Abstract
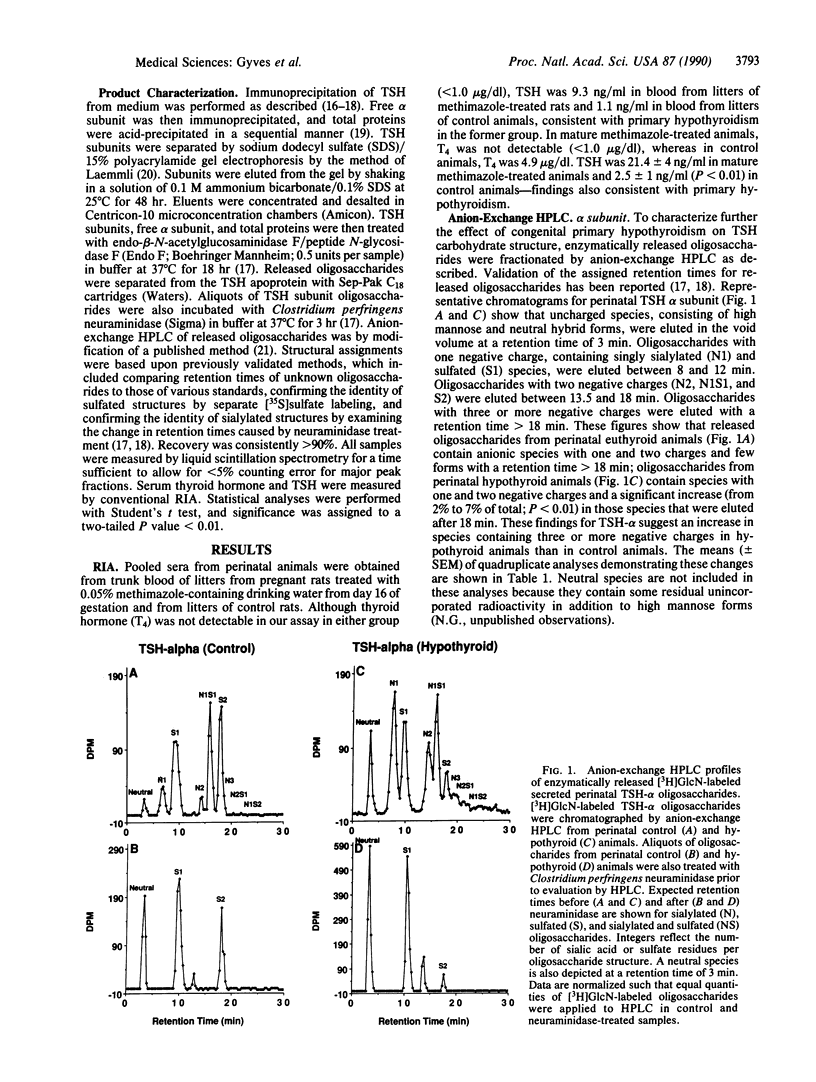
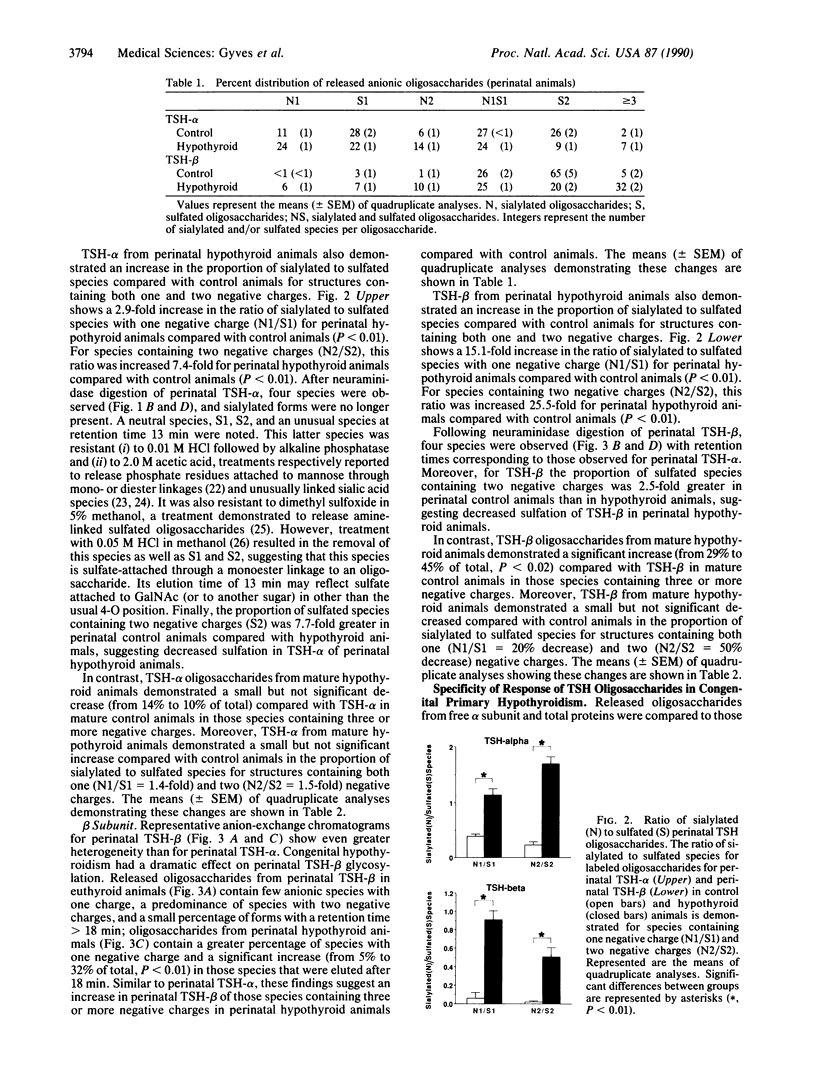
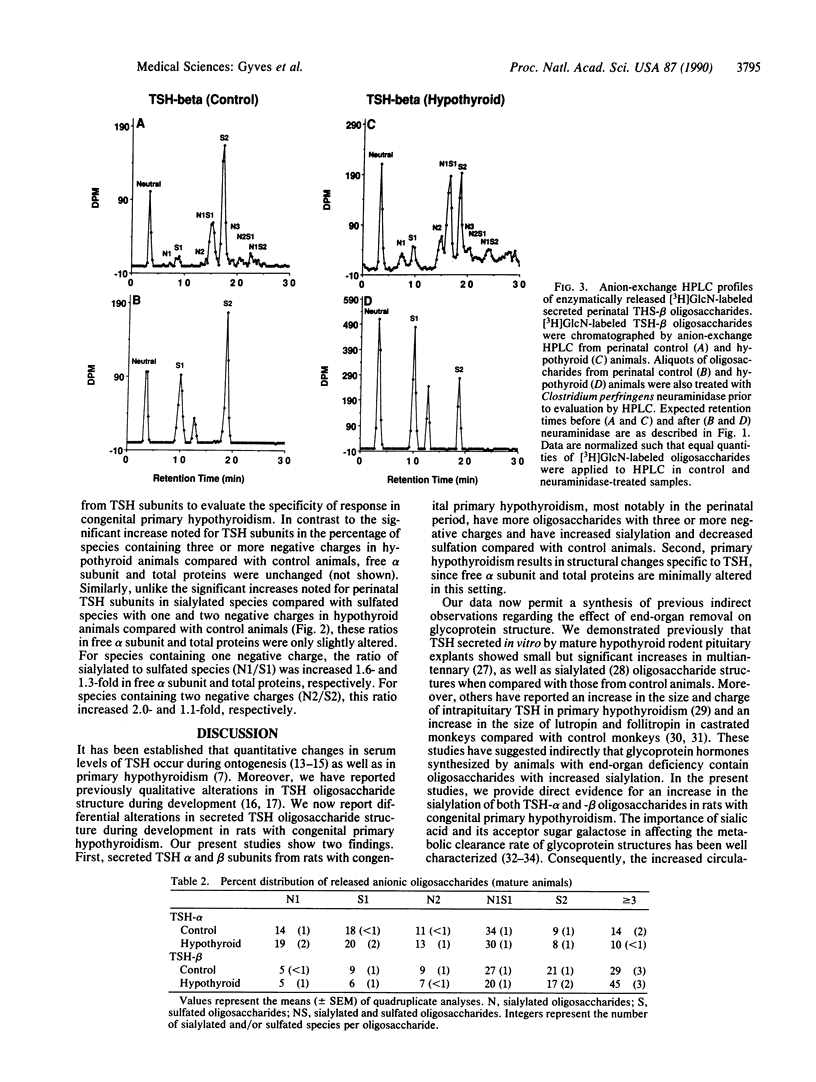
We have examined the oligosaccharide structure of secreted thyrotropin (TSH) in perinatal and mature rats with congenital primary hypothyroidism. Rat pituitaries from euthyroid control animals and those rendered hypothyroid by methimazole treatment were incubated with [3H]glucosamine in vitro. Secreted TSH was purified, and oligosaccharides were enzymatically released and characterized by anion-exchange HPLC. In perinatal hypothyroid animals compared with control animals, oligosaccharides from TSH alpha and beta subunits contained more species with three or more negative charges. Moreover, perinatal hypothyroid animals demonstrated a dramatic increase in the ratio of sialylated to sulfated species within oligosaccharides of the same negative charge (2.9- to 7.4-fold increase for TSH-alpha; 15.1- to 25.5-fold increase for TSH-beta). In mature hypothyroid 9-week-old animals compared with control animals, changes were less pronounced, suggesting that endocrine regulation of oligosaccharide structure is dependent upon the maturational state of the animal. These changes were specific for TSH because glycosylation of free alpha subunit (synthesized by the thyrotroph and gonadotroph) and of total glycoproteins was minimally altered by hypothyroidism. Together, these data provide direct evidence and characterization of specific changes in the structure of a secreted pituitary glycoprotein hormone occurring as a result of in vivo endocrine alterations during early development. Moreover, they provide a potential structural basis to explain the delayed clearance of both TSH and the gonadotropins with end-organ deficiency, which may have important implications for the in vivo biological activities of these hormones. Specifically, such posttranslational changes may be an important adaptive response to prevent the consequences of endocrine deficiency during early development.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir S. M., Kubota K., Tramontano D., Ingbar S. H., Keutmann H. T. The carbohydrate moiety of bovine thyrotropin is essential for full bioactivity but not for receptor recognition. Endocrinology. 1987 Jan;120(1):345–352. doi: 10.1210/endo-120-1-345. [DOI] [PubMed] [Google Scholar]
- Amr S., Menezez-Ferreira M., Shimohigashi Y., Chen H. C., Nisula B., Weintraub B. D. Activities of deglycosylated thyrotropin at the thyroid membrane receptor-adenylate cyclase system. J Endocrinol Invest. 1985 Dec;8(6):537–541. doi: 10.1007/BF03348556. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Natowicz M. Rapid separation of anionic oligosaccharide species by high performance liquid chromatography. Anal Biochem. 1981 Apr;112(2):357–361. doi: 10.1016/0003-2697(81)90305-5. [DOI] [PubMed] [Google Scholar]
- Berman M. I., Thomas C. G., Jr, Manjunath P., Sairam M. R., Nayfeh S. N. The role of the carbohydrate moiety in thyrotropin action. Biochem Biophys Res Commun. 1985 Dec 17;133(2):680–687. doi: 10.1016/0006-291x(85)90958-1. [DOI] [PubMed] [Google Scholar]
- Carr F. E., Burnside J., Chin W. W. Thyroid hormones regulate rat thyrotropin beta gene promoter activity expressed in GH3 cells. Mol Endocrinol. 1989 Apr;3(4):709–716. doi: 10.1210/mend-3-4-709. [DOI] [PubMed] [Google Scholar]
- Constant R. B., Weintraub B. D. Differences in the metabolic clearance of pituitary and serum thyrotropin (TSH) derived from euthyroid and hypothyroid rats: effects of chemical deglycosylation of pituitary TSH. Endocrinology. 1986 Dec;119(6):2720–2727. doi: 10.1210/endo-119-6-2720. [DOI] [PubMed] [Google Scholar]
- DeCherney G. S., Gesundheit N., Gyves P. W., Showalter C. R., Weintraub B. D. Alterations in the sialylation and sulfation of secreted mouse thyrotropin in primary hypothyroidism. Biochem Biophys Res Commun. 1989 Mar 15;159(2):755–762. doi: 10.1016/0006-291x(89)90059-4. [DOI] [PubMed] [Google Scholar]
- Dussault J. H., Labrie F. Development of the hypothalamic-pituitary-thyroid axis in the neonatal rat. Endocrinology. 1975 Nov;97(5):1321–1324. doi: 10.1210/endo-97-5-1321. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Dussault J. H., Foley T. P., Jr, Klein A. H., LaFranchi S., Larsen P. R., Mitchell M. L., Murphey W. H., Walfish P. G. Screening for congenital hypothyroidism: results of screening one million North American infants. J Pediatr. 1979 May;94(5):700–705. doi: 10.1016/s0022-3476(79)80133-x. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Dussault J. H., Sack J., Chopra I. J. Ontogenesis of hypothalamic--pituitary--thyroid function and metabolism in man, sheep, and rat. Recent Prog Horm Res. 1976;33:59–116. doi: 10.1016/b978-0-12-571133-3.50010-6. [DOI] [PubMed] [Google Scholar]
- Gesundheit N., Gyves P. W., DeCherney G. S., Stannard B. S., Winston R. L., Weintraub B. D. Characterization and charge distribution of the asparagine-linked oligosaccharides on secreted mouse thyrotropin and free alpha-subunits. Endocrinology. 1989 Jun;124(6):2967–2977. doi: 10.1210/endo-124-6-2967. [DOI] [PubMed] [Google Scholar]
- Gesundheit N., Magner J. A., Chen T., Weintraub B. D. Differential sulfation and sialylation of secreted mouse thyrotropin (TSH) subunits: regulation by TSH-releasing hormone. Endocrinology. 1986 Aug;119(2):455–463. doi: 10.1210/endo-119-2-455. [DOI] [PubMed] [Google Scholar]
- Green E. D., Baenziger J. U. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. I. Structural elucidation of the sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem. 1988 Jan 5;263(1):25–35. [PubMed] [Google Scholar]
- Green E. D., Baenziger J. U. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. II. Distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem. 1988 Jan 5;263(1):36–44. [PubMed] [Google Scholar]
- Gurr J. A., Kourides I. A. Thyroid hormone regulation of thyrotropin alpha- and beta-subunit gene transcription. DNA. 1985 Aug;4(4):301–307. doi: 10.1089/dna.1985.4.301. [DOI] [PubMed] [Google Scholar]
- Gyves P. W., Gesundheit N., Stannard B. S., DeCherney G. S., Weintraub B. D. Alterations in the glycosylation of secreted thyrotropin during ontogenesis. Analysis of sialylated and sulfated oligosaccharides. J Biol Chem. 1989 Apr 15;264(11):6104–6110. [PubMed] [Google Scholar]
- Gyves P. W., Gesundheit N., Taylor T., Butler J. B., Weintraub B. D. Changes in thyrotropin (TSH) carbohydrate structure and response to TSH-releasing hormone during postnatal ontogeny: analysis by concanavalin-A chromatography. Endocrinology. 1987 Jul;121(1):133–140. doi: 10.1210/endo-121-1-133. [DOI] [PubMed] [Google Scholar]
- Hickman J., Ashwell G., Morell A. G., van den Hamer C. J., Scheinberg I. H. Physical and chemical studies on ceruloplasmin. 8. Preparation of N-acetylneuraminic acid-1-14C-labeled ceruloplasmin. J Biol Chem. 1970 Feb 25;245(4):759–766. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Irvine R. A., Sternlieb I., Scheinberg I. H., Ashwell G. Physical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J Biol Chem. 1968 Jan 10;243(1):155–159. [PubMed] [Google Scholar]
- Mori M., Ohshima K., Fukuda H., Kobayashi I., Wakabayashi K. Changes in the multiple components of rat pituitary TSH and TSH beta subunit following thyroidectomy. Acta Endocrinol (Copenh) 1984 Jan;105(1):49–56. doi: 10.1530/acta.0.1050049. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Inoue Y., Kamata T. Solvolytic desulfation of glycosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1977 Sep;58(1):47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- Nissim M., Lee K. O., Petrick P. A., Dahlberg P. A., Weintraub B. D. A sensitive thyrotropin (TSH) bioassay based on iodide uptake in rat FRTL-5 thyroid cells: comparison with the adenosine 3',5'-monophosphate response to human serum TSH and enzymatically deglycosylated bovine and human TSH. Endocrinology. 1987 Oct;121(4):1278–1287. doi: 10.1210/endo-121-4-1278. [DOI] [PubMed] [Google Scholar]
- Peckham W. D., Knobil E. Qualitative changes in the pituitary gonadotropins of the male Rhesus monkey following castration. Endocrinology. 1976 Apr;98(4):1061–1064. doi: 10.1210/endo-98-4-1061. [DOI] [PubMed] [Google Scholar]
- Peckham W. D., Knobil E. The effects of ovariectomy, estrogen replacement, and neuraminidase treatment on the properties of the adenohypophysial glycoprotein hormones of the Rhesus monkey. Endocrinology. 1976 Apr;98(4):1054–1060. doi: 10.1210/endo-98-4-1054. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Chin W. W., Habener J. F., Ridgway E. C. Transcriptional regulation of the thyrotropin subunit genes by thyroid hormone. J Biol Chem. 1985 Mar 10;260(5):2900–2903. [PubMed] [Google Scholar]
- Slomiany B. L., Slomiany A. Isolation and characterization of the sulfated neolactotetraosylceramide from hog gastric mucosa. J Biol Chem. 1978 May 25;253(10):3517–3520. [PubMed] [Google Scholar]
- Stannard B. S., Gesundheit N., Ronin C., Burnside J., Weintraub B. D. Differential carbohydrate processing and secretion of thyrotropin and free alpha subunit. Effects of 1-deoxynojirimycin. J Biol Chem. 1988 Jun 15;263(17):8309–8317. [PubMed] [Google Scholar]
- Strbák V., Michalicková J. Hypothalamic-pituitary-thyroid system during suckling period in rat and man. Endocrinol Exp. 1984 Sep;18(3):183–196. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Biosynthetic intermediates of beta-glucuronidase contain high mannose oligosaccharides with blocked phosphate residues. J Biol Chem. 1980 Jul 25;255(14):6633–6639. [PubMed] [Google Scholar]
- Taylor T., Gesundheit N., Gyves P. W., Jacobowitz D. M., Weintraub B. D. Hypothalamic hypothyroidism caused by lesions in rat paraventricular nuclei alters the carbohydrate structure of secreted thyrotropin. Endocrinology. 1988 Jan;122(1):283–290. doi: 10.1210/endo-122-1-283. [DOI] [PubMed] [Google Scholar]
- Wondisford F. E., Farr E. A., Radovick S., Steinfelder H. J., Moates J. M., McClaskey J. H., Weintraub B. D. Thyroid hormone inhibition of human thyrotropin beta-subunit gene expression is mediated by a cis-acting element located in the first exon. J Biol Chem. 1989 Sep 5;264(25):14601–14604. [PubMed] [Google Scholar]


