Abstract
In this issue of Blood, Subramaniam et al showed that complement proteins affect platelet activation and tissue factor (TF) procoagulant activity (PCA) in an inferior vena cava (IVC) ligation model of venous thrombosis in mice.1
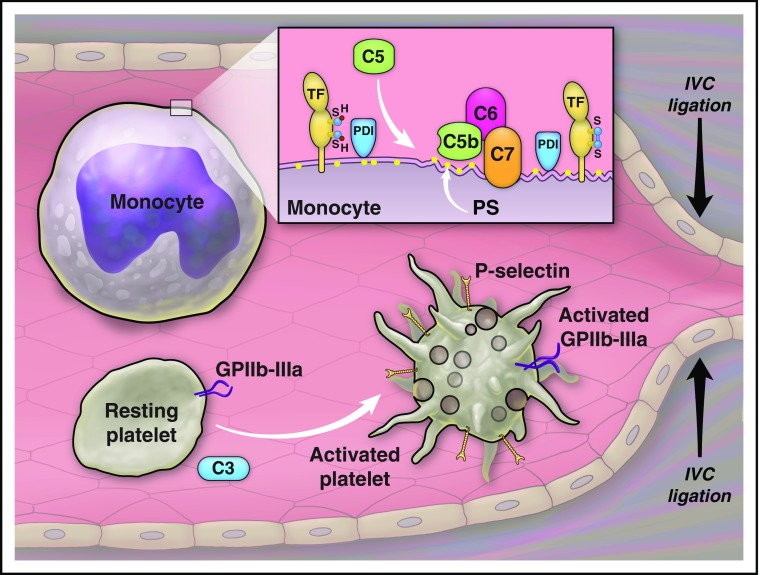
Complement proteins participate in venous thrombosis after ligation of the IVC. C3 has a role in activation of platelets. C5 has a role in activation of TF on leukocytes. The effect of C5 on TF activation depends on the assembly of C5b-7, expression of phosphatidylserine (PS), and activity of protein disulfide isomerase (PDI) on the cell membrane. Illustration by The University of Texas MD Anderson Cancer Center.
The complement system is a component of innate immunity that preceded adaptive immunity during evolution, and comprises a cascade of cysteine proteases similar to the organization of the coagulation cascade. The complement system and hemostatic factors interact at several points during the initiation, propagation, and regulation of complement activation and blood coagulation. Previous studies showed that a complement activation end product, C5b-9 (membrane attack complex), activates platelets,2,3 and C5a (anaphylatoxin) increases expression of TF on leukocytes,4 linking complement activation to hemostasis. More recently, it has been shown that platelets not only become activated as a result of complement activation, but also are able to activate and regulate the complement system via assembling complement-activating protein complexes and expressing or anchoring complement regulatory proteins on their surface.5-7 The interactions between the complement system and hemostatic factors maintain hemostasis under physiologic conditions and promote thrombosis under the pathologic conditions as manifested in thrombotic complications of paroxysmal nocturnal hemoglobinuria and atypical hemolytic syndrome.
In the article, Subramaniam et al advance our understanding about the role of complement proteins in hemostasis by studying platelet function and fibrin deposition in C3−/− and C5−/− mice after ligation of the IVC.
In a prior study on C3−/− mice, we showed a prolongation in the tail-bleeding time and a reduction in platelet aggregation in response to a protease-activated receptor-4 agonist peptide in vitro.8 In their article, Subramaniam et al confirmed prolongation of tail-bleeding time and additionally showed a reduction in the convulxin (GPVI agonist)-induced platelet activation (expression of P-selectin, von Willebrand factor binding, and activation of αIIbβ3 integrin) in C3−/− mice. Interestingly, the authors did not detect any abnormality in the convulxin-induced platelet activation in C5−/− mice, supporting a role for C3, or C3 degradation products, in platelet activation independent of activation of complement proteins downstream to C3 (see figure). The mechanism of the complement activation-independent effect of C3 on platelet aggregation is not known and requires additional studies. Time to the first cessation of bleeding after the tail cut in C5−/− mice was similar to that in strain-matched controls, but a longer total bleeding time and a higher rate of rebleeding in C5−/− mice were consistent with the instability of thrombi in these mice.
Another important finding of this article is that complement proteins participate in venous thrombosis after IVC ligation. A prolonged arteriolar occlusion time after the photochemical injury to the cremasteric microvessels in C3−/− mice was reported previously.8 In the current study, the authors measured thrombus size (weight and length) and the amount of fibrin deposition after IVC ligation in C3−/− mice. Ligation of IVC restricted blood flow by 85% to 90% and resulted in visible thrombi in <30% of C3−/− mice vs in about 80% of wild-type controls. C3 deficiency reduced fibrin deposition and transient and firm adhesion of platelets to the vessel wall, but did not affect leukocyte rolling and firm attachment. C5−/− mice also had smaller thrombi and less fibrin deposition after IVC ligation compared with the wild-type controls; however, adhesion or rolling of platelets and leukocytes was not affected by C5 deficiency. If C5 deficiency does not affect platelet activation or adhesion, then how does it reduce thrombus size? The authors investigated the effect of C5 on coagulation and identified a reduction in the TF procoagulant activity of leukocytes in C5−/− mice. The same group has previously shown that complement activation and expression of PS play important roles in decryption of TF on monocytes induced by antithymocyte globulin (ATG).9 Although C5b-9 was reported to increase PS expression on platelets,10 C9 was not required for the ATG-induced activation of TF on monocytes.9 In their article, the authors confirmed that C5 is important for activation of TF on leukocytes (see figure). C5 did not affect TF expression on monocytes after exposure to lipopolysaccharide (LPS), but was crucial for regulating LPS-induced TF PCA. Blocking C5, C6, and C7, but not C9, reduced TF activity. From these findings, the authors concluded that assembly of C5b-7 is important for TF PCA in thrombosis and inflammation. How does C5 regulate TF activity? In their article, the authors showed that C5 is important for regulation of PS expression on monocytes after IVC ligation. TF activity also depends on PDI activity, and an inhibitor of PDI, rutin, decreased LPS and ATG-induced TF activation. From these observations and the previous report on C5 involvement in the ATG-induced TF activation, one may speculate that, during venous thrombosis, C5 regulates TF activity in a PDI-dependent manner. The authors’ observation that the effect of complement on TF activity is independent of C9, and as a result independent of membrane attack complex deposition, is interesting but raises several important questions, such as how a partial activation of the complement system might be connected to PS expression and PDI activity.
In this study, Subramaniam et al make important observations on the role of complement in venous thrombosis and discover novel interactions between complement proteins and hemostatic factors. Their findings raise important, and yet unanswered, questions that can be answered with additional mechanistic studies.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Subramaniam S, Jurk K, Hobohm L, et al. . Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017;129(16):2291-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polley MJ, Nachman RL. Human complement in thrombin-mediated platelet function: uptake of the C5b-9 complex. J Exp Med. 1979;150(3):633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263(34):18205-18212. [PubMed] [Google Scholar]
- 4.Ritis K, Doumas M, Mastellos D, et al. . A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177(7):4794-4802. [DOI] [PubMed] [Google Scholar]
- 5.Del Conde I, Crúz MA, Zhang H, López JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201(6):871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peerschke EI, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost. 2006;4(9):2035-2042. [DOI] [PubMed] [Google Scholar]
- 7.Hamad OA, Ekdahl KN, Nilsson PH, et al. . Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J Thromb Haemost. 2008;6(8):1413-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gushiken FC, Han H, Li J, Rumbaut RE, Afshar-Kharghan V. Abnormal platelet function in C3-deficient mice. J Thromb Haemost. 2009;7(5):865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer F, Spath B, Fischer C, et al. . Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121(12):2324-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CP, Zhao J, Wiedmer T, Sims PJ. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J Biol Chem. 1993;268(10):7171-7178. [PubMed] [Google Scholar]