Abstract
Prosopagnosia is a selective visual agnosia characterized by the inability to recognize the identity of faces. There are both acquired forms secondary to brain damage and developmental forms without obvious structural lesions. In this review, we first discuss the diagnosis of acquired and developmental prosopagnosia, and the challenges present in the latter case. Second, we discuss the evidence regarding the selectivity of the prosopagnosic defect, particularly in relation to the recognition of other objects, written words (another visual object category requiring high expertise), and voices. Third, we summarize recent findings about the structural and functional basis of prosopagnosia from studies using magnetic resonance imaging, functional magnetic resonance imaging, and event-related potentials. Finally, we discuss recent attempts at rehabilitation of face recognition in prosopagnosia.
Keywords: face recognition, perception, fusiform gyrus, anterior temporal, review
Introduction
Face recognition is usually effortless and rapid. In different places and times, despite changes in expression, hairstyle, and clothing, we easily recognize colleagues, friends, and family. Our visual expertise with faces likely exceeds that for any other type of object, and this ability to identify people is a cornerstone of our social interactions as human beings. Subjects with prosopagnosia, however, cannot recognize that they have seen a face before, an impairment that affects both faces well known to them and those recently encountered. This is not due to more general problems with vision, object recognition, or memory. The term “impaired face recognition” should be used rather than “prosopagnosia” when this symptom is part of a broader problem, as with macular degeneration, general memory problems in Alzheimer’s disease, and cognitive issues in schizophrenia, for example. These subjects realize that a face is a face and not a car or a tree, but simply cannot say whether they have seen it before or whose face it is. These subjects rely on other cues to identity, such as hairstyle, gait, or voice, and make mistakes if these cues change (eg, hairstyle). They relate surprising and sometimes embarrassing stories, such as not recognizing themselves in a mirror, or walking past siblings or spouses as if they were strangers.
Prosopagnosia can be either acquired or developmental. In acquired prosopagnosia, poor face recognition is the result of brain injury. While the first case of acquired prosopagnosia was reported 150 years ago,1,2 the modern study of this condition began with Bodamer’s3 report in 1947, which described impaired face recognition in wounded soldiers. Subsequently, it has been recognized that acquired prosopagnosia can arise from many different pathologies, including trauma, stroke, encephalitis, tumors, degenerative atrophy, or temporal lobe resections.4
Developmental prosopagnosia has been more recently described and is less well understood. Subjects with this condition fail to develop face recognition skills despite otherwise normal vision and memory, and do not have obvious lesions on brain imaging.5,6 Developmental prosopagnosia may have a genetic basis. It can run in families, with some pedigrees showing as many as ten affected members across two generations,7–9 observations that parallel findings that normal face recognition skills also have a heritable component, with monozygotic twins having more similar face recognition abilities than dizygotic twins.10,11 While the acquired form is rare, the developmental form may be relatively common. Some suggest that as many as 2.5% of the population has developmental prosopagnosia,12,13 although this number will vary with the statistical criteria used and may confound those subjects with a developmental problem with those on the low end of normal face recognition ability (see Barton and Corrow14 for a discussion on the prevalence and diagnosis of developmental prosopagnosia).
Prosopagnosia has significant implications for those who have it. Adults with developmental prosopagnosia often report that their failure to recognize others creates traumatic social experiences, leading to chronic anxiety, feelings of embarrassment and guilt, and a limited social circle.15 Our subjects with acquired prosopagnosia acknowledge similar difficulties. Children with developmental prosopagnosia and their parents describe the same problems, but with additional implications for the school environment and safety.16
Models of face recognition
Face recognition is a multistage process ending with the identification of a person. These stages are reflected in cognitive models of face recognition, the most influential being that of Bruce and Young17 (Figure 1). Each box in the model represents a distinct cognitive process: while it is not necessary that these different stages occur in separate anatomic structures, some neuroanatomic models suggest that this may be the case.4,18 The model begins with creating a “facial percept”, the encoding of the structural information about the face. This percept is matched to stores of face memories, termed “face recognition units”, to determine whether the face has been seen before. Some argue that a correct match at this stage produces a feeling of familiarity with the face.19–21 A correct match also activates a “person identity node”, which allows access to semantic information and the name of the person to whom the face belongs. This model continues to be useful and has been elaborated to incorporate parallel sources of information from other cues (eg, voice),22,23 hemispheric lateralization of these cues,20 and more extensive bidirectional influences between modules.19,22–25
Figure 1.
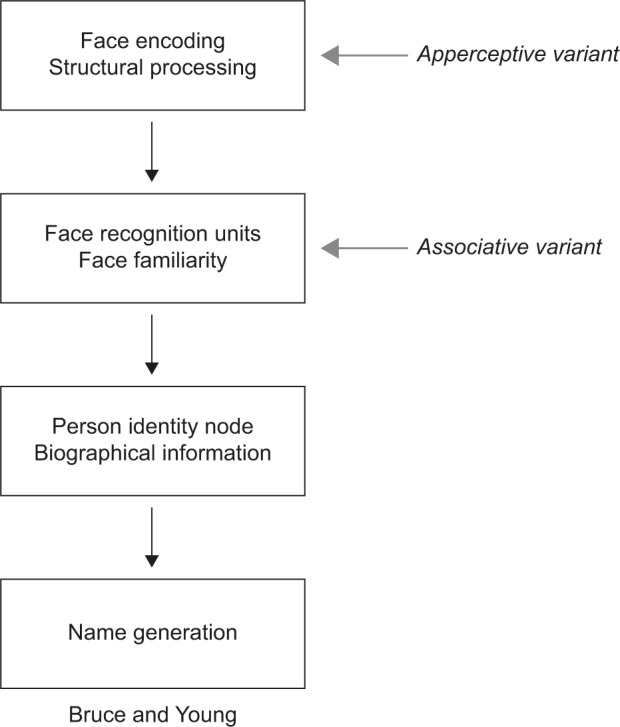
Adaption of the Bruce and Young model.
Notes: In the associative variant of prosopagnosia, face encoding is thought to be intact, represented by the ability to accurately discriminate between faces. However, faces are not seen as familiar suggesting a failure to activate face recognition units, subsequently affecting later stages in the face-processing stream. In the apperceptive variant, face encoding is thought to be impaired, affecting all later stages in the model when faces are the cue to identity. Gray arrows indicate the first stage of the model showing the greatest deficit in apperceptive and associative variants of prosopagnosia, respectively. © 1986 The British Psychological Society. Adapted from Bruce V, Young A. Understanding face recognition. Br J Psychol. 1986;77(Pt 3):305–327 with permission John Wiley and Sons.17
These models are reflected in our concepts about prosopagnosia. There are functional variants that may correspond to dysfunction of different cognitive stages.26 Impairments in the ability to see differences between faces, or their structures, suggest an “apperceptive variant”, a failure in encoding the facial percept. Other prosopagnosic subjects can perceive facial structure accurately but on tests of facial imagery cannot recall the faces of familiar people, indicating an “associative or amnestic variant”. However, this is a relative rather than absolute dichotomy: subtle defects in face perception can be seen in patients with an associative variant,27–30 while those with an apperceptive variant have milder deficits on face imagery tests.4 Nonetheless, this distinction remains useful, and these variants have distinct neural correlates (see “Neuroimaging” section).4
Diagnosis
Tests of face familiarity
The hallmark of prosopagnosia is the reduced ability of subjects to realize that they have seen a face before: hence, key diagnostic tests probe the sense of familiarity for previously seen faces. Earlier tests of face recognition may have been less sensitive because their stimuli could allow subjects to use alternative strategies to circumvent poor face recognition, such as remembering hairstyles and clothing.31,32 Newer tests have addressed those limitations by minimizing those extraneous cues. The most commonly used test of familiarity for recently viewed faces is the Cambridge Face Memory Test (CFMT),33 a test with high internal reliability.34 While the original version of this test used only adult Caucasian faces, other versions have been created, such as the CFMT-Chinese,35 CFMT-Australian,36 and pediatric versions, such as the CFMT-C37 and the CFMT-Kids.38
Tests that use anonymous faces like the CFMT have the advantage that, as none of the faces are familiar to subjects prior to learning, all subjects taking the test have the same degree of short-term familiarity with the faces seen during the test. Tests of familiarity for famous faces are also used, but such tests depend on the person having seen those celebrities before, and are therefore affected by age, education, and cultural background. In prosopagnosic subjects, this can be compounded by the fact that these subjects may lose interest in films and television because they cannot keep track of the characters, thus limiting their exposure to newer celebrities.
Tests of face perception
Tests of face perception – that is, the ability to perceive differences between faces – do not establish the diagnosis of prosopagnosia. What they can do is demonstrate if prosopagnosia is due to impaired encoding of the facial structure, and therefore is an apperceptive variant, or if such encoding is intact, which would point to an associative variant. Deficits in face perception have been measured by the Cambridge Face Perception Test39 and the Glasgow Face Matching Test,40 which involve sorting or matching faces by their identity with minimal demands on memory. The Dartmouth Face Perception Test is useful for children.41
Questionnaires of social impact
Questionnaires can evaluate everyday experiences with face recognition. There is a 15-item self-report questionnaire13 that contains questions on face recognition, attractiveness judgments, and expression recognition, and a more recent 20-item questionnaire for face identity (Prosopagnosia Index, “PI20”,42). However, these should be supplemented by objective tests for diagnosis.
Exclusionary tests
Establishing impaired face recognition is not sufficient for the diagnosis of prosopagnosia. One must also show that this is not due to more general problems with vision and memory. The assessment of acuity and visual fields can exclude low-level impairments of vision as a cause of poor face recognition: indeed one of the problems of subjects with macular degeneration is difficulty recognizing faces.43 Beyond this, to exclude a more general visual agnosia, subjects with prosopagnosia should have normal object recognition at a “basic” level (ie, identifying that an object is a face, a bicycle, a lamp, etc). Some may have difficulties identifying specific examples of these objects (ie, which bicycle or which lamp): this is not grounds for rejecting a diagnosis of prosopagnosia, but is relevant to the debate about whether the recognition problem in prosopagnosia is truly specific for faces alone (see “Face Specificity” section). For this reason, challenging tests of object recognition that include measures of reaction time and premorbid expertise44 are useful (see “Objects” section).
Finally, face identity recognition deficits can occur in the context of other disorders, and the diagnostic process should consider whether any of these are present. In children, this includes conditions such as autism45–48 and Turner’s syndrome,49 while in adults impaired face recognition has been reported in schizophrenia,50,51 Alzheimer’s disease,52–54 and Parkinson’s disease,55 for example. The diagnosis of prosopagnosia should be reserved for cases in which poor face recognition cannot be explained by one of these other conditions. Suggested criteria for the diagnosis of acquired and developmental prosopagnosia are outlined in Table 1. Greater detail regarding guidelines and available tests can be found in a recent review.56
Table 1.
Suggested inclusion and exclusion criteria for the diagnosis of acquired and developmental prosopagnosia
| Inclusion criteria | Exclusion criteria | Clarification questions |
|---|---|---|
| • Difficulty with faces evident in everyday life (PI20) • Impairment on at least two measures of face familiarity (CFMT) • Confirmation of lesion by MRI or CT scan (AP cases only) |
• Low-level visual impairment that could otherwise explain prosopagnosia • General visual agnosia • General memory impairment • Neuropsychological disorders associated with face recognition impairment • Visible lesion on MRI (DP cases only) |
• Does the individual have associative or apperceptive subtype? (Cambridge Face Perception Test or Glasgow Face Matching Test) • Is the disorder prosopagnosia or a multimodal person recognition disorder? (Tests of name and voice familiarity) |
Note: Suggested tests for adults are indicated in parentheses, and brackets indicate criteria specific to either acquired prosopagnosia (AP) or developmental prosopagnosia (DP).
Abbreviations: CFMT, Cambridge Face Memory Test; MRI, magnetic resonance imaging; CT, computed tomography.
Face specificity
Are prosopagnosic subjects impaired in the recognition of faces only? Here, we comment on four aspects of this question about specificity. First, a long-standing debate in face research is whether the mechanisms used to process faces are dedicated to faces alone, ie, “face specific”, or if they are involved in processing other objects, particularly those for which we possess perceptual expertise.57–59 Second, new theories have proposed that words and faces, two visual classes for which literate humans have great expertise, share and compete for resources, leading to predictions that prosopagnosic subjects may have subtle deficits in word processing.60,61 Third, questions have arisen as to whether some prosopagnosic subjects may actually have a multimodal problem in recognizing people.19,62,63 If so, they should also have impairment of recognition of people by voice and name; however, voice recognition has seldom been objectively evaluated in prosopagnosia. Finally, an issue of less theoretical but some practical interest is the array of other visual deficits that likely reflect damage to neighboring structures and networks, particularly with acquired prosopagnosia.
Objects
All objects share visual processing in the striate and early extrastriate cortex: whether the processing of faces and objects diverges later is the question. Neuroimaging studies of healthy individuals show that face processing depends on a cortical network of regions that is partially overlapping but distinct from areas involved in object processing.64–67 Transcranial magnetic stimulation has demonstrated a double dissociation between face and object processing: stimulation of face areas interrupts face processing more than object processing and stimulation of object areas results in the reverse.68
The contribution of prosopagnosia research to this debate is mixed. While there are studies that report intact ability to distinguish between members of other object categories,28,69–76 others describe cases who have difficulty.63,77 If prosopagnosia is about expert processing, though, a notable omission from many of these studies is the failure to consider the premorbid expertise of the prosopagnosic subject for the objects being used in the testing. A recent advance is the development of a method to use verbal semantic knowledge about a type of object as an index of their premorbid expertise and to adjust visual recognition scores for the degree of expertise. When this was done, nine of ten subjects with acquired prosopagnosia were impaired in expertise-adjusted car recognition.44,78
Similar mixed results have been obtained in adults with developmental prosopagnosia, with several studies describing cases in which the recognition deficit affected only faces9,79–84 and some cases in which the recognition of other objects was also impaired.8,9,79,80,85–88 This is true for studies of children too.9,49,89–92 A study of six children with developmental prosopagnosia found face-specific deficits in four, and more general deficits for both faces and objects in one.93 These differences across cases and studies may reflect a real heterogeneity rather than methodological issues.
One study has also attempted to evaluate the effect of object expertise on recognition ability in developmental prosopagnosia.34 Using the Cambridge Car Memory Test,94 this study reported that, at the group level, those with developmental prosopagnosia did not differ from controls after controlling for car expertise. However, individual data were analyzed before controlling for expertise, making it difficult to know whether expertise-adjusted car recognition was intact in each subject.
Words
Next to faces, words may be the stimulus category for which we have the highest degree of visual expertise. Although face processing is more active in the right hemisphere and word processing on the left, both show bilateral networks that overlap.95 A recent theory proposes that face processing and word processing compete for neural resources during development and that incomplete hemispheric lateralization is a result of this competition.60,96,97 The prediction of this theory is that prosopagnosic subjects should have subtle impairments in the processing of words, even if their lesions are limited to the right hemisphere.
Several recent studies have tested this prediction in acquired prosopagnosia. One study found subtle impairments in word processing in three subjects,61 but these may have had a more general integrative visual agnosia rather than prosopagnosia.81,98 A second study of five subjects found normal performance on seven different reading tasks.99 A third study100 found that only prosopagnosic subjects with bilateral fusiform lesions showed an increased word-length effect (the time taken to read a word as a function of the number of letters), and slow sorting of printed cards by their word content. On the other hand, even subjects with right hemisphere lesions alone were impaired when they had to sort the same cards by their font or handwriting. This suggested that the right hemisphere makes a critical contribution to the processing of stylistic properties of written text, rather than analyzing their word content. However, a similar recent study in developmental prosopagnosia has not found any deficit in processing the words or style of writing.101 This may indicate that the style-processing impairments in acquired prosopagnosia are related to damage to adjacent processing areas rather than damage to the mechanisms involved in face processing.
Voices
Another question of specificity has examined whether individuals with prosopagnosia have difficulty in only the recognition of faces, or whether they struggle with person recognition more generally, such as the recognition of voices. On the other hand, years of relying on voice cues to recognize others may produce superior voice recognition in prosopagnosic subjects.102
A recent study of acquired prosopagnosia found that only subjects with bilateral anterior temporal lesions had deficits in the recognition of voices, and therefore were better classified as having a multimodal disorder of person recognition.62 However, the recognition deficit was specific to faces and did not involve voices or names in those with right anterior temporal lesions alone or occipitotemporal lesions. A second study of 12 subjects with developmental prosopagnosia found impaired voice recognition in only one subject;103 nevertheless, this provides more evidence for heterogeneity in the developmental variant.
Other deficits from damage to adjacent structures
The classic tetrad found with acquired prosopagnosia, particularly when due to occipitotemporal lesions, is superior field deficits, dyschromatopsia and topographic disorientation. Cerebral dyschromatopsia is associated with damage to the lingual and fusiform gyri, in the vicinity of the collateral sulcus, almost always with bilateral but rarely with right unilateral lesions.104,105 It is characterized primarily by an accentuation of the tritanopic-like patterns seen in healthy subjects.104 This last study did not find color impairments in subjects with developmental prosopagnosia.
Topographic disorientation, a disorder in which subjects get lost in familiar surroundings, is commonly reported with acquired prosopagnosia. A recent review found mention of topographic difficulty in 29% of 147 cases.106 One possible explanation is the close proximity of the parahippocampal place area,107 an area activated when viewing scenes, to the fusiform face area (FFA),108 which is activated by viewing faces. In developmental prosopagnosia, there are anecdotal reports of both impaired94–96,109 and preserved84,110,111 navigational abilities. A recent study found that most patients with acquired prosopagnosia, regardless of lesion location, were impaired in scene and landmark recognition, while those with occipitotemporal lesions were also impaired in the ability to form cognitive maps,112 and either deficit was rare in developmental prosopagnosia.
Neuroimaging
The advent of functional imaging has revolutionized cognitive brain science. In face research, it has delineated networks of regions active during face perception. This includes a core face network that includes the FFA,108 the occipital face area (OFA), and the posterior portion of the superior temporal sulcus113,114 (Figure 2). There is also an extended network that includes the anterior temporal face area and other regions such as the inferior frontal gyrus and precuneus.4,115 While faces activate these areas in both hemispheres,95,114 the effect is stronger on the right.108
Figure 2.
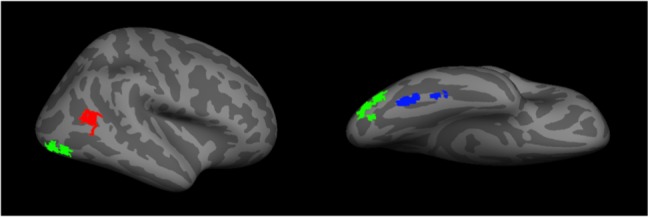
A representation of the core face network – including the fusiform face area (blue), the occipital face area (green), and the posterior superior temporal sulcus (red).
Studies of acquired prosopagnosia have been advanced by the improved functional and structural capabilities of magnetic resonance imaging (MRI). A key fact is that a variety of lesions can cause prosopagnosia,116 an observation that makes sense when one considers the widely distributed networks involved in face processing. Two key observations have been made from the study of acquired prosopagnosia. First, lesions may be bilateral or unilateral, and when unilateral they are far more likely to be on the right.4,117,118 A few prosopagnosic subjects with left-sided lesions have been described, but most have been left-handed,119–121 raising the possibility that they may have had anomalous hemispheric lateralization to begin with. Second, there is a useful division between occipitotemporal and anterior temporal damage. Recent functional MRI work has shown that occipitotemporal damage is associated with loss of activation of core components such as the FFA and OFA,78 while activation in anterior areas may be spared.122 Conversely, activation of the FFA and OFA may be spared in individuals with anterior temporal lesions.78
These modern neuroimaging observations have generated structural correlates for functional variants of prosopagnosia that had long been hypothesized (see “Models of face recognition” section and Figure 1).26 Recent studies show that those with fusiform lesions are more likely to have the apperceptive variant,4,78,123 whereas those with anterior temporal lesions are more likely to have the associative variant4,78,124,125 (Figure 3). The main conclusion is that acquired prosopagnosia is not a single disorder, but a family of disorders with different mechanisms and different lesions that nevertheless lead to the same end result of impaired face recognition.78
Figure 3.
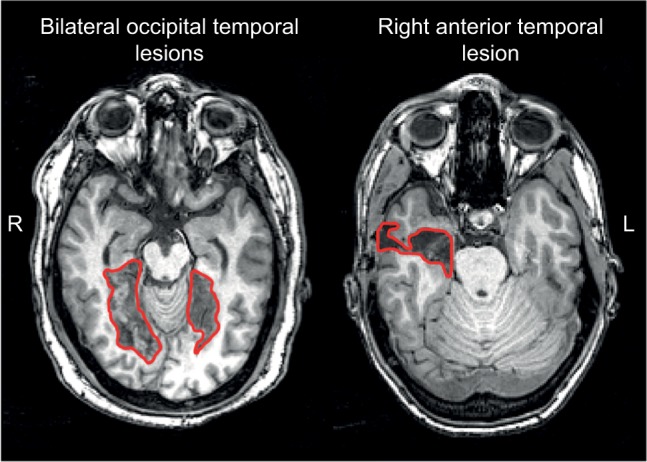
Examples of lesions that produce acquired prosopagnosia.
Notes: Approximate lesions, as can be seen on a single slice, are outlined in red. Patients with bilateral occipital temporal lesions (left) often experience apperceptive prosopagnosia and those with anterior temporal lesions (right) often experience associative prosopagnosia. These cases demonstrate that acquired prosopagnosia is a family of disorders with different mechanisms and different lesion locations that lead to the same end result of prosopagnosia.
The structural correlates of developmental prosopagnosia are still debated. By definition, there is no obvious structural lesion, and early studies examining the evidence of abnormal activation of the core face network produced mixed results, with some reporting normal activation126,127 and another reporting activation for faces that did not differ from activation for other object types.126–128 Recent work with more advanced imaging methods has begun to uncover both structural and functional anomalies in developmental prosopagnosia, but there is disagreement. Some have suggested that there are anatomical84 or functional129–131 abnormalities in the FFA and localized differences in white matter fibers around the right FFA.132,133 Others maintain that the core face network is largely normal and that abnormalities lie instead in the anterior temporal cortex,134,135 other regions of the extended face network,136 or the long-range white matter tracts that connect the core regions in occipitotemporal cortex with the anterior temporal face area, namely the inferior longitudinal fasciculus.134,137 Both groups claim that the degree of altered white matter connectivity in their results correlated with behavioral measures of impaired face recognition.133,137 Whether these discrepancies reflect a real heterogeneity that exists in developmental prosopagnosia remains to be determined.
Event-related potentials
While event-related potentials do not have as good spatial resolution as MRI, they have a much finer resolution in time and can advance our understanding of the temporal dynamics of face recognition. Studies of face recognition in healthy subjects identify three components. The N170 component is prominent in right lateral occipitotemporal areas: it shows larger responses to faces than other objects and is associated with perceptual aspects of face processing.138–140 The N250 is also right-dominant and is the first component to show effects linked to the appearance of a specific facial identity, rather than just faces in general.18,141,142 The P600 is seen when subjects can recognize a person by stating their name or providing information about them.142,143
Studies of acquired prosopagnosia support the association of the N170 with both an occipitotemporal location and perceptual aspects of face processing. Dalrymple et al144 found that the face-selective aspect in the N170 was absent in subjects with apperceptive prosopagnosia whose lesions included at least two components of the core network (eg, FFA and OFA). However, it was intact in those with associative prosopagnosia whose lesions were restricted to anterior temporal cortex. Another study supported this finding by demonstrating preserved N170 face-selectivity in a subject with a right OFA lesion but preserved right fusiform gyrus,145 and other studies reported its absence in a subject with impaired face perception.143,146
The findings in developmental prosopagnosia are less straightforward.139 There are reports of both normal147–149 and abnormal N170 components,111,129,147,150 including one on the analogous M170 component detected by magnetoencephalography.151 Larger studies have found heterogeneous results across subjects that can explain this inconsistency.147,152–154 It is also possible that there are more subtle abnormalities in the N170 component. For example, the amplitude of the N170 component is usually larger when viewing upside-down faces, likely because it is harder to process them, but one study found that the majority of 16 subjects with developmental prosopagnosia failed to show an orientation effect in the N170 amplitude.152
With regard to the later potentials, a study of 12 subjects with developmental prosopagnosia found normal N250 and P600 components on the few trials on which these subjects did identify a face, suggesting relatively normal processing when face recognition is successful. About half also exhibited N250 components for famous faces they did not recognize, which may indicate some unconscious processing. There was no P600 component under those circumstances, implying that this potential reflects conscious face identification.155 Even though these studies indicate that the processes indexed by these later potentials can still be activated in developmental prosopagnosia, a recent Event Related Potential study claimed that these components are delayed156 (see Towler et al157 for a recent review of ERP findings in developmental prosopagnosia).
Treatment and rehabilitation
Can training improve prosopagnosia? The answer may be that it depends. In acquired prosopagnosia, one might speculate that the efficacy of any training could be affected by age at onset, duration since onset, and lesion size, laterality, and location, particularly with regard to how much of the face network and its connections are compromised.158 Given the rarity of acquired prosopagnosia, it will be very difficult to establish the impact of each of these factors. To date, there have been few remedial attempts for the acquired variant, and most focus on enhancing coping strategies to circumvent poor face recognition.158,159 Only one published study attempted to improve face recognition, in a child with diffuse damage after meningococcal meningitis: 18 months of training did not improve matters.160 More recently, two training studies have been reported at conferences. DeGutis et al159,161 attempted to train a 46-year-old with a right occipital-temporal lesion to categorize faces based on the distances between face parts. Unfortunately, this did not help. A second study trained 12 subjects to discriminate increasingly subtler differences in face shape across variations in expression and viewpoint, over 11 weeks. Some improvement was found, but this was more modest for the recognition of faces not used during training.162
Given the lack of overt brain damage, one might wonder whether training may be more effective in developmental prosopagnosia. One group trained 25 subjects with developmental prosopagnosia163,164 to perceive the spacing between facial features and found improvements that generalized to new faces but did not help recognition when viewpoint varied.164 A different therapeutic approach was used in a randomized, placebo-controlled, double-blind study examining the effect of intranasal inhalation of oxytocin, a drug associated with the regulation of social behaviors, on face identity processing.165 The authors reported transient improvement of face perception and recognition in ten subjects with developmental prosopagnosia after oxytocin administration.
While these reports are encouraging, there may be limitations. Given the heterogeneity of deficits in prosopagnosia, it may be that a specific training program will not be appropriate for all subjects. How much of a residual face network one needs in acquired prosopagnosia to benefit from training is unknown. The belief that the subtler structural alterations of developmental prosopagnosia imply a better chance of having the neural substrate to generate benefit from training is unproven.
Conclusion
A prevailing theme in prosopagnosia research is the heterogeneity of findings across both acquired and developmental prosopagnosia. There is heterogeneity in the mechanism of prosopagnosia (ie, apperceptive versus associative), the location, lateralization, and extent of structural damage in the acquired form, and the presence or absence of impairments in other perceptual domains (eg, object, word, and voice processing). Heterogeneity is expected when one is dealing with a complex process such as person recognition, but it does create challenges that require particular care and rigor in experimental study and analysis.
For one, it is important to ensure that heterogeneity is not the inadvertent result of experimental factors. To this end, care is required in establishing the diagnosis of prosopagnosia and excluding other conditions (Table 1). First, besides excluding more general failures in object recognition and memory, tests of voice and name recognition are needed to establish where a patient is more accurately characterized as having a multi-modal disorder of person recognition, whose mechanisms may differ from prosopagnosia. Second, uniform diagnostic criteria are needed. This is particularly an issue for developmental prosopagnosia. Currently, there is no diagnostic consensus: inclusion criteria range from purely self-report measures12,13 to various conglomerations of self-report, behavioral tests of face familiarity, and tests of face naming/identification,156,166 and few require imaging to exclude brain lesions that would point to an early-onset acquired variant rather than developmental prosopagnosia. Another diagnostic issue that reflects the current lack of definitive genetic or radiologic markers for the developmental form is the challenge of distinguishing subjects with true pathology resulting from aberrant development of face recognition networks from those who are simply at the low end of a spectrum of normal face-processing skill (see Barton and Corrow14 for a discussion).
Nevertheless, it remains a possibility that there is real heterogeneity in developmental prosopagnosia, just as there is in acquired prosopagnosia. This accounts for the current trend to use single-subject methods of analysis, using Crawford’s T tests, for example. However, it may be difficult for subtle anomalies to achieve statistical significance at the individual level, as illustrated by recent ERP work.151,152 Further work may benefit from the definition of more homogeneous variants, and supplementing the single-subject methods with group analyses on these subgroups. This will necessitate the collection of larger samples of these patients. Such efforts should advance our knowledge of the neuroanatomic and functional origins of these intriguing conditions.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure
This work was supported by CIHR operating grant (MOP-102567) to JB. JB was supported by a Canada Research Chair and the Marianne Koerner Chair in Brain Diseases. SC was supported by National Eye Institute of the National Institutes of Health under award number F32 EY023479-02 and Loan Repayment Program. The authors report no other conflicts of interest in this work.
References
- 1.Quaglino A, Borelli G. Emiplegia sinistra con amaurosi–guarigione–perdita totale della percezione dei colori e della memoria della configurazione degli oggetti. [Left hemiplegia with amaurosis–healing–total loss of color perception and the configuration memory of the objects] Giornale di Oftalmologia Italiano. 1867;10:106–117. Italian. [Google Scholar]
- 2.Della Sala S, Young AW. Quaglino’s 1867 case of prosopagnosia. Cortex. 2003;39(3):533–540. doi: 10.1016/s0010-9452(08)70263-6. [DOI] [PubMed] [Google Scholar]
- 3.Bodamer J. Die Prosop-Agnosie. [The Prosopagnosic] Archiv fur Psychiatrie und Nervenkrankheiten. 1947;179:6–53. doi: 10.1007/BF00352849. [DOI] [PubMed] [Google Scholar]
- 4.Barton JJ. Structure and function in acquired prosopagnosia: lessons from a series of 10 patients with brain damage. J Neuropsychol. 2008;2(Pt 1):197–225. doi: 10.1348/174866407x214172. [DOI] [PubMed] [Google Scholar]
- 5.Duchaine B, Nakayama K. Developmental prosopagnosia: a window to content-specific face processing. Curr Opin Neurobiol. 2006;16(2):166–173. doi: 10.1016/j.conb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Susilo T, Duchaine B. Advances in developmental prosopagnosia research. Curr Opin Neurobiol. 2013;23(3):423–429. doi: 10.1016/j.conb.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Schmalzl L, Palermo R, Coltheart M. Cognitive heterogeneity in genetically based prosopagnosia: a family study. J Neuropsychol. 2008;2(1):99–117. doi: 10.1348/174866407x256554. [DOI] [PubMed] [Google Scholar]
- 8.Duchaine B, Germine L, Nakayama K. Family resemblance: ten family members with prosopagnosia and within-class object agnosia. Cogn Neuropsychol. 2007;24(4):419–430. doi: 10.1080/02643290701380491. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Duchaine B, Wilson HR, Nakayama K. Three cases of developmental prosopagnosia from one family: detailed neuropsychological and psychophysical investigation of face processing. Cortex. 2010;46(8):949–964. doi: 10.1016/j.cortex.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Wilmer JB, Germine L, Chabris CF, et al. Human face recognition ability is specific and highly heritable. Proc Natl Acad Sci U S A. 2010;107(11):5238–5241. doi: 10.1073/pnas.0913053107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Q, Song Y, Hu S, et al. Heritability of the specific cognitive ability of face perception. Curr Biol. 2010;20(2):137–142. doi: 10.1016/j.cub.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Kennerknecht I, Grueter T, Welling B, et al. First report of prevalence of non-syndromic hereditary prosopagnosia (HPA) Am J Med Genet A. 2006;140(15):1617–1622. doi: 10.1002/ajmg.a.31343. [DOI] [PubMed] [Google Scholar]
- 13.Kennerknecht I, Ho NY, Wong VC. Prevalence of hereditary prosopagnosia (HPA) in Hong Kong Chinese population. Am J Med Genet A. 2008;146A(22):2863–2870. doi: 10.1002/ajmg.a.32552. [DOI] [PubMed] [Google Scholar]
- 14.Barton JJ, Corrow SL. The problem of being bad at faces. Neuropsychologia. 2016;89:119–124. doi: 10.1016/j.neuropsychologia.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yardley L, McDermott L, Pisarski S, Duchaine B, Nakayama K. Psychosocial consequences of developmental prosopagnosia: a problem of recognition. J Psychosom Res. 2008;65(5):445–451. doi: 10.1016/j.jpsychores.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Dalrymple KA, Fletcher K, Corrow S, et al. “A room full of strangers every day”: the psychosocial impact of developmental prosopagnosia on children and their families. J Psychosom Res. 2014;77:144–150. doi: 10.1016/j.jpsychores.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce V, Young A. Understanding face recognition. Br J Psychol. 1986;77(Pt 3):305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- 18.Schweinberger SR, Burton AM. Covert recognition and the neural system for face processing. Cortex. 2003;39(1):9–30. doi: 10.1016/s0010-9452(08)70071-6. [DOI] [PubMed] [Google Scholar]
- 19.Barton JJ, Corrow SL. Recognizing and identifying people: a neuropsychological review. Cortex. 2016;75:132–150. doi: 10.1016/j.cortex.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gainotti G. Cognitive models of familiar people recognition and hemispheric asymmetries. Front Biosci (Elite Ed) 2014;6:148–158. doi: 10.2741/e698. [DOI] [PubMed] [Google Scholar]
- 21.Farah MJ, O’Reilly RC, Vecera SP. Dissociated overt and covert recognition as an emergent property of a lesioned neural network. Psychol Rev. 1993;100(4):571–588. doi: 10.1037/0033-295x.100.4.571. [DOI] [PubMed] [Google Scholar]
- 22.Belin P, Fecteau S, Bédard C. Thinking the voice: neural correlates of voice perception. Trends Cogn Sci. 2004;8(3):129–135. doi: 10.1016/j.tics.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Ellis HD, Jones DM, Mosdell N. Intra- and inter-modal repetition priming of familiar faces and voices. Br J Psychol. 1997;88(Pt 1):143–156. doi: 10.1111/j.2044-8295.1997.tb02625.x. [DOI] [PubMed] [Google Scholar]
- 24.Burton AM, Bruce V. I recognize your face but I can’t remember your name: a simple explanation? Br J Psychol. 1992;83(Pt 1):45–60. doi: 10.1111/j.2044-8295.1992.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 25.Bredart S, Valentine T, Calder A, Gassi L. An interactive activation model of face naming. Q J Exp Psychol A. 1995;48(2):466–486. doi: 10.1080/14640749508401400. [DOI] [PubMed] [Google Scholar]
- 26.De Renzi E, Faglioni P, Grossi D, Nichelli P. Apperceptive and associative forms of prosopagnosia. Cortex. 1991;27(2):213–221. doi: 10.1016/s0010-9452(13)80125-6. [DOI] [PubMed] [Google Scholar]
- 27.Busigny T, Van Belle G, Jemel B, Hosein A, Joubert S, Rossion B. Face-specific impairment in holistic perception following focal lesion of the right anterior temporal lobe. Neuropsychologia. 2014;56:312–333. doi: 10.1016/j.neuropsychologia.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Busigny T, Joubert S, Felician O, Ceccaldi M, Rossion B. Holistic perception of the individual face is specific and necessary: evidence from an extensive case study of acquired prosopagnosia. Neuropsychologia. 2010;48(14):4057–4092. doi: 10.1016/j.neuropsychologia.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 29.White D, Rivolta D, Burton AM, Al-Janabi S, Palermo R. Face matching impairment in developmental prosopagnosia. Q J Exp Psychol. 2016 Apr 22; doi: 10.1080/17470218.2016.1173076. Epub. [DOI] [PubMed] [Google Scholar]
- 30.Barton JJ, Zhao J, Keenan JP. Perception of global facial geometry in the inversion effect and prosopagnosia. Neuropsychologia. 2003;41(12):1703–1711. doi: 10.1016/s0028-3932(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 31.Duchaine BC, Nakayama K. Developmental prosopagnosia and the benton facial recognition test. Neurology. 2004;62(7):1219–1220. doi: 10.1212/01.wnl.0000118297.03161.b3. [DOI] [PubMed] [Google Scholar]
- 32.Duchaine BC, Weidenfeld A. An evaluation of two commonly used tests of unfamiliar face recognition. Neuropsychologia. 2003;41(6):713–720. doi: 10.1016/s0028-3932(02)00222-1. [DOI] [PubMed] [Google Scholar]
- 33.Duchaine B, Nakayama K. The Cambridge Face Memory Test: results from neurologically intact individuals and an investigation of its validity using inverted stimuli and prosopagnosic participants. Neuropsychologia. 2006;44(4):576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Esins J, Schultz J, Stemper C, Kennerknecht I, Bülthoff I. Face perception and test reliabilities in congenital prosopagnosia in seven tests. Iperception. 2016;7(1):1–37. doi: 10.1177/2041669515625797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKone E, Stokes S, Liu J, et al. A robust method of measuring other-race and other-ethnicity effects: the Cambridge Face Memory Test format. PLoS One. 2012;7(10):e47956. doi: 10.1371/journal.pone.0047956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKone E, Hall A, Pidcock M, et al. Face ethnicity and measurement reliability affect face recognition performance in developmental prosopagnosia: evidence from the Cambridge Face Memory Test-Australian. Cogn Neuropsychol. 2011;28(2):109–146. doi: 10.1080/02643294.2011.616880. [DOI] [PubMed] [Google Scholar]
- 37.Croydon A, Pimperton H, Ewing L, Duchaine BC, Pellicano E. The Cam-bridge Face Memory Test for Children (CFMT-C): a new tool for measuring face recognition skills in childhood. Neuropsychologia. 2014;62:60–67. doi: 10.1016/j.neuropsychologia.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Dalrymple K, Gomez J, Duchaine B. CFMT-Kids: a new test of face memory for children. J Vis. 2012;12(9):492. [Google Scholar]
- 39.Duchaine B, Yovel G, Nakayama K. No global processing deficit in the Navon task in 14 developmental prosopagnosics. Soc Cogn Affect Neurosci. 2007;2:104–113. doi: 10.1093/scan/nsm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burton A, White D, McNeill A. The Glasgow Face Matching Test. Behav Res Methods. 2010;42(1):286–291. doi: 10.3758/BRM.42.1.286. [DOI] [PubMed] [Google Scholar]
- 41.Dalrymple KA, Garrido L, Duchaine B. Dissociation between face perception and face memory in adults, but not children, with developmental prosopagnosia. Dev Cogn Neurosci. 2014;10:10–20. doi: 10.1016/j.dcn.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah P, Gaule A, Sowden S, Bird G, Cook R. The 20-item prosopagnosia index (PI20): a self-report instrument for identifying developmental prosopagnosia. R Soc Open Sci. 2015;2(6):140343. doi: 10.1098/rsos.140343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes CS, De l’Aune W, Schuchard RA. A test of face discrimination ability in aging and vision loss. Optom Vis Sci. 2011;88(2):188–199. doi: 10.1097/OPX.0b013e318205a17c. [DOI] [PubMed] [Google Scholar]
- 44.Barton JJ, Hanif H, Ashraf S. Relating visual to verbal semantic knowledge: the evaluation of object recognition in prosopagnosia. Brain. 2009;132(Pt 12):3456–3466. doi: 10.1093/brain/awp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barton JJ, Cherkasova MV, Hefter R, Cox TA, O’Connor M, Manoach DS. Are patients with social developmental disorders prosopagnosic? perceptual heterogeneity in the asperger and socio-emotional processing disorders. Brain. 2004;127(Pt 8):1706–1716. doi: 10.1093/brain/awh194. [DOI] [PubMed] [Google Scholar]
- 46.Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci Biobehav Rev. 2012;36(3):1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Duchaine B, Murray H, Turner M, White S, Garrido L. Normal social cognition in developmental prosopagnosia. Cogn Neuropsychol. 2009;26(7):620–634. doi: 10.1080/02643291003616145. [DOI] [PubMed] [Google Scholar]
- 48.Wilson CE, Palermo R, Schmalzl L, Brock J. Specificity of impaired facial identity recognition in children with suspected developmental prosopagnosia. Cogn Neuropsychol. 2010;27(1):30–45. doi: 10.1080/02643294.2010.490207. [DOI] [PubMed] [Google Scholar]
- 49.Hong D, Scaletta Kent J, Kesler S. Cognitive profile of turner syndrome. Dev Disabil Res Rev. 2009;15(4):270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frith CD, Stevens M, Johnstone EC, Owens DG, Crow TJ. Integration of schematic faces and other complex objects in schizophrenia. J Nerv Ment Dis. 1983;171(1):34–39. doi: 10.1097/00005053-198301000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Archer J, Hay DC, Young AW. Face processing in psychiatric conditions. Br J Clin Psychol. 1992;31(Pt 1):45–61. doi: 10.1111/j.2044-8260.1992.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 52.Roudier M, Marcie P, Grancher AS, Tzortzis C, Starkstein S, Boller F. Discrimination of facial identity and of emotions in Alzheimer’s disease. J Neurol Sci. 1998;154(2):151–158. doi: 10.1016/s0022-510x(97)00222-0. [DOI] [PubMed] [Google Scholar]
- 53.Bäckman L, Herlitz A. The relationship between prior knowledge and face recognition memory in normal aging and Alzheimer’s disease. J Gerontol. 1990;45(3):P94–P100. doi: 10.1093/geronj/45.3.p94. [DOI] [PubMed] [Google Scholar]
- 54.Hodges JR, Salmon DP, Butters N. Recognition and naming of famous faces in Alzheimer’s disease: a cognitive analysis. Neuropsychologia. 1993;31(8):775–788. doi: 10.1016/0028-3932(93)90128-m. [DOI] [PubMed] [Google Scholar]
- 55.Dewick HC, Hanley JR, Davies AD, Playfer J, Turnbull C. Perception and memory for faces in Parkinson’s disease. Neuropsychologia. 1991;29(8):785–802. doi: 10.1016/0028-3932(91)90072-g. [DOI] [PubMed] [Google Scholar]
- 56.Dalrymple KA, Palermo R. Guidelines for studying developmental prosopagnosia in adults and children. Wiley Interdiscip Rev Cogn Sci. 2016;7(1):73–87. doi: 10.1002/wcs.1374. [DOI] [PubMed] [Google Scholar]
- 57.Diamond R, Carey S. Why faces are and are not special: an effect of expertise. J Exp Psychol Gen. 1986;115(2):107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- 58.Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3(2):191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- 59.Xu Y, Liu J, Kanwisher N. The M170 is selective for faces, not for expertise. Neuropsychologia. 2005;43:588–597. doi: 10.1016/j.neuropsychologia.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Behrmann M, Plaut DC. Distributed circuits, not circumscribed centers, mediate visual recognition. Trends Cogn Sci. 2013;17(5):210–219. doi: 10.1016/j.tics.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Behrmann M, Plaut DC. Bilateral hemispheric processing of words and faces: evidence from word impairments in prosopagnosia and face impairments in pure alexia. Cereb Cortex. 2014;24(24):1102–1118. doi: 10.1093/cercor/bhs390. [DOI] [PubMed] [Google Scholar]
- 62.Liu R, Pancaroglu R, Hills CS, Duchaine B, Barton JJ. Voice recognition in face-blind patients. Cereb Cortex. 2016;26(4):1473–1487. doi: 10.1093/cercor/bhu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neuner F, Schweinberger SR. Neuropsychological impairments in the recognition of faces, voices, and personal names. Brain Cogn. 2000;44(3):342–366. doi: 10.1006/brcg.1999.1196. [DOI] [PubMed] [Google Scholar]
- 64.Grill-Spector K. The neural basis of object perception. Curr Opin Neurobiol. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 65.Haxby J, Gobbini M. Distributed neural systems for face perception. In: Calder A, Rhodes G, Johnson M, Haxby J, editors. The Oxford andbook of Face Perception. Oxford, United Kingdom: Oxford University Press; 2011. pp. 93–110. [Google Scholar]
- 66.Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 67.Kanwisher N. Domain specificity in face perception. Nat Neurosci. 2000;3(8):759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- 68.Pitcher D, Charles L, Devlin JT, Walsh V, Duchaine B. Triple dissociation of faces, bodies, and objects in extrastriate cortex. Curr Biol. 2009;19(4):319–324. doi: 10.1016/j.cub.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Farah MJ, Levinson KL, Klein KL. Face perception and within-category discrimination in prosopagnosia. Neuropsychologia. 1995;33(6):661–674. doi: 10.1016/0028-3932(95)00002-k. [DOI] [PubMed] [Google Scholar]
- 70.Susilo T, Yovel G, Barton JJ, Duchaine B. Face perception is category-specific: evidence from normal body perception in acquired prosopagnosia. Cognition. 2013;129(1):88–94. doi: 10.1016/j.cognition.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Rezlescu C, Pitcher D, Duchaine B. Acquired prosopagnosia with spared within-class object recognition but impaired recognition of degraded basic-level objects. Cogn Neuropsychol. 2012;29(4):325–347. doi: 10.1080/02643294.2012.749223. [DOI] [PubMed] [Google Scholar]
- 72.Rezlescu C, Barton JJ, Pitcher D, Duchaine B. Normal acquisition of expertise with greebles in two cases of acquired prosopagnosia. Proc Natl Acad Sci U S A. 2014;111(14):5123–5128. doi: 10.1073/pnas.1317125111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riddoch MJ, Johnston RA, Bracewell RM, Boutsen L, Humphreys GW. Are faces special? a case of pure prosopagnosia. Cogn Neuropsychol. 2008;25(1):3–26. doi: 10.1080/02643290801920113. [DOI] [PubMed] [Google Scholar]
- 74.McNeil JE, Warrington EK. Prosopagnosia: a face-specific disorder. Q J Exp Psychol A. 1993;46(1):1–10. doi: 10.1080/14640749308401064. [DOI] [PubMed] [Google Scholar]
- 75.Farah MJ, Wilson KD, Drain HM, Tanaka JR. The inverted face inversion effect in prosopagnosia: evidence for mandatory, face-specific perceptual mechanisms. Vision Res. 1995;35(14):2089–2093. doi: 10.1016/0042-6989(94)00273-o. [DOI] [PubMed] [Google Scholar]
- 76.Henke K, Schweinberger SR, Grigo A, Klos T, Sommer W. Specificity of face recognition: recognition of exemplars of non-face objects in prosopagnosia. Cortex. 1998;34(2):289–296. doi: 10.1016/s0010-9452(08)70756-1. [DOI] [PubMed] [Google Scholar]
- 77.Gauthier I, Berhmann M, Tarr MJ. Can face recognition really be dissociated from object recognition? J Cogn Neurosci. 1999;11(4):349–370. doi: 10.1162/089892999563472. [DOI] [PubMed] [Google Scholar]
- 78.Davies-Thompson J, Pancaroglu R, Barton J. Acquired prosopagnosia: structural basis and processing impairments. Front Biosci (Elite Ed) 2014;6:159–174. doi: 10.2741/e699. [DOI] [PubMed] [Google Scholar]
- 79.Duchaine B, Nakayama K. Dissociations of face and object recognition in developmental prosopagnosia. J Cogn Neurosci. 2005;17(2):249–261. doi: 10.1162/0898929053124857. [DOI] [PubMed] [Google Scholar]
- 80.Garrido L, Furl N, Draganski B, et al. Voxel-based morphometry reveals reduced grey matter volume in the temporal cortex of developmental prosopagnosics. Brain. 2009;132(Pt 12):3443–3455. doi: 10.1093/brain/awp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Susilo T, McKone E, Dennett H, et al. Face recognition impairments despite normal holistic processing and face space coding: evidence from a case of developmental prosopagnosia. Cogn Neuropsychol. 2010;27(8):636–664. doi: 10.1080/02643294.2011.613372. [DOI] [PubMed] [Google Scholar]
- 82.Tree J, Wilkie J. Face and object imagery in congenital prosopagnosia: a case series. Cortex. 2010;46(9):1189–1198. doi: 10.1016/j.cortex.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 83.Duchaine B, Yovel G, Butterworth E, Nakayama K. Prosopagnosia as an impairment to face-specific mechanisms: elimination of the alternative hypotheses in a developmental case. Cogn Neuropsychol. 2006;23(5):714–747. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- 84.Nunn J, Postma P, Pearson R. Developmental prosopagnosia: should it be taken at face value? Neurocase. 2001;7(1):15–27. doi: 10.1093/neucas/7.1.15. [DOI] [PubMed] [Google Scholar]
- 85.Behrmann M, Avidan G. Congenital prosopagnosia: face-blind from birth. Trends Cogn Sci. 2005;9(4):180–187. doi: 10.1016/j.tics.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 86.De Haan EH, Campbell R. A fifteen year follow-up of a case of developmental prosopagnosia. Cortex. 1991;27(4):489–509. doi: 10.1016/s0010-9452(13)80001-9. [DOI] [PubMed] [Google Scholar]
- 87.Duchaine BC, Nieminen-von Wendt T, New J, Kulomaki T. Dissociations of visual recognition in a developmental agnosic: evidence for separate developmental processes. Neurocase. 2003;9(5):380–389. doi: 10.1076/neur.9.5.380.16556. [DOI] [PubMed] [Google Scholar]
- 88.Duchaine BC, Parker H, Nakayama K. Normal recognition of emotion in a prosopagnosic. Perception. 2003;32(7):827–838. doi: 10.1068/p5067. [DOI] [PubMed] [Google Scholar]
- 89.Jones RD, Tranel D. Severe developmental prosopagnosia in a child with superior intellect. J Clin Exp Neuropsychol. 2001;23(3):265–273. doi: 10.1076/jcen.23.3.265.1183. [DOI] [PubMed] [Google Scholar]
- 90.Ariel R, Sadeh M. Congenital visual agnosia and prosopagnosia in a child. Cortex. 1996;12:76–82. doi: 10.1016/s0010-9452(96)80048-7. [DOI] [PubMed] [Google Scholar]
- 91.McConachie HR. Developmental prosopagnosia. A single case report. Cortex. 1976;12(1):76–82. doi: 10.1016/s0010-9452(76)80033-0. [DOI] [PubMed] [Google Scholar]
- 92.Brunsdon R, Coltheart M, Nickels L, Joy P. Developmental prosopagnosia: a case analysis and treatment study. Cogn Neuropsychol. 2006;23(6):822–840. doi: 10.1080/02643290500441841. [DOI] [PubMed] [Google Scholar]
- 93.Dalrymple KA, Elison JT, Duchaine B. Face-specific and domain-general visual processing deficits in children with developmental prosopagnosia. Q J Exp Psychol (Hove) 2016 May 4; doi: 10.1080/17470218.2015.1122642. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dennett HW, McKone E, Tavashmi R, et al. The Cambridge Car Memory Test: a task matched in format to the Cambridge Face Memory Test, with norms, reliability, sex differences, dissociations from face memory, and expertise effects. Behav Res Methods. 2012;44(2):587–605. doi: 10.3758/s13428-011-0160-2. [DOI] [PubMed] [Google Scholar]
- 95.Nestor A, Behrmann M, Plaut DC. The neural basis of visual word form processing: a multivariate investigation. Cereb Cortex. 2013;23(7):1673–1684. doi: 10.1093/cercor/bhs158. [DOI] [PubMed] [Google Scholar]
- 96.Dundas EM, Plaut DC, Behrmann M. The joint development of hemispheric lateralization for words and faces. J Exp Psychol Gen. 2013;142(2):348–358. doi: 10.1037/a0029503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dehaene S, Pegado F, Braga LW, et al. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- 98.Marotta JJ, McKeeff TJ, Behrmann M. The effects of rotation and inversion on face processing in prosopagnosia. Cogn Neuropsychol. 2002;19(1):31–47. doi: 10.1080/02643290143000079. [DOI] [PubMed] [Google Scholar]
- 99.Susilo T, Wright V, Tree JJ, Duchaine B. Acquired prosopagnosia without word recognition deficits. Cogn Neuropsychol. 2015;32(6):321–339. doi: 10.1080/02643294.2015.1081882. [DOI] [PubMed] [Google Scholar]
- 100.Hills CS, Pancaroglu R, Duchaine B, Barton JJ. Word and text processing in acquired prosopagnosia. Ann Neurol. 2015;78(2):258–271. doi: 10.1002/ana.24437. [DOI] [PubMed] [Google Scholar]
- 101.Rubino C, Corrow SL, Corrow JC, Duchaine B, Barton JJ. Word and text processing in developmental prosopagnosia. Cogn Neuropsychol. 2016;33:5–6. doi: 10.1080/02643294.2016.1204281. [DOI] [PubMed] [Google Scholar]
- 102.Hoover AE, Demonet JF, Steeves JK. Superior voice recognition in a patient with acquired prosopagnosia and object agnosia. Neuropsychologia. 2010;48(13):3725–3732. doi: 10.1016/j.neuropsychologia.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Liu RR, Corrow SL, Pancaroglu R, Duchaine B, Barton JJ. The processing of voice identity in developmental prosopagnosia. Cortex. 2015;71:390–397. doi: 10.1016/j.cortex.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moroz D, Corrow SL, Corrow JC, Barton AR, Duchaine B, Barton JJ. Localization and patterns of Cerebral dyschromatopsia: a study of subjects with prospagnosia. Neuropsychologia. 2016;89:153–160. doi: 10.1016/j.neuropsychologia.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bouvier SE, Engel SA. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cereb Cortex. 2006;16(2):183–191. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- 106.Schmidt D. Neuro-ophthalmological findings in patients with acquired prosopagnosia. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):333–334. doi: 10.1007/s00417-014-2890-1. [DOI] [PubMed] [Google Scholar]
- 107.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 108.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Temple CM. Developmental memory impairment: faces and patterns. In: Campbell R, editor. Mental Lives: Case Studies in Cognition. Oxford, United Kingdom: Blackwell; 1992. pp. 199–215. [Google Scholar]
- 110.Duchaine BC. Developmental prosopagnosia with normal configural processing. Neuroreport. 2000;11(1):79–83. doi: 10.1097/00001756-200001170-00016. [DOI] [PubMed] [Google Scholar]
- 111.Bentin S, Deouell LY, Soroker N. Selective visual streaming in face recognition: evidence from developmental prosopagnosia. Neuroreport. 1999;10(4):823–827. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- 112.Corrow JC, Corrow SL, Lee E, et al. Getting lost: topographic skills in acquired and developmental prosopagnosia. Cortex. 2016;76:89–103. doi: 10.1016/j.cortex.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45(1):32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 114.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 115.Jonas J, Rossion B, Brissart H, et al. Beyond the core face-processing network: intracerebral stimulation of a face-selective area in the right anterior fusiform gyrus elicits transient prosopagnosia. Cortex. 2015;72:140–155. doi: 10.1016/j.cortex.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 116.Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Annu Rev Neurosci. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- 117.Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology. 1982;32(4):331–341. doi: 10.1212/wnl.32.4.331. [DOI] [PubMed] [Google Scholar]
- 118.De Renzi E. Prosopagnosia in two patients with CT scan evidence of damage confined to the right hemisphere. Neuropsychologia. 1986;24(3):385–389. doi: 10.1016/0028-3932(86)90023-0. [DOI] [PubMed] [Google Scholar]
- 119.Mattson AJ, Levin HS, Grafman J. A case of prosopagnosia following moderate closed head injury with left hemisphere focal lesion. Cortex. 2000;36(1):125–137. doi: 10.1016/s0010-9452(08)70841-4. [DOI] [PubMed] [Google Scholar]
- 120.Tzavaras A, Merienne L, Masure MC. Prosopagnosie, amnésie et troubles du langage par lésion temporale gauche chez un sujet gaucher [Prosopagnosia, amnesia and language disorders by left temporal lesion in a left-handed subject] Encephale. 1973;62(4):382–394. French. [PubMed] [Google Scholar]
- 121.Barton JJ. Prosopagnosia associated with a left occipitotemporal lesion. Neuropsychologia. 2008;46(8):2214–2224. doi: 10.1016/j.neuropsychologia.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 122.Yang H, Susilo T, Duchaine B. The anterior temporal face area contains invariant representations of face identity that can persist despite the loss of right FFA and OFA. Cereb Cortex. 2016;26(3):1096–1107. doi: 10.1093/cercor/bhu289. [DOI] [PubMed] [Google Scholar]
- 123.Barton JJ, Press DZ, Keenan JP, O’Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002;58(1):71–78. doi: 10.1212/wnl.58.1.71. [DOI] [PubMed] [Google Scholar]
- 124.Kanwisher N, Barton J. The functional architecture of the face system: Integrating evidence from fMRI and patient studies. In: Rhodes G, Calder A, Johnson M, Haxby JV, editors. Oxford Handbook of Face Perception. Oxford; United Kingdom: 2011. [Google Scholar]
- 125.Barton JJ, Cherkasova M. Face imagery and its relation to perception and covert recognition in prosopagnosia. Neurology. 2003;61(2):220–225. doi: 10.1212/01.wnl.0000071229.11658.f8. [DOI] [PubMed] [Google Scholar]
- 126.Hasson U, Avidan G, Deouell LY, Bentin S, Malach R. Face-selective activation in a congenital prosopagnosic subject. J Cogn Neurosci. 2003;15(3):419–431. doi: 10.1162/089892903321593135. [DOI] [PubMed] [Google Scholar]
- 127.Avidan G, Hasson U, Malach R, Behrmann M. Detailed exploration of face-related processing in congenital prosopagnosia: 2. functional neuroimagining findings. J Cogn Neurosci. 2005;17(7):1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- 128.Hadjikhani N, de Gelder B. Neural basis of prosopagnosia: an fMRI study. Hum Brain Mapp. 2002;16(3):176–182. doi: 10.1002/hbm.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bentin S, DeGutis JM, D’Esposito M, Robertson LC. Too many trees to see the forest: performance, event-related potential, and functional magnetic resonance imaging manifestations of integrative congenital prosopagnosia. J Cogn Neurosci. 2007;19(1):132–146. doi: 10.1162/jocn.2007.19.1.132. [DOI] [PubMed] [Google Scholar]
- 130.Furl N, Garrido L, Dolan RJ, Driver J, Duchaine B. Fusiform gyrus face selectivity relates to individual differences in facial recognition ability. J Cogn Neurosci. 2011;23(7):1723–1740. doi: 10.1162/jocn.2010.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang J, Liu J, Xu Y. Neural decoding reveals impaired face configural processing in the right fusiform face area of individuals with developmental prosopagnosia. J Neurosci. 2015;35(4):1539–1548. doi: 10.1523/JNEUROSCI.2646-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gomez J, Pestilli F, Witthoft N, et al. Functionally defined white matter reveals segregated pathways in human ventral temporal cortex associated with category-specific processing. Neuron. 2015;85(1):216–227. doi: 10.1016/j.neuron.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song S, Garrido L, Nagy Z, et al. Local but not long-range micro-structural differences of the ventral temporal cortex in developmental prosopagnosia. Neuropsychologia. 2015;78:195–206. doi: 10.1016/j.neuropsychologia.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Avidan G, Tanzer M, Hadj-Bouziane F, Liu N, Ungerleider LG, Behrmann M. Selective dissociation between core and extended regions of the face processing network in congenital prosopagnosia. Cereb Cortex. 2014;24(6):1565–1578. doi: 10.1093/cercor/bht007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cereb Cortex. 2007;17(10):2354–2363. doi: 10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- 136.Avidan G, Behrmann M. Functional MRI reveals compromised neural integrity of the face processing network in congenital prosopagnosia. Curr Biol. 2009;19(13):1146–1150. doi: 10.1016/j.cub.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thomas C, Avidan G, Humphreys K, Jung KJ, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12(1):29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Towler J, Eimer M. Electrophysiological studies of face processing in developmental prosopagnosia: neuropsychological and neurodevelopmental perspectives. Cogn Neuropsychol. 2012;29(5–6):503–529. doi: 10.1080/02643294.2012.716757. [DOI] [PubMed] [Google Scholar]
- 140.Eimer M. The face-sensitive N170 component of the event-related brain potential. In: Calder AJ, Rhodes G, Johnson M, Haxby J, editors. The Oxford Handbook of Face Perception. Oxford, United Kingdom: Oxford University Press; 2011. pp. 329–344. [Google Scholar]
- 141.Tanaka JW, Curran T, Porterfield AL, Collins D. Activation of preexisting and acquired face representations: the N250 event-related potential as an index of face familiarity. J Cogn Neurosci. 2006;18(9):1488–1497. doi: 10.1162/jocn.2006.18.9.1488. [DOI] [PubMed] [Google Scholar]
- 142.Gosling A, Eimer M. An event-related brain potential study of explicit face recognition. Neuropsychologia. 2011;49(9):2736–2745. doi: 10.1016/j.neuropsychologia.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 143.Eimer M. Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clin Neurophysiol. 2000;111(4):694–705. doi: 10.1016/s1388-2457(99)00285-0. [DOI] [PubMed] [Google Scholar]
- 144.Dalrymple KA, Oruc I, Duchaine B, et al. The anatomic basis of the right face-selective N170 IN acquired prosopagnosia: a combined ERP/fMRI study. Neuropsychologia. 2011;49(9):2553–2563. doi: 10.1016/j.neuropsychologia.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 145.Prieto EA, Caharel S, Henson R, Rossion B. Early (N170/M170) face-sensitivity despite right lateral occipital brain damage in acquired prosopagnosia. Front Human Neurosci. 2011;5:138. doi: 10.3389/fnhum.2011.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eimer M, McCarthy RA. Prosopagnosia and structural encoding of faces: evidence from event-related potentials. Neuroreport. 1999;10(2):255–259. doi: 10.1097/00001756-199902050-00010. [DOI] [PubMed] [Google Scholar]
- 147.Harris AM, Duchaine BC, Nakayama K. Normal and abnormal face selectivity of the M170 response in developmental prosopagnosics. Neuropsychologia. 2005;43(14):2125–2136. doi: 10.1016/j.neuropsychologia.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 148.Rivolta D, Palermo R, Schmalzl L, Williams MA. Investigating the features of the M170 in congenital prosopagnosia. Front Human Neurosci. 2012;6:45. doi: 10.3389/fnhum.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.de Gelder B, Stekelenburg JJ. Naso-temporal asymmetry of the N170 for processing faces in normal viewers but not in developmental prosopagnosia. Neurosci Lett. 2005;376(1):40–45. doi: 10.1016/j.neulet.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 150.Kress T, Daum I. Event-related potentials reflect impaired face recognition in patients with congenital prosopagnosia. Neurosci Lett. 2003;352(2):133–136. doi: 10.1016/j.neulet.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 151.Lueschow A, Weber JE, Carbon C-C, et al. The 170ms response to faces as measured by MEG (M170) is consistently altered in congenital prosopagnosia. PLoS One. 2015;10(9):e0137624. doi: 10.1371/journal.pone.0137624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Towler J, Gosling A, Duchaine B, Eimer M. The face-sensitive N170 component in developmental prosopagnosia. Neuropsychologia. 2012;50(14):3588–3599. doi: 10.1016/j.neuropsychologia.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 153.Righart R, de Gelder B. Impaired face and body perception in developmental prosopagnosia. Proc Natl Acad Sci U S A. 2007;104(43):17234–17238. doi: 10.1073/pnas.0707753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Minnebusch DA, Suchan B, Ramon M, Daum I. Event-related potentials reflect heterogeneity of developmental prosopagnosia. Eur J Neurosci. 2007;25(7):2234–2247. doi: 10.1111/j.1460-9568.2007.05451.x. [DOI] [PubMed] [Google Scholar]
- 155.Eimer M, Gosling A, Duchaine B. Electrophysiological markers of covert face recognition in developmental prosopagnosia. Brain. 2012;135(2):542–554. doi: 10.1093/brain/awr347. [DOI] [PubMed] [Google Scholar]
- 156.Parketny J, Towler J, Eimer M. The activation of visual face memory and explicit face recognition are delayed in developmental prosopagnosia. Neuropsychologia. 2015;75:538–547. doi: 10.1016/j.neuropsychologia.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 157.Towler J, Fisher K, Eimer M. The cognitive and neural basis of developmental prosopagnosia. Q J Exp Psychol (Hove) 2016:1–29. doi: 10.1080/17470218.2016.1165263. [DOI] [PubMed] [Google Scholar]
- 158.Bate S, Bennetts RJ. The rehabilitation of face recognition impairments: a critical review and future directions. Front Hum Neurosci. 2014;8:491. doi: 10.3389/fnhum.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.DeGutis JM, Chiu C, Grosso ME, Cohan S. Face processing improvements in prosopagnosia: successes and failures over the last 50 years. Front Human Neurosci. 2014;8:561. doi: 10.3389/fnhum.2014.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ellis HD, Young A. Training in face-processing skills for a child with acquired prosopagnosia. Dev Neuropsychol. 1988;4(4):283–294. [Google Scholar]
- 161.DeGutis J, Cohan S, Kahn DA, Aguirre GK, Nakayama K. Facial expression training improves emotion recognition and changes neural tuning in a patient with acquired emotion recognition deficits and prosopagnosia. J Vis. 2013;13(9):993–993. [Google Scholar]
- 162.Davies-Thompson J, Fletcher K, Hills C, Corrow S, Pancaroglu R, Barton JJS. Perceptual training of faces in rehabilitation of acquired prosopagnosia; Paper presented at: 38th European Conference on Visual Percpetion; August 23–27; 2015; Liverpool, United Kingdom. [Google Scholar]
- 163.DeGutis JM, Bentin S, Robertson LC, D’Esposito M. Functional plasticity in ventral temporal cortex following cognitive rehabilitation of a congenital prosopagnosic. J Cogn Neurosci. 2007;19(11):1790–1802. doi: 10.1162/jocn.2007.19.11.1790. [DOI] [PubMed] [Google Scholar]
- 164.DeGutis J, Cohan S, Nakayama K. Holistic face training enhances face processing in developmental prosopagnosia. Brain. 2014;137(Pt 6):1781–1798. doi: 10.1093/brain/awu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bate S, Cook SJ, Duchaine B, Tree JJ, Burns EJ, Hodgson TL. Intranasal inhalation of oxytocin improves face processing in developmental prosopagnosia. Cortex. 2014;50:55–63. doi: 10.1016/j.cortex.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 166.Towler J, Parketny J, Eimer M. Perceptual face processing in developmental prosopagnosia is not sensitive to the canonical location of face parts. Cortex. 2016;74:53–66. doi: 10.1016/j.cortex.2015.10.018. [DOI] [PubMed] [Google Scholar]


