Abstract
Background
Placenta-derived mesenchymal stem cells (PMSCs) were isolated from placenta and had differentiation and self-renewal potential. We transfected PMSCs with glial cell line-derived neurotrophic factor (GDNF) and compared their effect for repairing spinal cord injury (SCI) with that of GDNF-transfected bone marrow-derived mesenchymal stem cell (BMSC).
Material/Methods
The PMSCs were isolated from Sprague-Dawley rat placenta; BMSCs were isolated from Sprague-Dawley rat thigh bone marrow. Primary cultured BMSCs and PMSCs were uniformly spindle-shaped. Flow cytometry indicated that both cell types were CD29- and CD90-positive and CD34- and CD45-negative, confirming that they were MSCs. The PMSCs and BMSCs were transfected with recombinant lentivirus containing the GDNF gene in vitro. PMSC and BMSC viability was increased after transfection, and GDNF expression was increased until 10 d after transfection. SCI was created in the rats (n=64) and was repaired using transfected PMSCs and BMSCs or untransfected PMSCs and BMSCs.
Results
The transfected PMSCs and BMSCs repaired the SCI. Flow cytometry, histology, immunohistochemical, kinesiology properties, and Basso-Beattie-Bresnahan locomotion score measurements determined no significant difference between transfected PMSCs and BMSCs at 7, 14, and 21 d post-transplantation (P>0.05); the injury healed better in transfected PMSCs and BMSCs than in untransfected PMSCs and BMSCs (P<0.05).
Conclusions
MSCs have similar biology characteristics and capacity for SCI repair to BMSCs and can be used as a new resource for treating SCI.
MeSH Keywords: Bone Marrow, Mesenchymal Stromal Cells, Spinal Cord Injuries
Background
Spinal cord injury (SCI) is one of the most serious spinal traumas and is the main cause of disability. At present, nerve repair and regeneration after SCI remains a worldwide problem [1,2]. Early high-dose hormone impact therapy, prompt intervention, minimally invasive surgery, and postoperative rehabilitation exercise all do not achieve a satisfactory effect. In recent years, advancement in stem cell transplantation has brought new hope for treating SCI. Many studies have confirmed that stem cell transplantation can repair neurons, improving the symptoms of nervous system diseases [3–5]. Bone marrow-derived mesenchymal stem cells (BMSCs) are an appealing resource for use in nerve repair and regeneration. However, very small number of cells can be derived from bone marrow, and only 1 mesenchymal stem cell (MSC) can be isolated from every 104–105 mononuclear cells. Worse, BMSCs numbers and differentiation and proliferation capability tend to decline with age [6,7]. Therefore, it is essential to find a new source of MSCs.
Recently, MSCs with differentiation and self-renewal potential were isolated from various sources, including muscle, fat, peripheral blood [8,9]. These tissues have a common feature in that they derive from the mesoderm in embryonic development. Many studies have demonstrated that various parts of the placenta, including umbilical cord blood, chorion, umbilical cord, and amnion, are great future sources of MSCs [10–12]. Beyond easier access and fewer ethical restrictions, MSCs isolated from the placenta have obviously greater growth potential than BMSCs because of their telomerase expression [13,14]. Because the require no invasive procedures, the discovery of PMSCs appears to have had a notable impact on stem cell therapy research.
Previously, we gained PMSCs from term human placental decidua basalis. Such PMSCs have multipotent differentiation and self-renewal ability under controlled conditions in vitro. Such cells have similar phenotypes as BMSCs. Besides, they are moderately positive (in the 33.5–44.6% range) for stage-specific embryonic antigen Oct-4, TRA-1-60, TRA-1-81, (SSEA)-1, SSEA-3, and SSEA-4, which are mainly expressed in embryonic stem cells [15].
Glial cell line-derived neurotrophic factor (GDNF) was first isolated from the B49 cell line by Lin in 1993 [16]; it can promote survival in dopaminergic, motor, sensory, and other neurons. Further research has indicated that GDNF has good application prospects for treating nerve injury and neurodegenerative disease [17–19].
In this study, we tried to repair SCI in Sprague-Dawley rats using GDNF-transfected PMSCs and BMSCs to construct a clinical research foundation for PMSC transplantation in nervous system damage.
Material and Methods
Isolation and cultivation of PMSCs and BMSCs
To get PMSCs, the pregnant SD rat (weighing approximately 300 g) was sacrificed. Then, we dissected decidua basalis from the placenta and washed it in NaCl/p\Pi (pH 7.4). The harvested tissue was chopped into approximately 1–2 mm3 pieces and digested with 0.25% trypsin and 0.1% collagenase IV (1: 1; Gibco, Grand Island, NY, USA) for 30 min. Digestion was terminated by adding 10% fetal bovine serum (HyClone, Rockford, IL, USA). We removed undigested tissues with a 100 mesh. The mixture was centrifuged at 250×g for 5 min, and the pellet was dissolved in 1 mL of Dulbecco’s modified Eagle’s medium (DMEM; Gibco) containing 20% fetal bovine serum. Next, we added 5mL lymphocyte separation liquid (Hengxin Chemical, Shanghai, China). The mixture was exposed to density gradient centrifugation at 800×g for 20 min, then we harvested the monocyte layer and dissolved in 1 mL DMEM containing 20% fetal bovine serum, and centrifuged it at 250×g for 5 min. The harvested cells were seeded into a culture bottle containing DMEM and 15% fetal bovine serum at 1×106 mL−1, then cultured in a 5% CO2 incubator (Sanyo, Osaka, Japan) at 37°C. We replaced medium every 2 d.
To get BMSCs, an adult SD rat was sacrificed. We isolated the femur and cut open its ends. The bone marrow was swilled out with low-glucose DMEM using a 1-mL syringe. We harvested the cells in a culture dish, dissolved using a Pasteur pipette, seeded into a flask containing DMEM and 15% fetal bovine serum, then cultured in an incubator. We replaced the medium 2 d later. When the cells were about 85% confluent, they were passaged with 0.1% EDTA and 0.25% trypsin (2: 1). We monitored cell growth under an inverted phase-contrast microscope (Olympus, Tokyo, Japan).
Identification of cell surface markers
Well-growing third-generation PMSCs and BMSCs were collected and washed twice using phosphate-buffered saline (PBS), and digested with 0.25% trypsin and 0.1% EDTA (1: 2). The digestion was terminated by adding 10% fetal bovine serum. The cell suspension was centrifuged at 250×g for 10 min, and the pellet was resuspended with a moderate amount of PBS to prepare a concentration of 1×105 mL−1. Mouse anti-rat monoclonal antibodies against CD90, CD29, CD34, and CD45 (20 μL; BD, Franklin Lakes, NJ, USA) were added to a 100 μL cell suspension, which was then incubated in 5% CO2 for 30 min. PBS (1.5 mL) was added and mixed, the cell suspension was centrifuged at 250×g for 10 min, resuspended in PBS, and transferred to the flow tubes. CD90, CD29, CD34, and CD45 expression was detected and analyzed.
GDNF gene transfection
Third-generation PMSCs and BMSCs were inoculated into 6-well plates at an inoculation density of 1×105 in 1 hole, cultured in a 5% CO2 incubator at 37°C, and then transfected with the GDNF overexpression lentivirus (Genechem, Shanghai, China). Each well contained a total volume of 2 mL with different volume fractions of serum-free DMEM and the recombinant adenovirus suspension; the respective infection scores (MOI) were 10, 50, 80, 100, 150, and 200. After 2-h incubation in a constant temperature incubator, the DMEM and adenovirus combination was replaced with 2 mL DMEM containing 10% fetal bovine serum and the culture was continued; we observed GFP expression every 12 h.
PMSC and BMSC viability post-transfection
PMSCs and BMSCs were transfected in 96-well plates at an inoculation density of 1×104 in 1 hole. After 24-h incubation, the PMSCs and BMSCs were rinsed twice in PBS and randomly seeded in 5 wells each in 96-well plates. After 1-, 3-, 5-, 7-, and 9-d culture, 20 μL MTT working solution was added to random wells in each group. After 4-h incubation in the dark, the solution was removed and 150 μL dimethyl sulfoxide per well was added, and the plates were placed on a shaker for 15 min. The absorbance was detected at 492 nm. The experiment was repeated 3 times and the data were collected and analyzed using analysis of variance to compare the differences between all groups of data.
Western blot analysis
GDNF-transfected PMSCs and BMSCs were seeded in 6-well plates that were cultured for 7 d. Total cellular extracts were obtained by lysing the cells in radioimmunoprecipitation assay lysis buffer. Protein concentrations of the cell lysates were determined by Coomassie blue dye-binding assay (Bio-Rad, CA, USA). Aliquots of cell lysates containing 50 μg protein were separated by 10% SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose filters. The filters were blocked with TBST buffer (10 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 0.05% Tween 20) containing 5% skimmed milk, incubated with rabbit anti-mouse GDNF antibody overnight, followed by the addition of horseradish peroxidase–linked anti-rabbit IgG and electrochemiluminescence visualization of the bands. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as the internal control to normalize the expression of other proteins.
Animal model
SD rats were selected randomly and anesthetized with 0.3% chloral hydrate anesthesia. Then, the rats were tied to the experiment board and the dorsal skin was shaved and sterilized. Next, the spinal cords were surgically exposed and struck with a fixed amount force of 10×1.25 g cm, where spasmodic trembling of the tail and congestion of the struck tissue could be observed. After confirming a successful strike, the back incision was sutured and subjected to kinematics analysis. Three days after surgery, the spinal cords were re-exposed and randomly divided into 4 groups (group A, untransfected PMSCs; group B, untransfected BMSCs; group C, transfected PMSCs; group D, transfected BMSCs, 16 rats in each group). Four cell suspensions were injected sequentially into the spinal cords injury area using a microinjection syringe, and there were 7 points for each sample injection. Every point was injected with 2.5 ul of cell suspensions with a concentration of 4×104/ul and dissolved by cell medium. Subsequently, normal feeding was resumed after the incision was sutured. The kinematics properties of each rat were assessed at 7, 14, and 28 d post-injection. After death, the rats were perfused with 4% formaldehyde. The spinal cord was removed and embedded in paraffin for HE staining and immunohistochemical staining. The recovery of the rat spinal cord from the SCI was observed.
BBB scores
The BBB scores were assessed at 7, 14, and 28 d post-surgery using the BBB locomotor rating scale [20], which was performed by personnel external to our laboratory but who were familiar with the scoring standard (they are not co-authors). Every experiment was repeated 3 times to obtain the average. The BBB scores was used to assess limb function after spinal cord injury.
Histology and immunohistochemistry
Spinal cord samples were fixed in 10% formalin solution at 7, 14, and 28 d post-surgery, and then cut into 4-μm thick paraffin sections for histology and immunohistochemistry analysis. Routine HE staining was performed. Briefly, paraffin sections were washed 3 times (every 5 min) in PBS, 15 min in 1 g L−1 Triton, and 3 times (every 5 min) in PBS, and then incubated with BSA (ZsBio, Beijing, China) at room temperature for 30 min. After the serum was discarded and anti-NSE (1: 200, Abcam, Cambridge, UK) antibody was added, the sections were embedded in a wet box at 4°C overnight. The sections were washed 4 times (every 5 min) in PBS and then horseradish peroxidase–labeled streptavidin–avidin solution was added. Finally, the sections were counterstained with the chromogenic solution diaminobenzidine (Boster, Wuhan, China), mounted, and observed under an inverted phase-contrast microscope. GFAP and NF-200 (Abcam, Cambridge, UK) expression was assessed using the same preparation as above; the concentration of the GFAP and NF-200 antibodies was 1: 100. The immunohistochemistry analysis of HE, NSE, and GFAP was assessed based on optical density and analyzed using Image-Pro Plus 5.1 software (Media cybernetics, MD, USA). Three sections were selected from each group and the optical density of positive cells per field was calculated to obtain the mean. The results for NF-200 were analyzed using Image-Pro Plus 5.1 software and the length of the nerve fibers was calculated to obtain the mean.
Statistical analysis
All samples were analyzed using a paired Student’s t-test. Therefore, all data are expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS 16.0 statistical software (Chicago, IL, USA); measurement data were analyzed using variance. The results were considered statistically significant at P<0.05.
Results
Primary culture of PMSCs and BMSCs
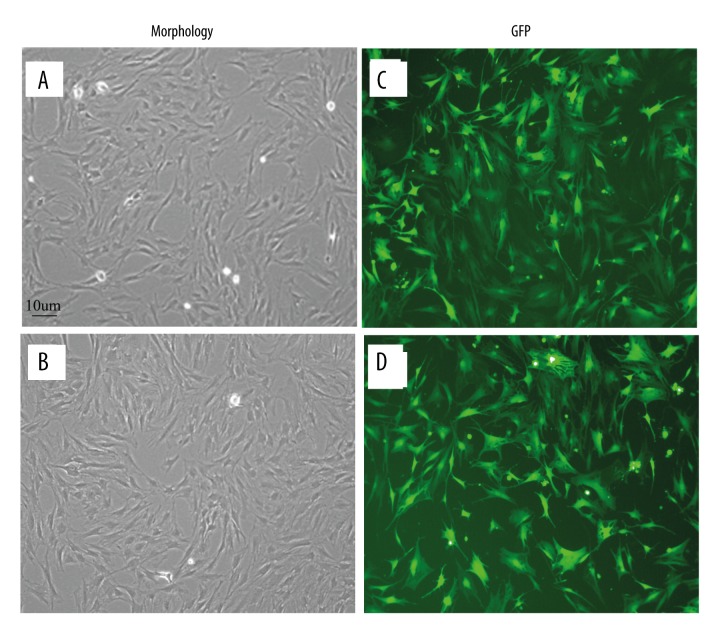
PMSCs and BMSCs were both isolated and cultivated successfully. Approximately 30% of cells began to adhere 48–72 h after seeding in primary cultures, where they grew and formed colonies. All cells had a long spindle-like shape after 4 d culturing. On day 5, the cells were passaged when they reached 80–90% confluence. Both PMSCs and BMSCs formed morphologically homogeneous populations of fibroblast-like cells in 5 d with a doubling time of 4–5d (Figure 1A, 1B).
Figure 1.
(A, B) PMSC and BMSC morphology under inverted phase-contrast microscopy (×100). Day 5 first-generation PMSCs (A) and BMSCs (B) are elongated spindle-shaped and followed similar patterns such as uniform fibroblast-like colony growth. (C, D) Morphology of GDNF-transfected PMSCs and BMSCs. Cells were observed under inverted phase-contrast microscopy (×100), MOI=100 12 h after transfection. GFP expression of PMSCs (C) and BMSCs (D) was significant and the cell morphology was intact without obvious cytopathic effects.
Identification of cell surface markers
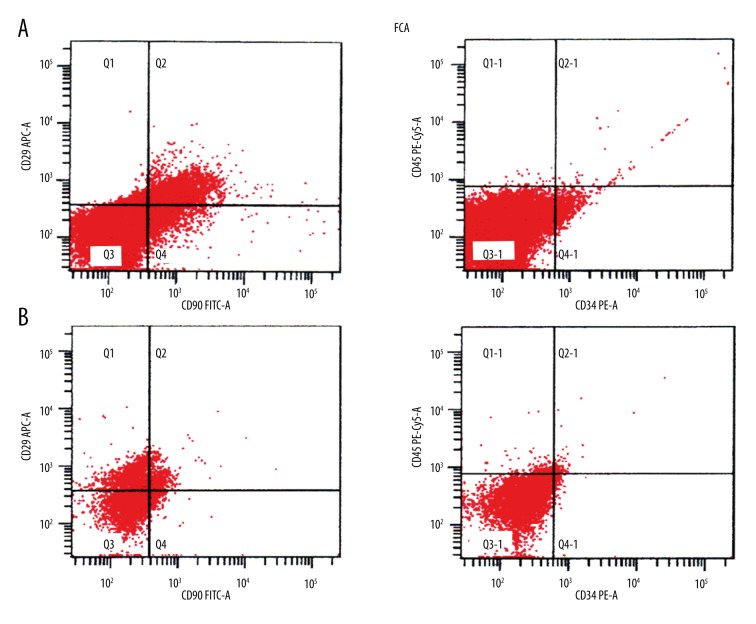
Flow cytometry showed high CD29 and CD90 expression in the PMSC and BMSC cell membrane and cytoplasm; the cells were negative for CD34 and CD45 expression, showing that both cell types were MSCs (Figure 2).
Figure 2.
Flow cytometry of PMSCs and BMSCs. CD29 and CD90 were highly expressed in PMSCs (A) and BMSCs (B), while CD34 and CD45 were not.
GDNF gene transfection
After 12-h transfection, both PMSCs and BMSCs had a multiplicity of infection (MOI) of 100 and expressed significant levels of green fluorescent protein (GFP). The morphological integrity of the cells was intact and there was no obvious cytopathic effect. When MOI <100, there were significantly fewer GFP-positive PMSCs and BMSCs than when MOI=100; when MOI >100, fluorescence intensity was strong, but varying degrees of cytopathic effects were observed. There was no GFP expression when MOI=0. Therefore, MOI=100 indicated the best infection numerically (Figure 1C, 1D).
PMSC or BMSC viability post-transfection
The tetrazolium (MTT) assay showed that both PMSCs and BMSCs were in the logarithmic growth phase at 3–5 d and grew slowly, plateauing at 6–7 d after transfection. Cell viability was not significantly different in groups A (untransfected PMSCs), B (untransfected BMSCs), C (GDNF-transfected PMSCs), and D (GDNF-transfected BMSCs) (P>0.05) at 24 h after transfection, but 3 d after transfection, the cell viability of groups C and D was slightly but significantly higher than that of groups A and B (P<0.05). Nevertheless, cell viability in groups C and D was not significantly different (P>0.05) (Figure 3).
Figure 3.
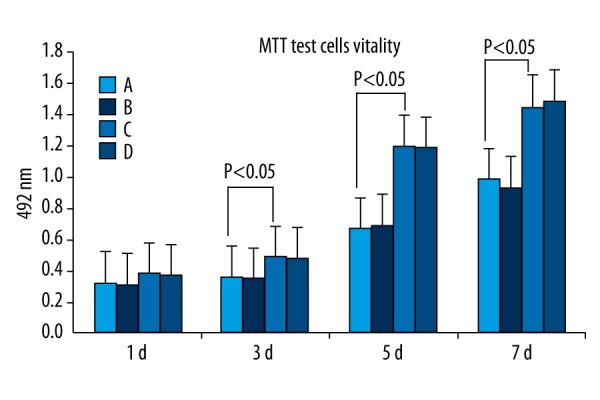
MTT assay of untransfected PMSCs (A) and untransfected BMSCs (B) and GDNF-transfected PMSCs (C) and GDNF-transfected BMSCs (D). After 3–5 d of transfection, PMSCs and BMSCs exhibited logarithmic growth and the growth rate slowed at 6–7 d. The cell viability of all 4 groups was not significantly different (P>0.05) at 24 h after transfection. At 3 d after transfection, the cell viability of GDNF-transfected-PMSCs and GDNF-transfected-BMSCs gradually became significantly higher than that of PMSCs and BMSCs (P<0.05). The cell viability of GDNF-transfected-PMSCs and GDNF-transfected-BMSCs was not significantly different (P > 0.05).
Western blot detection of GDNF expression in PMSCs and BMSCs after transfection
Western blotting showed that PMSCs and BMSCs expressed GDNF (Figure 4) at 7 d after transfection.
Figure 4.
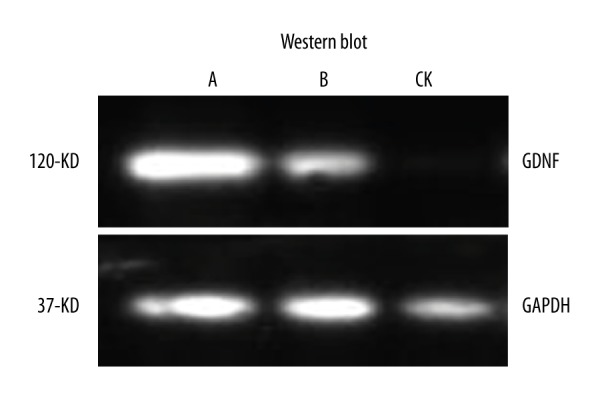
Western blotting showing that PMSCs (A) and BMSCs (B) express GDNF after 7-d GDNF transfection.
General observations
All rats revived within 2 h after surgery and could feed and move their forelimbs freely within 24 h. All surgical lesions healed without infection.
Basso-Beattie–Bresnahan (BBB) score after SCI
One day after SCI induction in the rats using the Allen hit device, all experimental groups (A, B, C, D) presented lower-limb paralysis; the BBB score was 0 points. After 7 d, the motor function in all 4 groups was slightly improved; the lower limbs of some of the rats in C and D had 1–2-joint activities. After 14 d, there was lower limb movement in group C and D rats, and some of the rats had better leg joint activity; lower-limb function recovery was slow in group A and B. After 28 d, there was lower limb motor function recovery in group C and D rats, and some of the rats could support their body weight with both lower limbs; group A and B rats showed slower limb recovery.
At 1, 2, and 4 weeks after surgery, the BBB scores of group C and D rats showed no significant difference (P>0.05). However, the scores of group A and B rats and group C and D rats were significantly different (P<0.05) (Figure 5), suggesting similar nerve regeneration abilities of the PMSCs and BMSCs and that GDNF promotes such abilities.
Figure 5.
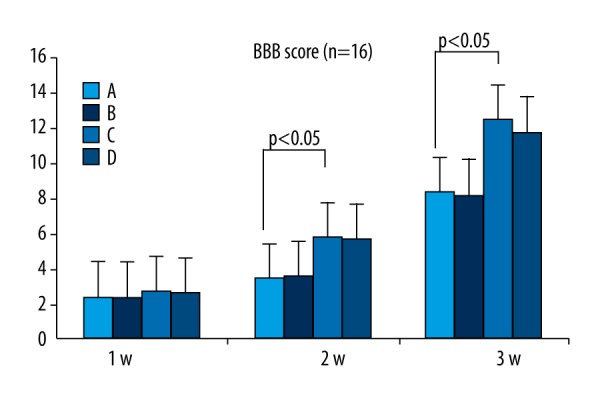
BBB score. At 1, 2, and 4 weeks after surgery, the BBB score between PMSCs (A) and BMSCs (B) and GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) were significantly different (P<0.05); the difference between GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) was not significant (P>0.05).
Histological observation
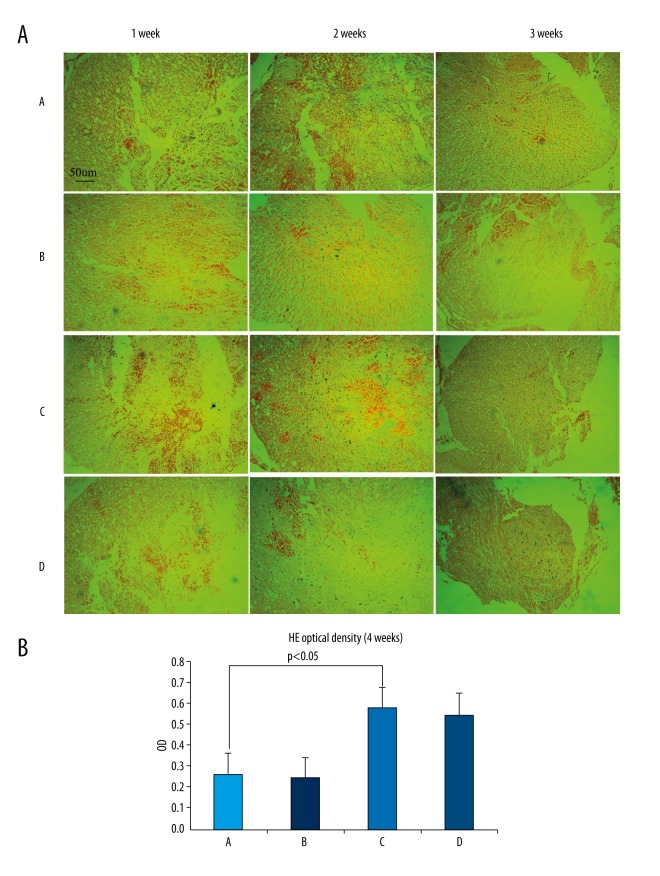
One week after surgery, microscopic observation of hematoxylin-eosin (HE)-stained sections revealed microcapsules and necrotic nerve fibers in the inflammatory cells, cavities, and white matter of the damaged rat spinal cord tissue. Nerve cell proliferation was observed in group C and D. After 2 weeks, all SCI tissues were filled with dense fibrous connective tissue, and astrocytes had proliferated and accumulated around the glial scar. There were significantly more proliferated nerve cells in group C and D rats than in group A and B rats. After 4 weeks, the scar tissue in each group remained visible, but the number of necrotic cysts was reduced, the tissue was denser, and the number of nerve cells had obviously increased. There were significantly increased proliferating nerve cells in group C and D rats, and some neural cell proliferation was observed in group A and B rats. Overall, there was no significant difference between group C and D, but group C and D were significantly different from group A and B, and injury repair was significantly better than in group A and B (Figure 6A, 6B).
Figure 6.
(A, B) HE staining observed under inverted phase-contrast microscopy (×100). One week after surgery, inflammatory cells, cavities, and nerve fiber necrosis can be observed in the SCI. Nerve cell proliferation can be also observed in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D). After 2 weeks and in all 4 groups, the SCI was filled with dense fibrous connective tissue. Astrocyte proliferation and accumulation in the glial scar is visible. There were obviously more proliferating cells in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) than in PMSCs (A) and BMSCs (B). After 4 weeks, the scar tissue can still be observed in each group but with reduced cysts and necrosis. The tissue is denser, while there are significantly increased nerve cells as compared to that at 2 weeks. There was significantly increased nerve cell proliferation in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) but it was much lower in PMSCs (A) and BMSCs (B). Overall, GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) did not differ greatly, but were significantly improved as compared to PMSCs (A) and BMSCs (B).
Glial fibrillary acidic protein (GFAP) expression at SCI site
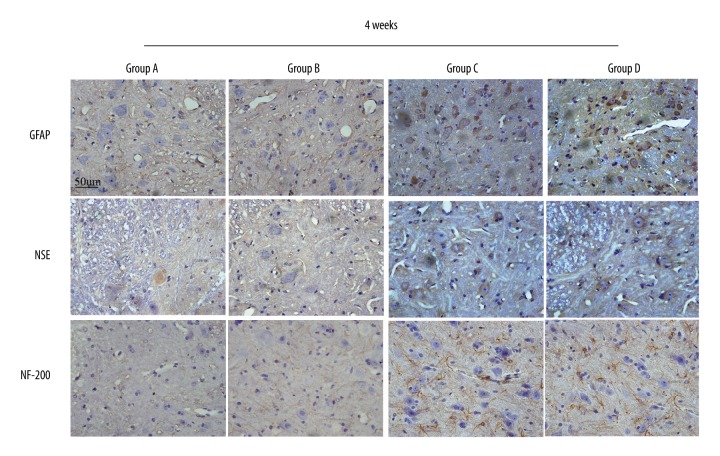
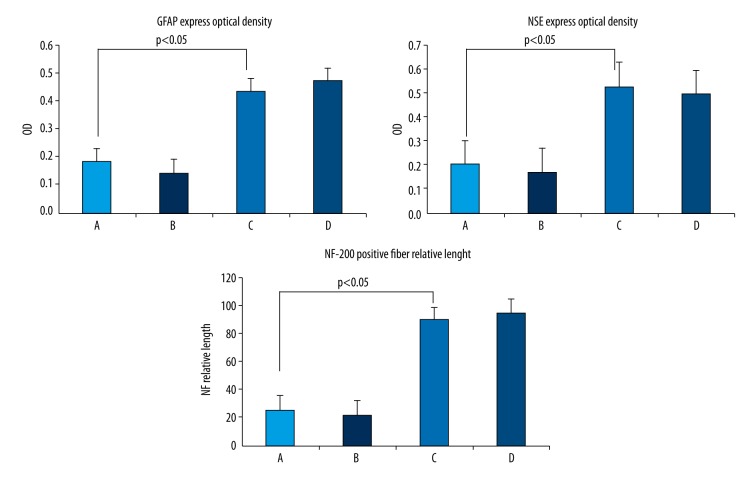
Four weeks after surgery, microscopy observation of immunohistochemically stained sections showed considerably increased GFAP expression in the SCI sites in group C and D that was significantly higher than that in group A and B (P<0.05) (Figure 7). Group C and D were not significantly different (P>0.05) (Figure 8).
Figure 7.
GFAP expression in the SCI area was significantly higher in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) than in PMSCs (A) and BMSCs (B). The blue nuclei are surrounded by the brown-stained cytoplasm. NSE expression in the SCI area was significantly higher in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs(D) than in PMSCs (A) and BMSCs (B). The blue nuclei are surrounded by brown-stained cytoplasm. The NF-200 nerve fibers in the SCI area were significantly longer in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) than in PMSCs (A) and BMSCs (B). GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) were not obviously different.
Figure 8.
The optical density of GFAP expression in the SCI area was higher in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) than in PMSCs (A) and BMSCs (B). The optical density of NSE expression in the SCI area was higher in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) than in PMSCs (A) and BMSCs (B). The relative length of NF-200–positive nerve fibers in the SCI area was greater in GDNF-transfected-PMSCs (C) and GDNF-transfected-BMSCs (D) than in PMSCs (A) and BMSCs (B).
Neuron-specific enolase (NSE) expression at SCI site
Four weeks after surgery, microscopy observation of immunohistochemically stained sections showed considerably increased NSE expression in the SCI sites in group C and D that was significantly higher than that in group A and B (P<0.05) (Figure 7). Group C and D were not significantly different (P>0.05) (Figure 8).
NF-200 expression at SCI site
Four weeks after surgery, microscopy observation of immunohistochemically stained sections showed significantly longer NF-200-positive nerve fibers in the SCI sites in group C and D than in group A and B (P<0.05) (Figure 7). Group C and D were not significantly different (P>0.05) (Figure 8).
Discussion
Recovering neurological function after spinal nerve injury remains a worldwide problem. In recent years, stem cell transplantation for treating neurological disease has gradually become a popular research topic. After glial precursor cell and Schwann cell transplantation to the SCI region, glial precursor cells can differentiate into oligodendrocytes and form new myelin over the damaged axons. Furthermore, SCI animals also recover nerve functions to a certain degree [21,22]. Some studies have also indicated that the transplantation of neural stem cells (NSC) to brain lesion regions is followed by increased expression of presynaptic protein and regeneration-associated protein at the injury site and significantly higher neuromotor function and selective behavior scores as compared to the control group [23].
MSCs were first isolated from bone marrow by Haynesworth et al. [24]. The cells have also been isolated from muscle, adipose tissue, periodontal ligament, peripheral blood, and other types of connective tissue [25–28]. MSCs have been isolated from amniotic fluid and the amnion and have demonstrated better amplification capability than BMSCs. PMSCs and BMSCs have similar phenotypes, such as positive CD166, CD105, CD29, and CD44 expression and negative CD117, CD45, CD34, and CD14 expression. They are also positive for human leukocyte antigen (HLA)-ABC expression, but negative for HLA-DR expression [29,30]. Some have reported that PMSCs have multiple differentiation potential and are able to differentiate into bone cells, fat cells, cartilage cells, liver cells, and pancreatic islet cells [31,32]. Our study confirms that PMSCs are similar to BMSCs, which have moderate positive expression (ranging 33.5–44.6%) of Oct-4, TRA-1-60, TRA-1-81, (SSEA)-1, SSEA-3, and SSEA-4, which are mainly expressed in embryonic stem cells [15]. The present study illustrates the fact that PMSC are primitive cells with multi-directional differentiation ability. As they are considered medical waste, there are fewer ethical restrictions and better practicability regarding their use in medical research; the placenta is viewed as an ideal source of MSCs, which can be used in clinical research and stem cell therapy.
In this study, SD rat PMSCs and BMSCs were isolated and cultivated successfully, presenting homogeneous fibroblast-like morphology and similar doubling times. Flow cytometry confirmed that the PMSCs and BMSCs have high CD29 and CD90 expression and low CD34 and CD45 expression, indicating successful transfection of the GDNF gene and good growth ability following transfection.
Neurotrophic factors include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and GDNF. Among them, GDNF was first discovered by Lin [16] as a target-derived neurotrophic factor that provides nutrition to specific neurons by retrograde transport or the paracrine effect. In nerve cell differentiation, GDNF stimulates neuron pathway survival and growth via the Ras/MARK pathway and induces neuronal lamellipodia formation through the phosphatidylinositol 3-kinase (P13K) signaling pathways, which are directly involved in the occurrence of axons and dopaminergic neuron differentiation. GDNF has tropism for many kinds of neurons [33–35], meaning it is a good prospect for treating neurological disorders.
The study of SCI has spanned a century. Many models have been created, including the cuts (transection/hemisection) model, the contusion and compression injury model, the ischemic injury model, and the photochemical damage model. Each model has a different focus and scope of application. Briefly, the cuts model is a cutting spinal cord transection injury wholly or in part, or even the removal of small pieces of the spinal cord, which is suitable for researching transplantation and regeneration in nerve tissue engineering [36]. However, it has drawbacks, such as poor concordance with clinical conditions, relatively demanding care, and high mortality of animal models [37]. The compression injury model can specifically simulate clinical disease subacute chronic SCI resulting from tumor compression and disc herniation, and has the advantage of more precise control of the area and extent of damage/injury; however, it fails to simulate SCIs with high clinical incidence and that are difficult to treat. The contusion model is a good mimic of SCI due to various traumas, which especially suits the variation of secondary pathophysiology after SCI [38,39]. In summary, the classic SCI model caused by heavy impact was chosen because of the simple equipment required, easy operation, and minor collateral injury. The impact apparatus used in this experiment is 10 mm high, weighs 10 g, and has a drop height of 1.25 cm. As the site and range of injuries in the modeling process can be controlled to maintain the integrity of the dura, no exogenous components can invade the damaged area, and exposure of the spinal cord and cerebrospinal fluid leakage can be prevented. Accordingly, the stability of the model can be ensured.
In recent years, many studies have found that stem cells can repair SCIs to some extent and improve neurological function after nerve injury. However, a series of problems exist, including survival of the transplanted stem cells at the injury site, the issue of directional differentiation, and integration of the structure and function between differentiation of nerve cells and host cells. A variety of strategies have been adopted to resolve these problems and have achieved good effects. First, stem cells are transplanted after pre-differentiation. Due to the pathophysiological microenvironment after SCI, the majority of stem cells differentiate into astrocytes with few neurons and oligodendrocytes after MSC transplantation into the spinal cord. Accordingly, in recent years, many studies have pre-differentiated MSCs into oligodendrocyte precursor cells or neuronal precursor cells [40], and then implanted them into injured spinal cords. The results show that this method promotes the recovery of spinal cord motor function. Second, transgenic stem cells are used for transplantation. The characteristics of stem cells mean that they are relatively easy to genetically modify. If gene transfection is successful, the stem cells can promote nerve cell survival and orientation differentiation, which will help solve existing problems in the current stem cell transplantation process. Recent studies on gene transfection have mainly involved GDNF, NGF, BDNF, NT-3, and molecules that can promote stem cell differentiation, such as Noggin and MASH1. Rat embryo-derived glial-limiting precursor cells were transfected with the BDNF and NT3 genes by retrovirus, and then implanted to the thoracic cord which had been injured 9 d before [41]. The implanted cells successfully differentiated into mature oligodendrocytes. Implanting stem cells transfected with the NT3 gene to chronic SCI that had formed a glial scar over 6 w also revealed that these cells can promote axon passage through the glial scar and reach the site of injury [42]. In the present study, PMSCs and BMSCs transfected GDNF by overexpressing lentivirus were implanted into SCI, and also achieved excellent nerve repair results. Moreover, cells United scaffolds were transplanted. After SCI, the injury site forms local tissue necrosis and chronic glial scars and cavities due to the primary mechanical damage and secondary pathological damage that constitute physical and chemical barriers to spinal cord regeneration. Therefore, filling scaffolds that can bridge the 2 parts of an injury into the site before glial scar and necrotic cavity formation can promote SCI repair. The scaffold can also prevent inflammatory cell invasion. Additionally, the modified scaffolds are beneficial for stem cell adhesion and nutritional factor agglomeration. A copolymer scaffold made of lactic acid and glycolic acid was first co-cultured with NSC for 4 d, and then was implanted to a T9/10 hemisection SCI. The cells in the scaffold were arranged in order and no scar tissue or aggregates formed.
The mechanism of PMSC differentiation into neurons is unclear. It may be a collective effect of a variety of mechanisms that alter PMSC expression. Subsequently, gene expression in the neural ectoderm is activated, resulting in PMSC differentiation into neurons. In recent years, studies have found that the Wnt/β-catenin pathway may play a regulatory role in PMSC differentiation into neuronal cells [43]. We found that PMSCs transplanted with the GDNF gene can promote nerve regeneration and significantly improve motor function.
Overall, several theories explain the main mechanism of MSC reduction of nerve damage, promotion of neuroprotection, and nerve regeneration. First, MSCs differentiate into nervous system cells after transplantation, which can take the place of apoptotic and necrotic neurons to promote axon myelination [44]. Second, MSCs can inhibit microglia activation after nerve injury, which can reduce the immune and inflammatory response [45]. Third, the secreted cytokines and neurotrophic factors, such as GDNF, NT-3, interleukin (IL)-6, BDNF, basic fibroblast growth factor (bFGF), and glucocorticoid-induced tumor necrosis factor-3 (TNF-3), have a neuroprotective effect [46,47]. Fourth, MSCs promote angiogenesis, on the one hand secreting vascular endothelial growth factor receptor-3 (VEGFR-3) and on the other hand differentiating into endothelial cells [45]. Lastly, MSCs may play a role in endogenous NSC activation and proliferation. These factors may act synergistically rather than alone [48]. Although considerable improvement has been made in using stem cells for treating SCI, where there is improved motor and sensory function after SCI, many remaining issues require further study. First, most studies are based on immunochemical methods to track stem cell proliferation and differentiation, lacking direct evidence proving that transplanted stem cells express voltage-gated ion channels, generate action potential, and have direct functional integration with the host spinal cord. Second, many measures play a role in promoting transplanted stem cell survival, differentiation, and physiological function; however, the efficiency remains relatively low in clinical terms. Further study would still focus on how efficiency can be improved.
Conclusions
In this study, we demonstrate that PMSCs have similar biological properties to BMSCs and can be successfully transfected with the GDNF gene. After transplantation, PMSCs can promote nerve regeneration and significantly improve motor function. Therefore, PMSCs can be used as a new source of cells in clinical and experimental research on nerve damage.
Acknowledgements
The authors are grateful to Li Ma for her technical assistance. We are also grateful to Prof. Quan Zhang, Comparative Medicine Center, Yangzhou University, for his guidance in the experiments.
Abbreviations
- BMSCs
bone marrow-derived mesenchymal stem cells
- PMSCs
placenta-derived mesenchymal stem cells
- GDNF
glial cell-derived neurotrophic factor
- GFP
green fluorescent protein
- SCI
spinal cord injury
- DMEM
Dulbecco’s modified Eagle’s medium
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
Footnotes
Competing of interests
None.
Source of support: Departmental sources
References
- 1.Anderberg L, Aldskogius H, Holtz A. Spinal cord injury – scientific challenges for the unknown future. Ups J Med Sci. 2007;112:259–88. doi: 10.3109/2000-1967-200. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell CS, Lee RH. Pathology dynamics predict spinal cord injury therapeutic success. J Neurotrauma. 2008;25:1483–97. doi: 10.1089/neu.2008.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae JS, Han HS, Youn DH. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells. 2007;5:1307–16. doi: 10.1634/stemcells.2006-0561. [DOI] [PubMed] [Google Scholar]
- 4.Dezawa M, Ishikawa H, Hoshino M. Potential of bone marrow stromal cells in applications for neuro-degenerative, neuro-traumatic and muscle degenerative disease. Curr Neuropharmacol. 2005;4:257–66. doi: 10.2174/157015905774322507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu D, Li Y, Wang L. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;8:813–19. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–47. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–30. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: Paradoxes of passaging. Exp Hematol. 2004;32:414–25. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Barry FP, Murphy JM. Mesenchymal stem cells: Clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 10.In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 11.Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–75. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 12.Fu YS, Cheng YC, Lin MY, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: Potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–24. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell KE, Weiss ML, Mitchell BM, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 14.Miao Z, Jin J, Chen L, et al. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–87. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Huang YC, Yang ZM, Chen XH, et al. Isolation of mesenchymal stem cells from human placenta decidua basalis and resistance to hypoxia and serum deprivation. Stem Cell Rev. 2009;5:247–55. doi: 10.1007/s12015-009-9069-x. [DOI] [PubMed] [Google Scholar]
- 16.Lin LF, Doherty DH, Lile JD, et al. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–32. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 17.Garbayo E, Montero-Menei CN, Ansorena E, et al. Effective GDNF brain delivery using microspheres – a promising strategy for Parkinson’s disease. J Control Release. 2009;135:119–26. doi: 10.1016/j.jconrel.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Deng LX, Hu JG, Liu NK, et al. GDNF modifies reactive astrogliosis allowing robust axonal regenerative through Schwann cell-seeded guidance channels after spinal cord injury. Exp Neurol. 2011;2:238–50. doi: 10.1016/j.expneurol.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang S, Gao Q, Bao L, et al. Striatal extracts promote the dopaminergic differentiation of GFP-bone mesenchymal stem cells. Neurosci Lett. 2012;2:115–20. doi: 10.1016/j.neulet.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 20.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU Weight-Drop device versus transection. Exp Neurol. 1996;139:244–56. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 21.Amemori T, Jendelová P, Růzicková K, et al. Co-transplantation of olfactory ensheathing glial and mesenchymal stromal cells does not have synergistic effects after spinal cord injury in the rat. Cytotherapy. 2010;12:212–25. doi: 10.3109/14653240903440103. [DOI] [PubMed] [Google Scholar]
- 22.Amenmori T, Romanyuk N, Jendelova P, et al. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res Ther. 2013;4:68–83. doi: 10.1186/scrt219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma H, Yu B, Kong L, et al. Transplantation of neural stem cells enhances expression of synaptic protein and promotes functional recovery in a rat model of traumatic brain injury. Mol Med Rep. 2011;5:849–56. doi: 10.3892/mmr.2011.510. [DOI] [PubMed] [Google Scholar]
- 24.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 25.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 26.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trubiani O, Di Primio R, Traini T, et al. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol. 2005;18:213–21. doi: 10.1177/039463200501800204. [DOI] [PubMed] [Google Scholar]
- 28.Shih DT, Lee DC, Chen SC, et al. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005;23:1012–20. doi: 10.1634/stemcells.2004-0125. [DOI] [PubMed] [Google Scholar]
- 29.Yen BL, Huang HI, Chien CC, et al. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 30.Fukuchi Y, Nakajima H, Sugiyama D, et al. Human placenta-derived cells have mesenchymal stem ⁄ progenitor cell potential. Stem Cells. 2004;22:649–58. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 31.Chien CC, Yen BL, Lee FK, et al. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells. 2006;24:1759–68. doi: 10.1634/stemcells.2005-0521. [DOI] [PubMed] [Google Scholar]
- 32.Chang CM, Kao CL, Chang YL, et al. Placenta-derived multipotent stem cells induced to differentiate into insulin-positive cells. Biochem Biophys Res Commun. 2007;357:414–20. doi: 10.1016/j.bbrc.2007.03.157. [DOI] [PubMed] [Google Scholar]
- 33.Iwakawa M, Mizoi K, Tessler A, Itoh Y. Intraspinal implants of fibrin glue containing glial cell line-derived neurotrophic factor promote dorsal root regeneration into spinal cord. Neurorehabil Neural Repair. 2001;15:173–82. doi: 10.1177/154596830101500304. [DOI] [PubMed] [Google Scholar]
- 34.Iwase T, Jung CG, Bae H, et al. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J Neurochem. 2005;94:1488–99. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- 35.Anastasía A, Wojnacki J, de Erausquin GA, Mascó DH. Glial cell-line derived neurotrophic factor is essential for electroconvulsive shock-induced neuroprotection in an animal model of Parkinson’s disease. Neuroscience. 2011;195:100–11. doi: 10.1016/j.neuroscience.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Xu XM, Guenard V, Kleitman N, et al. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–60. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 37.Talae R, Friedman JA, Moore MJ, et al. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25:1505–10. doi: 10.1016/s0142-9612(03)00497-6. [DOI] [PubMed] [Google Scholar]
- 38.Kwon BK, Oxland TR, Tetzlaff W. Animal models used in spinal cord regeneration researeh. Spine. 2002;27:1504–10. doi: 10.1097/00007632-200207150-00005. [DOI] [PubMed] [Google Scholar]
- 39.Metz GA, Curt A, van de Meent H, et al. Validation of the weight-drop contusion model in rats: A comparative study of human spinal cord injury. J Neurotrauma. 2000;17:1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- 40.Hendricks WA, Pak ES, Owensby JP, et al. Predifferentiated embryonic stem cells prevent chronic pain behaviors and restore sensory function following spinal cord injury in mice. Mol Med. 2006;12:34–46. doi: 10.2119/2006-00014.Hendricks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Q, Xu XM, Devries WH, et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–57. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu P, Jones LL, Tuszynski MH, et al. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203:8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 43.Teng YD, Lavik EB, Qu X, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99:3024–29. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Zhao W, Liu W, et al. Transplantation of placenta-derived mesenchymal stem cell-induced neuralstem cells to treat spinal cord injury. Neural Regen Res. 2014;9:2197–204. doi: 10.4103/1673-5374.147953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding DC, Shyu WC, Chiang MF, et al. Enhancement of neuroplasticity through up regulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27:339–53. doi: 10.1016/j.nbd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Yang CC, Shih YH, Ko MH, et al. Transplantation of human umbilical mesenchymal stem cells from wharton’s jelly after complete transection of the rat spinal cord. PLoS One. 2008;3(10):e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song S, Kamath S, Mosquera D, et al. Expression of brain natriuretic peptide by human bone marrow stromal cells. Exp Neurol. 2004;185:191–97. doi: 10.1016/j.expneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Keirstead HS, Coutts M. Stem cells for the treatment of spinal cord injury. Exp Neurol. 2008;209:368–77. doi: 10.1016/j.expneurol.2007.09.002. [DOI] [PubMed] [Google Scholar]