Abstract
Background
Prostate carcinoma (PCa) is often not diagnosed until advanced disease with bone metastasis. Predictive factors for bone metastasis are required to improve patient outcomes. The study aimed to analyze the factors associated with bone metastases in newly diagnosed patients with PCa.
Material/Methods
This was a retrospective study of 80 patients newly diagnosed with PCa by pathological examination between January 2012 and December 2014. Bone metastases were diagnosed by positron emission computed tomography. Clinical data, serological laboratory results, and pathological examination results were collected.
Results
Among the 80 patients, 45 (56%) had bone metastases. Age, serum alkaline phosphatase, prostate-specific antigen (PSA), erythrocyte sedimentation rate, PCa tissue Gleason score, androgen receptor (AR) expression, and Ki-67 expression were higher in patients with bone metastasis compared with those without (all P<0.05). Multivariate logistic regression showed that PSA (OR: 1.005; 95%CI: 1.001–1.010; P=0.016), Gleason score (OR: 4.095; 95%CI: 1.592–10.529; P=0.003), and AR expression (OR: 14.023; 95%CI: 3.531–55.6981; P=0.005) were independently associated with bone metastases. Cut-off values for PSA, Gleason score, and AR expression were 67.1 ng/ml (sensitivity: 55.6%; specificity: 97.1%), 7.5 (sensitivity: 75.6%; specificity: 82.9%), and 2.5 (sensitivity: 84.0%; specificity: 91.4%), respectively.
Conclusions
PSA, Gleason score, and AR expression in PCa tissues were independently associated with PCa bone metastases. These results could help identifying patients with PCa at high risk of bone metastases.
MeSH Keywords: Neoplasm Grading; Prostate-Specific Antigen; Prostatic Neoplasms; Receptors, Androgen
Background
Prostate cancer (PCa) ranks second among the most common types of solid tumors and the sixth among the leading causes of cancer deaths in men worldwide [1–3]. In the United States in 2015, there were 27,540 deaths resulting from an estimated 220,800 new cases of PCa [4,5], accounting for 25% of the incidence of the 3 most common cancers (prostate, lung and bronchus, and colorectal) [4,5]. The incidence of PCa in South-Central Asia is much lower than that in most of America, Oceania, and western and northern European countries [4,6,7]. The incidence of PCa is now rapidly increasing in China, probably due to significant changes in lifestyle (such as diet and smoking habits), ageing population, environmental factors (such as increased pollution), monitoring prostate-specific antigen (PSA), and advanced radiological investigations among the elderly population [6–10]. In Shanghai, the incidence of PCa has increased by more than 4.8-fold since 1980 and is now the most common genitourinary tumor in males in this region [11].
Metastases are present in 24–35% of patients with newly diagnosed PCa, with bone metastasis accounting for approximately 70% of these cases [12]. Among patients with PCa in the USA, the incidence of bone metastasis is 8% among Caucasian patients, and 14% among those of African American origin [13]. In Asian populations, studies reported an incidence of bone metastases of PCa of 14–36% [14–16]. Bone metastases in patients with PCa is associated with bone-related complications including pain at metastatic sites, brittle bones, and osteoporosis [2,3]. Severe disease may also induce pathological fracture, vertebral collapse, and symptoms of spinal cord compression caused by tumor direct invasion [2]. These symptoms have a serious impact on the quality of life of patients with PCa [11,12]. The prognosis of PCa patients with bone metastases is poor, with a survival of only 3–5 years [17]. This is due to the lack of effective treatments and poor health awareness. Furthermore, the absence of obvious clinical symptoms in the early stages of disease often mean that most patients are with advanced PCa and bone metastases at initial diagnosis [11,16,18]. Therefore, there is a need to identify factors associated with the presence of bone metastases at diagnosis.
Previous studies showed that age, alkaline phosphatase (ALP), erythrocyte sedimentation rate (ESR), Gleason score, and prostate tissue expression of androgen receptors (AR) and Ki-67 are associated with the presence of bone metastases in PCa [11,12], but the exact relationships vary among studies. The Gleason score is based on histopathological grading of tumor architecture and is the strongest clinical predictor of prostate cancer progression [19], but this grading system has changed over time, reducing its predictive value [20]. PSA is an antigen that is exclusively secreted by PCa cells [9] and PCa metastases keep this ability [2]. Ki-67 is a marker of cell proliferation and is generally thought to represent the aggressiveness of cancers [21,22]. Androgen receptors play a critical role in the development of PCa and are an important marker for efficacy of androgen deprivation therapies [23,24]. The diagnosis of bone metastasis is relatively straightforward using various imaging methods such as technetium-99m (99mTc) bone scintigraphy, positron emission computed tomography (ECT), and magnetic resonance imaging (MRI) [2]. However, these techniques are used for the detection of established metastases and are resource-consuming. Therefore, simple markers of bone metastases are necessary.
In the present retrospective study, the data of Chinese patients with PCa were reviewed in order to identify factors associated with bone metastases of PCa. These results could allow identifying patients at higher risk of bone metastases.
Material and Methods
Study design and patients
This was a retrospective study 80 Chinese patients (age range, 54–93 years; median, 74 years) newly diagnosed with PCa at the Department of Nuclear Medicine, Guangzhou First People’s Hospital, Guangzhou Medical University (Guangdong, China) between January 2012 and December 2014. Inclusion criteria were: 1) diagnosed with PCa confirmed by prostate tissue pathology of samples obtained by transrectal ultrasound-guided prostate biopsy or surgery; 2) available ECT data; 3) newly diagnosed PCa; and 4) no history of chemotherapy, radiotherapy, or hormone therapy. Patients with bone metastases resulting from other malignancies were excluded. The 80 patients were divided into 2 groups based on the presence (n=45) or absence (n=35) of bone metastases.
This study was approved by the Ethics Committee of the Guangzhou First People’s Hospital (No. 2015068).
Data collection
Demographic data (age and body mass index [BMI]), clinical symptoms (prostate volume [PV] and T stage), histological analysis (Gleason score, AR, and Ki-67 of PCa tissues), and biochemical examinations (serum ALP, PSA, and ESR) were retrieved from the medical charts.
Histological analysis
PCa tissues were obtained by transrectal ultrasound-guided prostate biopsy or surgery. The Gleason scores were evaluated as described previously [25,26]. AR and Ki-67 expressions in PCa tissues were analyzed by immunohistochemistry using rabbit anti-human AR monoclonal antibody (Clone EP120; lot number: 14620206; ZSGB-BIO, Beijing, China) and rabbit anti-human Ki-67 monoclonal antibody (Clone 30-9; lot number: E10075; Roche Diagnostics, Basel, Switzerland). Immunoreactive sites were visualized with diaminobenzidine (DAB), and counterstained with hematoxylin. The slides were randomly and blindly analyzed by 2 independent experienced pathologists. AR expression was evaluated using a semi-quantitative scoring method: 0–5% (negative expression, −, scored 0); 5–25% (weak positive expression, +, scored 1); 25–50% (moderate positive expression, ++, scored 2), and >50% (strong positive expression, +++, scored 3). The Ki-67 positive expression rate (%) was expressed as the percentage of immunoreactive tumor cells among the total counted tumor cells.
Diagnosis of bone metastasis by ECT
Single photon emission computed tomography (SPECT) (Millennium VG 5) with the Hawkeye system (GE Healthcare, Waukesha, WI, USA) and 99mTC-MDP as imaging agent were used to diagnose bone metastases.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) and were analyzed using the independent samples t-test. Categorical variables are presented as proportions and were analyzed using the Fisher’s exact test. Odds ratio (OR) and 95% confidence intervals (95%CIs) were determined by multivariate logistic regression analysis using stepwise methods. Selected variables with P-values <0.05 in the univariate analyses were considered for inclusion in the regression model, but because the sample size was small, only data for PSA, PCa tissue Gleason score, AR, and Ki-67 were included in the multivariate model. The discriminative abilities of PSA, Gleason score, and AR to identify bone metastases were verified using the area under the receiver operating curve (ROC). Data were analyzed using SPSS 17 (IBM, Armonk, NY, USA). Two-sided P-values <0.05 were considered statistically significant.
Results
Characteristics of the patients
The demographic and clinical characteristics of patients with PCa are shown in Table 1. Age, ALP, PSA, and ESR of patients with bone metastases were higher than in patients without bone metastasis (P=0.001, 0.028, 0.031, and 0.009, respectively), but PV, BMI, and T stage were comparable between the 2 groups (all P>0.05).
Table 1.
Characteristics of the patients.
| Variables | Bone metastases (n=45) | No bone metastasis (n=35) | P |
|---|---|---|---|
| Age (years) | 76.3±1.0 | 71.4±1.1 | 0.001 |
| PV | 51.6±25.7 | 53.4±21.6 | 0.428 |
| BMI (kg/m2) | 23.3±2.6 | 23.5±2.4 | 0.627 |
| ALP (U/L) | 199.6±49.6 | 75.9±3.0 | 0.031 |
| PSA (ng/ml) | 241.5±62.8 | 65.2±37.4 | 0.028 |
| ESR (mm/h) | 45.8±3.3 | 31.0±4.7 | 0.009 |
| Gleason score | 8.2±0.2 | 5.9±0.3 | <0.001 |
| Gleason score, n (%) | <0.001 | ||
| ≤6 | 4 (8.9) | 21 (60.0) | |
| 7 | 7 (15.6) | 8 (22.9) | |
| ≥8 | 34 (75.6) | 6 (17.1) | |
| AR | 2.8±0.1 | 1.4±0.1 | <0.001 |
| Ki-67 positive expression rate (%) | 34.2±3.8 | 11.4±2.5 | <0.001 |
| T stage, n (%) | 0.101 | ||
| T1 | 5 | 11 | |
| T2 | 33 | 18 | |
| T3 | 3 | 3 | |
| T4 | 4 | 3 |
Data are shown as mean ± standard deviation (SD) or percentage, as appropriate. PV – prostate volume; BMI – body mass index; ALP – alkaline phosphatase; PSA – prostate-specific antigen; ESR – erythrocyte sedimentation rate; AR – androgen receptor.
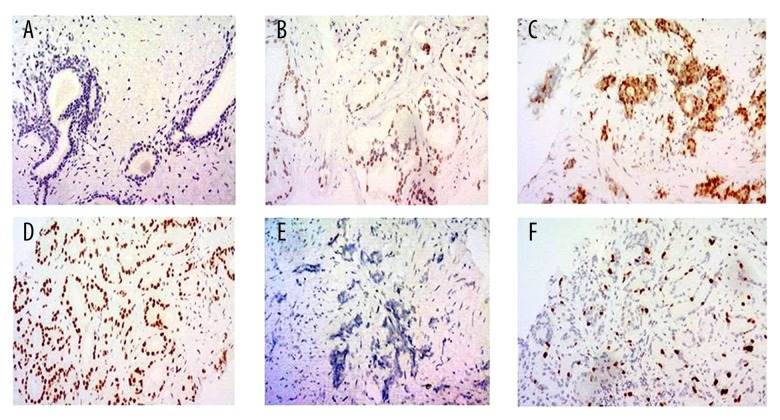
Representative immunohistochemistry images of AR and Ki-67 expression are shown in Figure 1. Gleason score and expressions of AR and Ki-67 were also significantly higher in patients with bone metastasis than in those without bone metastasis (all P<0.001).
Figure 1.
Androgen receptor (AR) and Ki-67 expressions in prostate carcinoma tissues were determined by immunohistochemistry. (A) AR negative (–); (B) AR weak positive (+); (C) AR moderate positive (++); (D) AR strong positive (+++); (E) Ki-67 negative; (F) Ki-67 positive. Magnification: ×100.
Univariate and multivariate analyses of factors associated with bone metastasis in patients with PCa
The results of univariate and multivariate logistic regression analyses are shown in Table 2. Univariate regression analysis indicated that PSA (OR=1.003; 95%CI: 1.000–1.007; P=0.014), Gleason score (OR=2.964; 95%CI: 1.841–4.773; P<0.001), AR expression (OR=8.966; 95%CI: 4.079–19.708; P<0.001), and Ki-67 expression (OR=1.054; 95%CI: 1.024–1.084; P<0.001) were associated with PCa bone metastasis. In multivariate regression analysis, PSA (OR=1.005; 95%CI: 1.001–1.010; P=0.016), Gleason score (OR=4.095; 95%CI: 1.592–10.529; P=0.003), and AR expression (OR=14.023; 95%CI: 3.531-55.6981; P=0.005) in PCa tissues were independently associated with PCa bone metastases.
Table 2.
Univariate and multivariate logistic regression analyses of the factors associated with bone metastases in patients with prostate carcinoma.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| PSA | 1.003 | 1.000–1.007 | 0.014 | 1.005 | 1.001–1.010 | 0.016 |
| Gleason score | 2.964 | 1.841–4.773 | <0.001 | 4.095 | 1.592–10.529 | 0.003 |
| AR | 8.966 | 4.079–19.708 | <0.001 | 14.023 | 3.531–55.691 | 0.005 |
| Ki-67 | 1.054 | 1.024–1.084 | <0.001 | 1.003 | 0.963–1.044 | 0.899 |
PSA – prostate-specific antigen; AR – androgen receptor; OR – odds ratio; 95% CI – 95% confidence intervals.
ROC curves
The 3 variables identified in the multivariate analysis were tested using the ROC curve method for the prediction of bone metastases in patients with PCa. Cut-off values for PSA, Gleason score, and AR expression were 67.1 ng/ml (AUC: 0.819; sensitivity: 55.6%; specificity: 97.1%), 7.5 (cut-off value: 7.5; AUC: 0.866; sensitivity: 75.6%; specificity: 82.9%) and 2.5 (cut-off value: 2.5; AUC: 0.894; sensitivity: 84.0%; specificity: 91.4%), respectively (Figure 2).
Figure 2.

Receiver operating characteristic (ROC) curves of prostate-specific antigen (PSA), Gleason score, and the prostate carcinoma tissue AR-positive expression for the prediction of bone metastasis in patients with prostate carcinoma. PSA (cut-off value: 67.1 ng/mL; AUC: 0.819; sensitivity: 55.6%; specificity: 97.1%). Gleason score (cut-off value: 7.5; AUC: 0.866; sensitivity: 75.6%; specificity: 82.9%). AR (cut-off value: 2.5; AUC: 0.894; sensitivity: 84.0%; specificity: 91.4%).
Discussion
With few clinical symptoms in the early stages, PCa is often not diagnosed until disease is advanced with bone metastasis. Therefore, the aim of the present study was to analyze the data of Chinese patients with PCa in order to identify factors associated with bone metastases of PCa. Results showed that PSA, Gleason score, and AR expression in PCa tissues were independently associated with PCa bone metastases. These results could allow identifying patients at higher risk of bone metastases at diagnosis.
PSA is secreted exclusively by prostate epithelial cells, thus exhibiting high tissue specificity, rather than cancer specificity. Under normal physiological conditions, there is a barrier between the prostate acinus that secretes PSA and the lymphatic system [27]. Consequently, only small amounts of PSA cross into the circulation and the serum concentration in males is very low. In PCa, this concentration increases with cancer cell overgrowth and PV and damage to the normal prostate tissue structure [28]. This effect is also observed when PCa cells that transfer to the bone in patients with bone metastasis [29]. Indeed, studies showed that PSA has predictive value for PCa bone metastases. PSA levels exceeding 100 ng/mL are associated with a 41.4–79.9% probability of bone metastasis, while the probability is low at PSA concentrations lower than 20 ng/L [30,31]. In this study, the mean PSA concentration for PCa patients with bone metastasis was significantly higher than that in the group of patients without metastasis. Multivariate regression analysis indicated that PSA was an independent factor for PCa bone metastasis. In addition, the cut-off value for PSA was 67.1 ng/mL (sensitivity: 55.6%; specificity: 97.1%).
The Gleason rating system is regarded as the best indicator of tumor staging and prognosis [19,32]. The main and secondary areas of prostate cancer lesions are classified into 5 levels, with the histological score obtained by the addition of those of the 2 levels ranging from 2 to 10. Gleason scores ≤6 indicate low risk, while 7 indicates moderate risk, and >8 indicates high risk [25]. The Gleason score is one of the main reference indicators for diagnosis, determination of treatment approaches, treatment effect, and prognosis [32]. Some studies showed that bone metastasis occurred in patients with advanced PCa with Gleason scores greater than 7 and local infiltration or positive bone scans were observed [33]. In the present study, 34 of the 45 patients with bone metastasis had Gleason scores ≥8. Among the 35 patients without bone metastasis, only 6 had Gleason scores ≥8. Multivariate regression analysis revealed that the Gleason score was an independent factor associated with PCa bone metastasis, with a cut-off value of 7.5 (sensitivity: 75.6%; specificity: 82.9%). The Gleason score has been shown to predict prognosis in patients with PCa and bone metastases [19]. Another study suggested that patients with a Gleason score ≤6 had a low risk of bone metastases [30].
Evaluation of degree of AR expression can predict the distribution of AR in PCa tissue, which guides disease classification, treatment approaches, and prognosis [23,24]. In this study, AR expression in PCa tissue was higher in patients with bone metastasis than in those without. In addition, the multivariate regression analysis revealed that AR expression in PCa tissue was an independent factor correlated with PCa bone metastasis, with a cut-off value of 2.5 (sensitivity: 84.0%; specificity: 91.4%). However, the studies of AR and prognosis are controversial. Indeed, some studies showed that AR was associated with prognosis of PCa [34–36], while other studies showed that AR was not [37,38]. These discrepancies may be due to a number of reasons including patient population and patient selection. Additional studies are still necessary.
Ki-67 is a nuclear antigen associated with proliferative cells and is commonly used in studies of many malignancies, especially PCa [21,22,38]. Ki-67 is higher in poorly differentiated adenocarcinoma tissues compared with that in well-differentiated adenocarcinoma tissues, suggesting that the degree of Ki-67 staining is correlated with histological grade [21]. Furthermore, the number of Ki-67-positive tumor cells (Ki-67 marking index) is usually correlated with the clinical course of disease and has important reference value in the evaluation of the diagnosis, treatment and prognosis of PCa [21,22,37]. In this study, the degree of Ki-67 expression was significantly higher in patients with bone metastasis than in those without. However, the degree of Ki-67 expression was not independently correlated with PCa bone metastasis. A number of reasons might be involved in this discrepancy. Indeed, there is no standardized measurement of Ki-67 [39], introducing an important bias among studies.
The limitations of this study should be noted. First, due to the retrospective design, the conclusion of this study may be affected by biases in the selection of the patients and data extraction. Second, this study was conducted in a relatively small sample size, with more patients with bone metastasis than those without. Third, the data were obtained from patients treated at a single center in China, which limits the generalizability of the results to the wider population. Finally, of course, the present study only examined factors that are routinely assessed in the clinical management of PCa, but novel markers such as aberrant DNA methylation [40,41], miRNA expression [42], and genes involved in inflammation [43] are promising and should be examined in future studies. Multicenter prospective studies including larger sample sizes are required to confirm the conclusions of this study.
Conclusions
The present study strongly suggests that PSA, Gleason score, and AR expression in the PCa tissues were independently correlated with PCa bone metastases at diagnosis. These results might be important in the prediction of disease progression and improving outcomes in Chinese patients with PCa.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 81372382 and 81273205), the Natural Science Foundation of Guangdong Province (S2013010013780, 2014A030313677, 2015A030313363, and 2015A030313731), and the Science and Technology Planning Project of Guangdong Province (2012B040304015)
References
- 1.Amato R, Stepankiw M, Gonzales P. A phase II trial of androgen deprivation therapy (ADT) plus chemotherapy as initial treatment for local failures or advanced prostate cancer. Cancer Chemother Pharmacol. 2013;71:1629–34. doi: 10.1007/s00280-013-2163-4. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer. Version 2.2014. Fort Washington: National Comprehensive Cancer Network; 2014. [Google Scholar]
- 3.Graham J, Kirkbride P, Cann K, et al. Prostate cancer: Summary of updated NICE guidance. BMJ. 2014;348:f7524. doi: 10.1136/bmj.f7524. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 5.Bell KJ, Del Mar C, Wright G, et al. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer. 2015;137:1749–57. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Wang HK, Qu YY, Ye DW. Prostate cancer in East Asia: Evolving trend over the last decade. Asian J Androl. 2015;17:48–57. doi: 10.4103/1008-682X.132780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowke JH, McLerran DF, Gupta PC, et al. Associations of body mass index, smoking, and alcohol consumption with prostate cancer mortality in the Asia Cohort Consortium. Am J Epidemiol. 2015;182:381–89. doi: 10.1093/aje/kwv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitagawa Y, Namiki M. Prostate-specific antigen-based population screening for prostate cancer: Current status in Japan and future perspective in Asia. Asian J Androl. 2015;17:475–80. doi: 10.4103/1008-682X.143756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asia Pacific Cohort Studies Collaboration. Huxley R, Ansary-Mohaddam A, et al. The impact of modifiable risk factors on mortality from prostate cancer in populations of the Asia-Pacific region. Asian Pac J Cancer Prev. 2007;8:199–205. [PubMed] [Google Scholar]
- 11.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88:2989–94. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74–79. doi: 10.3109/17453674.2011.645197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segamwenge IL, Mgori NK, Abdallahyussuf S, et al. Cancer of the prostate presenting with diffuse osteolytic metastatic bone lesions: A case report. J Med Case Rep. 2012;6:425. doi: 10.1186/1752-1947-6-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito K, Kubota Y, Suzuki K, et al. Correlation of prostate-specific antigen before prostate cancer detection and clinicopathologic features: Evaluation of mass screening populations. Urology. 2000;55:705–9. doi: 10.1016/s0090-4295(99)00568-3. [DOI] [PubMed] [Google Scholar]
- 15.Yang G, Zuo S, Ma C. The diagnostic correlations of bone scintigraphy, pathological grade and PSA for metastatic prostate cancers. The Chinese-German J Clinical Oncol. 2009;8:702–4. [Google Scholar]
- 16.Zaman MU, Fatima N, Sajjad Z. Metastasis on bone scan with low prostate specific antigen (</=20 ng/ml) and Gleason’s score (<8) in newly diagnosed Pakistani males with prostate cancer: Should we follow Western guidelines? Asian Pac J Cancer Prev. 2011;12:1529–32. [PubMed] [Google Scholar]
- 17.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–97. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 18.Wymenga LF, Boomsma JH, Groenier K, et al. Routine bone scans in patients with prostate cancer related to serum prostate-specific antigen and alkaline phosphatase. BJU Int. 2001;88:226–30. doi: 10.1046/j.1464-410x.2001.02275.x. [DOI] [PubMed] [Google Scholar]
- 19.Kambara T, Oyama T, Segawa A, et al. Prognostic significance of global grading system of Gleason score in patients with prostate cancer with bone metastasis. BJU Int. 2010;105:1519–25. doi: 10.1111/j.1464-410X.2009.09048.x. [DOI] [PubMed] [Google Scholar]
- 20.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–53. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 21.Verma R, Gupta V, Singh J, et al. Significance of p53 and ki-67 expression in prostate cancer. Urol Ann. 2015;7:488–93. doi: 10.4103/0974-7796.158507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tollefson MK, Karnes RJ, Kwon ED, et al. Prostate cancer Ki-67 (MIB-1) expression, perineural invasion, and gleason score as biopsy-based predictors of prostate cancer mortality: The Mayo model. Mayo Clin Proc. 2014;89:308–18. doi: 10.1016/j.mayocp.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Di Zazzo E, Galasso G, Giovannelli P, et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget. 2016;7:193–208. doi: 10.18632/oncotarget.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz J, Wheler JJ, Kurzrock R. Androgen receptors beyond prostate cancer: an old marker as a new target. Oncotarget. 2015;6:592–603. doi: 10.18632/oncotarget.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 26.Martin NE, Mucci LA, Loda M, Depinho RA. Prognostic determinants in prostate cancer. Cancer J. 2011;17:429–37. doi: 10.1097/PPO.0b013e31823b042c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21:383–91. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 28.Velonas VM, Woo HH, dos Remedios CG, Assinder SJ. Current status of biomarkers for prostate cancer. Int J Mol Sci. 2013;14:11034–60. doi: 10.3390/ijms140611034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamaleshwaran KK, Mittal BR, Harisankar CN, et al. Predictive value of serum prostate specific antigen in detecting bone metastasis in prostate cancer patients using bone scintigraphy. Indian J Nucl Med. 2012;27:81–84. doi: 10.4103/0972-3919.110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka N, Fujimoto K, Shinkai T, et al. Bone scan can be spared in asymptomatic prostate cancer patients with PSA of ≤20 ng/ml and Gleason score of ≤6 at the initial stage of diagnosis. Jpn J Clin Oncol. 2011;41:1209–13. doi: 10.1093/jjco/hyr118. [DOI] [PubMed] [Google Scholar]
- 31.Bruwer G, Heyns CF, Allen FJ. Influence of local tumour stage and grade on reliability of serum prostate-specific antigen in predicting skeletal metastases in patients with adenocarcinoma of the prostate. Eur Urol. 1999;35:223–27. doi: 10.1159/000019850. [DOI] [PubMed] [Google Scholar]
- 32.Harnden P, Shelley MD, Coles B, et al. Should the Gleason grading system for prostate cancer be modified to account for high-grade tertiary components? A systematic review and meta-analysis. Lancet Oncol. 2007;8:411–19. doi: 10.1016/S1470-2045(07)70136-5. [DOI] [PubMed] [Google Scholar]
- 33.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–74. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 34.Revelos K, Petraki C, Scorilas A, et al. Correlation of androgen receptor status, neuroendocrine differentiation and angiogenesis with time-to-biochemical failure after radical prostatectomy in clinically localized prostate cancer. Anticancer Res. 2007;27:3651–60. [PubMed] [Google Scholar]
- 35.Fujimura T, Takahashi S, Urano T, et al. Expression of androgen and estrogen signaling components and stem cell markers to predict cancer progression and cancer-specific survival in patients with metastatic prostate cancer. Clin Cancer Res. 2014;20:4625–35. doi: 10.1158/1078-0432.CCR-13-1105. [DOI] [PubMed] [Google Scholar]
- 36.Donovan MJ, Osman I, Khan FM, et al. Androgen receptor expression is associated with prostate cancer-specific survival in castrate patients with metastatic disease. BJU Int. 2010;105:462–67. doi: 10.1111/j.1464-410X.2009.08747.x. [DOI] [PubMed] [Google Scholar]
- 37.Miyake H, Muramaki M, Kurahashi T, et al. Expression of potential molecular markers in prostate cancer: correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urol Oncol. 2010;28:145–51. doi: 10.1016/j.urolonc.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Dunsmuir WD, Gillett CE, Meyer LC, et al. Molecular markers for predicting prostate cancer stage and survival. BJU Int. 2000;86:869–78. doi: 10.1046/j.1464-410x.2000.00916.x. [DOI] [PubMed] [Google Scholar]
- 39.Polley MY, Leung SC, Gao D, et al. An international study to increase concordance in Ki67 scoring. Mod Pathol. 2015;28:778–86. doi: 10.1038/modpathol.2015.38. [DOI] [PubMed] [Google Scholar]
- 40.Deng QK, Lei YG, Lin YL, et al. Prognostic value of protocadherin10 (PCDH10) methylation in serum of prostate cancer patients. Med Sci Monit. 2016;22:516–21. doi: 10.12659/MSM.897179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niu WB, Gui SL, Lin YL, et al. Promoter methylation of protocadherin8 is an independent prognostic factor for biochemical recurrence of early-stage prostate cancer. Med Sci Monit. 2014;20:2584–89. doi: 10.12659/MSM.893083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiaoli Z, Yawei W, Lianna L, et al. Screening of target genes and regulatory function of miRNAs as prognostic indicators for prostate cancer. Med Sci Monit. 2015;21:3748–59. doi: 10.12659/MSM.894670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han X, Zhang JJ, Yao N, et al. Polymorphisms in NFKB1 and NFKBIA genes modulate the risk of developing prostate cancer among Han Chinese. Med Sci Monit. 2015;21:1707–15. doi: 10.12659/MSM.893471. [DOI] [PMC free article] [PubMed] [Google Scholar]