Abstract
Background:
The quality of caregiving in mothers with substance abuse problems appears to be compromised. However, divergent findings, methodological variability, and sample characteristics point to the need for research synthesis.
Methods:
A comprehensive systematic search was undertaken. Studies were eligible if they (1) compared substance-misusing mothers with non–substance-misusing mothers, (2) involved children from birth to 3 years, and (3) maternal sensitivity and child responsiveness were measured using observational methodology.
Results:
A global meta-analysis for maternal sensitivity (n = 24 studies) and child responsiveness (n = 16 studies) on 3433 mother-infant dyads yielded significant population effect sizes and significant heterogeneity. Subgroup analyses found reduced heterogeneity when the meta-analysis was conducted on studies where groups were matched on key demographic characteristics; although the effect size was small, it was still significant for maternal sensitivity but not child responsiveness.
Conclusions:
Compromised quality of caregiving is found in high-risk, substance-misusing mothers, emphasising the importance of early intervention that draws from attachment-based interventions.
Keywords: Caregiving, substance, abuse, dependence, women, mothers
Introduction
The developmental outcomes of children living in families with illicit parental substance use are significantly compromised with difficulties initially identified in early infancy extending throughout childhood and into adolescence.1 Many areas of children’s functioning are compromised, including early interaction with caregivers resulting in elevated rates of insecure and disorganised attachment,2,3 and performance on tests of cognitive functioning4–6 and language.7,8 The compromised caregiving seen in mother-infant dyads9 has been implicated in poor outcome and high rates of disorganised and insecure attachment.10 These difficulties extend into childhood with evidence of difficulties in a range of executive functions11 and higher-than-normal rates of internalising and externalising disorders.12–14
Extensive research indicates that many of these difficulties are influenced by exposure to an early caregiving environment that lacks sensitive, contingent, and responsive maternal caregiving behaviour.15 For example, maternal insensitivity is associated with long-term difficulties,16,17 including conflict within parent-child relationships18,19 and internalising and externalising disorders at 5 years of age20 and beyond.21
There has been considerable investigation of the quality of caregiving in substance-misusing mothers, although there is also wide variability in the way in which the quality of caregiving has been measured across studies. Substantial variability in sample size, populations studied, and quality of the study design has contributed to mixed findings. An early narrative review of research studies published between 1990 and 1999 by Johnson9 provided an integrative synthesis of 23 studies in which mother-infant interaction had been measured. Fifteen of these studies were longitudinal or cross-sectional in design and focused on correlational associations between maternal characteristics and quality of caregiving. Notably, only 8 studies were included where there had been a direct comparison of mother-infant interaction in substance-misusing and non–substance-misusing mothers. Although 6 of these studies found poorer quality caregiving in the substance-misusing mothers, 2 found that substance-misusing mothers did not differ from a nonsubstance comparison group in their interactional style,22,23 with both studies using a comparison group that was matched on demographic characteristics.
Since Johnson’s9 review, studies have continued to show differences in the quality of caregiving.24,25 LaGasse and colleagues24 found that cocaine-using mothers were significantly poorer on 3 of 5 measures of maternal behaviour during feeding with their 1-month-old infant compared with non–substance-using mothers. Prenatal cocaine exposure was associated with poorer ratings of mother-child interactions measured at 3 years of age.26 Mother-child interactions were poorest for children with prenatal cocaine exposure whose mothers continued cocaine use postnatally, compared with children whose mothers did not use cocaine during pregnancy or at a 3-year follow-up visit. Poorer emotional availability was observed in a study of opioid-dependent mothers and their 7-month-old children compared with non–substance using mothers.27 Women who were polydrug and cocaine users during pregnancy have also shown greater dyadic conflict during feeding interactions.28 Contrary findings by Ukeje and colleagues29 found compromised care in both substance-misusing mothers and a matched comparison group. Thus, it is possible that the poor-quality caregiving relationship found in some studies may be more related to the accumulation of adverse environmental risk than maternal substance use per se.30,31
In summary, there is inconsistency in the results of studies addressing the quality of caregiving in mothers who have used illicit substances. One methodological issue that emerges from our reading of the literature that may potentially help explain discrepant results relates to study design. Studies with greatest methodological rigour have compared substance-misusing mothers with mothers facing similar environmental adversity, whereas those that are less methodologically robust have drawn the comparison group from a general population of mothers. However, studies also varied on other factors, such as the age of children, when the quality of caregiving was assessed. Finally, mothers who are engaged in treatment, and particularly residential treatment, may show less compromised caregiving as many treatment services, particularly residential programmes, may have addressed parenting as part of the treatment process. Thus, associated improvements in well-being32,33 and parenting practices34 may influence the quality of caregiving.
The aim of this study is to assess the extent to which mothers with substance misuse have compromised caregiving. This builds on existing narrative reviews and extends this literature by providing a comprehensive systematic review and meta-analysis of studies that have compared the quality of parent-child interactions (maternal sensitivity and child responsiveness) in illicit substance-misusing and nonmisusing groups. Illicit substance use was the focus of this review as there is a range of legal, environmental, and lifestyle risks accompanying illicit substance use in women which makes them qualitatively different from women with tobacco or alcohol problems.35 The primary aim of this study was to investigate the quality of caregiving relationship in mothers with substance use problems (including those on opioid replacement therapy) by comparing measures of maternal sensitivity and child responsiveness with mothers who did not have a substance misuse problem. The second aim was to examine factors (moderators) that could influence the quality of the caregiving relationship.
Methods
Studies
Studies were included in the review if they included all of the following elements: mothers of children aged birth to 3 years, mothers who were current illicit substance misusers and/or were on opioid replacement therapy due to a history of opioid dependence and/or were in residential treatment due to a history of illicit substance use, and a comparison group of non–substance-using mothers; there was an assessment of maternal-child interactions using an observational method that was videotaped and subsequently coded to assess the quality of maternal caregiving.
Outcomes
The primary outcome measure was maternal sensitivity. This was operationalised as a maternal response to infant or child cues related to maternal warmth in situations of low frustration rather than during situations of frustration or negative affect.36 The measure of maternal sensitivity was extracted from a range of observational tasks that included free play, structured play, and infant feeding observations. Thus, we selected scores on observational coding systems that explicitly measured maternal behaviour and affect using terms such as ‘talks to infant’, ‘shows pleasure towards infant’ and ‘appears cheerful’,37(p4) ‘responding to the child’s activity and interests (sensitivity/pacing), positive feelings shown to the child’.38(p557) The secondary outcome measure was child responsiveness. This was also required to have been explicitly included as a scale or construct measured in the observational system that rated infant or child responsiveness directly, such as ‘involvement with the mother, positive feelings shown to mother’,38(p557) and ‘child responsiveness indicates how well infant responds to maternal bids and expressions’.27(p250)
Given the diversity of study populations, we identified 3 potential sources of heterogeneity across studies to test in subgroup analyses: (1) study design (groups matched on key demographic variables vs studies comparing substance-misusing mothers with a general population group), (2) age of child (less than 12 months vs more than 12 months and up to 40 months), and (3) treatment (not in treatment vs currently in outpatient treatment, including opioid replacement therapy and/or residential treatment).
Search strategy
Search terms were identified by (1) an examination of indexing terms in relevant databases, and (2) a preliminary scoping of eligible studies prior to the systematic search. Search terms were combined together with Boolean OR and each set of search terms was then combined with Boolean AND to search across title, abstract, and keywords in each search location. There were no restrictions placed on document type. Search terms were (‘maternal substance use’ OR ‘maternal drug use’ OR ‘substance-using mothers’ OR ‘drug-using mothers’) AND (‘caregiving’ OR ‘care giving’ OR ‘interaction’). Studies were included if they were in English-language publications and the date range was from 1995 to 2015 (updated April 2016; see Figure 1) as this 20-year period reflects the steady increase in the use of cannabis and the growing use of crack cocaine and heroin that began in the early 1990s.39 Studies were identified by searching the following electronic databases: Scopus, MEDLINE, ScienceDirect, PsycINFO, SpringerLink, and Google Scholar. The reference lists of existing reviews and eligible studies were harvested after completion of systematic screening to ensure capture of all eligible studies.
Figure 1.
Selection process for eligible papers for systematic review and meta-analysis (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 2009 flow diagram.
Study selection and data extraction
The literature search identified 2028 studies. Figure 1 provides a description of the complete selection and exclusion process using Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)40 guidelines. The PRISMA Statement was developed to enhance clarity and transparency of reporting in systematic reviews. It includes a detailed 27-item checklist that provides explanations of the key components that need to be determined in a systematic review and an additional 4-phase flow diagram that documents the decision-making process in the selection of studies for the review.41 Search results were exported into EndNote Version X7 for Windows, and duplicates and ineligible document types (eg, books) were removed. The remaining records were imported into systematic review management software, SysReview,42 for initial eligibility screening. Titles/abstracts/keywords were screened and the record was excluded if the title and/or abstract and/or keyword indicated that the document was not an eligible document type. Following the completion of title and abstract screening, a full-text review was undertaken and data were extracted in a standardised format following the PRISMA guidelines.40 Of the remaining 84 articles, a further 52 documents were eliminated (level 2 exclusion), reducing the pool to 32 documents. One further study was identified during an updated literature search (April 2016) and included (see Figure 1).
These 33 documents reported the results of 30 unique research studies as 3 studies had reported on maternal sensitivity across different research reports. Finally, 6 studies were not included in the meta-analysis as data were not reported or not available from authors due to the passage of time (descriptive characteristics are included in Table 1). Therefore, data from 24 studies were included in the final analysis.
Table 1.
Participant characteristics including primary drug used, age, race, recruitment site, and description of maternal sensitivity and child responsiveness measures.
| Author | Substance-using mothers | Comparison of non–substance-using mothers | Diagnostic assessment and measures of substance use | Age of infant | Measure of maternal sensitivity to child | Measure of child responsiveness to mother |
|---|---|---|---|---|---|---|
| Groups matched on demographic characteristics | ||||||
| Eiden et al37 | Mean age: 31.6 (5.6) y (USA) Cocaine use screened – excluded if positive for opioids and benzodiazepines Total sample 72% African American 70% family assistance Recruited from hospital post delivery |
Mean age: 29.3 (5.2) y No illicit drug use – THC use declined post delivery Matched on same hospital recruitment site, maternal education, age, race, and infant gender |
Cocaine use measured by TLFB for quantity and frequency: confirmed by urine toxicology 99% and hair analysis 79% | 1-2 mo | MIFS: derived a factor score of maternal sensitivity during feeding | Not measured |
| Eiden et al43 | Mean age: 30.9 (6.0) y (USA) Excluded on screening if positive for drugs other than cocaine and THC Total sample 74% African American 71% family assistance Recruited from hospital post delivery |
Mean age: 28.6 (5.6) y Matched on same hospital recruitment site, maternal education, age, race, and infant gender |
Cocaine use measured by TLFB for quantity and frequency: confirmed for 98% of sample using urine toxicology and hair analysis | 7 mo | 5 min free play coded using the Clark et al (1980): sensitivity measure | Not measured |
| Eiden et al44 | Mean age: 31.1 (6.0) y (USA) Excluded on screening if positive for drugs other than cocaine and THC Total sample 70% African American 81% family assistance Recruited from 2 local hospitals post delivery |
Mean age: 27.9 (5.6) y Matched on same hospital recruitment site, maternal education, age, race, and infant gender |
Cocaine use measured by TLFB for quantity and frequency: confirmed by urine toxicology 55% and 79% hair analysis | 13 mo | 10-min free play coded using PC-ERA: sensitivity measure | PC-ERA: responsiveness |
| Fraser et al45 | Mean age: 27.0 y(USA) Total sample 60% African American 100% Medicaid eligible Recruited from services aimed to support substance-using mothers |
Recruited from same 2 recruitment sites – no differences on age, race, and education | Clinical assessment to arrive at substance use disorder: 95% substance-related disorder, 67% cocaine-related disorder, and 67% reported polysubstance use | 3.5 mo | 10-min free play coded using EAS: sensitivity measure | EAS: responsiveness |
| French et al46 | Age >20 y (USA) 66% alcohol, THC and/or cocaine THC only (33%) Total sample 67% African American 97% single Recruited at initial prenatal visit |
Recruited from same antenatal clinic – matched on parity, age, race, marital status, and education | Prenatal urine drug toxicology | Within 24 h after delivery | Feeding observation using NCAFS prior to intervention: total sensitive scale | NCAFS: total responsiveness |
| Goodman et al38 | Age range: 18 to 35 y (USA) OMT; Total sample 100% African American Recruited from prenatal clinics |
Recruited from same prenatal clinics – no differences on race, age, SES, years of education, parity | Self-report of methadone maintained for a minimum period of 6 mo prior to pregnancy | 12 mo | 40 min of structured and unstructured activities: composite score of caregiver communication | PCOG: composite score of infant communication |
| Hans et al47 | Mean age: 27.7 (3.9) y (USA) OMT Total sample 100% African American Recruited from prenatal clinic |
Mean age: 25.7 (3.9) y Recruited from same prenatal clinic – no differences on race and SES |
Clinic records identified mothers receiving methadone maintenance | Authors report: standardised and summed 4, 12, and 24 mo | 40 min structured and unstructured age appropriate activities: sensitivity responsiveness measure | PCOG: child perception of mother: accepting |
| Minnes et al48 | Mean age: 29.3 (5.0) y (USA) 100% cocaine + alcohol (87%), THC 53%), amphetamine (1%) Total sample – 81% ‘nonwhite’ All mothers recruited prenatally from a large urban county hospital |
Mean age: 25.8 (5.0) y Alcohol (66%), THC (13%), and amphetamine (1%) Recruited from same antenatal clinic – no differences on race, SES status, and education attained |
Severity of substance use assessed by maternal report and toxicology; heavy use = maternal report >17.5 units of use or >70th percentile cocaine metabolite in meconium sample | 12 mo | Feeding session videoed and coded using NCAFS – sensitive to cues measured | No means provided |
| Schuler et al49 | Mean age: 27.2 (6.4) y (USA) 100% cocaine + 45% heroin + 35% THC 85% African American 95% single Total sample recruited from same postnatal wards |
Mean age: 24.5 (5.0) y 90% African American 90% single Did not differ on age, race, education, and marital status |
Maternal self-report and infant urine toxicology results | 2 wk | Free play mother-infant interaction coded using PC-ERA: composite scores on positive maternal involvement | PC-ERA: composite scores on positive infant involvement |
| Singer et al50 | Mean age: 27.1 (4.0) y (USA) 100% cocaine + alcohol (46%), THC (36%), and barbiturates (12%) Total sample 100% African American 63% in care of biological mother All recruited from infant admission to NICU for VLBW |
Mean age: 25.6 (6.0) y Alcohol (6%), THC (0%), and barbiturates (9%) 95% in care of biological mother No differences on age, race, and SES |
Maternal self-report and infant urine toxicology | Close to 40 wk (gestational age) | Feeding interaction was coded using NCAFS: sensitive to cues | NCAFS: responsiveness |
| Tronick et al25 | 70.3% aged between 26 and 35 y (USA) Predominantly cocaine 77.5% African American 81.4% single 89.8% Medicaid Total sample recruited from 4 maternity hospitals (MLS study) |
4.95% aged between 26 and 35 y 75.2% African American 70.3% single 74.3% Medicaid No differences on race, sex, and gestational age |
Maternal self-report and meconium sampling for cocaine or opiate metabolites | 4 mo | 6-7 min FFSF paradigm: structured interactions coded for maternal engagement | FFSF: reported infant distancing (avoidance) |
| Uhlhorn et al51 | 68.8% aged between 26 and 35 y (USA) 100% cocaine + alcohol (71%), THC (39%) 72.9% African American 81.3% single Total sample Recruited from 4 maternity hospitals (MLS study) |
39.0% aged between 26 and 35 y Alcohol (35%), THC (4%) 80.5% African American 71.4% single No differences on education level, marital status, SES, or race |
Maternal self-report or meconium toxicology for cocaine metabolites | 18 mo | 10-min unstructured play coded using Ainsworth’s Sensitivity Rating Scale (1974): maternal sensitivity | Ainsworth: positivity |
| Ukeje et al29 | No mean provided (USA) Predominantly cocaine Total sample 100% African American Recruited from 2 prenatal maternity hospital clinics or just after delivery |
Recruited from same demographics: no difference on race | Maternal report of substance use via a structured interview (quantity and frequency) confirmed by meconium toxicology | 12 mo | 5-min free play interaction, then 2-min separation and 2-min reunion. Adapted from Ainsworth (1978): maternal warmth | Ainsworth: positive affect |
| Veira et al52 | Mean age: 31.0 (6.0) y (USA) Predominantly cocaine use. Total sample 74% African American 71% receive welfare support 60% single All recruited from 2 maternity hospitals after delivery |
Mean age: 27.7 (5.6) y Closest matching comparison family recruited matched on maternal education, maternal race, and infant gender |
Maternal self-report 2% and 98% via hair sampling | Authors report a mean of 7, 13, and 24 mo | 10-min free play interaction coded using Clark et al (1980): maternal sensitivity | Not measured |
| Groups not matched on demographic characteristics | ||||||
| Johnson et al26 | Mean age: 32.0 (4.8) y (USA) 100% cocaine + alcohol (45%), THC (26%) Total sample 100% African American Recruited from hospital post delivery 81% unemployed |
Mean age: 27.2 (5.6) y Alcohol (40%), THC (11%) 64% unemployed |
Structured and standardised interview conducted for quantity and frequency. Confirmed by maternal and infant urine and meconium toxicology metabolites | Mean age: 40 mo | 15-min semistructured play interaction using Egeland et al (1995): quality of instruction | Not measured |
| LaGasse et al24 | Mean age: 30.5 (4.8) y (USA) Predominantly cocaine 83% African American Total sample Recruited after hospital discharge by 4 major universities 90.1% single 78.6% below poverty line 48.4% education > high school 19.7% no antenatal care |
Mean age: 26.4 (5.8) y 78.8% African American 78.2% single 63.2% below poverty line 32.1% education > high school 4.3% no antenatal care |
Mothers completed the MISU and ASI. Substance use exposure confirmed by maternal self-report, structured interview, and meconium toxicology for metabolites | 1 mo | 15 min of a feeding session and coded using maternal flexibility | Not measured |
| Molitor and Mayes53 | Mean age: 29.3 (4.2) y (USA) Cocaine + alcohol, THC 92.9% African American 100% receiving welfare support 99.5% single 69.2% education >12 y Total sample Recruited during routine prenatal appointments |
Mean age: 25.8 (4.9) y 75.0% African American 83.3% receiving welfare 85% single 77.8% education >12 y |
Maternal self-report (lifetime exposure, frequency, and quantity), urine toxicology | 18 mo | 5-min child play without mother’s attention and 5-min free play with mother coded using RSIS: maternal interactive competence | RSIS responsiveness |
| Pajulo et al54 | Mean age: 25.4 (5.6) y (Finland) Residential treatment for drug (THC, amphetamine, and heroin) or alcohol misuse Recruited from 3 treatment units 58% single 17% education >12 y 92% unemployed |
Mean age: 27.3 (3.4) y Recruited from child health care centres in South Finland 0% single 100% education >12 y 25% unemployed |
Admission to a residential treatment for severe substance use | 3 and 6 mo | Feeding and a free play interaction were videoed and coded using PC-ERA: total score of maternal sensitivity | Not measured |
| Perry et al55 | Mean age: 29.2 (4.3) y (Australia) OMT 61% homeless 9% aboriginal 45% single 63.6% homeless Total sample Recruited during routine prenatal appointments |
Mean age: 28.8 (4.9) y 0% homeless 0% aboriginal 7% single 0% homeless |
Structured interview for history of substance use (Psychosocial Assessment Interview) and supported by treatment for heroin addiction with opioid substitution | Less than 18 mo | 15 min unstructured free play interaction was videoed and coded using EAS: total score of maternal sensitivity | EAS: responsiveness |
| Salo et al27 | Mean age: 28 (2.8) y (Finland) ORT Recruited during pregnancy from maternity hospital 100% children had child welfare involvement |
Mean age: 29.08 (3.20) y Recruited through well-baby clinics 0% children had child welfare involvement |
Current treatment for opioid dependence with buprenorphine | 36 mo | 5-min free play interaction and coded using EAS: maternal sensitivity | EAS: responsiveness |
| Salo et al56 | Mean age: 28.3 (3.4) y (Finland) ORT + THC (40%), alcohol 40%, amphetamine 40% 66.7% has criminal record 40% mother fostered as child Recruited during pregnancy from maternity hospital |
Mean age: 29.9 (3.2) y 0% criminal record 0% mother fostered Recruited randomly through well-baby clinics |
Current treatment for opioid dependence with buprenorphine | 7 mo | 4-min free play interaction coded using EAS: maternal sensitivity | EAS: responsiveness |
| Sarfi et al57 | Mean age: 32.4 y (Norway) All on ORT 34% unemployed 10 y of education 38.8% single Recruited during pregnancy while in treatment |
Mean age: 32.5 y Volunteered 0% unemployed 16 y of education 0% single Recruited via leaflets at local health centres |
Prenatal assessment using European ASI with pregnant women enrolled in OMT programmes | 6 mo | 15 min free play coded using NICHD modified by Cox and Crnic (2003): maternal style | Not measured |
| Savonlahti et al58 | Mean age: 26.7 y (Finland) Residential treatment 93% unemployed 78% single 86% <12 y education Recruited during pregnancy while in treatment from 3 sites |
Mean age: 27.3 y 0% unemployed 0% single 0% <12 y education Recruited from well-baby clinics |
Admission to a residential treatment for severe substance use | 6 mo | 5-min feeding and free play interactions videoed and coded using PC-ERA: composite score of dyadic interactive capacity | PC-ERA: mutuality of affect in play situation |
| Siqveland and Moe59 | Mean age: 26 y (Norway) Residential treatment and detoxified during pregnancy 77% single Recruited prenatally from residential treatment centres |
Mean age: 33.3 y Voluntary participation 0% single Recruited from well-baby clinics |
Prenatal assessment using European ASI with pregnant women admitted to residential treatment for severe substance use and detoxed during pregnancy | 3 and 12 mo | 15-min semistructured play interactions at 12 mo coded using PC-ERA: expressed affect | PC-ERA: child involvement |
| Studies not included in meta-analysis | ||||||
| Mayes et al60 | Mean age: 25.6 (4.4) y (USA) Predominantly cocaine 58.1% completed high school Total sample 86% African American 90% single parents 100% receiving welfare support Recruited prior to and post delivery |
Mean age: 26.6 (5.3) y No drug use 85.7% completed high school |
Maternal report and urine toxicology | 3 and 6 mo | 3-min interaction with infant with infant seated facing mother coded using Clark and Seifer – RSIS: maternal attentiveness | RSIS: infant readiness |
| Burns et al61 | Mean age: 27.3 (4.45) y (USA) Recruited clinics for substance-using mothers 11.2 mean years of education 70% African American Total sample Family income: <$15 000 |
Mean age: 26.1 (6.3) y Recruited from a public health clinic 12.6 y of education 80% African American |
Maternal report and/or toxicology records | Mean age: 10 mo | 2 × 5 min (structured and unstructured) coded using PC-ERA: sensitivity and responsiveness to cues | PC-ERA: responsiveness to maternal social behaviour |
| Ball et al62 | Mean age: 28.2 (4.8) y (USA) 100% cocaine use Total sample 81.8% African American 89.3% single parent 94.6% unemployed 61.3% high school educated Recruited antenatally in hospital examining room |
Mean age: 26.2 (4.6) y Repeated negative on urine toxicology |
Structured interview for lifetime and recent substance use, ASI, and urine toxicology | 3, 6, 12, and 18 mo | 10- to 15-min free play interaction while child sitting on mother’s lap as part of a developmental assessment (BSID): maternal attentiveness | Not measured |
| Eiden28 | Mean age: 31.53 (6.34) y (USA) Predominantly cocaine and polydrug Total sample 91% African American 72% single parents 60% receiving aid to families with dependents Recruited postnatally |
Mean age: 26.04 (5.18) y No illicit drug use |
Maternal report, chart review, and maternal urine toxicology | 2 mo | 10- to 20-min feeding task; MIFS: maternal sensitivity | MIFS: affect |
| Belt et al63 | Mean age: 25.53 (4.16) y (Finland) 19% single parent 46% basic education 38.5% unemployed Recruited from 2 addiction outpatient psychiatry clinics |
Mean age: 29.24 (5.02) y 4% single parent 12% basic education 6% unemployed Recruited from maternal outpatient clinics |
Maternal report and semistructured assessment and urine toxicology | 4 and 12 mo | 7-10 min assessed at T(2) and T(3) using EAS: maternal sensitivity | EAS: child responsiveness |
| Lewis et al64 | Mean age: 27 (5.7) y (USA) Predominantly cocaine 37.8% African American 53.3% Latina 11 (1.6) y of education 5.3 (2.1) income logged Total sample Recruited from high-risk prenatal clinic |
Mean age: 23.4 (4.1) y Noncocaine using 26.9% African American 61.5% Latina 11.8 (2.3) y of education 5.5 (2.4) income logged |
Maternal report, interview, and urine toxicology | 6 mo | 3 min interaction using FFSF: caregiver sensitivity | Not measured |
Abbreviations: ASI, Addiction Severity Index; BSID, The Bayley Scales of Infant Development–Revised; EAS, Emotional Availability Scale; FFSF, Face-to Face Still-Face; MIFS, Mother Infant Feeding Scale; MISU, Maternal Inventory of Substance Use; MLS, Maternal Lifestyle Study; NCAFS, Nursing Child Assessment Feeding Scale; NICHD, National Institute Child Health and Human Development Network; NICU, Neonatal Intensive Care Unit; OMT, Opioid Maintenance Therapy; ORT, Opioid Replacement Therapy; PC-ERA, Parent-Child Early Relational Assessment; PCOG, Parent-Child Observation Guides for Program Planning; RSIS, Rating Scale of Interaction Style; SES, socioeconomic status; THC, Marijuana; TLFB, Timeline Follow Back; VLBW, Very low birth weight.
Data synthesis, study quality appraisal, and analyses
Study quality was assessed using 9 items from the 14-item Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (National Institute of Health).65 The excluded items related to aspects of study quality that required a follow-up component (eg, attrition, number of follow-up points). The retained items are listed in Supplementary Table 1. All meta-analytical calculations were performed using Review Manager 5.66 Standardised effect sizes (d) were calculated for the included studies. When data on maternal sensitivity were reported at 2 time points in the same document, data from the second time point were used (ie, 3 and 6 months54; 3 and 12 months59). On both occasions, the second time point was used as this was closer to the mean age of children across all studies. Two studies24,25 divided their sample into ‘heavy’ and ‘some use’, and we selected data from the heavy group as this reflected substance use patterns reported in other papers.
A random-effects model was used to calculate effect size due to expected heterogeneity in the studies.67 Three potential sources of heterogeneity were identified a priori and 2 were subsequently investigated. The latter were as follows: (1) study design (groups matched on key demographic variables vs studies comparing substance-misusing mothers with general population) and (2) age of child (less than 12 vs more than 12 months and up to 40 months) in accordance with recommendations regarding subgroup analyses (Cochrane Handbook; Chapter 9.6.6).68 It was not possible to test whether treatment was a moderator as most of the studies that were classified as ‘in treatment’ were also included in the subgroup ‘unmatched’.
A forest plot was calculated in Review Manager 5 (version: 5.3.5), and the heterogeneity between studies was assessed using the Q statistic and I2 index. Subgroup analyses were conducted to investigate sources of heterogeneity. Finally, sensitivity analyses using a priori weight functions were conducted to determine whether the estimates of effect size were likely to be influenced by publication bias.69,70
Results
Study characteristics and quality appraisal
Twenty-four studies that included a total of 3433 mother-child dyads met final inclusion for the meta-analysis. Of these, 15 studies reported that the mother’s primary drug of use was cocaine or a combination of cocaine and other drugs, whereas 9 studies reported that the primary drug was an opioid. Studies did not typically use diagnostic nomenclature to describe the study population. Thirteen studies provided information on quantity and frequency plus urine toxicology, hair analysis, and/or meconium testing; 1 study used clinical assessment to arrive at a diagnosis; 9 studies reported that mothers were being prescribed opioid replacement therapy as either an outpatient or were currently in a residential treatment facility; and 1 study used prenatal maternal urine toxicology reports to verify substance use. Of all the 17 studies reporting maternal substance use conducted in the United States, only 3 studies had a focus on mothers in treatment. Conversely, all studies conducted outside the United States were conducted in either residential treatment or outpatient opioid replacement clinics (ie, Finland, 4 studies; Norway, 2 studies; Australia, 1 study). The timing, identification, and assessment of substance use also differed between studies. Twelve studies identified and assessed mothers for substance misuse antenatally, 2 studies reported assessment of substance use both antenatally and postnatally, and the remaining (n = 10) assessed substance use postnatally. Studies were divided relatively equally between infants less than 1 year (n = 13) and infants more than 1 year (n = 11). The observational measures all included a measure of the construct of maternal sensitivity and consisted of Ainsworth’s Maternal Sensitivity Measure (n = 2),71 the Parent-Child Early Relational Assessment (n = 6) (PC-ERA),72 the Emotional Availability Scales (n = 4) (EAS),73 and Still-Face Paradigm (n = 1),25 and 11 studies used purposely designed measures of maternal sensitivity (see Table 1 for a description of studies).
There was relatively little variability in study quality, with all studies scoring a YES on key elements of design (eg, clearly stated research question, clearly defined population, and clearly defined independent and dependent variables (see Supplementary Table 1). The 1 item that showed variability related to study design, namely, were participants recruited from the same or similar populations. Fourteen studies scored a YES indicating that the samples have been drawn from the same population. However, 10 of the studies reported variations in sample characteristics and thus scored NO. This item was subsequently used to classify studies on the basis of matched or unmatched samples for later subgroup analyses.
Global analyses of maternal sensitivity and child responsiveness
A global meta-analysis of the 24 studies reporting maternal sensitivity yielded an overall population effect size of 0.46 (95% confidence interval [CI]: 0.31-0.61, Z = 5.99, P < .00001), indicating that maternal sensitivity was higher in non–substance-using mothers compared with substance-misusing mothers. Notably, however, the proportion of total variability explained by heterogeneity was high (Q(23) = 73.53, P < .00001; I2 = 69%).
Similar findings were obtained for a second global meta-analysis of the 16 studies reporting child responsiveness. The overall population effect size was 0.32 (95% CI: 0.06-0.59, Z = 2.37, P = .02), once again indicating that child responsiveness was higher in non–substance-using mothers compared with substance-misusing mothers. There was also significant heterogeneity across studies (Q(15) = 65.05), P < .00001), and the proportion of total variability explained by heterogeneity was high (I2 = 77%).
Moderation analyses to identify sources of heterogeneity
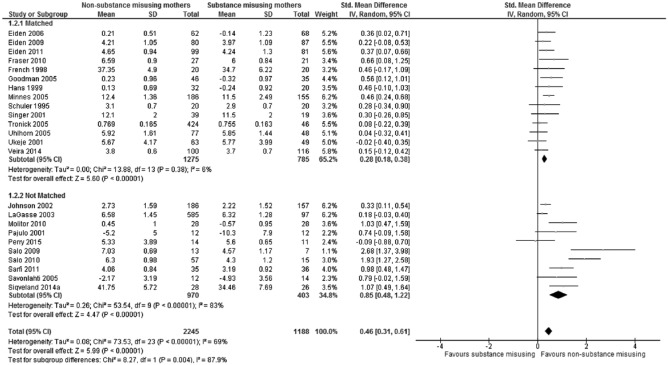
The significant heterogeneity did not allow any meaningful interpretation of the distribution of effect sizes across studies.77 To investigate potential sources of heterogeneity, subgroup analyses were undertaken. The first of these was design, as the precision of effect sizes is related to methodological quality, including the matching of study groups.78 Subgroup analysis for study design found that the overall effect size of maternal sensitivity for the matched subgroup was statistically significant (Z = 5.60, P < .00001) but small (0.28, 95% CI: 0.18-0.38; see Figure 2), whereas for the nonmatched subgroups, the overall effect size remained large (0.85, 95% CI: 0.48-1.22) and also statistically significant (Z = 4.47, P < .00001). A test for subgroup differences found that the lower estimate of effect size within the matched subgroup was statistically significant (Q(1) = 8.27, P = .004), and heterogeneity within the matched subgroup was significantly reduced and no longer statistically significant (Q(13) = 13.88, P = .38; I2 = 6%), whereas heterogeneity within the nonmatched subgroup remained high and statistically significant (Q(9) = 53.54, P < .00001; I2 = 83%).
Figure 2.
Forest plot of maternal sensitivity for total sample (n = 24) grouped by study design (matched and nonmatched).
A similar but nonsignificant pattern was found for child responsiveness. The overall effect size of child responsiveness for the matched subgroup was not statistically significant (Z = 1.59, P = .11) and small (0.13, 95% CI: –0.03–0.29; see Figure 3), whereas for the nonmatched subgroup, the overall effect size remained large (0.79, 95% CI: 0.00-1.58) and statistically significant (Z = 1.96, P = .05). A test for subgroup differences found that the lower estimate of effect size within the matched subgroup was not statistically significant (Q(1) = 2.57, P = .11). Although heterogeneity within the matched subgroup was reduced, it was not statistically significant (Q(9) = 12.19(9), P = .20; I2 = 26%) and heterogeneity within the nonmatched subgroup remained high and statistically significant (Q(5) = 36.82(5), P < .00001; I2 = 86%).
Figure 3.
Forest plot of child responsiveness for total sample (n = 16) grouped by study design (matched and nonmatched).
Overall, these results show significantly reduced levels of heterogeneity for estimates of effect size for both maternal sensitivity and child responsiveness when samples of substance-misusing mothers were compared with mothers matched on factors such as socioeconomic status, level of education, and (for US studies) eligibility for Medicaid. However, when substance-misusing mothers were compared with mothers drawn from the general population, the differences between the groups on maternal sensitivity and child responsiveness were observed to be significantly larger.
There were no effects of age on heterogeneity. Furthermore, consideration of the variable treatment (mothers in treatment vs not in treatment) was not pursued as the studies of mothers in treatment were also those with nonmatched design, indicating that any finding relating to heterogeneity would be confounded by design.
Publication bias
The existence of publication bias was evaluated by inspection of the funnel plots for maternal sensitivity and child responsiveness. A funnel plot is a scatterplot of effect size (x-axis) graphed against sample size (y-axis) centred on the true population effect size. In the absence of publication bias, studies with larger sample sizes would be expected to be closer to the true population effect size with greater variability of effect size estimates in studies with smaller sample sizes. Thus, when the values of effect size estimates are plotted, the values will be symmetrically distributed around the population effect size in the shape of a funnel. Visual inspection of the funnel plot for maternal sensitivity showed that 3 studies did not fall within the expected funnel shape. The Kendall τ, a test to detect the presence of publication bias, was found to be significant τ(N = 24) = .38, P = .01, suggesting publication bias. To assess whether the publication bias would differ when adjusted for publication bias, we used the procedure by Vevea and Woods70 as described in Field and Gillett.69 This procedure assesses how effect size estimates would change if selection bias was present using several models of possible selection bias. Adjusted parameter estimates ranged from .52 to .58, suggesting slightly lower overall effect size for maternal sensitivity after adjusting for publication bias.
Discussion
This systematic review and meta-analysis examined 24 studies with a combined total of 3433 mother-child dyads to compare quality of caregiving in mothers who were using illicit substances or were currently in treatment and/or prescribed opioid replacement therapy with the quality of caregiving in non–substance-using women. These findings provide a synthesis of the literature on the quality of caregiving in substance-misusing mothers and is the first quantitative analysis of caregiving quality in substance-misusing mothers.9
Overall, the composite effect size based on the meta-analysis of all 24 studies indicated that maternal sensitivity and child responsiveness were higher in mothers who had not used illicit substances. However, we found considerable heterogeneity that limited meaningful interpretation of the results.79 Therefore, we undertook an examination of potential moderators that might be influencing the variability in effect sizes between studies using subgroup analyses. The first moderator to be tested was design. Smaller effect size values were observed for maternal sensitivity but not for child responsiveness in studies in which substance-misusing mothers were drawn from the same population and thus shared similar demographic characteristics such as socioeconomic status, single parenthood, level of education, and eligibility for Medicaid compared with those studies that were not matched groups. This finding raises important questions about the interplay between environmental risk and maternal substance use on a key moderator of child outcome: the quality of the caregiving relationship.80 It is clear that the participants in the matched group of studies were recruited from high-risk populations: they were all of low income, drawn from geographical areas associated with severe financial disadvantage, had low levels of education, and in the case of the US studies, were predominantly from ethnic minority groups who were in receipt of Medicaid. All of these are well-recognised risk factors that have a cumulative rather than additive effect on child outcome.81,82 Thus, these families are at high risk of poor child outcome. The addition of maternal illicit substance use appeared to increase risk; there was still a significant, albeit small, difference in the quality of caregiving that favoured the non–substance-using group for maternal sensitivity. Thus, the additional risk of maternal substance use is likely to confer even greater vulnerability for these children who are already exposed to a significant number of socioenvironmental risks.83,84
We also tested age of child as a potential moderator. Typically, maternal sensitivity and associated constructs are relatively stable across time.85 However, family stress and adversity have been found to influence a range of maternal behaviours, including sensitivity.86 Thus, it is plausible that for families with maternal substance use, and at least for those matched in sociodemographic features, age of the child may be associated with poorer sensitivity and child responsiveness. The third moderator that we had aimed to assess was whether mothers who were currently in treatment for opioid replacement therapy and/or residential treatment differed from mothers who were not in treatment. However, it is notable that most of the studies that met this criterion were also nonmatched. As design took precedence over testing treatment as a moderator, we are unable to answer the question. Thus, the question remains one for further research.
Implications for research, practice, and policy
These results have important research, practice, and policy implications. First, the quality of the caregiving relationship in substance-misusing mothers is poorer than for mothers facing similar environmental adversity. Although these differences are not large, they underscore the potential impact substance misuse has on a mother’s capacity to provide sensitive and nurturing caregiving.
What is also striking, however, is the number of risk factors present in the matched non–substance-using group of mothers. The extensive literature linking multiple risk exposure to poor child outcomes dates from the seminal work of Rutter and colleagues.81 More contemporary models of socioenvironmental risk emphasise the importance of cumulative risk rather than the identification of specific risk factors.87 Thus, within this model, maternal substance misuse should be viewed as one further risk that, in combination with a range of other risk factors such as poverty,88 will be associated with compromised child outcome. This leads us to question whether there is a disproportionate focus on maternal substance use as a risk factor independent of the broader contextual environment of impoverished families. Substance use is one of the key reasons families are referred to child protection services in both pregnancy and the postnatal period.89 Although women with substance use problems have complex lives and histories, making them a high-risk group, these meta-analysis results raise issues about the potential failure to identify families where the quality of caregiving is poor, but maternal substance misuse is not necessarily present.90
Second, results highlight the importance of providing parenting support to substance-misusing mothers that focuses on enhancing maternal sensitivity and responsivity to maximise child outcomes. This is an area of growing research and clinical focus, and a number of studies have shown the benefits of providing attachment-based interventions for high-risk mothers and their children.91–93 However, improving maternal sensitivity will also require a focus on helping mothers develop emotional regulation skills and additional support to address real-world problems such as housing and access to material resources. Contemporary parenting programmes, such as the Parents Under Pressure,91,94 draw from conceptual models of affect regulation and integrate these within a parenting framework.
Third, it is notable that most of the studies identified have focused on cocaine (sometimes in combination with other drugs of abuse), with only a few focusing on opioids but in the context of replacement therapy. Drug use patterns across much of Europe, Australia, and North America are changing with a growing use of psychomotor stimulants such as ‘ice’ and ‘crystal meth’.95 These substances may influence the quality of caregiving in qualitatively different ways. For example, amphetamine abuse is more likely to be associated with a pattern of interaction with the child that may be hostile and/or unpredictable, given that these are both behaviours associated with ongoing amphetamine abuse.96 This environment may be qualitatively different due to the direct effects of the substance, compared, for example, with cannabis and could result in poorer outcomes, including insecure/disorganised attachment strategies that occur in the context of hostile and unpredictable parent-child relationships,15 leading to even greater risk of psychopathology.12 Thus, future research should be conducted that investigates the relationship between the type of substances, combination of substances, and child outcome, ensuring that adverse environmental risk is controlled for by careful matching of comparison groups.
Conclusions
This meta-analysis is the first study to bring together literature spanning 20 years to assess both the impact of maternal substance use on quality of caregiving and factors that might moderate this relationship. The clear operationalisation of maternal sensitivity and child responsiveness enabled us to systematically identify and meta-analyse data from 24 studies and undertake subgroup analyses that enabled us to look at the potential impact of study design and infant age. The results show that maternal illicit substance use is significantly related to caregiving quality in the first 3 years of a child’s life. The subgroup analyses have highlighted that this difference, although significant, is nonetheless a small effect. These findings highlight the importance of addressing the quality of caregiving for substance-using mothers and draw attention to the need for future studies to ensure that substance-using mothers are compared with mothers who also face a range of environmental adversity.
Supplementary Material
Acknowledgments
All contributions to the authorship of this paper have been acknowledged.
Footnotes
Peer review:Eight peer reviewers contributed to the peer review report. Reviewers’ reports totalled 2725 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author Denise Hatzis gratefully acknowledges receiving a Griffith University Postgraduate Research Scholarship awarded to her during the period 2011-2014 (CRICOS provider number: 00233E), which was provided to support candidature in the Doctor of Philosophy in Clinical Psychology (062206F) in the Applied School of Psychology.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: The systematic review as presented was designed and conducted by the first author DH who produced the first draft of the manuscript. DH, SD, and JB coded identified articles for inclusion into the meta-analysis. The second author SD provided constructive critical feedback and revision of the manuscript. PH contributed by assisting with analysing the data for the meta-analysis. JB made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
Disclosures and Ethics: As a requirement of publication, author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Dawe S, Frye SA, Best D, et al. Drug Use in the Family: Impacts and Implications for Children. Canberra, ACT: Australian National Council on Drugs; 2007. [Google Scholar]
- 2. Bergin C, McCollough P. Attachment in substance-exposed toddlers: the role of caregiving and exposure. Inf Mental Hlth J. 2009;30:407–423. [DOI] [PubMed] [Google Scholar]
- 3. Beeghly M, Frank DA, Rose-Jacobs R, Cabral H, Tronick E. Level of prenatal cocaine exposure and infant-caregiver attachment behavior. Neurotoxicol Teratol. 2003;25:23–38. [DOI] [PubMed] [Google Scholar]
- 4. Arendt RE, Short EJ, Singer LT, et al. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Behav Pediatr. 2004;25:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiriboga CA. Fetal alcohol and drug effects. Neurologist. 2003;9:267–279. [DOI] [PubMed] [Google Scholar]
- 6. Tronick E, Beeghly M. Prenatal cocaine exposure, child development, and the compromising effects of cumulative risk. Clin Perinatol. 1999;26:151–171. [PubMed] [Google Scholar]
- 7. Bandstra ES, Morrow CE, Vogel AL, et al. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. 2002;24:297–308. [DOI] [PubMed] [Google Scholar]
- 8. Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Subst Use Misuse. 2004;39:25–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson MO. Mother-infant interaction and maternal substance use/abuse: an integrative review of research literature in the 1990s. Online J Knowl Synth Nurs. 2001;8:2. [PubMed] [Google Scholar]
- 10. Van Ijzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: meta-analysis of precursors, concomitants, and sequelae. Dev Psychopathol. 1999;11:225–250. [DOI] [PubMed] [Google Scholar]
- 11. Bridgett DJ, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: the effects of gender and risk and subsequent aggressive behavior. Neurotoxicol Teratol. 2011;33:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bennett DS, Marini VA, Berzenski SR, Carmody DP, Lewis M. Externalizing problems in late childhood as a function of prenatal cocaine exposure and environmental risk. J Pediatr Psychol. 2013;38:296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dixon DR, Kurtz PF, Chin MD. A systematic review of challenging behaviors in children exposed prenatally to substances of abuse. Res Dev Disabil. 2008;29:483–502. [DOI] [PubMed] [Google Scholar]
- 14. Fearon RP, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Lapsley AM, Roisman GI. The significance of insecure attachment and disorganization in the development of children’s externalizing behavior: a meta-analytic study. Child Dev. 2010;81:435– 456. [DOI] [PubMed] [Google Scholar]
- 15. Bakermans-Kranenburg M, Van Ijzendoorn MH, Juffer F. Disorganized infant attachment and preventive interventions: a review and meta-analysis. Inf Mental Hlth J. 2005;26:191–216. [DOI] [PubMed] [Google Scholar]
- 16. Skovgaard AM. Mental health problems and psychopathology in infancy and early childhood. Dan Med Bull. 2010;57:B4193. [PubMed] [Google Scholar]
- 17. Skovgaard AM, Olsen EM, Christiansen E, et al. Predictors (0-10 months) of psychopathology at age 1½ years – a general population study in The Copenhagen Child Cohort CCC 2000. J Child Psychol Psychiatry. 2008;49:553– 562. [DOI] [PubMed] [Google Scholar]
- 18. DeGangi GA. Pediatric Disorders of Regulation in Affect and Behavior. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 19. DeGangi GA, Breinbauer C, Roosevelt JD, Porges S, Greenspan S. Prediction of childhood problems at three years in children experiencing disorders of regulation during infancy. Inf Mental Hlth J. 2000;21:156–175. [Google Scholar]
- 20. Keenan K, Shaw D, Delliquadri E, Giovannelli J, Walsh B. Evidence for the continuity of early problem behaviors: application of a developmental model. J Abnorm Child Psychol. 1998;26:441–452. [DOI] [PubMed] [Google Scholar]
- 21. Hemmi MH, Wolke D, Schneider S. Associations between problems with crying, sleeping and/or feeding in infancy and long-term behavioural outcomes in childhood: a meta-analysis. Arch Dis Child. 2011;96:622–629. [DOI] [PubMed] [Google Scholar]
- 22. Schuler ME, Black MM, Starr J, Raymond H. Determinants of mother-infant interaction: effects of prenatal drug exposure, social support, and infant temperament. J Clin Child Psychol. 1995;24:397–405. [Google Scholar]
- 23. Neuspiel DR, Hamel SC, Hochberg E, Greene J, Campbell D. Maternal cocaine use and infant behavior. Neurotoxicol Teratol. 1991;13:229–233. [DOI] [PubMed] [Google Scholar]
- 24. LaGasse L, Messinger D, Lester B, et al. Prenatal drug exposure and maternal and infant feeding behaviour. Arch Dis Child Fetal Neonatal Ed. 2003;88:F391–F399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tronick E, Messinger D, Weinberg M, et al. Cocaine exposure is associated with subtle compromises of infants’ and mothers’ social-emotional behavior and dyadic features of their interaction in the face-to-face still-face paradigm. Dev Psychol. 2005;41:711. [DOI] [PubMed] [Google Scholar]
- 26. Johnson AL, Morrow CE, Accornero VH, Xue L, Anthony JC, Bandstra ES. Maternal cocaine use: estimated effects on mother-child play interaction in the preschool period. J Dev Behav Pediatr. 2002;23:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salo S, Kivistö K, Korja R, et al. Emotional availability, parental self-efficacy beliefs, and child development in caregiver-child relationships with buprenorphine-exposed 3-year-olds. Parenting. 2009;9:244–259. [Google Scholar]
- 28. Eiden R. Maternal substance use and mother-infant feeding interactions. Inf Mental Hlth J. 2001;22:497–511. [Google Scholar]
- 29. Ukeje I, Bendersky M, Lewis M. Mother-infant interaction at 12 months in prenatally cocaine-exposed children. Am J Drug Alcohol Abuse. 2001;27:203–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eiden R, Godleski S, Colder CR, Schuetze P. Prenatal cocaine exposure: the role of cumulative environmental risk and maternal harshness in the development of child internalizing behavior problems in kindergarten. Neurotoxicol Teratol. 2014;44:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kettinger LA, Nair P, Schuler ME. Exposure to environmental risk factors and parenting attitudes among substance-abusing women. Am J Drug Alcohol Abuse. 2000;26:1–11. [DOI] [PubMed] [Google Scholar]
- 32. Amato L, Davoli M, Perucci C, Ferri M, Faggiano F, Mattick R. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28:321–329. [DOI] [PubMed] [Google Scholar]
- 33. Mattick RP, Breen C, Kimber J, Davoli M. Methadone Maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niccols A, Milligan K, Smith A, Sword W, Thabane L, Henderson J. Integrated programs for mothers with substance abuse issues and their children: a systematic review of studies reporting on child outcomes. Child Abuse Neglect. 2012;36:308–322. [DOI] [PubMed] [Google Scholar]
- 35. Powis B, Gossop M, Bury C, Payne K, Griffiths P. Drug-using mothers: social, psychological and substance use problems of women opiate users with children. Drug Alcohol Rev. 2000;19:171–180. [Google Scholar]
- 36. Lohaus A, Keller H, Ball J, Elben C, Voelker S. Maternal sensitivity: components and relations to warmth and contingency. Parenting. 2001;1:267–284. [Google Scholar]
- 37. Eiden R, Stevens A, Schuetze P, Dombkowski LE. A conceptual model for maternal behavior among polydrug cocaine-using mothers: the role of postnatal cocaine use and maternal depression. Psychol Addict Behav. 2006;20:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodman G, Hans SL, Bernstein VJ. Mother expectation of bother and infant attachment behaviors as predictors of mother and child communication at 24 months in children of methadone-maintained women. Inf Mental Hlth J. 2005;26:549–569. [DOI] [PubMed] [Google Scholar]
- 39. United Nations Office on Drugs and Crime. World drug report. http://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf Published 2016. Accessed December 20, 2016.
- 40. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 41. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. SysReview [Systematic Review Management Software] [computer program]. Brisbane, QLD, Australia: The University of Queensland; 2014. [Google Scholar]
- 43. Eiden R, McAuliffe S, Kachadourian L, Coles C, Colder C, Schuetze P. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicol Teratol. 2009;31:60– 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eiden R, Schuetze P, Coles CD. Maternal cocaine use and mother-infant interactions: direct and moderated associations. Neurotoxicol Teratol. 2011;33:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fraser JG, Harris-Britt A, Thakkallapalli EL, Kurtz-Costes B, Martin S. Emotional availability and psychosocial correlates among mothers in substance-abuse treatment and their young infants. Inf Mental Hlth J. 2010;31:1–15. [DOI] [PubMed] [Google Scholar]
- 46. French ED, Pituch M, Brandt J, Pohorecki S. Improving interactions between substance-abusing mothers and their substance-exposed newborns. J Obstet Gynecol Neonatal Nurs. 1998;27:262– 269. [DOI] [PubMed] [Google Scholar]
- 47. Hans SL, Bernstein VJ, Henson LG. The role of psychopathology in the parenting of drug-dependent women. Devel Psychopathol. 1999;11:957– 977. [DOI] [PubMed] [Google Scholar]
- 48. Minnes S, Singer LT, Arendt R, Satayathum S. Effects of prenatal cocaine/polydrug use on maternal-infant feeding interactions during the first year of life. J Dev Behav Pediatr. 2005;26:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schuler ME, Black MM, Starr JRH. Determinants of mother-infant interaction: effects of prenatal drug exposure, social support, and infant temperament. J Clin Child Psychol. 1995;24:397– 405. [Google Scholar]
- 50. Singer LT, Hawkins S, Huang J, Davillier M, Baley J. Developmental outcomes and environmental correlates of very low birthweight, cocaine-exposed infants. Early Hum Dev. 2001;64:91–103. [DOI] [PubMed] [Google Scholar]
- 51. Uhlhorn SB, Messinger DS, Bauer CR. Cocaine exposure and mother–toddler social play. Infant Behav Dev. 2005;28:62–73. [Google Scholar]
- 52. Veira Y, Finger B, Schuetze P, Colder CR, Godleski S, Eiden R. Child behavior problems: role of cocaine use, parenting, and child exposure to violence. Psychol Violence. 2014;4:266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Molitor A, Mayes LC. Problematic dyadic interaction among toddlers and their polydrug-cocaine-using mothers. Inf Mental Hlth J. 2010;31:121–140. [DOI] [PubMed] [Google Scholar]
- 54. Pajulo M, Savonlahti E, Sourander A, Ahlqvist S, Helenius H, Piha J. An early report on the mother-baby interactive capacity of substance-abusing mothers. J Subst Abuse Treat. 2001;20:143–151. [DOI] [PubMed] [Google Scholar]
- 55. Perry N, Newman LK, Hunter M, Dunlop A. Improving antenatal risk assessment in women exposed to high risks. Clin Child Psychol Psychiatry. 2015;20;84–105. [DOI] [PubMed] [Google Scholar]
- 56. Salo S, Politi J, Tupola S, et al. Early development of opioid-exposed infants born to mothers in buprenorphine-replacement therapy. J Reprod Infant Psyc. 2010;28:161–179. [Google Scholar]
- 57. Sarfi M, Smith L, Waal H, Sundet JM. Risks and realities: dyadic interaction between 6-month-old infants and their mothers in opioid maintenance treatment. Infant Behav Dev. 2011;34:578–589. [DOI] [PubMed] [Google Scholar]
- 58. Savonlahti E, Pajulo M, Ahlqvist S, et al. Interactive skills of infants with their high-risk mothers. Nord J Psychiatry. 2005;59:139–147. [DOI] [PubMed] [Google Scholar]
- 59. Siqveland TS, Moe V. Longitudinal development of mother-infant interaction during the first year of life among mothers with substance abuse and psychiatric problems and their infants. Child Psychiatry Hum Dev. 2014;45:408–421. [DOI] [PubMed] [Google Scholar]
- 60. Mayes LC, Feldman R, Granger R, Haynes O, Bornstein MH, Schottenfeld R. The effects of polydrug use with and without cocaine on mother-infant interaction at 3 and 6 months. Infant Behav Dev. 1997;20:489–502. [Google Scholar]
- 61. Burns K, Chethik L, Burns WJ, Clark R. The early relationship of drug abusing mothers and their infants: an assessment at eight to twelve months of age. J Clin Psychol. 1997;53:279– 287. [DOI] [PubMed] [Google Scholar]
- 62. Ball SA, Maves LC, DeTeso JA, Schottenfeld RS. Maternal attentiveness of cocaine abusers during child-based assessments. Am J Addict. 1997;6:135–143. [PubMed] [Google Scholar]
- 63. Belt RH, Flykt M, Punamäki RL, Pajulo M, Posa T, Tamminen T. Psychotherapy groups and individual support to enhance mental health and early dyadic interaction among drug-abusing mothers. Inf Mental Hlth J. 2012;33:520–534. [DOI] [PubMed] [Google Scholar]
- 64. Lewis MW, Phillips G, Bowser M, DeLuca S, Johnson HL, Rosen TS. Cocaine-exposed infant behavior during Still-Face: risk factor analyses. Am J Orthopsychiatry. 2009;79:60– 70. [DOI] [PubMed] [Google Scholar]
- 65. National Heart Lung and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Published 2014. Accessed December 19, 2016.
- 66. Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 67. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 68. Higgins JPT, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. http://handbook.cochrane.org. Published March 2011. Accessed December 20, 2016.
- 69. Field AP, Gillett R. How to do a meta-analysis. Brit J Math Stat Psy. 2010;63:665–694. [DOI] [PubMed] [Google Scholar]
- 70. Vevea JL, Woods CM. Publication bias in research synthesis: sensitivity analysis using a priori weight functions. Psychol Methods. 2005;10:428–443. [DOI] [PubMed] [Google Scholar]
- 71. Ainsworth M, Blehar M, Walters E, Wall S. Patterns of Attachment. Hillsdale, NY: Lawrence Erlbaum; 1978. [Google Scholar]
- 72. Clark R. The Parent-Child Early Relational Assessment Manual. Madison, WI: Department of Psychiatry, Medical School, University of Wisconsin; 1985. [Google Scholar]
- 73. Biringen Z. The Emotional Availability (EA) Scales. 4th ed. Boulder, CO: Emotional Availability; 2008. [Google Scholar]
- 74. Clark R, Musick J, Scott F, Klehr K. The mother’s project rating scale of mother-child intervention. Unpublished manuscript. 1980.
- 75. Egeland B, Weinfield N, Heister M, et al. Teaching Tasks Administration and Scoring Manual. University of Minnesota. May, 1995. [Google Scholar]
- 76. Cox M, Crnic K. Qualitative ratings for parent -child interaction at 3–15 months of age. Unpublished manuscript. 2003.
- 77. Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 78. Rose S, Laan MJ. Why match? investigating matched case-control study designs with causal effect estimation. Int J Biostat. 2009;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Groenwold R, Rovers M, Lubsen J, Heijden G. Subgroup effects despite homogeneous heterogeneity test results. BMC Med Res Methodol. 2010;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bakermans-Kranenburg M, Van Ijzendoorn MH, Juffer F. Less is more: meta-analyses of sensitivity and attachment interventions in early childhood. Psychol Bull. 2003;129:195–215. [DOI] [PubMed] [Google Scholar]
- 81. Rutter M. Protective factors in children’s responses to stress and disadvantage. In: Kent MW, Rolf JE. eds. Primary Prevention of Psychopathology: Social Competence in Children. Vol 3 Hanover, NH: University of New England Press; 1979:49–74. [Google Scholar]
- 82. Sameroff AJ, Seifer R, Barocas R, Zax M, Greenspan S. Intelligence quotient scores of 4-year-old children: social-environmental risk factors. Pediatrics. 1987;79:343–350. [PubMed] [Google Scholar]
- 83. Roy AL, Raver CC. Are all risks equal? early experiences of poverty-related risk and children’s functioning. J Fam Psychol. 2014;28:391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yumoto C, Jacobson SW, Jacobson JL. Fetal substance exposure and cumulative environmental risk in an African American cohort. Child Dev. 2008;79:1761–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kemppinen K, Kumpulainen K, Raita-Hasu J, Moilanen I, Ebeling H. The continuity of maternal sensitivity from infancy to toddler age. J Reprod Infant Psyc. 2006;24:199–212. [Google Scholar]
- 86. Belsky J, Fearon R. Infant-mother attachment security, contextual risk, and early development: a moderational analysis. Dev Psychopathol. 2002;14:293–310. [DOI] [PubMed] [Google Scholar]
- 87. Sameroff AJ. Environmental risk factors in infancy. Pediatrics. 1998;102:1287–1292. [PubMed] [Google Scholar]
- 88. Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. [DOI] [PubMed] [Google Scholar]
- 89. McGlade A, Ware R, Crawford M. Child protection outcomes for infants of substance-using mothers: a matched-cohort study. Pediatrics. 2009;124:285–293. [DOI] [PubMed] [Google Scholar]
- 90. Daniel B, Taylor J, Scott J. Recognition of neglect and early response: overview of a systematic review of the literature. Child Fam Soc Work. 2010;15:248–257. [Google Scholar]
- 91. Dawe S, Harnett P. Reducing potential for child abuse among methadone-maintained parents: results from a randomized controlled trial. J Subst Abuse Treat. 2007;32:381–390. [DOI] [PubMed] [Google Scholar]
- 92. Pajulo M, Suchman N, Kalland M, Mayes L. Enhancing the effectiveness of residential treatment for substance abusing pregnant and parenting women: focus on maternal reflective functioning and mother-child relationship. Inf Mental Hlth J. 2006;27:448–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Suchman N, Pajulo M, Decoste C, Mayes L. Parenting interventions for drug-dependent mothers and their young children: the case for an attachment-based approach. Fam Relat. 2006;55:211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barlow J, Sembi S, Gardner F, et al. An evaluation of the parents under pressure programme: a study protocol for an RCT into its clinical and cost effectiveness. Trials. 2013;14:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chomchai C, Chomchai S. Global patterns of methamphetamine use. Curr Opin Psychiatry. 2015;28:269–274. [DOI] [PubMed] [Google Scholar]
- 96. Dawe S, Davis P, Lapworth K, McKetin R. Mechanisms underlying aggressive and hostile behavior in amphetamine users. Curr Opin Psychiatry. 2009;22:269–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.