with compound 9) and inhibits cAMP-mediated EPAC2 GEF activity with IC50 of 1 µM, showing no significant inhibition of EPAC1 at 100 µM.
Fig. 6.
The chemical structures of compounds 18 and 19.
To further identify non-cyclic nucleotide EPAC1 selective inhibitors, the most recent study by this team was focused on compound 14 (Fig. 4).85 Compound 14 was identified as a non-cyclic nucleotide EPAC1 (IC50 = 3.2 µM) and EPAC2 (IC50 = 7.0 µM) dual inhibitor.89 In the meantime, it did not show any effect on PKA even at high concentrations. Furthermore, it is involved in EPAC-mediated Rap1 activation, AKT phosphorylation, pancreatic beta cells insulin production and secretion.1
Although previous work found that the levels of EPAC1 was overexpressed in human pancreatic ductal adenocarcinoma (PDA), the mechanism was unclear.90 Cheng and co-workers62 revealed that compound 14 inhibited EPAC1-mediated adhesion of AsPC-1 and PANC-1 cells without affecting cell proliferation and viability. Furthermore, in an orthotopic metastatic mouse model, with an injection dose of 10 mg/kg once daily for 3 weeks, compound 14 prevented local and distant MIA PaCa-2 cell spread and significantly reduced metastasis to the liver.63 This result indicates that EPAC1 is a promising target for pancreatic cancer migration and invasion. Cronstein and co-workers91 revealed that compound 14 completely blocked RANKL-induced osteoclast differentiation at 10 µM, and it might diminish bone destruction in inflammatory arthritis. In vivo, compound 14 dose-dependently inhibited Freund’s adjuvant (CFA)-induced mechanical hyperalgesia without influencing mechanical sensitivity of control mice, suggesting that compound 14 may be developed as a potential therapy for chronic pain.50
The essential roles of cAMP-mediated signaling pathways in modulating leptin production/secretin and regulating metabolic homeostasis were previously explored, and the importance of EPAC-cAMP pathway in leptin resistance has been recently discovered.92 Further studies to investigate EPAC-cAMP pathway in obesity regulation were reported by Cheng and co-workers.93, 94 Their investigation implies that EPAC inhibitor compound 14 can inhibit leptin production and secretion, in vitro and in vivo. These findings offer strong evidence that EPAC1 could act as a novel pharmacological target for diabetes and obesity therapies.
Recently, Tao et al.95 found the capacity of compound 14 at a nontoxic concentration (10 µM) to protect Calu-3 cells against MERS-CoV and SARS-CoV infection via inhibiting the replication of viral RNA and expression of MERS-CoV and SARS-CoV protein without affecting virus binding to Calu-3 and expression and localization of EPAC protein. Compared wild-type C57BL/6 mice treated with compound 14 (10 mg/kg, once daily, i.p. for 12 days) group to control group, the survival rate was significantly improved after Rickettsia australis infection by the mechanism that compound 14 could completely block rickettsial attachment and/or invasion at an early step.68 Monje and co-workers reported that compound 14 could regulate schwann cell proliferation and differentiation by inhibiting EPAC.96 In blood stage, compound 14 prevents Plasmodium falciparum merozoite invasion of human erythrocytes by inhibiting parasite growth in blood stage.97 Moreover, Compound 14 can regulate the re-establishment of endothelial and recovery of EC barrier function after thrombin induced hyperpermeability.98
In addition, PK studies on 14 indicate that it displays excellent bioavailability, pharmacological and toxicological parameters.1, 68 A concern was initially raised about compound 14 that it may cause EPAC denaturing properties and false positives.99 To address this issue, Cheng and co-workers100 have conducted a battery of biochemical and pharmacological characterizations of compound 14, and further validated this compound as an EPAC specific inhibitor. Compound 14 has been widely used as a useful chemical probe to elucidate the role of EPAC in anti-cancer migration, anti-inflammation, anti-obesity, antivirus, anti-bacteria and other diseases.
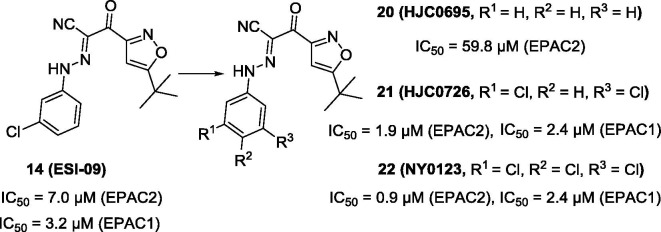
A preliminary structure-activity relationship (SAR) of compound 14 revealed that choro atom at C3-position of phenyl ring was essential for the EPAC inhibitory activities. Replacing the choro atom at C3-position of phenyl ring with hydrogen atom led to compound 20 (IC50 = 59.8 µM) (Fig. 7 ), with about 6-fold activity loss of EPAC2 inhibition. Adding another Cl atom at 5-position of compound 14 led to compound 21 (Fig. 7). The IC50 value of compound 21 (IC50 = 1.9 µM for EPAC2) was about 5-fold improvement compared to compound 14. Further, Zhou and co-workers.101 systematically investigated SAR of compound 14. Alteration of the linker and modification of the substitutes on isoxazole ring resulted in a dramatic activity loss. However, a slightly modification on the phenyl ring by addition of a third choro atom on compound 21 afforded compound 22 (Fig. 7). Compound 22 was proved to be a potent compound with an IC50 value of 0.9 µM for EPAC2 inhibition. In addition, it also showed a high EPAC1 inhibitory effect with an IC50 value of 2.4 µM. Molecular docking studies show that compound 22 could form hydrophobic and hydrogen bond interactions with EPAC2 CBD.1, 101 The motif on the isoxazole ring can stretch into the hydrophobic pocket and form hydrophobic interactions with residues of Phe367, Leu406, Ala407 and Ala415, while the phenyl moiety occupies and interacts with the hydrophobic pocket consisting of Val386 and Leu397. Moreover, there is a crucial hydrogen bond between NH of the linker and the side oxygen atom of residue Asp402.
Fig. 7.
Chemical structures of compounds 20–22.
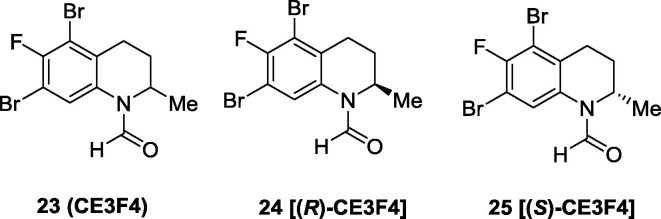
Meanwhile, Courilleau et al. identified a tetrahydroquinoline derivative, named as CE3F4 (23), from the chimiothèque essentielle compound library (Fig. 8 ) as an EPAC inhibitor.102 In intact cells, it showed certain inhibitory activity in EPAC1 GEF without affecting the PKA activity. Further, compound 23 was reported to exhibit modest selectivity on EPAC1 versus EPAC2, providing an EPAC1 selective inhibitor.103 An early SAR study indicated that the substituents on the tetrahydroquinoline pharmacophore was requisite for the EPAC1 inhibitory activity. The stereochemistry also plays an important role in EPAC1 inhibitory activity, and its (R)-enantiomer (24) was found to be 10-fold more potent than its (S)-enantiomer (25) in EPAC1 GEF inhibition in vitro. In addition, compound 24 exhibits 10-fold selectivity for EPAC1 versus EPAC2. Compound 24 may serve as a useful chemical probe for elucidating biological functions of EPAC1, while more relevant studies are needed to further validate its target-specificity and therapeutic potential.
Fig. 8.
Chemical structures of tetrahydroquinoline derivative CE3F4 and its enantiomers as EPAC1 selective inhibitors.
Conclusions and future perspectives: It is almost two decades since the discovery of EPAC as an important regulator of the cAMP-mediated biological function processes. Major advances have been made in understanding the function roles of EPACs with the aid of genetic knockout and pharmacological manipulations in various pathogenic models. Moreover, recent findings reveal the critical roles of EPACs in the manifestation of diverse maladies including cancer, diabetes and obesity, heart failure, inflammation, pain, infections, and CNS disorders, suggesting that EPAC presents a promising target for the treatment of various diseases.
X-ray co-crystal analysis and NMR spectroscopy not only provide the molecular clues of EPAC activation, but also give researcher a clear vision of the binding modes of EPAC proteins with their modulators. For example, the first breakthrough work on development of EPAC activator compound 4 through rational design facilitated by the co-crystal structural analysis of compound 1 and EPAC2. Although compound 4 and its prodrug have been useful as pharmacological tools for probing cAMP-mediated signaling, the big challenge is that it exhibits cross-target activities and off-target effects. Thus, the therapeutic applications of such cAMP analogues as EPAC agonists are potentially limited.
Another milestone in the field is the de novo discovery of EPAC2 and EPAC1 specific inhibitors through fluorescence-based HTS assays. Due to the excellent EPAC/PKA selectivity of compound 14, it has been widely applied as a useful chemical probe to discriminate EPAC related signaling pathways and biological functions. In addition, compound 14 and its analogues exert excellent in vivo PK and toxicity profiles and are promising lead compounds for therapeutic applications. Nevertheless, more extensive chemical optimizations are imperative to improve the potency (ideally submicromolar to nanomolar IC50) and the isoform-selectivity (EPAC1 vs. EPAC2) of this class of EPAC inhibitors. Therefore, EPAC-isoform specific agonists or antagonists with high potency and selectivity are still in urgent need, and to this end, modern drug discovery approaches (e.g. HTS, fragment-based drug discovery104, 105, 106 and computer-aided drug design107 may pave the way. Of note, EPAC1 and EPAC2 may play significant and distinct biological roles in a variety of human diseases, especially in cancer, inflammation, cardiovascular diseases, CNS disorders (e.g. drug addiction, and pain), infections and diabetes. Hence, potent EPAC agonists or antagonists with high EPAC1 or EPAC2-isoform selectivity, as well as ideal pharmacokinetic profiles are highly appreciated for further clinical development towards a viable therapeutic strategy for various human diseases.
Acknowledgments
This work was supported by grants R01 GM106218, R01 GM066170, and R01 AI111464 from the National Institutes of Health, John Sealy Memorial Endowment Fund, and the Center for Addiction Research (CAR) at UTMB.
References
- 1.Chen H., Wild C., Zhou X., Ye N., Cheng X., Zhou J. J Med Chem. 2014;57:3651–3665. doi: 10.1021/jm401425e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lezoualc'h F., Fazal L., Laudette M., Conte C. Circ Res. 2016;118:881–897. doi: 10.1161/CIRCRESAHA.115.306529. [DOI] [PubMed] [Google Scholar]
- 3.Fujita T., Umemura M., Yokoyama U., Okumura S., Ishikawa Y. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2336-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beavo J.A., Brunton L.L. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 5.de Rooij J., Zwartkruis F.J.T., Verheijen M.H.G. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki H., Springett G.M., Mochizuki N. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 7.Sugawara K., Shibasaki T., Takahashi H., Seino S. Gene. 2016;575:577–583. doi: 10.1016/j.gene.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parnell E., Palmer T.M., Yarwood S.J. Trends Pharmacol Sci. 2015;36:203–214. doi: 10.1016/j.tips.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almahariq M., Mei F.C., Cheng X. Acta Biochim Biophys Sin (Shanghai) 2016;48:75–81. doi: 10.1093/abbs/gmv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt M., Dekker F.J., Maarsingh H. Pharmacol Rev. 2013;65:670–709. doi: 10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee U., Cheng X. Gene. 2015;570:157–167. doi: 10.1016/j.gene.2015.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos J.L. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 13.Bos J.L. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Rehmann H., Das J., Knipscheer P., Wittinghofer A., Bos J.L. Nature. 2006;439:625–628. doi: 10.1038/nature04468. [DOI] [PubMed] [Google Scholar]
- 15.Rehmann H., Arias-Palomo E., Hadders M.A., Schwede F., Llorca O., Bos J.L. Nature. 2008;455:124–127. doi: 10.1038/nature07187. [DOI] [PubMed] [Google Scholar]
- 16.Chepurny O.G., Kelley G.G., Dzhura I. Am J Physiol Endocrinol Metab. 2010;298:E622–633. doi: 10.1152/ajpendo.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang G., Leech C.A., Chepurny O.G., Coetzee W.A., Holz G.G. J Physiol. 2008;586:1307–1319. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almahariq M., Mei F.C., Cheng X. Trends Endocrinol Metab. 2014;25:60–71. doi: 10.1016/j.tem.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzhura I., Chepurny O.G., Leech C.A. Islets. 2011;3:121–128. doi: 10.4161/isl.3.3.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira L., Metrich M., Fernandez-Velasco M. J Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C., Katoh M., Shibasaki T. Science. 2009;325:607–610. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto K., Shibasaki T., Yokoi N. J Biol Chem. 2002;277:50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 23.Kwan E.P., Xie L., Sheu L., Ohtsuka T., Gaisano H.Y. Diabetes. 2007;56:2579–2588. doi: 10.2337/db06-1207. [DOI] [PubMed] [Google Scholar]
- 24.Kai A.K., Lam A.K., Chen Y. FASEB J. 2013;27:4122–4135. doi: 10.1096/fj.13-230433. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki N., Shibasaki T., Kashima Y. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes H.B., Riordan S., Nomura T. J Neurosci. 2015;35:6544–6553. doi: 10.1523/JNEUROSCI.0314-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen A.E., Selheim F., de Rooij J. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 28.Kiermayer S., Biondi R.M., Imig J. Mol Biol Cell. 2005;16:5639–5648. doi: 10.1091/mbc.E05-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emery A.C., Eiden M.V., Eiden L.E. J Biol Chem. 2014;289:10126–10139. doi: 10.1074/jbc.M113.529321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Shu X., Liu D. Neuron. 2012;73:774–788. doi: 10.1016/j.neuron.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava D.P., Jones K.A., Woolfrey K.M. J Neurosci. 2012;32:11864–11878. doi: 10.1523/JNEUROSCI.1349-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oestreich E.A., Wang H., Malik S. J Biol Chem. 2007;282:5488–5495. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- 33.Oestreich E.A., Malik S., Goonasekera S.A. J Biol Chem. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulucan C., Wang X., Baljinnyam E. Am J Physiol Heart Circ Physiol. 2007;293:H1662–1672. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- 35.Metrich M., Lucas A., Gastineau M. Circ Res. 2008;102:959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 36.Pereira L., Cheng H.Q., Lao D.H. Circulation. 2013;127:913–922. doi: 10.1161/CIRCULATIONAHA.12.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira L., Rehmann H., Lao D.H. Proc Natl Acad Sci U S A. 2015;112:3991–3996. doi: 10.1073/pnas.1416163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber K.T., Sun Y., Bhattacharya S.K., Ahokas R.A., Gerling I.C. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 39.Roberts O.L., Dart C. Biochem Soc Trans. 2014;42:89–97. doi: 10.1042/BST20130253. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama U., Minamisawa S., Quan H. Am J Physiol Heart Circ Physiol. 2008;295:H1547–1555. doi: 10.1152/ajpheart.01317.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eid A.H. Cell Physiol Biochem. 2012;30:247–258. doi: 10.1159/000339061. [DOI] [PubMed] [Google Scholar]
- 42.Grandoch M., Hoffmann J., Röck K. Basic Res Cardiol. 2013;108:340. doi: 10.1007/s00395-013-0340-6. [DOI] [PubMed] [Google Scholar]
- 43.Purves G.I., Kamishima T., Davies L.M., Quayle J.M., Dart C. J Physiol. 2009;587:3639–3650. doi: 10.1113/jphysiol.2009.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts O.L., Kamishima T., Barrett-Jolley R., Quayle J.M., Dart C. J Physiol. 2013;591:5107–5123. doi: 10.1113/jphysiol.2013.262006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato Y., Yokoyama U., Yanai C. Arterioscler Thromb Vasc Biol. 2015;35:2617–2625. doi: 10.1161/ATVBAHA.115.306534. [DOI] [PubMed] [Google Scholar]
- 46.Wang H., Robichaux W.G., Wang Z. Sci. Rep. 2016;6:36552. doi: 10.1038/srep36552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sands W.A., Woolson H.D., Milne G.R., Rutherford C., Palmer T.M. Mol Cell Biol. 2006;26:6333–6346. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehrke M., Kahles F., Makowska A. J Mol Cell Cardiol. 2015;81:23–33. doi: 10.1016/j.yjmcc.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Wang H., Heijnen C.J., van Velthoven C.T. J Clin Invest. 2013;123:5023–5034. doi: 10.1172/JCI66241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singhmar P., Huo X., Eijkelkamp N. Proc Natl Acad Sci U S A. 2016;113:3036–3041. doi: 10.1073/pnas.1516036113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Billington C.K., Hall I.P. Br J Pharmacol. 2012;166:401–410. doi: 10.1111/j.1476-5381.2011.01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Billington C.K., Ojo O.O., Penn R.B., Ito S. Pulm Pharmacol Ther. 2013;26:112–120. doi: 10.1016/j.pupt.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dekkers B.G.J., Racké K., Schmidt M. Pharmacol Ther. 2013;137:248–265. doi: 10.1016/j.pharmthera.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Grandoch M., López de Jesús M., Oude Weernink P.A., Weber A.A., Jakobs K.H., Schmidt M. Cell Signal. 2009;21:609–621. doi: 10.1016/j.cellsig.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Moon E.Y., Lee G.H., Lee M.S., Kim H.M., Lee J.W. Life Sci. 2012;90:373–380. doi: 10.1016/j.lfs.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 56.Flacke J.P., Flacke H., Appukuttan A. J Biol Chem. 2013;288:3126–3135. doi: 10.1074/jbc.M112.403279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Misra U.K., Pizzo S.V. PLoS ONE. 2013;8:e63150. doi: 10.1371/journal.pone.0063150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Lee J.W., Lee J., Moon E.Y. Anticancer Res. 2014;34:3447–3455. [PubMed] [Google Scholar]
- 59.Harper K., Arsenault D., Boulay-Jean S., Lauzier A., Lucien F., Dubois C.M. Cancer Res. 2010;70:4634–4643. doi: 10.1158/0008-5472.CAN-09-3813. [DOI] [PubMed] [Google Scholar]
- 60.Baljinnyam E., Umemura M., De Lorenzo M.S. BMC Cancer. 2011;11:256. doi: 10.1186/1471-2407-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bastian P., Balcarek A., Altanis C. Cancer Lett. 2009;274:218–224. doi: 10.1016/j.canlet.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 62.Almahariq M., Tsalkova T., Mei F.C. Mol Pharmacol. 2013;83:122–128. doi: 10.1124/mol.112.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almahariq M., Chao C., Mei F.C. Mol Pharmacol. 2015;87:142–149. doi: 10.1124/mol.114.095158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Almahariq M., Mei F.C., Wang H. Biochem J. 2015;465:295–303. doi: 10.1042/BJ20140952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Onodera Y., Nam J.M., Bissell M.J. J Clin Invest. 2014;124:367–384. doi: 10.1172/JCI63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonough K.A., Rodriguez A. Nat Rev Microbiol. 2011;10:27–38. doi: 10.1038/nrmicro2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng X., Ji Z., Tsalkova T., Mei F. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong B., Shelite T., Mei F.C. Proc Natl Acad Sci U S A. 2013;110:19615–19620. doi: 10.1073/pnas.1314400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwede F., Maronde E., Genieser H., Jastorff B. Pharmacol Ther. 2000;87:199–226. doi: 10.1016/s0163-7258(00)00051-6. [DOI] [PubMed] [Google Scholar]
- 70.Enserink J.M., Christensen A.E., de Rooij J. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 71.Dao K.K., Teigen K., Kopperud R. J Biol Chem. 2006;281:21500–21511. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- 72.Hucho T.B., Dina O.A., Levine J.D. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fechner L., Baumann O., Walz B. Cell Calcium. 2013;53:94–101. doi: 10.1016/j.ceca.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Clemente M.I., Álvarez S., Serramía M.J., Martínez-Bonet M., Munoz-Fernández M.A. PLoS ONE. 2014;9:e85230. doi: 10.1371/journal.pone.0085230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X., Chen Y., Tong J. J Neurosci. 2016;36:4802–4815. doi: 10.1523/JNEUROSCI.3186-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vliem M.J., Ponsioen B., Schwede F. ChemBioChem. 2008;9:2052–2054. doi: 10.1002/cbic.200800216. [DOI] [PubMed] [Google Scholar]
- 77.Poppe H., Rybalkin S.D., Rehmann H. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 78.Schwede F., Bertinetti D., Langerijs C.N. PLoS Biol. 2015;13:e1002038. doi: 10.1371/journal.pbio.1002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conti M., Beavo J. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 80.Keravis T., Lugnier C. Curr Pharm Des. 2010;16:1114–1125. doi: 10.2174/138161210790963760. [DOI] [PubMed] [Google Scholar]
- 81.Waidmann O., Pleli T., Dvorak K. J Biol Chem. 2009;284:32256–32263. doi: 10.1074/jbc.M109.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Enyeart J.A., Liu H., Enyeart J.J. Mol Pharmacol. 2010;77:469–482. doi: 10.1124/mol.109.061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herfindal L., Nygaard G., Kopperud R., Krakstad C., Doskeland S.O., Selheim F. Biochem Biophys Res Commun. 2013;437:603–608. doi: 10.1016/j.bbrc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Tsalkova T., Mei F.C., Cheng X. PLoS ONE. 2012;7:e30441. doi: 10.1371/journal.pone.0030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsalkova T., Mei F.C., Li S. Proc Natl Acad Sci U S A. 2012;109:18613–18618. doi: 10.1073/pnas.1210209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen H., Tsalkova T., Mei F.C., Hu Y., Cheng X., Zhou J. Bioorg Med Chem Lett. 2012;22:4038–4043. doi: 10.1016/j.bmcl.2012.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen H., Tsalkova T., Chepurny O.G. J Med Chem. 2013;56:952–962. doi: 10.1021/jm3014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wild C.T., Zhu Y., Na Y. ACS Med Chem Lett. 2016;7:460–464. doi: 10.1021/acsmedchemlett.5b00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H., Ding C.Y., Wild C. Tetrahedron Lett. 2013;54:1546–1549. doi: 10.1016/j.tetlet.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lorenz R., Aleksic T., Wagner M., Adler G., Weber C.K. Pancreas. 2008;37:102–103. doi: 10.1097/MPA.0b013e318160748f. [DOI] [PubMed] [Google Scholar]
- 91.Mediero A., Perez-Aso M., Cronstein B.N. FASEB J. 2014;28:4901–4913. doi: 10.1096/fj.14-255703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukuda M., Williams K.W., Gautron L., Elmquist J.K. Cell Metab. 2011;13:331–339. doi: 10.1016/j.cmet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu Y., Robichaux W.G., Mei F.C. Mol Cell Biol. 2016;36:2440–2450. doi: 10.1128/MCB.01034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan J., Mei F.C., Cheng H. Mol Cell Biol. 2013;33:918–926. doi: 10.1128/MCB.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tao X., Mei F., Agrawal A. J Virol. 2014;88:3902–3910. doi: 10.1128/JVI.03001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bacallao K., Monje P.V. PLoS ONE. 2013;8:e82354. doi: 10.1371/journal.pone.0082354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dawn A., Singh S., More K.R. PLoS Pathog. 2014;10:e1004520. doi: 10.1371/journal.ppat.1004520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aslam M., Tanislav C., Troidl C., Schulz R., Hamm C., Gunduz D. Physiol Rep. 2014;2:e12175. doi: 10.14814/phy2.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rehmann H. Sci Rep. 2013;3:3032. doi: 10.1038/srep03032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu Y., Chen H., Boulton S. Sci Rep. 2015;5:9344. doi: 10.1038/srep09344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ye N., Zhu Y., Chen H. J Med Chem. 2015;58:6033–6047. doi: 10.1021/acs.jmedchem.5b00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Courilleau D., Bisserier M., Jullian J.C. J Biolog Chem. 2012;287:44192–44202. doi: 10.1074/jbc.M112.422956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Courilleau D., Bouyssou P., Fischmeister R., Lezoualc'h F., Blondeau J.P. Biochem Biophys Res Commun. 2013;440:443–448. doi: 10.1016/j.bbrc.2013.09.107. [DOI] [PubMed] [Google Scholar]
- 104.Chen H., Yang Z., Ding C. Eur J Med Chem. 2013;62:498–507. doi: 10.1016/j.ejmech.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen H., Zhou X., Wang A., Zheng Y., Gao Y., Zhou J. Drug Discov Today. 2015;20:105–113. doi: 10.1016/j.drudis.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hajduk P.J., Greer J. Nat Rev Drug Discov. 2007;6:211–219. doi: 10.1038/nrd2220. [DOI] [PubMed] [Google Scholar]
- 107.Chen H., Wang C.Z., Ding C. ChemMedChem. 2013;8:226–230. doi: 10.1002/cmdc.201200554. [DOI] [PMC free article] [PubMed] [Google Scholar]