Abstract
The prevalence of severe obesity in both the general and the chronic kidney disease (CKD) populations continues to rise, with more than one-fifth of CKD patients in the United States having a body mass index of ≥35 kg/m2. Severe obesity has significant renal consequences, including increased risk of end-stage renal disease (ESRD) and nephrolithiasis. Bariatric surgery represents an effective method for achieving sustained weight loss, and evidence from randomized controlled trials suggests that bariatric surgery is also effective in improving blood pressure, reducing hyperglycemia, and even inducing diabetes remission. There is also observational evidence suggesting that bariatric surgery may diminish the long-term risk of kidney function decline and ESRD. Bariatric surgery appears to be relatively safe in patients with CKD, with postoperative complications only slightly higher than in the general bariatric surgery population. The use of bariatric surgery in patients with CKD might help prevent progression to ESRD or enable selected ESRD patients with severe obesity to become candidates for kidney transplantation. However, there are also renal risks in bariatric surgery, namely, acute kidney injury, nephrolithiasis, and, in rare cases, oxalate nephropathy, particularly in types of surgery involving higher degrees of malabsorption. Although bariatric surgery may improve long-term kidney outcomes, this potential benefit remains unproved and must be balanced with potential adverse events.
Keywords: bariatric surgery, glomerular filtration rate, kidney, morbid obesity, obesity
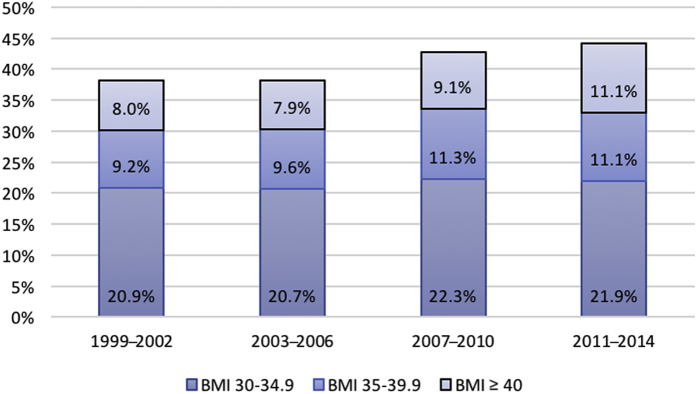
Worldwide, the prevalence of obesity (body mass index [BMI] ≥ 30 kg/m2) in the general population has risen dramatically over the past few decades; this trend is paralleled in the chronic kidney disease (CKD) population.1, 2 Among adults with CKD in the United States, for example, the prevalence of obesity increased from 38.1% in 1999 to 2002 to 44.1% in 2011 to 2014 (P = 0.004 for linear trend) (Figure 1).2 The increase in obesity prevalence occurred primarily in World Health Organization (WHO) class II and III obesity3 (BMI ≥ 35 and ≥ 40 kg/m2, respectively), which increased from 17.2% in 1999 to 2002 to 22.2% in 2011 to 2014 (P = 0.01 for linear trend).
Figure 1.
Trends* in class I, II, and III obesity over time in the US adult chronic kidney disease (CKD) population (National Health and Nutrition Examination Survey [NHANES] 1999−2014). *P values for linear trends over time ≤ 0.01 for body mass index (BMI) ≥ 30, BMI ≥ 35, and BMI ≥ 40 kg/m2.
An increasing prevalence of obesity in the CKD population is of particular concern due to evidence of associations between higher BMI and adverse renal outcomes. In observational studies, obesity has been associated with higher risk of incident CKD and end-stage renal disease (ESRD), as well as nephrolithiasis and renal cell cancer.4, 5, 6, 7 Potential mechanisms explaining the increased risk for CKD and ESRD include obesity-mediated hypertension, insulin resistance, glomerular hyperfiltration, activation of the renin−angiotensin−aldosterone system, inflammation, and adipocytokine dysregulation.8, 9 On the other hand, the risk associated with obesity may be reversible: a randomized controlled trial of patients with type 2 diabetes demonstrated that weight loss decreased the risk of adverse CKD outcomes.10 However, achieving sustained weight loss through lifestyle modification is challenging.
Bariatric surgery is a proven, effective method for sustained weight loss and is becoming more commonplace for patients with morbid or severe obesity. Consensus guidelines from a National Institutes of Health (NIH) conference include BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with obesity-related comorbidity as approved clinical indications for bariatric surgery.11 As a significant proportion of patients with CKD may qualify for bariatric surgery, it is increasingly important to understand the potential benefits and risks of bariatric surgery in regard to kidney function and other outcomes.
Overview of Bariatric Surgery Types
Contemporary bariatric surgery techniques are very effective in achieving sustained weight loss, with total weight loss averaging 20% to 35% of total body weight.12, 13 Several surgical procedures to promote weight loss have been developed over the past few decades. These procedures vary in terms of the amount of gastric surface area restriction, intended nutrient malabsorption, effects on gastrointestinal hormones, weight loss outcomes, and risk of complications (Table 1).14 Initial efforts in bariatric surgery started in the 1970s with the jejunoileal bypass, which was a purely malabsorptive procedure, bypassing most of the small intestine.15 The jejunoileal bypass has since been abandoned due to the high rate of complications, which included deficiency of fat-soluble vitamins, bacterial overgrowth, calcium oxalate nephrolithiasis, and kidney and liver failure.
Table 1.
Comparison of the most common surgical procedures for weight loss
| RYGB | LSG | LAGB | |
|---|---|---|---|
| Weight loss | Highest | Moderate | Lowest |
| Gastric emptying | ↑ or ↓ | ↑ | No change |
| Plasma GLP-1 levels | ↑ | ↑ | No change |
| Plasma PYY levels | ↑ | ↑ | No change |
| Plasma ghrelin levels | Variable | ↑ | ↓ |
| Plasma leptin levels | ↓ | ↓ | ↓ |
| Plasma bile acid levels | ↑ | ↑ | No change |
| Fat malabsorption/fat-soluble vitamin deficiency | ↑ | No change | No change |
| Nephrolithiasis risk | ↑ | No change | No change |
| Diabetes remission | Highest | Moderate | Lowest |
| Short-term complications | Higher | Lower | Lower |
| Need for reoperation | Lower | Lower | Higher |
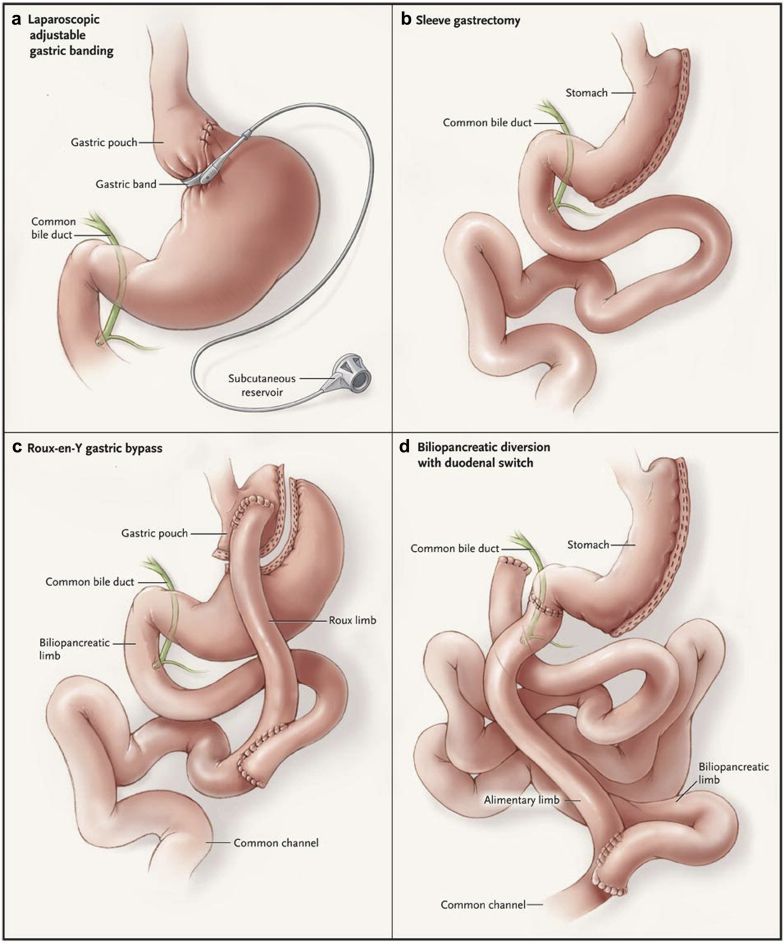
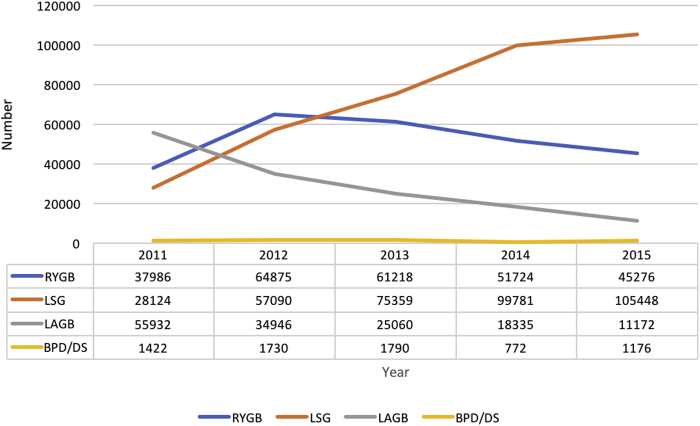
Currently, the most common bariatric procedures performed worldwide are laparoscopic Roux-en-Y gastric bypass (RYGB) and laparoscopic vertical sleeve gastrectomy (LSG).16, 17 The RYGB surgery involves both malabsorption and restriction. First, the stomach is divided into an upper stomach pouch (15−30 ml) and a lower gastric remnant (Figure 2). The stomach pouch is then anastomosed to the mid-jejunum, and a jejuno-jejunal anastomosis is created to reconnect the biliopancreatic limb and the gastric remnant, thereby allowing gastric, pancreatic, and biliary secretions to mix with food in the jejuno-jejunal anastomosis.14 LSG is a restrictive surgery that involves the removal of 70% to 80% of the lateral stomach. Due to its success in achieving weight loss and perhaps better safety profile compared to RYGB, LSG has become more common in the past few years and has eclipsed RYGB in the United States (Figure 3).18 However, some patients who undergo LSG may require subsequent conversion to RYGB or duodenal switch surgery, in which biliopancreatic secretions are diverted from the food until the last portion of the small bowel to increase malabsorption. Reported reasons for conversion of LSG to RYGB or duodenal switch surgery include weight regain and intractable acid reflux.19
Figure 2.
Surgical procedures for weight loss include (a) laparoscopic adjustable gastric banding, (b) sleeve gastrectomy, (c) Roux-en-Y gastric bypass, and (d) biliopancreatic diversion with duodenal switch. See text for details of these procedures.
Reprinted with permission from Maria EJ. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–2183.76
Figure 3.
Estimated number of surgical procedures for weight loss in the United States from 2011 to 2015.
Adapted from the American Society of Metabolic Surgery (ASMBS) estimations.18 BPD/DS, biliopancreatic diversion with duodenal switch; LAGB, laparoscopic-assisted gastric banding; LSG, laparoscopic sleeve gastrectomy; RYGB, Roux-en-y gastric bypass.
Laparoscopic adjustable gastric banding (LAGB) is another purely restrictive procedure that involves the insertion of an adjustable ring immediately below the gastroesophageal junction on the proximal stomach. Due to lower success in achieving weight loss and high risk of reoperation, LAGB has fallen out of favor during the past few years and is now much less commonly performed than RYGB or LSG. All 3 procedures are now almost exclusively done laparoscopically; the proportion of laparoscopic bariatric surgery procedures in a worldwide survey increased from 63% in 2003 to 96% in 2013.16, 20 Unlike gastric banding, RYGB and LSG have favorable effects on various hormones linked to hunger, satiety, and food preferences (Table 1).14 Other procedures that induce more weight loss by increased malabsorption are less commonly performed and include the biliopancreatic diversion with duodenal switch (Figure 2). A variation of the RYGB involves increasing the length of the Roux limb to upwards of 200 cm, resulting in increased malabsorption.21 Although increasing malabsorption results in greater weight loss, risks of nephrolithiasis and oxalate nephropathy may be increased.
Effect of Bariatric Surgery on CKD Risk Factors
Obesity greatly increases the risk of hypertension and diabetes, the 2 most common reported causes of ESRD.22 A systematic review of bariatric surgery studies with long-term follow-up reported remission rates for type 2 diabetes of 66.7% and 28.6% for RYGB and LAGB, respectively.23 In a randomized trial of obese patients with uncontrolled type 2 diabetes who were randomized to either bariatric surgery plus intensive medical therapy or intensive medical therapy alone, bariatric surgery resulted in better glycemic control, defined by achievement of glycated hemoglobin level of 6% or less after 3 years (RYGB, 38%; sleeve gastrectomy, 24%; medical therapy, 5%; P ≤ 0.01 for both comparisons).24 Bariatric surgery also reduced the number of glucose-lowering and antihypertensive medications, and improved quality of life compared to intensive medical therapy (P < 0.05 for all comparisons). Impressive effects on blood pressure have also been reported. Among adolescents undergoing bariatric surgery, elevated blood pressure remitted in 75% of patients.25 In the aforementioned systematic review of bariatric surgery studies with long-term follow-up, remission rates for hypertension were 60.4% for RYGB and 22.7% for LAGB.23
Associations Between Bariatric Surgery and Long-term Risk of Death
Over the long-term, observational studies suggest that bariatric surgery is associated with a 30% to 45% lower risk of death compared to similar patients with severe obesity who did not undergo bariatric surgery.26, 27, 28 Most of the observed reduction in mortality risk is due to lower risks of deaths from cardiovascular disease, diabetes, and cancer. However, caution should be advised in interpreting these observational findings, as they are subject to selection bias: patients who undergo bariatric surgery are likely healthier than their severely obese peers. Patients undergoing surgery go through a rigorous, multistep process that includes evaluations and multiple appointments with a multidisciplinary team, and require successful weight loss prior to bariatric surgery, thereby displaying their commitment and motivation. It is important to note that 1 study found a 58% higher rate of non−disease-related deaths (e.g., accidents, suicide) among patients who underwent bariatric surgery compared to matched controls.27 Further research from randomized trials, if possible, is needed to evaluate whether bariatric surgery provides a long-term mortality benefit as well as to elucidate the long-term risks of surgery.
Bariatric Surgery and Risk of Perioperative and Postoperative Complications
There are nontrivial perioperative and postoperative risks of bariatric surgery, including infection, respiratory failure, acute kidney injury (AKI), and death. Recent reports from a prospective observational cohort study in the United States and an Italian national registry found that modern bariatric surgery mortality rates were approximately 0.3%, similar to those for laparoscopic cholecystectomy.29, 30 In an analysis of 27,736 bariatric surgery patients from 2006 to 2008 in the American College of Surgeons National Surgical Quality Improvement Program, the risk of 30-day mortality was not significantly different across levels of estimated glomerular filtration rate (eGFR) (eGFR ≥ 90, 0.1%; eGFR 60−89, 0.2%; eGFR 30−59, 0.3%; eGFR <30, 0.0%; P = 0.2); however, postoperative complications were more common with lower levels of kidney function (4.6%, 6.1%, 7.7%, 7.5%, and 9.9%, respectively; P < 0.001 for linear trend).31 After multivariate adjustment, each increase in CKD stage was associated with an 18% higher odds of postoperative complications. Another American College of Surgeons National Surgical Quality Improvement Program study examined outcomes in 138 dialysis-dependent ESRD patients who underwent bariatric surgery betweeen 2006 and 2011 (34% LAGB, 49% RYGB, 17% LSG). Reassuringly, this study found that 30-day mortality was relatively low (0.7%) and noted a shift from LABG to LSG in more recent years, similar to overall trends in bariatric surgery.32
Effect of Bariatric Surgery on Measured Kidney Function in Patients With Preserved GFR
Change in kidney function after bariatric surgery was first described in 1980 in a study in which 8 obese patients had GFR measured (mGFR) using [51Cr] ethylenediaminetetraacetic acid (EDTA) before and 12 months after jejunoileal bypass (Table 2).33 Mean unindexed mGFR significantly decreased from 153 to 123 ml/min, but because body surface area (BSA) also decreased from 2.33 m2 to 1.93 m2, there was no change in mGFR indexed to BSA (114 to 110 ml/min/1.73 m2). Similar findings have been seen in other studies measuring GFR or creatinine clearance in individuals with preserved kidney function.34, 35, 36, 37, 38, 39 Since nephron number is fixed at birth, a decrease in unindexed GFR in this high range may be interpreted as improvement in single nephron glomerular hyperfiltration.34, 40 A meta-analysis of surgical weight loss studies found that bariatric surgery reduced unindexed mGFR in patients with normal GFR or high GFR by a mean of 25.6 ml/min.41 In support of a beneficial effect of bariatric surgery on future renal risk, a number of studies and meta-analyses have shown that albuminuria and proteinuria decrease after bariatric surgery, although it is unclear whether this is a direct effect of weight loss or mediated by improvements in blood pressure and insulin resistance.42, 43 Two case reports observed complete resolution of proteinuria after bariatric surgery in patients with obesity-related FSGS.44, 45 However, all of these studies were of short duration (1−2 years), and often lacked comparison groups.
Table 2.
Short-term studies measuring glomerular filtration rate by exogenous filtration markers or creatinine clearance (24-h urine) before and after bariatric surgery
| First author, year | n | Type of surgery (n) | Follow-up (mo) | GFR assessment | Presurgery GFR, CrCl, albuminuria |
Follow-up GFR, CrCl, albuminuria |
|---|---|---|---|---|---|---|
| Patients with normal or increased GFR | ||||||
| Brochner, 1980 | 8 | Intestinal bypass | 12 | mGFR (EDTA) | GFRunindexed 153 ml/min GFRindexed 114 ml/min/1.73 m2 |
GFRunindexed 123 ml/min GFR 110indexed ml/min/1.73 m2 |
| Chagnac, 2004 | 8 | Gastroplasty | 12 | mGFR (inulin) | GFRunindexed 145 ml/min UAE 16 μg/min |
GFRunindexed 110 ml/min UAE 5 μg/min |
| Navarro-Diaz, 2006 | 61 | Gastric bypass | 24 | 24-h CrCl | CrCl 140 ml/min UAE ≥ 30 mg/d 42.6% |
CrCl 118 ml/min UAE ≥ 30 mg/d 14.8% |
| Serpa, 2009 | 140 | RYGB | 8 | CrCl | CrCl 148 ml/min UAE ≥ 30 mg/d 43.6% |
CrCl 114 ml/min UAE ≥ 30 mg/d 21.4% |
| Saliba, 2010 | 35 | RYGB | 12 | CrCl | Diabetic patients: CrCl 155 ml/min UAE 26 mg/d Nondiabetic patients: CrCl 148 ml/min UAE 10 mg/d |
Diabetic patients: CrCl 132 ml/min UAE 15 mg/d Nondiabetic patients: CrCl 117 ml/min UAE 14 mg/d |
| Lieske, 2014 | 11 | RYGB (9), BPD/DS (2) | 12 | mGFR (iothalamate), CrCl | GFRunindexed 121 ml/min GFRindexed 95 ml/min/1.73 m2 CrCl 120 ml/min UAE 20.5 mg/d |
GFRunindexed 90 ml/min GFRindexed 85 ml/min/1.73 m2 CrCl 98 ml/min UAE 17.1 mg/d |
| Friedman, 2014 | 36 | Gastric bypass | Mean 10 | mGFR (iohexol) | GFRunindexed 117 ml/min GFRindexed 87 ml/min/1.73 m2 |
GFRunindexed 100 ml/min GFRindexed 87 ml/min/1.73 m2 |
| Patients with CKD | ||||||
| Navaneethan, 2015 | 15 | RYGB (7), LAGB (3), LSG (3) | 12 | mGFR (iothalamate) | GFRunindexed 82 ml/min GFRindexed 50 ml/min/1.73 m2 Proteinuria 0.60 g/d |
GFRunindexed 81 ml/min GFRindexed 64 ml/min/1.73 m2 Proteinuria 0.43 g/d |
BPD/DS, biliopancreatic diversion with duodenal switch; CKD, chronic kidney disease; CrCl, creatinine clearance; EDTA, ethylenediaminetetraacetic acid; GFR, glomerular filtration rate; LAGB, laparoscopic-assisted gastric banding; LSG, laparoscopic sleeve gastrectomy; mGFR, measured GFR; RYGB, Roux-en-y gastric bypass; UAE, urinary albumin excretion.
Effect of Bariatric Surgery on Measured Kidney Function in Patients With CKD
A limited number of studies have examined the effect of bariatric surgery in patients with CKD. In a study led by Navaneethan et al., 13 patients with serum creatinine ≥ 1.3 mg/dl underwent measurement of GFR using iothalamate clearance before surgery and 3, 6, and 12 months after bariatric surgery (RYGB, 7; LAGB, 3; LSG, 3).47 In contrast to the studies in patients with preserved GFR, unindexed mGFR in this CKD cohort remained unchanged at 12 months (82.0−80.5 ml/min, P = 0.3). When mGFR was indexed to BSA, mGFR actually increased from 50 to 64 ml/min/1.73 m2 over the 12-month period (P = 0.02). Interestingly, increased indexed and unindexed mGFR were significantly associated with decreases in leptin. A randomized trial comparing LSG to optimal medical management was conducted in 11 patients with stage 3 to 4 CKD (LSG, 5; control, 6), but was unable to draw firm conclusions due to small sample size and lack of measured GFR.46
Matched Observational Cohort Studies Examining Long-term Outcomes After Bariatric Surgery
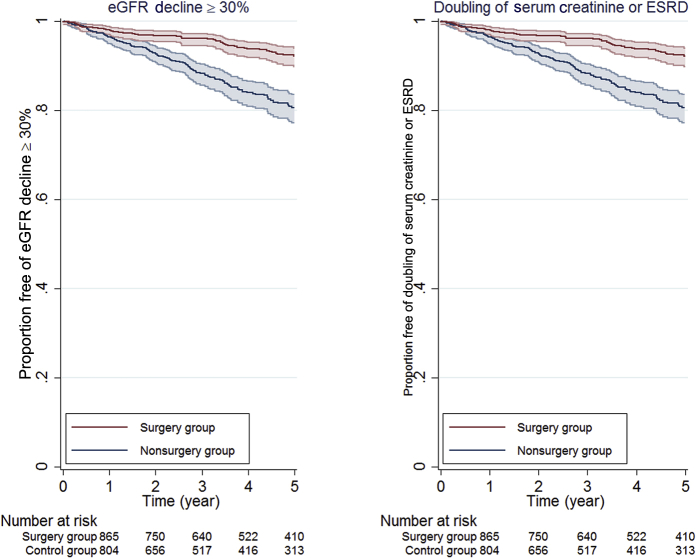
The association between bariatric surgery and kidney outcomes was evaluated in a large health system from central and northeast Pennsylvania with up to 9 years of follow-up.48 Using propensity scores that included demographic, comorbidity, laboratory, and previous nutrition clinic use data, 985 patients who underwent bariatric surgery (97% RYGB) were matched with 985 morbidly obese patients. Bariatric surgery was associated with a 58% lower risk of eGFR decline of ≥ 30% and a 57% lower risk of doubling of serum creatinine or ESRD (Figure 4). The beneficial association of bariatric surgery with kidney outcomes was similar among patients with and without eGFR < 90 ml/min/1.73 m2, hypertension, and diabetes. However, only 91 patients had eGFR <60 ml/min/1.73 m2, limiting power to examine CKD patients. Investigators at Kaiser Permanante reported a similar association between bariatric surgery and improved eGFR outcomes after bariatric surgery (RYGB, 58%; LSG, 42%) in 714 surgery patients and 714 matched controls with stage 3 or 4 CKD.49 Patients who underwent RYGB experienced greater weight loss and greater improvements in eGFR compared to LSG patients.
Figure 4.
Kaplan−Meier curves estimating time to kidney outcomes by surgery group (n = 985) and control group (n = 985). Figure from Chang et al.48 Estimated glomerular filtration (eGFR) decline ≥ 30% outcome was defined as having a follow-up outpatient eGFR ≥ 30% lower than the baseline eGFR value. End-stage renal disease (ESRD) was defined as eGFR < 15 ml/min/1.73 m2 or treated ESRD per US Renal Data System Registry. Shaded areas represent 95% confidence interval bounds.
Filtration Markers in Estimating GFR After Bariatric Surgery
There are important limitations of all observational studies of kidney disease and bariatric surgery, including potential residual confounding and the use of creatinine-based eGFR, which correlates with muscle mass. Loss of muscle mass with massive weight loss might result in overestimation of eGFR after bariatric surgery.35, 36, 50 Friedman et al. measured GFR by iothalamate clearance, serum creatinine, and cystatin C in 33 patients with normal or supranormal kidney function.36 Cystatin C correlated better with mGFR than creatinine both before and after surgery, although neither had very good accuracy in estimating GFR alone. Use of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) combined creatinine-cystatin C equation estimated GFR within 30% of mGFR more than 80% of the time before and after surgery. This suggests that the use of multiple filtration markers could be beneficial in future research studying kidney function changes after bariatric surgery.
Renal Risks of Bariatric Surgery
There exist a number of renal risks of bariatric surgery, including perioperative AKI, and long-term risks of nephrolithiasis and oxalate nephropathy. AKI is fairly common after bariatric surgery, with reports ranging from 2.9% to 8.5% in published studies, which have used varying definitions of AKI.51, 52, 53 Risk factors for AKI after bariatric surgery include higher BMI, lower eGFR, preoperative use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and intraoperative hypotension. Bariatric surgery patients may be prone to dehydration and higher risk for prerenal AKI in the long term, although this risk has not been quantified.
Obesity is a well-recognized risk factor for nephrolithiasis,54, 55 and thus one would hope that weight loss may reduce the risk of nephrolithiasis. However, risk of kidney stones may increase after certain types of bariatric surgery and appears to be related to the degree of fat malabsorption achieved. This complication was well recognized with the jejunoileal bypass: the 15-year risk of developing renal stones was 29%.15 Although less fat malabsorption occurs in RYGB than in the jejunoileal bypass, fecal fat has also been shown to increase 6 and 12 months after RYGB.56 Steatorrhea is thought to result in hyperoxaluria by increasing formation of calcium fatty acids salts, leading to decreased binding of calcium to oxalate, and then increased oxalate absorption.56 Several studies have found that patients who undergo RYGB have greater urinary risk factors for nephrolithiasis after surgery compared to before surgery.21, 57, 58, 59 Urinary oxalate and calcium oxalate supersaturation increase, whereas urinary citrate and total urine volume decrease. Supplementation with calcium citrate is recommended routinely after RYGB surgery60 and would be expected to reduce risk of oxaluria. However, to our knowledge, no data exist on the effect of calcium supplementation on urinary supersaturation for calcium oxalate and risk of nephrolithiasis and oxalate nephropathy.
In an observational study of 762 patients who underwent bariatric surgery and 762 matched nonsurgery patients in Olmsted County, Minnesota, the risk of nephrolithiasis was 11.1% in bariatric surgery patients compared to 4.3% in nonsurgery patients.61 When examined by type of bariatric surgery, the risk of nephrolithiasis was 315% higher for the procedures causing the most malabsorption (biliopancreatic diversion with duodenal switch or very long limb RYGB), and 113% higher for standard RYGB, compared to that in nonsurgery patients. In contrast, restrictive procedures (LAGB or LSG) were not associated with increased risk of nephrolithiasis. This study also found a 96% increased risk of CKD for biliopancreatic diversion with duodenal switch or very long limb RYGB surgeries, as well as a nonsignificant trend toward reduced risk of CKD for RYGB. Important limitations of this study include the use of International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes to ascertain nephrolithiasis and CKD, as well as the possibility that bariatric surgery patients may receive more medical care and thus more recorded diagnoses of nephrolithiasis and CKD than nonsurgery patients.
Oxalate nephropathy is the most severe renal complication of bariatric surgery and has been reported in patients after jejunoileal bypass and RYGB surgery. A case series of 11 patients with oxalate nephropathy after RYGB demonstrated acute and chronic tubulointerstitial nephropathy and calcium oxalate deposits (mean, 3.5 deposits per glomerulus).62 Time from surgery to AKI ranged from 4 to 96 months, and the majority of patients progressed to ESRD. More research is needed to determine how commonly oxalate nephropathy occurs after RYGB, and whether compliance with calcium citrate supplementation can prevent this complication or calcium oxalate nephrolithiasis. Reversal of RYGB may be considered to reduce hyperoxaluria, although it is unclear whether this improves long-term outcomes.63
Bariatric Surgery in ESRD Patients and Kidney Transplantation Candidates
Although policies vary by kidney transplantation center, BMI ≥ 35 to 40 kg/m2 is generally considered a contraindication for transplantation due to worse outcomes compared to those in patients with lower BMI.64 Therefore, bariatric surgery may play an important role in improving access to kidney transplantation for severely obese patients. In a 2004 retrospective study, investigators at the University of Cincinnati reported that RYGB (open, 97%; laparoscopic, 3%) was safe and effective at achieving weight loss in 30 morbidly obese patients with CKD or ESRD.65 Of the 10 patients who were on dialysis before RYGB, 3 patients received a kidney transplant, 4 were scheduled for a living donor transplant, and 3 were on the waitlist. Only 1 complication was reported (abdominal wound infection). There were no perioperative deaths; the only death reported was due to cardiovascular disease, occurring 7.9 years after RYGB and 6.1 years after transplantation.
The same group conducted a prospective study from 2011 to 2014, during which all kidney transplant candidates meeting National Institutes of Health (NIH) criteria for bariatric surgery were referred to a multidisciplinary clinic that included a bariatric surgeon, dietitian, and coordinator.66 These individuals received concurrent workup for laparoscopic LSG and renal transplantation, and were required to undergo a 6-month medical weight loss program, involving monthly physician-supervised visits with the dietitian and bariatric surgery coordinator. Of 170 patients deemed appropriate for both LSG and kidney transplantation, 102 were in evaluation for LSG, and 52 patients (47 dialysis, 5 CKD stage 4) had undergone LSG by the end of 2014. Among the 52 patients who underwent LSG, the mean BMI decreased from 43.0 to 36.4 kg/m2. The majority of patients (55.8%) achieved BMI < 35 kg/m2 and were able to be placed on the waitlist, with 6 patients receiving renal transplants after LSG. The only reported complication was an episode of supraventricular tachycardia, and no perioperative deaths (<30 days) occurred.
Another single-center study reported similar weight loss outcomes with LSG in 9 CKD (5 dialysis) patients but more common postoperative complications.67 The median BMI decreased from 44.2 kg/m2 to 34.7 kg/m2, and 4 of 5 dialysis patients achieved their target weight, enabling them to be placed on the waitlist. One of these listed patients was later made inactive due to infection and gastric leak. Other complications included fistula thrombosis on postoperative day 1, which was stented; AKI due to dehydration 2 weeks postoperatively, requiring hospitalization; and myocardial infarction 3 weeks postsurgery.
Lentine et al. identified Medicare billing claims for bariatric surgery among renal transplant candidates and recipients in the United States Renal Data System registry from 1991 to 2004.68 Of 188 cases of bariatric surgery identified, 72 surgeries were performed pre-listing, 29 while on the waitlist, and 87 posttransplantation. Of 29 waitlisted patients, 20 later proceeded to kidney transplantation after bariatric surgery. Perioperative mortality (30-day) was 3.5% for patients on the waitlist at the time of bariatric surgery and 3.5% for posttransplantation patients. Mortality between 30 and 90 days after bariatric surgery was 3.5% for posttransplantation patients, and 0% for patients on the waitlist at the time of bariatric surgery. However, all of these procedures were open procedures, and most were RYGB surgeries. Because the vast majority of surgeries are now laparoscopic LSG, further research is needed to examine outcomes of transplantation candidates and patients using these methods. Whether the pharmacokinetics of immunosuppressive drugs is altered in the setting of bariatric surgery is another open question and requires further study to draw firm conclusions.69, 70, 71, 72, 73
Conclusions
With the rising prevalence of morbid obesity overall and in the CKD population, there is an urgent need to better understand the effects of bariatric surgery on kidney-related outcomes. Bariatric surgery is extremely effective for achieving weight loss and improving CKD risk factors such as hypertension and diabetes. It also reduces proteinuria and glomerular hyperfiltration, which, in the long term, could impart beneficial effects. In observational studies, bariatric surgery is associated with improvement in creatinine-based renal outcomes, although additional long-term studies are needed using other filtration markers less correlated with body mass. In any case, other risks associated with surgery such as AKI and nephrolithiasis should be weighed before referring patients for surgery. Bariatric surgeries featuring higher malabsorption appear to increase the risk of nephrolithiasis and oxalate nephropathy, and further research is needed to identify those individuals who are at highest risk, as well as effective management strategies.
Disclosure
All the authors declared no competing interests.
Acknowledgments
ARC is supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K23 DK106515-01.
References
- 1.Ng M., Fleming T., Robinson M. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–881. doi: 10.1016/S0140-6736(14)60460-8. Erratum in: Lancet. 2014;384(9945):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang A.R., Appel L.J., Grams M.E. Abstract MP95: Prevalence of general and abdominal obesity in adults with chronic kidney disease: results from NHANES 2007-2012. Circulation. 2015;131(suppl 1):AMP95. [Google Scholar]
- 3.World Health Organization Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 4.Wang Y., Chen X., Song Y. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 5.Hsu C.Y., McCulloch C.E., Iribarren C. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Vivante A., Golan E., Tzur D. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172:1644–1650. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox C.S., Larson M.G., Leip E.P. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 8.Bagby S.P. Obesity-initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol. 2004;15:2775–2791. doi: 10.1097/01.ASN.0000141965.28037.EE. [DOI] [PubMed] [Google Scholar]
- 9.Griffin K.A., Kramer H., Bidani A.K. Adverse renal consequences of obesity. Am J Physiol Renal Physiol. 2008;294:F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 10.Look AHEAD Research Group Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2:801–809. doi: 10.1016/S2213-8587(14)70156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55(2 suppl):615S–619S. doi: 10.1093/ajcn/55.2.615s. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien P.E., McPhail T., Chaston T.B., Dixon J.B. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032–1040. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 13.Brethauer S.A., Hammel J.P., Schauer P.R. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5:469–475. doi: 10.1016/j.soard.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Miras A.D., le Roux C.W. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10:575–584. doi: 10.1038/nrgastro.2013.119. [DOI] [PubMed] [Google Scholar]
- 15.Requarth J.A., Burchard K.W., Colacchio T.A. Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch Surg. 1995;130:318–325. doi: 10.1001/archsurg.1995.01430030088018. [DOI] [PubMed] [Google Scholar]
- 16.Angrisani L., Santonicola A., Iovino P. Bariatric surgery worldwide 2013. Obes Surg. 2015;25:1822–1832. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 17.Esteban Varela J., Nguyen N.T. Laparoscopic sleeve gastrectomy leads the U.S. utilization of bariatric surgery at academic medical centers. Surg Obes Relat Dis. 2015;11:987–990. doi: 10.1016/j.soard.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 18.ASMBS. Estimate of Bariatric Surgery Numbers, 2011-2015. 2016. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed January 12, 2017.
- 19.Felsenreich D.M., Langer F.B., Kefurt R. Weight loss, weight regain, and conversions to Roux-en-Y gastric bypass: 10-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:1655–1662. doi: 10.1016/j.soard.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Buchwald H., Oien D.M. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 21.Lieske J.C., Kumar R., Collazo-Clavell M.L. Nephrolithiasis after bariatric surgery for obesity. Semin Nephrol. 2008;28:163–173. doi: 10.1016/j.semnephrol.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 23.Puzziferri N., Roshek T.B., 3rd, Mayo H.G. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312:934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schauer P.R., Bhatt D.L., Kirwan J.P., STAMPEDE Investigators Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inge T.H., Courcoulas A.P., Jenkins T.M. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374:113–123. doi: 10.1056/NEJMoa1506699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjostrom L., Narbro K., Sjostrom C.D. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 27.Adams T.D., Gress R.E., Smith S.C. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 28.Arterburn D.E., Olsen M.K., Smith V.A. Association between bariatric surgery and long-term survival. JAMA. 2015;313:62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 29.Morino M., Toppino M., Forestieri P. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg. 2007;246:1002–1007. doi: 10.1097/SLA.0b013e31815c404e. [discussion: 1007–1009] [DOI] [PubMed] [Google Scholar]
- 30.Smith M.D., Patterson E., Wahed A.S. Thirty-day mortality after bariatric surgery: independently adjudicated causes of death in the longitudinal assessment of bariatric surgery. Obes Surg. 2011;21:1687–1692. doi: 10.1007/s11695-011-0497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turgeon N.A., Perez S., Mondestin M. The impact of renal function on outcomes of bariatric surgery. J Am Soc Nephrol. 2012;23:885–894. doi: 10.1681/ASN.2011050476. [DOI] [PubMed] [Google Scholar]
- 32.Mozer A.B., Pender J.R., 4th, Chapman W.H. Bariatric surgery in patients with dialysis-dependent renal failure. Obes Surg. 2015;25:2088–2092. doi: 10.1007/s11695-015-1656-0. [DOI] [PubMed] [Google Scholar]
- 33.Brochner-Mortensen J., Rickers H., Balslev I. Renal function and body composition before and after intestinal bypass operation in obese patients. Scand J Clin Lab Invest. 1980;40:695–702. doi: 10.3109/00365518009095584. [DOI] [PubMed] [Google Scholar]
- 34.Chagnac A., Weinstein T., Herman M. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 35.Lieske J.C., Collazo-Clavell M.L., Sarr M.G. Gastric bypass surgery and measured and estimated GFR in women. Am J Kidney Dis. 2014;64:663–665. doi: 10.1053/j.ajkd.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman A.N., Moe S., Fadel W.F. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol. 2014;39:8–15. doi: 10.1159/000357231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarro-Diaz M., Serra A., Romero R. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17(12 suppl 3):S213–S217. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 38.Serpa Neto A., Bianco Rossi F.M., Dal Moro Amarante R. Effect of weight loss after Roux-en-Y gastric bypass, on renal function and blood pressure in morbidly obese patients. J Nephrol. 2009;22:637–646. [PubMed] [Google Scholar]
- 39.Saliba J., Kasim N.R., Tamboli R.A. Roux-en-Y gastric bypass reverses renal glomerular but not tubular abnormalities in excessively obese diabetics. Surgery. 2010;147:282–287. doi: 10.1016/j.surg.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delanaye P., Radermecker R.P., Rorive M. Indexing glomerular filtration rate for body surface area in obese patients is misleading: concept and example. Nephrol Dial Transplant. 2005;20:2024–2028. doi: 10.1093/ndt/gfh983. [DOI] [PubMed] [Google Scholar]
- 41.Navaneethan S.D., Yehnert H., Moustarah F. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afshinnia F., Wilt T.J., Duval S. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25:1173–1183. doi: 10.1093/ndt/gfp640. [DOI] [PubMed] [Google Scholar]
- 43.Li K., Zou J., Ye Z. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS One. 2016;11:e0163907. doi: 10.1371/journal.pone.0163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowler S.M., Kon V., Ma L. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol. 2009;24:851–855. doi: 10.1007/s00467-008-1024-6. [DOI] [PubMed] [Google Scholar]
- 45.Huan Y., Tomaszewski J.E., Cohen D.L. Resolution of nephrotic syndrome after successful bariatric surgery in patient with biopsy-proven FSGS. Clin Nephrol. 2009;71:69–73. doi: 10.5414/cnp71069. [DOI] [PubMed] [Google Scholar]
- 46.Navaneethan S.D., Malin S.K., Arrigain S. Bariatric surgery, kidney function, insulin resistance, and adipokines in patients with decreased GFR: a cohort study. Am J Kidney Dis. 2015;65:345–347. doi: 10.1053/j.ajkd.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacLaughlin H.L., Hall W.L., Patel A.G. Weight loss, adipokines, and quality of life after sleeve gastrectomy in obese patients with stages 3-4 CKD: a randomized controlled pilot study. Am J Kidney Dis. 2014;64:660–663. doi: 10.1053/j.ajkd.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Chang A.R., Chen Y., Still C. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90:164–171. doi: 10.1016/j.kint.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iman TH, Fischer H, Jing B, et al. Estimated GFR before and after bariatric surgery in CKD [e-pub ahead of print]. Am J Kidney Dis. http://dx.doi.org/10.1053/j.ajkd.2016.09.020. [DOI] [PMC free article] [PubMed]
- 50.Chang A.R., Greene T., Wang X. The effects of weight change on glomerular filtration rate. Nephrol Dial Transplant. 2015;30:1870–1877. doi: 10.1093/ndt/gfv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thakar C.V., Kharat V., Blanck S., Leonard A.C. Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol. 2007;2:426–430. doi: 10.2215/CJN.03961106. [DOI] [PubMed] [Google Scholar]
- 52.Weingarten T.N., Gurrieri C., McCaffrey J.M. Acute kidney injury following bariatric surgery. Obes Surg. 2013;23:64–70. doi: 10.1007/s11695-012-0766-1. [DOI] [PubMed] [Google Scholar]
- 53.Abdullah H.R., Tan T.P., Vaez M. Predictors of perioperative acute kidney injury in obese patients undergoing laparoscopic bariatric surgery: a single-centre retrospective cohort study. Obes Surg. 2016;26:1493–1499. doi: 10.1007/s11695-015-1938-6. [DOI] [PubMed] [Google Scholar]
- 54.Taylor E.N., Stampfer M.J., Curhan G.C. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 55.Semins M.J., Shore A.D., Makary M.A. The association of increasing body mass index and kidney stone disease. J Urol. 2010;183:571–575. doi: 10.1016/j.juro.2009.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar R., Lieske J.C., Collazo-Clavell M.L. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149:654–661. doi: 10.1016/j.surg.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park A.M., Storm D.W., Fulmer B.R. A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol. 2009;182:2334–2339. doi: 10.1016/j.juro.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 58.Asplin J.R., Coe F.L. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565–569. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 59.Sinha M.K., Collazo-Clavell M.L., Rule A. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100–107. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 60.Allied Health Sciences Section Ad Hoc Nutrition Committee. Aills L., Blankenship J., Buffington C. ASMBS allied health nutritional guidelines for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4(5 suppl):S73–S108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Lieske J.C., Mehta R.A., Milliner D.S. Kidney stones are common after bariatric surgery. Kidney Int. 2015;87:839–845. doi: 10.1038/ki.2014.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nasr S.H., D'Agati V.D., Said S.M. Oxalate nephropathy complicating Roux-en-Y gastric bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3:1676–1683. doi: 10.2215/CJN.02940608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agrawal V., Khan I., Rai B. The effect of weight loss after bariatric surgery on albuminuria. Clin Nephrol. 2008;70:194–202. doi: 10.5414/cnp70194. [DOI] [PubMed] [Google Scholar]
- 64.Gore J.L., Pham P.T., Danovitch G.M. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357–363. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 65.Alexander J.W., Goodman H.R., Gersin K. Gastric bypass in morbidly obese patients with chronic renal failure and kidney transplant. Transplantation. 2004;78:469–474. doi: 10.1097/01.tp.0000128858.84976.27. [DOI] [PubMed] [Google Scholar]
- 66.Freeman C.M., Woodle E.S., Shi J. Addressing morbid obesity as a barrier to renal transplantation with laparoscopic sleeve gastrectomy. Am J Transplant. 2015;15:1360–1368. doi: 10.1111/ajt.13116. [DOI] [PubMed] [Google Scholar]
- 67.MacLaughlin H.L., Hall W.L., Patel A.G., Macdougall I.C. Laparoscopic sleeve gastrectomy is a novel and effective treatment for obesity in patients with chronic kidney disease. Obes Surg. 2012;22:119–123. doi: 10.1007/s11695-011-0448-4. [DOI] [PubMed] [Google Scholar]
- 68.Modanlou K.A., Muthyala U., Xiao H. Bariatric surgery among kidney transplant candidates and recipients: analysis of the United States renal data system and literature review. Transplantation. 2009;87:1167–1173. doi: 10.1097/TP.0b013e31819e3f14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan G., Garneau P., Hajjar R. The impact and treatment of obesity in kidney transplant candidates and recipients. Can J Kidney Health Dis. 2015;2 doi: 10.1186/s40697-015-0059-4. 26-015-0059-4. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szomstein S., Rojas R., Rosenthal R.J. Outcomes of laparoscopic bariatric surgery after renal transplant. Obes Surg. 2010;20:383–385. doi: 10.1007/s11695-009-9969-5. [DOI] [PubMed] [Google Scholar]
- 71.Arias R.H., Mesa L., Posada J.G., Velez J.P. Kidney transplantation and gastric bypass: a better control of comorbidities. Obes Surg. 2010;20:851–854. doi: 10.1007/s11695-010-0165-4. [DOI] [PubMed] [Google Scholar]
- 72.Golomb I., Winkler J., Ben-Yakov A. Laparoscopic sleeve gastrectomy as a weight reduction strategy in obese patients after kidney transplantation. Am J Transplant. 2014;14:2384–2390. doi: 10.1111/ajt.12829. [DOI] [PubMed] [Google Scholar]
- 73.Rogers C.C., Alloway R.R., Alexander J.W. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281–291. doi: 10.1111/j.1399-0012.2007.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J.F., Lai D.D., Ni B., Sun K.X. Comparison of laparoscopic Roux-en-Y gastric bypass with laparoscopic sleeve gastrectomy for morbid obesity or type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Can J Surg. 2013;56:E158–E164. doi: 10.1503/cjs.026912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tice J.A., Karliner L., Walsh J. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885–893. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 76.DeMaria E.J. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–2183. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]