Abstract
Multidrug-resistant Neisseria gonorrhoeae is a top threat to public health. In November 2015, UCLA Health introduced a rapid gyrase A (gyrA) genotypic assay for prediction of Neisseria gonorrhoeae susceptibility to ciprofloxacin. We found a significant reduction in ceftriaxone use with a concomitant increase in targeted therapy.
Keywords: gyrA, Neisseria gonorrhoeae, ciprofloxacin, antibiotic resistance.
Over the past decades, Neisseria gonorrhoeae has developed resistance to all antibiotics used for treatment [1]. The propensity for developing antimicrobial resistance is promoted by the misuse of antibiotics, which is hastened by the lack of timely methods for susceptibility testing to guide antibiotic selection [1, 2]. Recently, the identification and spread of multidrug-resistant N. gonorrhoeae has caused great concern [2] and was declared one of the top 3 urgent threats to public health [3].
In response to that threat, we developed a molecular assay for the prediction of N. gonorrhoeae ciprofloxacin susceptibility based on the detection of mutation at codon Ser91 of the Neisseria gonorrhoeae gyrase A (gyrA) gene directly from N. gonorrhoeae–positive specimens; the results of the assay were 100% concordant with traditional phenotypic agar dilution methods for detecting ciprofloxacin susceptibility [4]. A recent summary of the literature demonstrated that 11 other studies using the genotypic gyrA assay found similar concordance with susceptibility results [5]. Because approximately 80% of N. gonorrhoeae infections in the United States are susceptible to ciprofloxacin [6], targeted use of ciprofloxacin therapy for the treatment of susceptible N. gonorrhoeae may be an effective strategy [7].
We adapted the assay for performance in a clinical laboratory using remnant N. gonorrhoeae nucleic acids extracted by a commonly used commercial nucleic acid amplification assay for routine clinical testing, thereby allowing same-day reflex testing of the gyrA genotype for all N. gonorrhoeae–positive specimens [8]. The assay performance was verified in accordance with Clinical Laboratory Improvement Amendments (CLIA) requirements for use on urine, genital swab, rectal swab, and liquid cytology specimens. The method was launched as a laboratory-developed test for routine clinical use in November 2015 at the UCLA Health Clinical Microbiology Laboratory (University of California, Los Angeles (UCLA)).
METHODS
UCLA Health is comprised of 2 hospitals, 2 emergency departments, and >150 primary care clinics serving approximately 500 000 patient-visits each year. At UCLA, cases of N. gonorrhoeae are detected by the Cobas 4800 CT/NG assay (Roche Molecular Systems, Pleasanton, California). Beginning in November 2015, all N. gonorrhoeae–positive specimens were reflexed to the real-time polymerase chain reaction gyrA genotypic susceptibility assay, coupled with high-resolution melt analysis using fluorescence resonance energy transfer probes that target the gyrA gene. Specimens from all anatomic sites were treated the same. Subsequent laboratory reports of positive gonorrhea tests included genotype results and treatment recommendations of ciprofloxacin 500 mg once for wild-type strains [9]. Prior to the launch of the assay, physician education was provided describing the genotyping method and treatment recommendations. Beginning in May 2016, for all positive cases, providers were contacted electronically with reminders of the genotype results and recommended therapy.
We reviewed de-identified patient records of all N. gonorrhoeae infections between 1 January 2015 and 29 July 2016 at UCLA Health. Data on gender, anatomic site of infection, human immunodeficiency virus (HIV) coinfection, time to treatment, gyrA genotype, and treatment selection were collected. Cases were defined as a diagnosis of N. gonorrhoeae infection on a single date; 1 case may have multiple anatomic site-specific infections. Empiric treatment was defined as treatment within 1 day of diagnosis. Wild-type strains were defined as the most prevalent gene sequence. The most prevalent gene sequence is nonmutated and predicts full susceptibility to ciprofloxacin.
The primary outcomes of interest were time in days from specimen collection to therapy (defined as prescription of at least 1 antibiotic with activity against N. gonorrhoeae) and the use of ceftriaxone or ciprofloxacin in the therapeutic regimen. Our primary explanatory variable was the period before (January 2015–November 2015) and after (November 2015–July 2016) assay implementation. We compared each outcome between the 2 periods using Student t test, χ2 test, or Fisher exact test. Analyses were performed using Stata software version 12 (StataCorp, College Station, Texas). The University of California Institutional Review Board determined that the review of de-identified patient data was not human subjects research.
RESULTS
Among 251 patients during the study period, there were 283 cases and 314 N. gonorrhoeae infections. Of the 130 patients prior to assay implementation, 108 (83.1%) were men compared with 134 of 153 (87.6%) patients after assay implementation (P = .28). The proportion of patients with HIV infection was 34.6% (n = 45) before assay implementation vs 32.7% (n = 50) after assay implementation (P = .73). Of all N. gonorrhoeae cases during the study period, 113 (39.9%) were treated the same day as specimen collection: 58 of 130 (44.6%) cases before assay implementation compared with 55 of 153 (35.9%) cases after assay implementation (P = .14). The mean time to treatment among nonempirically treated cases before assay implementation (n = 78) was 5.5 days (SD, 5.5 days), similar to the mean duration of 4.7 days (SD, 3.5 days) after assay implementation (n = 114) (0.8-day difference, P = .87).
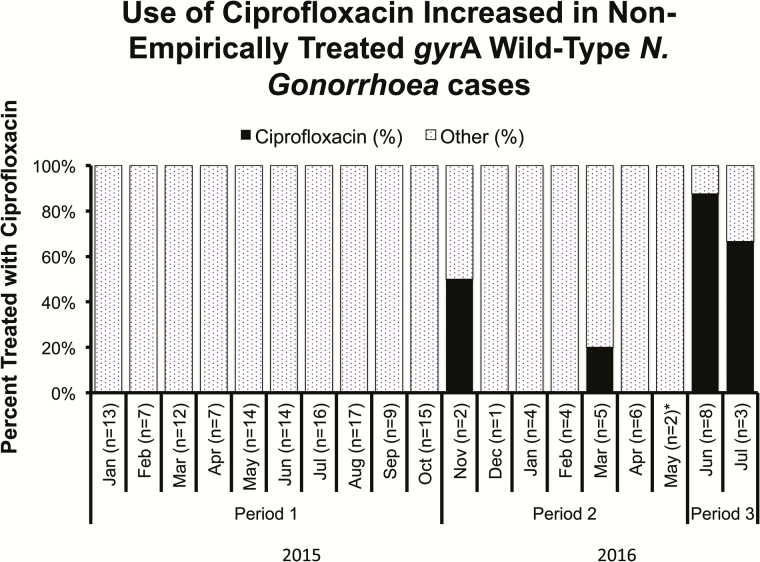
Among cases of nonempirically treated N. gonorrhoeae, 68 of 72 (94.4%) were treated with ceftriaxone before assay introduction compared with 77 of 98 (78.6%) after assay introduction (P = .004). Among 35 cases with wild-type gyrA genotype N. gonorrhoeae infection treated at least 2 days after specimen collection, the use of ciprofloxacin increased from 3 of 24 (12.5%) cases in the first 7 months of assay implementation to 9 of 11 (81.8%) cases in the final 2 months after electronic reminder notifications began (P < .001), compared with no ciprofloxacin use among non-empirically treated cases prior to assay implementation (P < .001) (Figure 1).
Figure 1.
Percent of non-empirically treated gyrA wild-type Neisseria gonorrhoeae cases treated with ciprofloxacin at UCLA Health between 1 January 2015 and 29 July 2016. Period 1 is the period before assay implementation (1 January 2015 – 31 October 2015), during which time there were no genotype results available. Period 2 is the initial seven months of assay implementation prior to sending electronic reminders of genotype results to providers (1 November 2015 – 26 May 2016). Period 3 is the final two months of assay implementation after electronic reminder notifications began (27 May 2016– 29 July 2016). *Electronic reminders began 27 May 2016
Of 176 infections detected between November 2015 and July 2016, 121 (68.8%) were successfully genotyped. Of those, 72 (59.5%) were wild-type gyrA and 49 (40.5%) were mutant. Among the remaining 55 infections, 49 could not be genotyped (6 were not attempted). Of the 62 pharyngeal N. gonorrhoeae infections, 40 (64.5%) could not be genotyped, in comparison to 9 of 114 (7.9%) samples from other anatomic locations (4 urine, 3 rectal, 2 vaginal/cervical) (P < .001). The proportion of gyrA mutant N. gonorrhoeae infection (N=49) did not vary significantly by anatomic site (pharyngeal 33%, rectal 45.7%, vaginal/cervical 57.1%, urine 38.6%; P = .67).
DISCUSSION
Within a large health system, we successfully implemented routine gyrA genotype testing on N. gonorrhoeae–positive clinical specimens. We found an increase in ciprofloxacin treatment among patients with wild-type gyrA infections, and a concomitant reduction in ceftriaxone treatment. Additionally, electronic reminder notifications of genotype results to providers significantly augmented treatment with ciprofloxacin among wild-type gyrA infections. The proportion of those with HIV infection, male gender, and who received empiric treatment did not significantly differ before or after the implementation of the molecular assay. For nonempirically treated cases, time to treatment was not significantly different before and after assay implementation, and was of sufficient duration to allow for the performance of the assay and timely communication of test results to providers.
Our findings are important because (1) the evolution of drug-resistant N. gonorrhoeae is ongoing and interventions to mitigate this continued emergence are urgently needed [1, 2], and (2) there are only a few new antimicrobials for N. gonorrhoeae infections in development [5]. Reintroduction of antibiotics previously thought to be ineffective is a novel approach that may reduce the continued emergence of resistance [7], made possible by rapid molecular methods for targeted therapy. Targeted therapy with oral agents enables use of simple, less costly medications, facilitating partner treatment, and can provide options for persons intolerant to recommended empiric therapy.
We also found that successful genotyping was dependent on the anatomic site of infection, with pharyngeal N. gonorrhoeae infections being significantly less likely to be genotyped compared with other anatomic sites. Reduced sensitivity for pharyngeal N. gonorrhoeae genotyping has been noted previously; however, the cause is uncertain [8]. Improving the performance of the assay for pharyngeal infections is a priority because prior studies have shown a higher prevalence of drug-resistant N. gonorrhoeae among pharyngeal specimens [10], thought to be the result of transformation of resistance elements between commensal Neisseria species [10].
Finally, we found that the use of electronic reminder notifications for providers regarding genotype results significantly augmented appropriate treatment selection. Such notification may help assure that assay implementation is associated with changes in prescribing behavior. A prior study has shown similar benefits of introducing electronic notifications in implementation of antibiotic stewardship interventions [11].
Our study had several limitations. Primarily, this was a single-center study in a well-resourced health system. Implementation of the assay into other health systems is necessary to demonstrate reproducibility of our results. Additionally, in our study we did not measure clinical outcomes; however, ciprofloxacin has been shown to be >99% effective for the treatment of phenotypically susceptible N. gonorrhoeae infections [12]. A clinical trial to evaluate outcomes among patients with wild-type gyrA N. gonorrhoeae infections treated with ciprofloxacin is underway at UCLA [13]. Furthermore, the study populations were not randomly assigned and therefore causality cannot be determined. Finally, as the molecular assay was a laboratory-developed test, smaller health systems may not have the capabilities to develop and implement the assay within the guidelines of CLIA.
CONCLUSIONS
The implementation of a rapid N. gonorrhoeae genotype assay in a large health system reduced the empiric use of ceftriaxone. Such a reduction in ceftriaxone use could slow the emergence of antibiotic-resistant N. gonorrhoeae, one of the top 3 urgent threats to public health. Use of ciprofloxacin in wild-type strains was augmented by electronic notification of providers regarding genotype results. Successful genotyping, however, may depend on the anatomic site of infection. Strong consideration should be made to the introduction and evaluation of the assay in other clinical settings.
Notes
Financial support. This work was supported by the David Geffen School of Medicine at UCLA and the National Institutes of Health (grant numbers R21AI117256 and R21AI109005).
Potential conflicts of interest. All authors: No reported conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27:587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T 2015; 40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Atlanta, GA: CDC, 2013. [Google Scholar]
- 4. Siedner MJ, Pandori M, Castro L, et al. Real-time PCR assay for detection of quinolone-resistant Neisseria gonorrhoeae in urine samples. J Clin Microbiol 2007; 45:1250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allan-Blitz L, Klausner JD. Neisseria gonorrhoeae susceptibility to ciprofloxacin. MLO Med Lab Obs 2016; 48:30. [PubMed] [Google Scholar]
- 6. Kirkcaldy RD, Harvey A, Papp JR, et al. Neisseria gonorrhoeae antimicrobial susceptibility surveillance—the Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveill Summ 2016; 65:1–19. [DOI] [PubMed] [Google Scholar]
- 7. Buono SA, Watson TD, Borenstein LA, Klausner JD, Pandori MW, Godwin HA. Stemming the tide of drug-resistant Neisseria gonorrhoeae: the need for an individualized approach to treatment. J Antimicrob Chemother 2015; 70:374–81. [DOI] [PubMed] [Google Scholar]
- 8. Hemarajata P, Yang S, Soge OO, Humphries RM, Klausner JD. Performance and verification of a real-time PCR assay targeting the gyrA gene for prediction of ciprofloxacin resistance in Neisseria gonorrhoeae. J Clin Microbiol 2016; 54:805–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis 1995; 20(suppl 1:S47–65. [DOI] [PubMed] [Google Scholar]
- 10. Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 2012; 7:1401–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 2015; 61:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Echols RM, Heyd A, O’Keeffe BJ, Schacht P. Single-dose ciprofloxacin for the treatment of uncomplicated gonorrhea: a worldwide summary. Sex Transm Dis 1994; 21:345–52. [DOI] [PubMed] [Google Scholar]
- 13. Klausner JD. Clinical validation of a molecular test for ciprofloxacin-susceptibility in Neisseria gonorrhoeae. Bethesda, MD: National Library of Medicine; Available at: https://www.clinicaltrials.gov/ct2/show/NCT02961751). Accessed 23 November 2016. [Google Scholar]