Abstract
The tendon enthesis originates from a specific pool of hedgehog-active Gli1+ progenitor cells that differentiate and produce mineralized fibrocartilage. The current study investigated the regenerative capacity of this cell population by comparing the responses of early postnatal and mature entheses to injury. Lineage tracing studies demonstrated that the original Gli1+ cell population had the capacity to heal immature entheses after injury, but this capacity was lost after the cells differentiated into mature fibrochondrocytes. To further examine the involvement of Gli1+ cells and hedgehog signaling in enthesis healing, Gli1 expression was examined via lineage tracing approaches and the effect of Smo deletion was examined in the injured entheses. Immature injured entheses retained high levels of Gli1 expression, a marker of hedgehog activation, consistent with non-injured controls. In contrast, injured mature entheses had few Gli1+ cells early in the healing process, with limited recovery of the cell population later in the healing process. These results suggest that the presence of activated hedgehog signaling in enthesis cells early in the healing process may enhance healing of enthesis injuries by mimicking developmental processes.
KEY WORDS: Enthesis, Fibrocartilage, Hedgehog, Regeneration, Stem cell, Tendon
Summary: Tendon enthesis progenitor cells are capable of healing enthesis injuries in immature mice, in a hedgehog pathway-dependent manner. This capacity is lost in mature animals.
INTRODUCTION
The mechanical function of the tendon-to-bone attachment (the ‘enthesis’) relies on the formation of a functionally graded extracellular matrix. Development of this attachment requires synergy between a number of biochemical signals and cell types (Lu and Thomopoulos, 2013; Thomopoulos et al., 2010). Unfortunately, the mature enthesis heals via a scar-mediated process that does not recapitulate the developmental program, resulting in a mechanically insufficient attachment (Galatz et al., 2015; Thomopoulos et al., 2015; Voleti et al., 2012). This lack of enthesis regeneration during healing results in failed healing clinically (Galatz et al., 2004). However, tendon injuries sustained in utero heal through a regenerative process that mimics normal development (Beredjiklian et al., 2003; Herdrich et al., 2010). Additionally, musculoskeletal injuries in young animals and children heal more readily than in adults (Bullard et al., 2003). Therefore, a better understanding of enthesis healing in immature animals may provide insights to improve healing in mature animals.
Enthesis injuries are typically accompanied by a significant decrease in the mineralized tissue within and underlying the tendon attachment site (Meyer et al., 2004). This loss of mineral contributes to the poor mechanical function of the healed tissue (Meyer et al., 2004). The hedgehog signaling pathway is a master regulator of endochondral mineralization and an attractive therapeutic target for enhanced tendon-to-bone healing. We have recently identified a population of cells in the neonatal enthesis that are positive for Gli1, a transcription factor that is a downstream target of the activated hedgehog (Hh) signaling pathway (Dahmane et al., 1997; Lee et al., 1997) but that in some cases also functions independently of Hh signaling (Aberger and Ruiz, 2014; Palle et al., 2015). This cell population and Hh pathway activation are required for the development of mineralized fibrocartilage in the enthesis (Breidenbach et al., 2015; Liu et al., 2013; Schwartz et al., 2015). However, in mature mineralized fibrocartilage, this cell population terminally differentiates and no longer expresses Gli1, which is likely to reduce the potential for enthesis regeneration after injury.
The current study investigated the potential for the Gli1+ cell population to regenerate enthesis fibrocartilage after injury. A healing process that progresses towards re-creating the natural morphology of the enthesis without first producing disorganized scar tissue is defined as regenerative. A healing process that produces disorganized scar tissue in response to injury is defined as being scar mediated. An enthesis injury model was developed and applied to early postnatal and mature Hh reporter mice. Lineage tracing was used to determine the involvement of Gli1+ progenitor cells in the healing process. The Gli1+ cell population was labeled before generating the injury in one set of experiments to track the participation of this cell lineage in healing, and the Gli1+ cell population was labeled after injury in a second set of experiments to track the potential activation of Gli1 during healing.
RESULTS AND DISCUSSION
Enthesis injury model
A needle punch enthesis injury model was developed and used to create injuries in immature [postnatal day (P)7] and mature (P42 and older) mouse supraspinatus entheses. The injury transected the mineralized enthesis fibrocartilage, including the region populated by the Gli1+ cell population (Fig. 1). Due to the small size of the murine enthesis, cells from adjacent tissues may participate in the healing response. However, these cells were not targeted by Gli1-CreERT2; using the mTmG fluorescent reporter model, infiltrating cells from the bone marrow or other sources could be distinguished from the native enthesis cell population.
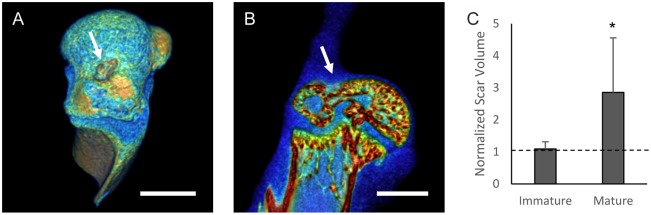
Fig. 1.
Needle punch injury results in reproducible enthesis damage and improved healing in immature entheses. (A) Three-dimensional and (B) cross-sectional views from a representative sample analyzed using microCT to illustrate the enthesis injury (white arrows) in a mature enthesis. The color scale indicates mineral content (blue=low, red=high). Scale bars: 1 mm. (C) The scar volume was significantly larger in mature entheses 3 weeks post injury relative to that of the contralateral non-injured shoulder. No difference in soft tissue volume was observed in the immature enthesis group relative to contralateral controls. *P<0.05, two-tailed paired t-test, mean±s.d., n=9 per group.
To validate the model, needle punch injuries were created in the entheses, animals were allowed to heal for 1-6 weeks, and the humeral heads and supraspinatus tendons were visualized using microcomputed tomography (microCT). Bony defects were apparent in the immature (4 out of 9) and mature (7 out of 9) enthesis groups 3 weeks after injury. If microCT visualization demonstrated that the defect was outside of the enthesis, the sample was excluded from further analysis. There was no difference in soft tissue volume at the enthesis between the injured and contralateral control limbs in immature animals; in contrast, the mature enthesis injury group had significant scar volume compared to contralateral controls (Fig. 1C).
Cells from the Gli1+ progenitor lineage participate in regenerating the immature enthesis
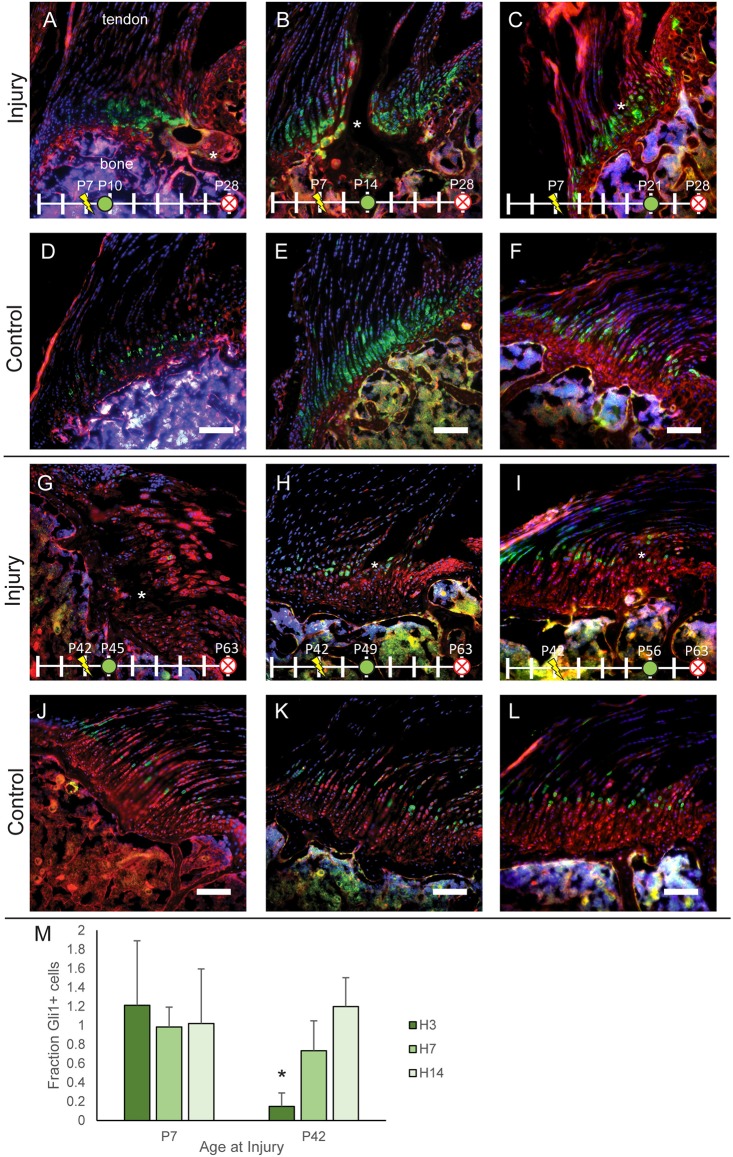
To investigate the role of cells from the Gli1+ lineage during enthesis healing, mice were treated with tamoxifen (TAM) on P4 to activate Gli1-CreERT2 and injuries to the enthesis were generated using a 28 G needle on P7 (immature) or P42 (mature). By labeling the Gli1+ enthesis progenitor cells before injury, the lineage of these cells and their participation in healing could be visualized. Large numbers of Gli1+ cells were observed adjacent to the defect site in the immature group at both 7 and 21 days post injury (Fig. 2A,E; note that healing at these time points was not yet complete, so a void remained at the injury site). Clusters of Gli1+ cells were observed near the borders of the injury, suggesting that these cells either proliferated or migrated to the injury during the healing process. In contrast, very few cells from the Gli1+ lineage were observed in the mature defect sites (Fig. 2B,F). At 21 days of healing (Fig. 2E,F), only a small defect remained in the immature enthesis (∼10% of the diameter of the original injury), with a well-organized enthesis morphology surrounding it. In contrast, the healing mature enthesis consisted of disorganized scar tissue, lacking the columnar arrays of Gli1+ cells seen in the healing immature enthesis. To further explore the behavior of the Gli1+ lineage cells, immunostaining of Ki67 was performed to label proliferating cells. Native enthesis cells do not proliferate rapidly (Dyment et al., 2015; Schwartz et al., 2015); however, some proliferative cells were observed in healing regions of injured enthesis. In immature entheses, proliferating cells corresponded to Gli1+ lineage cells one week after injury (Fig. 2I). In contrast, although many proliferating cells were observed in and around the healing regions of mature entheses, very few of these cells were derived from the Gli1+ lineage and are likely to represent the fibrovascular scar healing response typically seen during adult tendon-to-bone healing (Galatz et al., 2015) (Fig. 2J).
Fig. 2.
The enthesis progenitor population participates in remodeling immature but not mature entheses. Gli1-CreERT2;mTmG mice were injected with TAM on P4 to label the potentially Hh-responsive cell lineage (green) that populates the mature mineralized fibrocartilage. Mice were injured on P7 (A,C,E,G,I) or P42 (B,D,F,H,J) and killed 7 (A-D) or 21 (E-H) days later. Clusters of cells populating the injury regions (*) in A and E are green, indicating that they are part of the original enthesis cell lineage (contralateral controls in C,G). In contrast, cells populating the injury regions (*) in B and F are predominantly red, indicating that they are not derived from the original enthesis cell lineage. To determine the fraction of Gli1 lineage cells that were proliferating, Gli1-CreERT2 mice were crossed with Ai14 mice and stained for Ki67. (I,J) Proliferating cells (green, Ki67) colocalized with Gli1-Cre expression (red) in injured immature entheses (I) but not in mature entheses (J). Arrowheads indicate Ki67+ cells. Scale bars: 100 μm (A-H); 50 μm (I,J). n=5-7 per group. mTmG cells exhibit red fluorescence in the absence of Cre and green fluorescence in presence of Cre. Nuclei are shown in blue. Other colors (e.g. magenta, yellow) are the result of overlapping red, green and/or blue signals.
These results suggest that the cells from the enthesis Gli1+ progenitor lineage, which are established during late embryonic development, retain regenerative capacity through early postnatal time points. Cells from this lineage contributed to healing, but only when the injury was induced before maturation of the enthesis. When the injury was sustained after Gli1 lineage cells had terminally differentiated, these cells were unable to proliferate and participate in regenerating the enthesis. However, as healing in the immature enthesis occurred concurrently with the normal postnatal development and growth of the tissue, it remains unclear whether a regenerative program is initiated due to injury or whether the normal developmental program drives the healing response.
Gli1 expression is activated during early stages to regenerate the enthesis in immature animals
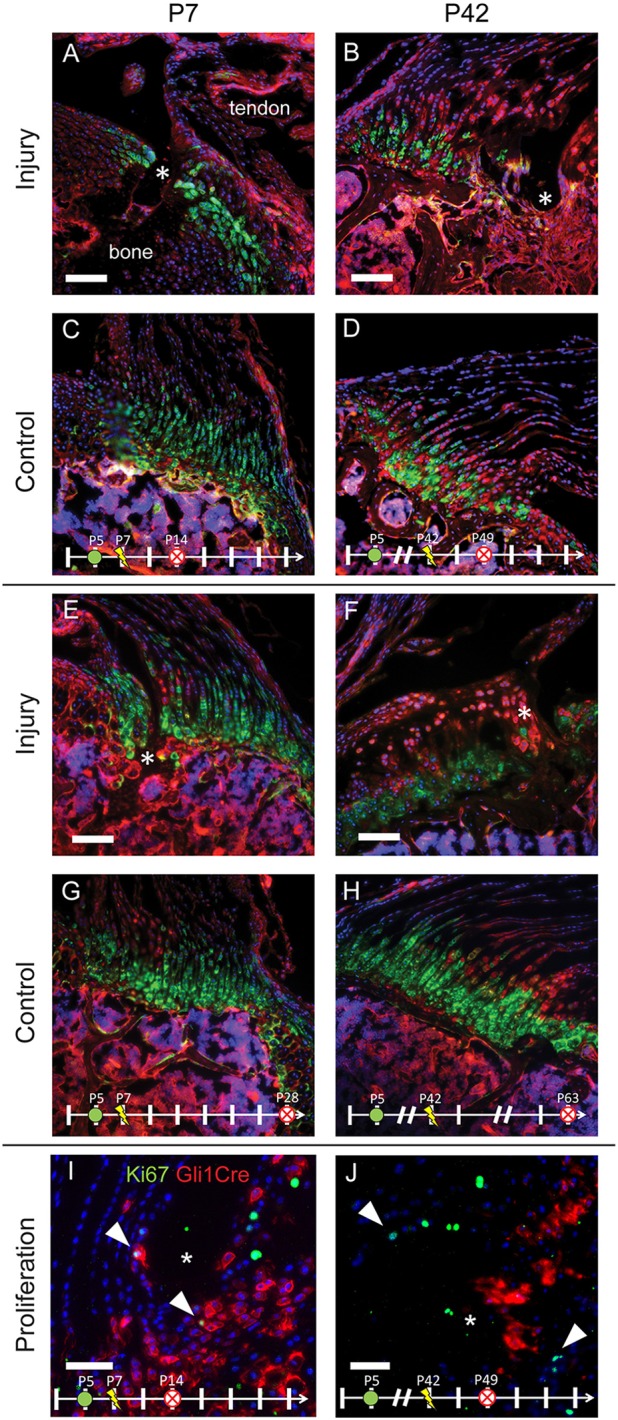
Active Hh signaling is required for the development of functional enthesis fibrocartilage (Schwartz et al., 2015). Furthermore, Ihh protein may be increased during tendon-to-bone healing (Carbone et al., 2015). Using lineage tracing, we investigated whether Gli1 expression, a downstream target of Hh signaling, was activated during enthesis healing and whether signaling patterns differed between immature and mature entheses. To accomplish this, mice from both immature (P7) and mature (P42) enthesis groups were treated with TAM at 3, 7 or 14 days after injury. All tissues were harvested 3 weeks post injury. By labeling the Gli1+ lineage cells after injury, the activation of Hh signaling due to healing could be visualized. In the immature group, Gli1+ lineage cells were observed adjacent to the injury and throughout the enthesis at all time points after injury (Fig. 3A-C). The number of Gli1+ lineage cells was consistent with the degree of labeling in contralateral controls (Fig. 3D-F). This result is consistent with previous results, which showed that Gli1 is expressed throughout the entire region of developing mineralized fibrocartilage during the first 4 weeks of postnatal development (Schwartz et al., 2015). As this cell population is actively forming the enthesis fibrocartilage during the healing time period, it is not surprising that the same cells are capable of participating in healing the injured enthesis. Importantly, the presence of injury did not alter the number of enthesis cells expressing Gli1. Quantification of Gli1+ lineage cell number demonstrated consistently high levels of positive cells throughout the 14 day healing period (Fig. 3M).
Fig. 3.
Gli1 expression is suppressed in mature healing entheses. (A-F) Entheses of Gli1-CreERT2;mTmG mice injured on P7 (A-C) with matching controls shown below (D-F). (G-L) Entheses of Gli1-CreERT2;mTmG mice injured on P42 (G-I) with matching controls shown below (J-L). TAM was injected at 3 (A,G), 7 (B,H) or 14 days (C,I) after injury to induce labeling of the Gli1+ (green) cell population. Clusters of Gli1+ cells were observed near the defect (*) at all time points after injury in immature entheses (A-C), but few Gli1+ cells were observed 3 days post injury in mature entheses. Scale bars: 100 μm. (M) Quantification of the number of Gli1+ cells in the injured enthesis normalized to that of the Gli1+ cells in contralateral controls after 3 (H3), 7 (H7) and 14 (H14) days of healing. *P<0.05 relative to contralateral controls, two-tailed paired t-test, mean±s.d., n=4-7 per group. mTmG cells exhibit red fluorescence in the absence of Cre and green fluorescence in presence of Cre. Nuclei are shown in blue. Other colors (e.g. magenta, yellow) are the result of overlapping red, green and/or blue signals.
In mature entheses, fewer Gli1+ lineage cells were observed 3 days after injury relative to the number present in contralateral controls (Fig. 3G,J). This is in sharp contrast to immature enthesis injuries, where Gli1 expression was maintained at high levels, equal to that of contralateral controls (Fig. 4M). The reduction of Gli1+ lineage cells after injury in mature entheses may be due to changes in the phenotype of the small Gli1+ cell population present in the mature healthy enthesis. Alternatively, these Gli1+ cells could have died and been replaced by infiltrating inflammatory cells or fibroblasts. However, as Gli1− cells were still evident in regions that corresponded to areas of Gli1+ cells in the contralateral controls, this case is unlikely. At 7 and 14 days after injury, Gli1 lineage cells were again observed in the injured enthesis (Fig. 3H,I), reaching levels comparable to those of contralateral controls at 14 days (Fig. 3J). Furthermore, genetic deletion of Smo in tendon and enthesis cells led to impaired healing and a reduction of enthesis cellularity 6 weeks after injury in mature entheses (Fig. S2), demonstrating the requirement of Hh signaling in enthesis healing.
Fig. 4.
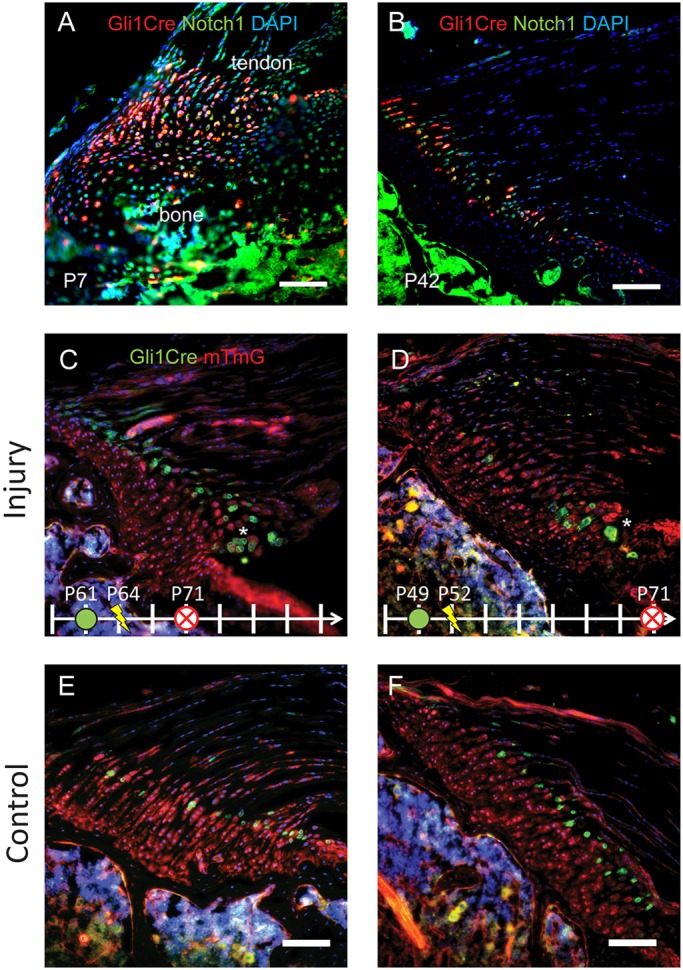
The small remaining Gli1+ cell population in the mature enthesis might retain a regenerative capacity. Notch1 immunostaining (green) was overlaid with expression of Gli1-CreERT2 (red) from P7 (A) and P42 (B) mice. Gli1-CreERT2;Ai14 mice were injected with TAM on P5 (A) or P39 (B) to label the Hh-responsive cell lineage. Notch1 expression was widespread in the early postnatal (P7) enthesis and also colocalized with the Gli1+ cell population (red). Notch1 expression was limited to the non-mineralized fibrocartilage in the mature enthesis and colocalized with the Gli1+ cell population. (C-F) Gli1-CreERT2;mTmG mice were injected with TAM 3 days before injury in order to label the Hh-responsive Gli1 cell lineage (green) in the mature mineralized fibrocartilage. Mice were injured on the indicated day and killed 7 (C) or 21 days (D) later. E and F show uninjured controls from the same animals shown in C and D, respectively. The small Gli1+ cell population that remained in the mature enthesis is evident in panels C and D. Clusters of green cells from this cell lineage in the injury site in panels C and D suggest that these cells proliferated after injury. Scale bars: 100 μm. n=4 per group. Note that Gli1-Cre is indicated in red on A and B and in green in C-F.
The injured mature enthesis retains a small number of Gli1+ lineage cells but is unable to regenerate
The majority of the original enthesis lineage cells terminally differentiate, stop expressing Gli1, and form the mineralized fibrocartilage in the mature enthesis (Dyment et al., 2015; Schwartz et al., 2015). This cell population, identified by Gli1 expression at P6 and their location in the mineralized fibrocartilage, did not contribute to healing injured mature enthesis in the current study. These cells did not express proliferation markers and were replaced by non-Gli1 lineage cells, indicating a scar-mediated healing response. However, there was a small population of Gli1+ cells in mature entheses located at the border between the non-mineralized and mineralized fibrocartilage. These cells could theoretically be activated to participate in repair and regeneration of the enthesis. To explore whether this small cell population had progenitor cell characteristics, immunostaining was performed for Notch1, an important stem cell regulator involved in skeletogenesis (Chen et al., 2014; Mead and Yutzey, 2012). In the mature enthesis, staining of Notch1 was limited to the small number of remaining Gli1+ cells (Fig. 4) and was notably excluded from the fully differentiated cells originating from a Gli1+ lineage in the mineralized fibrocartilage. In contrast, staining of Notch1 was widespread in the early postnatal enthesis and overlapped with the Gil1+ cell population. These results suggest that the small group of Gli1+ cells in the mature enthesis may retain characteristics of skeletal progenitor cells and that they could potentially be exploited in future therapeutic strategies. To probe this potential, mature animals (7-9 weeks old) were injected with TAM 3 days prior to enthesis injury and allowed to heal for 1 or 3 weeks. At both time points, clusters of Gli1+ cells were observed adjacent to the defect site in the enthesis (Fig. 4C,D). This was in contrast to contralateral controls (Fig. 4E,F), which contained mostly well-distributed single Gli1+ cells. Staining of Ki67 as marker for proliferation coincided with these clusters of Gli1+ cells (Fig. S1). Despite the overall scar-mediated healing of injuries in adult entheses, this result suggests that this small Gli1+ cell population might retain the ability to proliferate during the healing process and participate in healing. Future treatment approaches for enthesis injuries could focus on promoting this population of cells while concurrently suppressing the scar-mediated healing response. Furthermore, future studies should evaluate this response in older animals, as the current study used young adult animals for the ‘mature’ group.
Conclusions
The Gli1+ cell lineage, which is crucial for mineralized fibrocartilage development, participates in enthesis remodeling when the injury is sustained during early postnatal development. In the mature enthesis, this cell lineage is present, but the majority of cells do not express Gli1, and these cells lose the capacity to participate in the repair and remodeling of an injury. Furthermore, in immature entheses, the number of Gli1+ cells was unaffected during the healing process. In mature entheses, the number of Gli1+ cells was reduced during the initial healing process. Therefore, healing occurs via different mechanisms in mature entheses compared to immature entheses. The decrease in the size of the Gli1+ cell population at the mineralization front in mature entheses was associated with a decrease in mineralization and impaired healing of the injury, in comparison to the situation observed in younger animals. Temporally controlled activation of the Hh pathway that more closely mimics patterns seen during development could therefore be beneficial to healing.
MATERIALS AND METHODS
Animal model
The use of animals was approved by the Animal Studies Committee at Washington University. To evaluate Hh pathway activity in tendon healing, Gli1-CreERT2 mice (Ahn and Joyner, 2004) were crossed with Rosa26-mT/mG mice or Ai14 mice (Jackson Labs) to enable fluorescent identification of Cre-positive cells and Scx-Cre (Blitz et al., 2009), mice were crossed with Smofl/fl (Long et al., 2001) mice to delete Hh signaling in tendon and enthesis cells (Schwartz et al., 2015). For reporter experiments (Gli1-CreERT2; mT/mG and Gli1-CreERT2; Ai14 mice), a unilateral fibrocartilage injury was generated in animals (n=37 males, n=37 females) at 1 week of age (immature group, n=31) or 6-9 weeks of age (mature group, n=43). For deletion experiments (Scx-Cre;Smofl/fl mice), a unilateral fibrocartilage injury was generated in animals (n=7 males, n=8 females) at P42. Animals were killed 1 or 3 weeks after injury for reporter experiments and 6 weeks after injury for deletion experiments.
Needle punch enthesis injury model
A small incision was made in the right shoulder. The limb was externally rotated to bring the supraspinatus insertion into the surgical field. A needle was inserted into the humeral head to make a punch defect into the supraspinatus enthesis, which completely bisected the mineralized fibrocartilage into the marrow cavity. When establishing the injury model, 28 G needles were used to create injuries at P7 and 23 G needles were used to create injuries at P42. The two sizes of needle were used to account for the different sizes of the humeral heads in P7 compared to P42 animals. Representative results from this injury model are shown in Fig. 1. All subsequent injuries (Figs 2-4; Figs S1-S2) were created with 28 G needles, regardless of animal age. This allowed for better consistency in injury creation and healing evaluation on histologic sections. Furthermore, the use of a smaller needle in the P42 animals reinforces the conclusions of the paper, as the size of the injury was relative to the size of P7 animals, and not scaled to the larger shoulders of the P42 animals. The skin incision was closed with suture and animals were allowed to heal for 1 or 3 weeks.
Tamoxifen labeling
Mice were injected subcutaneously with 100-200 μg/g body weight of TAM to probe Hh activation in a temporally controlled manner: on P4 (to label the Gli1+ enthesis progenitor population prior to injury); or 3 days before, or 3, 7 or 14 days post injury.
MicroCT
Supraspinatus muscle-tendon-humerus samples were isolated, fixed in 4% paraformaldehyde overnight and washed with PBS. Samples were scanned using a Scanco µCT50 instrument. Images were generated using Osirix software (Rosset et al., 2004). Soft tissue volume, spanning a 1 mm axial region centered on the proximal end of the humeral head and aligned with the tendon and shaft of the humerus, was determined using Scanco software. Soft tissue volume for injured entheses was normalized to the volume of the equivalent region in the non-injured contralateral limb.
Histology and immunofluorescence
Samples were decalcified using 14% EDTA, washed with PBS, equilibrated with 30% sucrose and embedded in OCT (Sakura Finetek). 8 µm cryo-sections were prepared and mounted for epifluorescence imaging using Fluoromount II (EMS) to stain nuclei. Immunofluorescence staining was performed using anti-Ki67 (Abcam #16667, 1:200) or anti-Notch1 (Abcam #65297, 1:100) antibodies. Frozen sections were permeabilized with 0.5% Triton X-100, stained with primary antibody in 5-10% serum overnight, incubated with AlexaFluor488 or -647 (Abcam #150077, #150083, 1:400) for 1 h and mounted. At least three slides were imaged from each animal for analysis.
Acknowledgements
Ioannis Kormpakis assisted with animal surgeries and Dan Leib assisted with microCT analysis.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.G.S., S.T.; Methodology: A.G.S., L.M.G., S.T.; Investigation: A.G.S., L.M.G.; Writing - original draft preparation: A.G.S.; Writing - review and editing: A.G.S., L.M.G., S.T.; Funding acquisition: A.G.S., L.M.G., S.T.
Funding
This work was funded by the National Institutes of Health (R01 AR055580, T32 AR060719 and P30 AR057237). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.139303.supplemental
References
- Aberger F. and Ruiz i Altaba A. (2014). Context-dependent signal integration by the GLI code: the oncogenic load, pathways, modifiers and implications for cancer therapy. Semin. Cell Dev. Biol. 33, 93-104. 10.1016/j.semcdb.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S. and Joyner A. L. (2004). Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118, 505-516. 10.1016/j.cell.2004.07.023 [DOI] [PubMed] [Google Scholar]
- Beredjiklian P. K., Favata M., Cartmell J. S., Flanagan C. L., Crombleholme T. M. and Soslowsky L. J. (2003). Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann. Biomed. Eng. 31, 1143-1152. 10.1114/1.1616931 [DOI] [PubMed] [Google Scholar]
- Blitz E., Viukov S., Sharir A., Shwartz Y., Galloway J. L., Pryce B. A., Johnson R. L., Tabin C. J., Schweitzer R. and Zelzer E. (2009). Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861-873. 10.1016/j.devcel.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach A. P., Aschbacher-Smith L., Lu Y., Dyment N. A., Liu C.-F., Liu H., Wylie C., Rao M., Shearn J. T., Rowe D. W. et al. (2015). Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J. Orthop. Res. 33, 1142-1151. 10.1002/jor.22899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard K. M., Longaker M. T. and Lorenz H. P. (2003). Fetal wound healing: current biology. World J. Surg. 27, 54-61. 10.1007/s00268-002-6737-2 [DOI] [PubMed] [Google Scholar]
- Carbone A., Carballo C., Ma R., Wang H., Deng X., Dahia C. and Rodeo S. (2015). Indian hedgehog signaling and the role of graft tension in tendon-to-bone healing: evaluation in a rat ACL reconstruction model. J. Orthop. Res. 34, 641-649. 10.1002/jor.23066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lee B. H. and Bae Y. (2014). Notch signaling in skeletal stem cells. Calcif. Tissue Int. 94, 68-77. 10.1007/s00223-013-9773-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N., Lee J., Robins P., Heller P. and Ruiz i Altaba A. (1997). Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389, 876-881. 10.1038/39918 [DOI] [PubMed] [Google Scholar]
- Dyment N. A., Breidenbach A. P., Schwartz A. G., Russell R. P., Aschbacher-Smith L., Liu H., Hagiwara Y., Jiang R., Thomopoulos S., Butler D. L. et al. (2015). Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol. 405, 96-107. 10.1016/j.ydbio.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatz L. M., Ball C. M., Teefey S. A., Middleton W. D. and Yamaguchi K. (2004). The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Joint Surg. Am. 86, 219-224. 10.2106/00004623-200402000-00002 [DOI] [PubMed] [Google Scholar]
- Galatz L. M., Gerstenfeld L., Heber-Katz E. and Rodeo S. A. (2015). Tendon regeneration and scar formation: the concept of scarless healing. J. Orthop. Res. 33, 823-831. 10.1002/jor.22853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdrich B. J., Danzer E., Davey M. G., Bermudez D. M., Radu A., Zhang L., Zhang Z., Soslowsky L. J. and Liechty K. W. (2010). Fetal tendon wound size modulates wound gene expression and subsequent wound phenotype. Wound Repair Regen. 18, 543-549. 10.1111/j.1524-475X.2010.00615.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Platt K. A., Censullo P. and Ruiz i Altaba A. (1997). Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 124, 2537-2552. [DOI] [PubMed] [Google Scholar]
- Liu C.-F., Breidenbach A., Aschbacher-Smith L., Butler D. and Wylie C. (2013). A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS ONE 8, e65411 10.1371/journal.pone.0065411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F., Zhang X. M., Karp S., Yang Y. and McMahon A. P. (2001). Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 128, 5099-5108. [DOI] [PubMed] [Google Scholar]
- Lu H. H. and Thomopoulos S. (2013). Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 15, 201-226. 10.1146/annurev-bioeng-071910-124656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead T. J. and Yutzey K. E. (2012). Notch signaling and the developing skeleton. Adv. Exp. Med. Biol. 727, 114-130. 10.1007/978-1-4614-0899-4_9 [DOI] [PubMed] [Google Scholar]
- Meyer D. C., Fucentese S. F., Koller B. and Gerber C. (2004). Association of osteopenia of the humeral head with full-thickness rotator cuff tears. J. Shoulder Elbow Surg. 13, 333-337. 10.1016/j.jse.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Palle K., Mani C., Tripathi K. and Athar M. (2015). Aberrant GLI1 activation in DNA damage response, carcinogenesis and chemoresistance. Cancers 7, 2330-2351. 10.3390/cancers7040894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosset A., Spadola L. and Ratib O. (2004). OsiriX: an open-source software for navigating in multidimensional DICOM images. J. Digit. Imaging 17, 205-216. 10.1007/s10278-004-1014-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. G., Long F. and Thomopoulos S. (2015). Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 142, 196-206. 10.1242/dev.112714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S., Genin G. M. and Galatz L. M. (2010). The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing. J. Musculoskelet. Neuronal Interact. 10, 35-45. [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S., Parks W. C., Rifkin D. B. and Derwin K. A. (2015). Mechanisms of tendon injury and repair. J. Orthop. Res. 33, 832-839. 10.1002/jor.22806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti P. B., Buckley M. R. and Soslowsky L. J. (2012). Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng. 14, 47-71. 10.1146/annurev-bioeng-071811-150122 [DOI] [PubMed] [Google Scholar]