ABSTRACT
Among a broad range of hypotheses on the molecular nature of transmissible spongiform encephalopathy or scrapie agents discussed in 1960s was a hypothesis of self-replicating polysaccharides. While the studies of the past 40 years provided unambiguous proof that this is not the case, emerging evidence suggests that carbohydrates in the form of sialylated N-linked glycans, which are a constitutive part of mammalian prions or PrPSc, are essential in determining prion fate in an organism. The current extra-view article discusses recent advancements on the role of N-linked glycans and specifically their sialylation status in controlling prion fate. In addition, this manuscript introduces a new concept on the important role of strain-specific functional carbohydrate epitopes on the PrPSc surface as main determinants of strain-specific biologic features. According to this concept, individual strain-specific folding patterns of PrPSc govern selection of PrPC sialoglycoforms expressed by a host that can be accommodated within particular PrPSc structures. Strain-specific patterns of functional carbohydrate epitopes formed by N-linked glycans on PrPSc surfaces define strain-specific biologic features. As a constitutive part of PrPSc, the individual strain-specific patterns of carbohydrate epitopes propagate faithfully within a given host as long as individual strain-specific PrPSc structures are maintained, ensuring inheritance of strain-specific biologic features.
KEYWORDS: carbohydrate epitopes, microglia, N-linked glycans, prion, prion diseases, sialylation, sialic acid, secondary lymphoid organs
Among a broad range of ideas on the molecular nature of transmissible spongiform encephalopathy or scrapie agents discussed in 1960s was a hypothesis of self-replicating poly-saccharides.1 The studies of the past 40 years provided unambiguous proof that the scrapie agents or prions consist of misfolded, self-replicating states of a sialoglycoprotein called the prion protein or PrPC.2 Nevertheless, emerging evidence suggests that carbohydrates in the form of sialylated N-linked glycans, which are constitutive part of prions or PrPSc, are essential in determining prion fate in an organism. While the fact that PrPSc N-linked glycans are sialylated has been known for more than 30 years,3 little has been discovered about the role sialylation plays in prion diseases until recently.
Our recent studies demonstrated that PrPSc with reduced sialylation status does not transmit prion disease to wild type animals.4,5 For producing PrPSc with reduced sialylation, Protein Misfolding Cyclic Amplification with beads (PMCAb) was conducted using PrPC as a substrate that was partially desialylated by treatment with bacterial sialidases (dsPMCAb). All animals inoculated intracranially or intraperitoneally with brain-derived 263K developed scrapie with substantial amounts of PrPSc found in their brains and spleens. Remarkably, no animals inoculated intracranially or intraperitoneally with dsPMCAb-derived 263K developed the disease.4,5 Moreover, no PrPSc was detected in brains or spleens of animals from these groups by Western blot or by calibrated serial PMCAb that can detect a single PrPSc particle,6 arguing that these animals were free of scrapie.4,5 Control groups of animals inoculated with PMCAb-derived 263K developed disease at slightly longer incubation times relative to the control group that received brain-derived PrPSc. Such delay is attributed to a moderate shift in the sialylation pattern of PMCAb-derived 263K relative to that of brain-derived 263K.4 In the course of PMCAb, PrPC molecules with reduced sialylation status were preferentially recruited into PrPSc, producing a shift in sialylation status of PMCAb-derived material.4 In subsequent experiments, trafficking of brain-, PMCAb-, and dsPMCAb-derived PrPSc to secondary lymphoid organs was monitored after intraperitoneal administration. Colonization and replication of prions in secondary lymphoid organs, including spleen and lymph nodes, typically occur before and are important for neuroinvasion (reviewed in.7,8). While brain- and PMCAb-derived PrPSc were found in spleen and lymph nodes, dsPMCAb-derived PrPSc was targeted predominantly to the liver.5 In summary, sialylation was found to be critical for effective trafficking of PrPSc to SLOs and infecting a host, whether it is administered via itraperitoneal or intracranial routes.
To establish a cause-and-effect relationship between sialylation of PrPSc and its infectivity and to test whether this effect is limited to 263K or not, the sialylation status of a strain of synthetic origin SSLOW generated in our laboratory9,10 was altered using a similar approach. First, the sialylation status of SSLOW PrPSc was reduced by replicating brain-derived SSLOW in serial PMCAb using sialidase-treated PrPC and then restored to the original levels by replicating using non-treated PrPC (rsPMCAb). Remarkably, all animals that received PMCAb- or rsPMCAb-derived products with original or restored sialylation levels, respectively, were infected and showed PrPSc in brains and spleens.11 None of the animals that received PrPSc with reduced sialylation (dsPMCAb) were infected. Again, the brains and spleens of animals from the dsPMCAb group were completely cleared of prions, as assessed by Western blot and quantitative serial PMCAb.11 Analysis of SSLOW PrPSc purified from brain and PMCAb/dsPMCAb/rsPMCAb reactions using FTIR revealed that the structures of PMCAb-, dsPMCAb-, and rsPMCAb-derived SSLOW were indistinguishable as much as this technique can detect, whereas only dsPMCAb SSLOW with reduced sialylation failed to infect the animals.11 Similarly, FTIR analysis of PMCAb- and dsPMCAb-derived 263K revealed that their structures were also indistinguishable, whereas only dsPMCAb-263K failed to induce disease.5 In summary, these studies revealed that prion infectivity could be switched off and on in a reversible manner via altering the sialylation status of PrPSc.
Our work suggests that sialylation of PrPSc is one of the key determinants underlying prion infectivity. Several hypotheses could explain the observed effects.12 According to one hypothesis, terminal sialic acid residues are required for PrPSc trafficking to SLOs and its long-term stability in the periphery and CNS.12 An alternative hypothesis proposes that it is the lack of terminal sialylation, i.e. exposed terminal galactose, that controls the life-time of PrPSc and its trafficking.12 Thus, asialo-PrPSc displays an “eat me” signal in the form of exposed galactose, which is recognized by professional and non-professional macrophages, including Kupffer cells and microglia.13,14 According to the first hypotheses, the interacting partners of PrPSc involve proteins that recognize sialic acid residues, whereas the second hypothesis assumes that galactose-binding proteins are in control of PrPSc fate. A third possibility that both PrPSc life-time and trafficking are controlled by several classes of carbohydrate-binding proteins, including those that recognize sialic acids, galactose and possibly other carbohydrate residues found in PrPSc N-linked glycans (fucose, sulfate, mannose), should not be ignored.
In previous studies, highly infectious PrPSc was produced in vitro using recombinant PrP and co-factors.15,16 Because entire N-linked glycans were missing in recombinant PrPSc, the “eat me” signal in the form of exposed galactose was also absent, preventing identification of recombinant PrPSc by the innate immune system in the same manner as it might deal with asialo-PrPSc with displays an “eat me” signal. Upon inoculation, recombinant PrPSc recruits glycosylated PrPC expressed by the host, and therefore the fate of PrPSc seeded in vivo by recombinant PrPSc is likely to depend on its sialylation status.
Regardless of the specific mechanism behind the relationship between PrPSc sialylation and infectivity, our studies highlighted the important role of N-linked glycans in determining PrPSc fate. In intact PrPSc particles, the N-linked glycans are directed outwards exposing terminal residues that are accessible for interaction with other proteins. The range of proteins with which PrPSc could potentially interact is determined by the structural diversity of the functional carbohydrate epitopes on the PrPSc surface, which could be generated via several means. First, there are 2 types of sialic acid residues in mammals: N-acetylneuraminic acid or Neu5Ac and N-glycolylneuraminic acid or Neu5Gc.17 Humans and ferrets can synthesize only Neu5Ac, whereas the rest of mammalian species synthetize both Neu5Ac and Neu5Gc, with predominantly Neu5Ac produced in CNS and both Neu5Ac and Neu5Gc produced in peripheral tissues.12 Second, in PrPSc sialic acid residues can be attached to galactose via α2–3 or α2–6 linkages.18 The linkage type contributes to recognition specificity and selectivity by carbohydrate-binding proteins. Third, several natural modifications including O-acetyl, N-glycolyl, O-lactyl, O-sulfate, O-phosphate, hydroxyl, or O-methyl were found at several carbon positions in sialic acid residues.19 Whether any of these modifications are present in PrPSc has not been investigated; nevertheless, structural diversity of surface epitopes could be multiplied by these modifications. Fourth, combinations of sialic acid residues with other neighboring carbohydrate groups, including fucose and sulfate, result in a range of functional epitopes, some of which are found in PrPSc.18,20 Fifth, the N-linked glycans of PrPSc exhibit several types of branching patters including bisected and nonbisected bi-, tri-, and tetraantennary types that determine the density of sialic acid and other residues on the surface of PrPSc particles.18,20,21 (Fig 1A). Tetraantennary structures that accommodate up to 4 sialic acid residues per glycan are the most branched glycans that were assigned to PrPC/PrPSc.18,20,21. However, the fact that up to 5 sialic acid residues per glycan were found using mass spectrometry analysis21 and even higher number of sialic acid residues per glycan is predicted from analysis by 2D Western blots opens the possibility that a small fraction of PrPC/PrPSc is modified with more complex and/or unconventional N-glycans (Fig 1B). Structural analysis of PrP N-linked glycans conducted almost 30 y ago using mass spectrometry described over 400 different PrP glycoforms.18 The actual diversity of glycoforms could be even greater considering that the assignment to specific structures could only be made for the glycans known at that time and that numerous new glycan structures including unconventional structures have been identified since then. However, not all PrPC glycoforms are recruited proportionally into PrPSc. The size of N-linked glycans and electrostatic repulsion between sialic acid residues at the glycan terminal positions impose spatial and electrostatic constraints that control glycoform ratios and sialylation status within PrPSc in a strain-specific manner.4,22 (Fig 2A).
FIGURE 1.
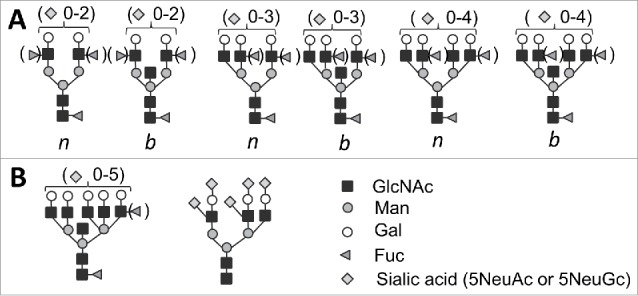
(A) Structures of bi-, tri-, and tetraantennary N-linked glycans found in PrPC and PrPSc that could be bisected (b) or nonbisected (n).18,20,21 Facultative fucosialtion and sialylation are shown within parenthesis with several sialic acid residues per glycan indicated. (B) Structures of pentatantennary and nonconventional N-liked glycans that can accommodate up to 5 sialic acid residues.28
FIGURE 2.
(A) Schematic diagram illustrating that PrPSc strains recruit PrPC isoforms selectively according to PrPC glycosylation and sialylation status (adopted from12). Strain #1 recruits sialoglycoforms of PrPC without noticeable preferences. Hypersialylated and diglycosylated PrPC are preferentially excluded from the strain #2 and even more so from the strain #3. As a result, the proportion of hyper- versus hyposialylated molecules within PrPSc (illustrated by the 2D Western blots), as well as the ratios of di-, mono- and unglycosylated glycoforms (shown on 1D Western blots on right hand side), changes in a strain-specific manner. PrPC molecules are shown as blue circles and sialic acid residues - as red diamonds. (B) Schematic diagram illustrates that species-specific amino acid sequences of PrPC determine the range of strain-specific folding patterns, which define the strain-specific patterns of functional carbohydrate epitopes on PrPSc surfaces within a particular host. Patterns of functional carbohydrate epitopes determine strain-specific biologic properties. PrPSc folding patterns are expected to be quite different for 2 groups of strains: (i) a group that can accommodate diglycosylated glycoforms and (ii) another group that selectively excludes diglycosylated glycoforms.
Here we propose a new hypothesis on the flow of information from species-specific amino acid sequences of PrPC to functional carbohydrate epitopes on the PrPSc surface that define strain-specific biologic properties. As was proposed previously, species-specific amino acid sequences of PrPC define the range of strain-specific folding patterns of PrPSc accessible for individual sequences (Fig 2B).23 Individual folding patterns govern selection of sialoglycoforms that can be accommodated within strain-specific PrPSc structures and, as a result, define the strain-specific pattern of functional carbohydrate epitopes on PrPSc surfaces (Fig 2B). Using this information, prion strains could be classified into 2 large categories. The first one includes strains with minimal structural constraints. Strains of this group are predominantly diglycosylated, as their PrPSc structures can accommodate diglycosylated and highly sialylated PrP molecules (for instance, the majority of hamster strains and variant Creutzfieldt-Jakob Disease, Figure 2B). The second group exhibits much greater structural constraints. Strains of this group are predominantly monoglycosylated, as they selectively exclude diglycosylated and highly sialylated PrP molecules (the majority of mouse strains and sporadic Creutzfieldt-Jakob Disease, Figure 2B).22 PrPSc folding patterns are expected to be quite different for each of the above groups. In addition, because the range of N-linked glycans synthetized in different hosts is likely to be species-specific, the result of selection of PrPC sialoglycoforms is not only determined by the constraints imposed by strain-specific PrPSc structures, but also shaped by the specific host. To illustrate, transmission of the same strain to hamsters and transgenic mice expressing hamster PrPC might result in different patterns of functional carbohydrate epitopes. Moreover, we speculate that other characteristics of N-linked glycans, including their size, branching pattern, and the presence of other charged groups, such as sulfate, contribute to strain-specific selection of PrPC glycoforms. Nevertheless, as a result of selective recruitment, unique strain-specific patterns of functional carbohydrate epitopes are formed on the surface of PrPSc particles. As a constitutive part of PrPSc, the strain-specific patterns of functional carbohydrate epitopes propagate faithfully as long as the host and individual strain-specific PrPSc structures are maintained, ensuring inheritance of strain-specific biologic features. Functional carbohydrate epitopes on the PrPSc surface determine the range of carbohydrate-binding molecules that can interact with PrPSc and, as a result, define the strain-specific biologic features, including cell-, neuro- and lymphotropisms. Several dozen if not hundreds of proteins that specifically recognize carbohydrate groups have been identified, including siglecs, selectins, galectins, asialoglycoprotein receptors, mannose receptors, and complement factors (reviewed in.24,25). Because the majority of carbohydrate-binding molecules have multivalent binding sites, the strength and selectivity of binding depends not only on the composition of functional carbohydrate epitopes but also their density and specific configuration of carbohydrate groups. To summarize, with the guidance of a strain-specific PrPSc template, the information encoded in the amino acid sequence of PrPC is transformed into self-replicating patterns of functional carbohydrate epitopes on the PrPSc surface that define strain-specific biologic properties.
Many questions need to be addressed in future studies. What factors controls sialylation of PrPSc? How can one manipulate PrPSc sialylation status? In previous studies, modulating the activity of sialyltransferases was found to be more effective than targeting sialidases for modulating the sialylation status of PrPC.26 Moreover, in secondary lymphoid organs, PrPSc was shown to be subject to enhanced post-conversion sialylation that might ensure extra-protection from the innate immune system.27 Therefore, identification of sialyltransferases responsible for sialylation of PrPC and PrPSc might represent an important future goal. Additionally, does the difference in sialic acids expressed in human and other mammals contribute to the mammal-to-human transmission barrier? Does the age-dependent decline in sialic acid content contribute to the etiology of sporadic prion diseases? Moreover, the hypothesis on functional carbohydrate epitopes has to be tested. Can we decode the relationship between composition of carbohydrate epitopes and strain-specific biologic features? However, perhaps the most important question is whether new therapeutic strategies against prion diseases could be developed by manipulating PrPSc sialylation status.
ABBREVIATIONS
- PrPC
normal cellular isoform of the prion protein
- PrPSc
abnormal, disease-associated isoform of the prion protein
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENT
We thank Pamela Wright for editing the manuscript.
FUNDING
This work was supported by NIH grant NS045585 to IVB.
REFERENCES
- [1].Field EJ. Transmission experiments with multiple sclerosis: an interim report. Br Med J 1966; 2:564-5; PMID:5950508; http://dx.doi.org/ 10.1136/bmj.2.5513.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136-44; PMID:6801762; http://dx.doi.org/ 10.1126/science.6801762 [DOI] [PubMed] [Google Scholar]
- [3].Bolton DC, Meyer RK, Prusiner SB. Scrapie PrP 27–30 is a sialoglycoprotein. J Virol 1985; 53:596-606; PMID:3918176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Katorcha E, Makarava N, Savtchenko R, D'Azzo A, Baskakov IV. Sialylation of prion protein controls the rate of prion amplification, the cross-species barrier, the ratio of PrPSc glycoform and prion infectivity. PLOS Pathog 2014; 10:e1004366; PMID:25211026; http://dx.doi.org/ 10.1371/journal.ppat.1004366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Srivastava S, Katorcha E, Daus ML, Lasch P, Beekes M, Baskakov IV. Sialylation controls prion fate in vivo. J Biol Chem 2017; 292:2359-68; PMID:27998976; http://dx.doi.org/ 10.1074/jbc.M116.768010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Makarava N, Savtchenko R, Alexeeva I, Rohwer RG, Baskakov IV. Fast and ultrasensitive method for quantitating prion infectivity titer. Nature Commun 2012; 3:741; http://dx.doi.org/ 10.1038/ncomms1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Aguzzi A, Nuvolone M, Zhu C. The immunology of prion diseases. Nat Rev Immunology 2013; 13:888-902; PMID:24189576; http://dx.doi.org/ 10.1038/nri3553 [DOI] [PubMed] [Google Scholar]
- [8].Mabbott NA. Prion pathogenesis and secondary lymphoid organs. Prion 2012; 6:322-33; PMID:22895090; http://dx.doi.org/ 10.4161/pri.20676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Makarava N, Kovacs GG, Bocharova OV, Savtchenko R, Alexeeva I, Budka H, Rohwer RG, Baskakov IV. Recombinant prion protein induces a new transmissible prion disease in wild type animals. Acta Neuropathol 2010; 119:177-87; PMID:20052481; http://dx.doi.org/ 10.1007/s00401-009-0633-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Makarava N, Kovacs GG, Savtchenko R, Alexeeva I, Budka H, Rohwer RG, Baskakov IV. Stabilization of a prion strain of synthetic origin requires multiple serial passages. J Biol Chem 2012; 287:30205-14; PMID:22807452; http://dx.doi.org/ 10.1074/jbc.M112.392985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Katorcha E, Daus ML, Gonzalez-Montalban N, Makarava N, Lasch P, Beekes M, Baskakov IV. Reversible off and on switching of prion infectivity via removing and reinstalling prion sialylation. Sci Rep 2016; 6:33119; PMID:27609323; http://dx.doi.org/ 10.1038/srep33119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baskakov IV, Katorcha E. Multifaceted role of sialylation in prion diseases. Front Neurosci 2016; 10:358; PMID:27551257; http://dx.doi.org/ 10.3389/fnins.2016.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aminoff D, Bruegge WF, Bell WC, Sarpolis K, Williams R. Role of sialic acid in survival of erythrocytes in the circulation: interaction of neuraminidase-treated and untreated erythrocytes with spleen and liver at the cellular level. Proc Acad Natl Sci U S A 1977; 74:1521-4; http://dx.doi.org/ 10.1073/pnas.74.4.1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Linnartz B, Kopatz J, Tenner AJ, Neumann H. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J Neurosci 2012; 32:946-52; PMID:22262892; http://dx.doi.org/ 10.1523/JNEUROSCI.3830-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang F, Wang X, Yuan CG, Ma J. Generating a prion bacterially expressed recombinant prion protein. Science 2010; 327:1132-5; PMID:20110469; http://dx.doi.org/ 10.1126/science.1183748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deleault NR, Piro JR, Walsh DJ, Wang F, Ma J, Geoghegan JC, Supattapone S. Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids. Proc Acad Natl Sci U S A 2012; 109:8546-51; http://dx.doi.org/ 10.1073/pnas.1204498109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Varki A. Sialic Acids In: Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J, eds. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1999:195-210. [Google Scholar]
- [18].Endo T, Groth D, Prusiner SB, Kobata A. Diversity of oligosaccharide structures linked to asparagines of the scrapie prion protein. Biochemistry 1989; 28:8380-8; PMID:2574992; http://dx.doi.org/ 10.1021/bi00447a017 [DOI] [PubMed] [Google Scholar]
- [19].Schauer R, Srinivasan GV, Wipfer D, Kniep B, Schwartz-Albiez R. O-Acetylated Sialic Acids and Their Role in Immune Defence In: Wu AM, ed. The Molecular Immunology of Complex Carbohydrates-3. New York, Dordrecht, Heidelberg, London: Sprinenger Science, 2011:525-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stimson E, Hope J, Chong A, Burlingame AL. Site-specific characterization of the N-linked glycans of murine prion protein by high-performance liquid chromatography/electrospray mass spectrometry and exoglycosidase digestions. Biochemistry 1999; 38:4885-95; PMID:10200178; http://dx.doi.org/ 10.1021/bi982330q [DOI] [PubMed] [Google Scholar]
- [21].Rudd PM, Endo T, Colominas C, Groth D, Wheeler SF, Harvey DJ, Wormald MR, Serban H, Prusiner SB, Kobata A, et al.. Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc Natl Acad Sci U S A 1999; 96:13044-9; PMID:10557270; http://dx.doi.org/ 10.1073/pnas.96.23.13044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Katorcha E, Makarava N, Savtchenko R, Baskakov IV. Sialylation of the prion protein glycans controls prion replication rate and glycoform ratio. Sci Rep 2015; 5:16912; PMID:26576925; http://dx.doi.org/ 10.1038/srep16912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baskakov IV, Breydo L. Converting the prion protein: What makes the protein infectious. Biochim Biophys Acta (Molecular Basis of Disease) 2007; 1772:692-703; http://dx.doi.org/ 10.1016/j.bbadis.2006.07.007 [DOI] [PubMed] [Google Scholar]
- [24].Rabinovich GA, Croci DO. Regulatori Circuits Mediated by Lectin-Glycan Interaction in Autoimmunity and Cancer. Immunity 2012; 36:322-35; PMID:22444630; http://dx.doi.org/ 10.1016/j.immuni.2012.03.004 [DOI] [PubMed] [Google Scholar]
- [25].Linnartz B, Bodea L-G, Neumann H. Microglia carbohydrate-binding receptors for neural repair cell tissue repair. Cell Tissue Res 2012; 349:215-27. [DOI] [PubMed] [Google Scholar]
- [26].Katorcha E, Klimova N, Makarava N, Savtchenko R, Pan X, Annunziata I, Takahashi K, Miyagi T, Pshezhetsky AV, d'Azzo A, et al.. Knocking out of cellular neuraminidases Neu1, Neu3 or Neu4 does not affect sialylation status of the prion protein. PLoS One 2015; 10:e0143218; PMID:26569607; http://dx.doi.org/ 10.1371/journal.pone.0143218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Srivastava S, Makarava N, Katorcha E, Savtchenko R, Brossmer R, Baskakov IV. Post-conversion sialylation of prions in lymphoid tissues. Proc Acad Natl Sci U S A 2015; 112:E6654-62; http://dx.doi.org/ 10.1073/pnas.1517993112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci 2004; 5:195-208; PMID:14976519; http://dx.doi.org/ 10.1038/nrn1349 [DOI] [PubMed] [Google Scholar]


