Abstract
Aims
To determine tolerability and the optimal dose regimen of the soluble guanylate cyclase stimulator vericiguat in patients with chronic heart failure and preserved ejection fraction (HFpEF).
Methods and results
SOCRATES-PRESERVED was a prospective, randomized, placebo-controlled double-blind, Phase 2b dose-finding study in patients with HFpEF (ejection fraction ≥ 45%). Patients received vericiguat once daily at 1.25 or 2.5 mg fixed doses, or 5 or 10 mg titrated from a 2.5 mg starting dose, or placebo for 12 weeks. The two primary endpoints were change from baseline in log-transformed N-terminal pro-B-type natriuretic peptide (NT-ProBNP) and left atrial volume (LAV) at 12 weeks. Patients (N = 477; 48% women; mean age 73 ± 10 years; baseline atrial fibrillation 40%) were randomized within 4 weeks of HF hospitalization (75%) or outpatient treatment with intravenous diuretics for HF (25%) to vericiguat (n = 384) or placebo (n = 93). In the pooled three highest dose arms change in logNT-proBNP (vericiguat: +0.038 ± 0.782 log(pg/mL), n = 195; placebo: −0.098 ± 0.778 log(pg/mL), n = 73; one-sided P = 0.8991, two-sided P = 0.2017), and change in LAV [vericiguat: −1.7 ± 12.8 mL (n = 194); placebo: −3.4 ± 12.7 mL (n = 67), one-sided P = 0.8156, two-sided P = 0.3688] were not different from placebo. Vericiguat was well tolerated (adverse events: vericiguat 10 mg arm, 69.8%; placebo, 73.1%), with low discontinuation rates in all groups, and no changes in blood pressure at 10 mg compared with placebo. The pre-specified exploratory endpoint of Kansas City Cardiomyopathy Questionnaire Clinical Summary Score improved in the vericiguat 10 mg arm by mean 19.3 ± 16.3 points [median 19.8 (interquartile range 10.4–30.7)] from baseline (mean difference from placebo 9.2 points).
Conclusion
Vericiguat was well tolerated, did not change NT-proBNP and LAV at 12 weeks compared with placebo but was associated with improvements in quality of life in patients with HFpEF. Given the encouraging results on quality of life, the effects of vericiguat in patients with HFpEF warrant further study, possibly with higher doses, longer follow-up and additional endpoints.
Keywords: Heart failure with preserved ejection fraction, Soluble guanylate cyclase stimulator, Vericiguat
Introduction
Insufficient generation of cyclic guanosine monophosphate (cGMP) by soluble guanylate cyclase (sGC) may contribute to the pathophysiology of heart failure with preserved ejection fraction (HFpEF) via cardiac, vascular, and peripheral mechanisms.1,2 Direct stimulators of sGC differ from other agents targeting the cGMP pathway in their nitric oxide (NO)-independent capacity to increase sGC activity,3 and could be promising therapeutic agents in HFpEF.2 New agents are particularly important in the setting of worsening chronic heart failure (HF) requiring hospitalization where, after discharge, cardiovascular event rates are high4–7 and there are no evidence-based therapies for patients with HFpEF.8
The novel, once-daily sGC stimulator vericiguat was studied in the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES) programme, which consisted of two parallel Phase 2 dose-finding studies in patients stabilized after hospitalization or IV diuretic therapy for HF in patients with heart failure with reduced ejection fraction (HFrEF, SOCRATES-REDUCED) and SOCRATES-PRESERVED in patients with HFpEF.9 In SOCRATES-REDUCED, vericiguat added on top of standard of care showed a clinically significant reduction in N-terminal pro-B-type natriuretic peptide (NT-proBNP) and a trend towards less hospitalizations for HF, supporting further exploration in a Phase 3 study.10 Conducted in parallel, the SOCRATES-PRESERVED study aimed to characterize the safety, tolerability, and pharmacodynamic effects of vericiguat in patients with HFpEF.9 In the absence of previous successful clinical outcome trials in HFpEF, no short-term predictors of long-term efficacy have been established.11 NT-proBNP and left atrial volume (LAV) were chosen as two primary endpoints, combining NT-proBNP as a marker of short-term wall stress and LAV as a measure of chronic elevations in left ventricular filling pressures.9
Methods
Study design and patient population
SOCRATES-PRESERVED was a multinational, randomized, double-blind, placebo-controlled, dose-finding clinical trial in patients ≥ 18 years of age with symptomatic worsening chronic HF and left ventricular ejection fraction (LVEF) ≥ 45%. Patients were enrolled within 4 weeks of clinical stabilization following HF hospitalization or IV diuretic treatment for worsening HF. The full study design has been described previously.9 Briefly, patients with HF classified as New York Heart Association (NYHA) class II–IV, with LVEF ≥ 45%, increased B-type natriuretic peptide (BNP) levels ≥ 100 pg/mL or NT-proBNP levels ≥ 300 pg/mL, (or BNP ≥200 pg/mL or NTproBNP ≥ 600 pg/mL if patients were in atrial fibrillation) at randomization, and with left atrial enlargement determined by echocardiography, were randomized 1:1:1:1:1 to 12 weeks of treatment with one of four vericiguat regimens (1.25, 2.5, 5, or 10 mg target doses) or placebo. Randomization was stratified by sinus rhythm vs. atrial fibrillation from an electrocardiogram (ECG) performed at baseline.
There were two fixed-dose vericiguat treatment arms (1.25 mg or 2.5 mg) and two up-titrated treatment arms (2.5–5 mg and 2.5–10 mg). The dose was increased by two blinded dose-doublings or sham titration at Week 2 (visit 2) and Week 4 (visit 3), according to blood pressure and tolerability, to reach target doses of 5 mg or 10 mg once a day. Patients received sham titrations when randomized to the 1.25 mg or 2.5 mg fixed dose arms.
A major protocol violation occurred during the study owing to an erroneous software update of the drug dispensation system (Interactive voice/web response system, IxRS), resulting in assignment of incorrect doses of study drug to patients in the 5 mg and 10 mg target dose groups, who consequently received lower doses than planned. The study complied with the Declaration of Helsinki and the approval for this study was obtained from all required ethical committees and regulatory authorities. All patients provided written informed consent.
Study endpoints
The two primary endpoints were change from baseline to Week 12 in log-transformed NT-proBNP and change from baseline to Week 12 in LAV. LAV and other echocardiographic parameters were centrally analysed by the cardiac imaging core lab at Charité Berlin (EP-K). Exploratory efficacy endpoints included assessments of patients’ health status by the disease-specific Kansas City Cardiomyopathy Questionnaire [KCCQ] and the generic health-related quality of life EQ-5D instrument,12 mortality and morbidity, and echocardiography at rest.
Statistical analysis
For the pre-specified analysis of the two primary endpoints, the three highest vericiguat dose arms (2.5 mg, 2.5–5 mg, and 2.5–10 mg) were pooled with the intention of increasing power for the detection of significant differences compared with placebo using pre-specified one-sided two-sample t-tests. The 1.25 mg dose arm was assumed to have no or minimal effect and was not included in the pool of presumed effective dose arms. The Hochberg procedure was used in order to determine whether either of the two primary endpoints was significant. The Hochberg procedure is a multiplicity adjustment that allows testing of the two primary endpoints in a way that the study is positive if at least one of the two primary endpoints meets the pre-specified significance level in the primary analysis. Either both tests need to be significant at the one-sided alpha level of 5% (two-sided 10%), or one test needs to be significant at the one-sided 2.5% level (two-sided 5%). The study was planned to detect a difference in at least one of the two primary endpoints with 90% power, assuming independence between the two primary endpoints.
The full analysis set (FAS) included all patients randomized to treatment and was used to display baseline characteristics. The safety analysis set (SAF) included all patients in the FAS who received at least one dose of the study drug. For the analyses of exploratory efficacy endpoints, patients with incorrectly assigned doses were excluded from the FAS (Figure 1), because the lower doses they received were disconnected from the randomized dose regimen, as well as from dose-titration principles. The per protocol sets (PPS) included all patients randomized to treatment who were alive with valid measurement of NT-proBNP at baseline and Week 12, or of LAV at baseline and Week 12, and had no major protocol deviations. The PPS were used for primary and secondary efficacy analyses of the primary endpoints. All other efficacy analyses were exploratory and confidence intervals are provided as indicated. No multiplicity adjustment was performed for exploratory efficacy analyses. Data are summarized by mean ± standard deviation or mean ± standard error as indicated, or median (interquartile range) for continuous data, and by frequencies and percentages for categorical data. Between-group comparisons were performed by t-tests or Wilcoxon rank-sum tests as indicated. Dose–response was assessed by linear regression models. SAS (version 9.2) was used for all analyses.
Figure 1.
Patient flow and validity of analysis sets. Detailed reasons for exclusion from per protocol sets are provided in Supplementary material online, Table S1. BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; FAS, full analysis set; LAV, left atrial volume; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SAF, safety analysis set.
Results
Of 632 patients screened, 477 patients were randomized (Figure 1) between November 2013 and May 2015 at 145 sites in Europe, North America, and Asia. Patients were randomized at 12.9 ± 9.0 (mean ± SD) days after clinical stabilization following hospitalization for worsening chronic heart failure (n = 358) or based on outpatient treatment with IV diuretics for worsening chronic HF (n = 117) (Table 1). For primary endpoint analysis, 345 patients (72% of the total randomized population) fulfilled validity criteria for per protocol analysis of NT-proBNP, and 338 patients (71%) fulfilled validity criteria for per protocol analysis of LAV (Figure 1, see Supplementary material online, Table S1). Owing to the IxRS error, 48 patients (5 mg group, n = 20; 10 mg group, n = 28) received lower than planned study drug doses and were excluded from analysis in the main results section (Figure 1). Actual doses of study drug at, and after, the Week 8 visit in this analysis set are shown in Supplementary material online, Table S2. In 8 patients (1.7%), vital status was not known at the end of the study.
Table 1.
Baseline characteristics (full analysis set)
| Placebo |
Vericiguat |
|||||
|---|---|---|---|---|---|---|
| 1.25 mg | 2.5 mg | 2.5–5 mg | 2.5–10 mg | Total | ||
| (n = 93) | (n = 96) | (n = 96) | (n = 96) | (n = 96) | (n = 477) | |
| Age (years), mean (SD) | 74 (9) | 74 (10) | 72 (11) | 74 (8) | 73 (10) | 73 (10) |
| Female gender, N (%) | 46 (49.5%) | 51 (53.1%) | 43 (44.8%) | 43 (44.8%) | 44 (45.8%) | 227 (47.6%) |
| Baseline body mass index (kg/m2), mean (SD) | 30.1 (6.5) | 29.6 (6.5) | 30.7 (6.3) | 30.1 (5.6) | 30.4 (5.0) | 30.2 (6.0) |
| LVEF (%), median (IQR) | 57 (53–62) | 56 (53–60) | 57 (52–62) | 58 (53–62) | 56 (53–60) | 57 (53–61) |
| Initial worsening HF presentation, N (%) | ||||||
| Hospitalization | 72 (77.4%) | 73 (76.0%) | 68 (70.8%) | 75 (78.1%) | 70 (72.9%) | 358 (75.1%) |
| Intravenous diuretic | 20 (21.5%) | 23 (24.0%) | 27 (28.1%) | 21 (21.9%) | 26 (27.1%) | 117 (24.5%) |
| Missing | 1 (1.1%) | 0 | 1 (1.0%) | 0 | 0 | 2 (0.4%) |
| Time from stabilization to randomization (days), mean (SD) | 11.9 (10) | 11.9 (8.6) | 12.6 (8.0) | 14.6 (8.9) | 13.7 (9.5) | 12.9 (9.0) |
| NYHA Class III/IV, N (%) | 39 (41.9%) | 46 (47.9%) | 44 (45.8%) | 41 (42.7%) | 46 (47.9%) | 216 (45.3%) |
| KCCQ-CSS, mean (SD) | 54.1 (23.0) | 56.0 (22.5) | 57.3 (22.3) | 55.7 (24.2) | 53.1 (21.5) | 55.2 (22.6) |
| EQ-5D US index score, mean (SD) | 0.73 (0.21) | 0.71 (0.20) | 0.72 (0.20) | 0.73 (0.21) | 0.72 (0.21) | 0.72 (0.20) |
| Systolic blood pressure (mmHg), mean (SD) | 133 (15) | 133 (14) | 132 (15) | 131 (14) | 134 (13) | 133 (14) |
| Heart rate (bpm), mean (SD) | 68 (11) | 69 (11) | 71 (12) | 70 (12) | 70 (12) | 70 (12) |
| NT-proBNP (pg/mL), median (IQR) | 975 (531–2576) | 1161 (401–2568) | 1140 (393–2271) | 1343 (358–3399) | 1458 (470–2653) | 1174 (433–2576) |
| LAV (mL), mean (SD) | 88 (47) | 88 (44) | 87 (31) | 84 (30) | 85 (26) | 86 (36) |
| Diabetes mellitus, N (%) | 47 (50.5%) | 48 (50.0%) | 46 (47.9%) | 47 (49.0%) | 44 (45.8%) | 232 (48.6%) |
| eGFR (mL/min/1.73m2), mean (SD), | 52.3 (20.6) | 52.8 (23.0) | 57.4 (20.8) | 54.2 (17.3) | 57.4 (19.3) | 54.8 (20.3) |
| Atrial fibrillation in baseline ECG, N (%) | 35 (37.6%) | 41 (42.7%) | 40 (41.7%) | 38 (39.6%) | 36 (37.5%) | 190 (39.8%) |
| Baseline therapies, N (%) | ||||||
| Diuretics | 85 (91.4%) | 91 (94.8%) | 85 (88.5%) | 88 (92.6%) | 90 (93.8%) | 439 (92.2%) |
| Beta-Blockers | 76 (81.7%) | 73 (76.0%) | 76 (79.2%) | 73 (76.8%) | 82 (85.4%) | 380 (79.8%) |
| ACE inhibitor | 40 (43.0%) | 42 (43.8%) | 41 (42.7%) | 33 (34.7%) | 35 (36.5%) | 191 (40.1%) |
| ARB | 32 (34.4%) | 32 (33.3%) | 33 (34.4%) | 31 (32.6%) | 34 (35.4%) | 162 (34.0%) |
| MRA | 39 (41.9%) | 37 (38.5%) | 34 (35.4%) | 35 (36.8%) | 33 (34.4%) | 178 (37.4%) |
| Calcium channel blocker | 30 (32.3%) | 38 (39.6%) | 40 (41.7%) | 30 (31.6%) | 33 (34.4%) | 171 (35.9%) |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; bpm, beats per minute; CSS, Clinical Summary Score; eGFR, estimated glomerular filtration rate; EQ-5D, 5-dimension EuroQol questionnaire; IQR, interquartile range; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LAV, left atrial volume; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
Baseline characteristics and medication use in the FAS are shown in Table 1 (for additional baseline characteristics see Supplementary material online, Table S3). At baseline, almost all (92%) patients were receiving diuretics, 40% angiotensin-converting enzyme (ACE) inhibitors, 34% angiotensin II receptor blockers, 80% β-blockers, 37% mineralocorticoid receptor antagonists, and 36% calcium channel blockers. Baseline NT-proBNP was high, with a median (interquartile range) of 1174 pg/mL (433–2576) in the entire group; levels were highest in the 10 mg target dose arm (median 1458 pg/mL), and lowest in the placebo group (median 975 pg/mL). In line with the higher cut-off for patients in atrial fibrillation at baseline, NT-proBNP at baseline [median (IQR)] was 1983 pg/mL (1170–3754) in these patients compared with 650 pg/mL (279–1619) in patients in sinus rhythm. Baseline LAV was large with a mean of 86 ± 36 mL in the entire group. Clinical characteristics were generally well balanced across treatment groups at baseline (Table 1, see Supplementary material online, Table S3).
Primary endpoints
In the respective PPS, the changes in logNT-proBNP and LAV from baseline to 12 weeks were small and did not differ in the primary analysis between the pooled three highest vericiguat dose arms and placebo or in the pre-specified secondary analyses between any vericiguat group and placebo (Table 2).
Table 2.
Primary endpoints [per protocol analysis (PPS-NT-proBNP and PPS-LAV)]
| Primary analysisa | Baseline | 12 weeks (Visit 5) | Treatment comparison |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Mean change from baseline (SD) | Difference (Treat-Plac) [Back- transformedb] | 90% Confidence interval [Back- transformedb] |
P-valuec |
||||
| One- sided | Two- sided | ||||||||
| LAV (mL) | Placebo | 67 | 89.075 (51.059) | −3.361 (12.654) | |||||
| Pooled 2.5/5/10 mg | 194 | 87.083 (30.204) | −1.732 (12.808) | 1.6291 | −1.36 to 4.62 | 0.8156 | 0.3688 | ||
| log(NT-proBNP) [log(pg/mL)] | Placebo | 73 | 6.897 (1.203) | −0.098 (0.778) | |||||
| Pooled 2.5/5/10 mg | 195 | 6.945 (1.297) | 0.038 (0.782) | 0.1372 [1.147] | −0.04 to 0.31 [0.96–1.37] | 0.8991 | 0.2017 | ||
| Secondary analysisd | Baseline | 12 weeks (visit 5) |
Treatment comparison |
Regressione | |||||
| n | Mean (SD) | Mean change from baseline (SD) | Difference (Treat-Plac) [Back- transformedb] | 95% Confidence interval [Back- transformedb] |
P-valuec |
Slope (one-sided P-value; two-sided P-valuec) | |||
| One- sided | Two- sided | ||||||||
| LAV (mL) | 2.5–10 mg | 59 | 87.741 (27.027) | −1.654 (10.245) | 1.7071 | −2.39 to 5.80 | 0.7945 | 0.4109 | 0.141 (0.7722; 0.4555) |
| 2.5–5 mg | 57 | 86.662 (32.598) | −1.252 (16.139) | 2.1093 | −3.01 to 7.23 | 0.7917 | 0.4165 | ||
| 2.5 mg | 78 | 86.892 (31.033) | −2.142 (11.931) | 1.2192 | −2.82 to 5.26 | 0.7241 | 0.5518 | ||
| 1.25 mg | 77 | 89.464 (46.805) | −2.163 (7.895) | 1.1983 | −2.23 to 4.63 | 0.7546 | 0.4908 | ||
| log(NT-proBNP) [log(pg/mL)] | 2.5–10 mg | 60 | 7.170 (1.240) | −0.023 (0.705) | 0.0758 [1.079] | −0.18 to 0.33 [0.83–1.40] | 0.7194 | 0.5611 | 0.006 (0.6809; 0.6383) |
| 2.5–5 mg | 57 | 7.025 (1.372) | 0.057 (0.819) | 0.1561 [1.169] | −0.12 to 0.43 [0.88–1.54] | 0.8653 | 0.2695 | ||
| 2.5 mg | 78 | 6.713 (1.262) | 0.071 (0.818) | 0.1706 [1.186] | −0.09 to 0.43 [0.92–1.53] | 0.9041 | 0.1917 | ||
| 1.25 mg | 77 | 6.824 (1.498) | −0.047 (0.788) | 0.0519 [1.053] | −0.20 to 0.30 [0.82–1.36] | 0.6572 | 0.6855 | ||
LAV, left atrial volume; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PPS, per-protocol set; SD, standard deviation.
Assessed by Hochberg procedure.
Back-transformation from logarithmic to original scale. This is equivalent to the ratio of geometric means on the original scale.
t-test.
Secondary analyses are exploratory only due to non-significance of the primary analysis. All secondary tests were to be assessed at the one-sided 2.5% level (two-sided 5%).
Linear regression with dose group as explanatory variable.
Adjustment of changes in NT-proBNP and LAV by baseline values did not reveal any treatment effects compared with placebo (see Supplementary material online, Table S4). Exploratory subgroup analyses of both primary endpoints by baseline heart rhythm (atrial fibrillation vs. no atrial fibrillation) showed no major differences in the pooled treatment groups from the placebo group (see Supplementary material online, Table S5).
Exploratory endpoints
Changes in patient-reported outcomes (PRO) were pre-specified as a key exploratory outcome.9 Results for the KCCQ Clinical Summary Score (CSS), which quantifies patients’ perceptions of their symptoms and physical limitations, are shown in Table 3. The CSS improved in a time- and dose-dependent fashion, with a clinically meaningful difference of ≥ 5 points13 in the change from baseline to 12 weeks in the 10 mg target dose arm compared with placebo. Changes from week 4 are also reported in Table 3 to account for the initial titration period. These effects were consistent with changes in other scores and subdomains of the KCCQ, the summary score of the EQ-5D and the Euroqol visual analog scale, and trends in NYHA class as well as clinical signs of congestion. For detailed results of KCCQ scores and other PRO variables see parallel publication.12
Table 3.
Kansas City cardiomyopathy questionnaire-clinical summary score (full analysis set excluding patients with incorrectly assigned doses)
| Baseline |
12 weeks (Visit 5) |
Treatment comparison |
Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) |
Mean change from | Mean change from | Change at 12 weeks | Change between | Slope (SD), P-valueb | ||||||
| baseline (SD) |
Week 4 (SD) |
from baseline |
4 and 12 weeks |
||||||||
| Difference (Treat-Plac) | P-valuea | Difference (Treat-Plac) | P-valuea | ||||||||
| n | n | n | |||||||||
| 2.5–10 mg | 68 | 52.3 (20.4) | 61 | 19.3 (16.3) | 60 | 6.2 (15.7) | 9.2 | 0 .016 | 5.7 | 0.0465 | 0.92 (0.29), P=0.0017 |
| 2.5–5 mg | 75 | 52.9 (24.0) | 61 | 12.3 (18.9) | 60 | 7.4 (13.6) | 2.1 | 0 .5065 | 6.9 | 0.0046 | |
| 2.5 mg | 95 | 57.3 (22.3) | 83 | 8.7 (18.4) | 83 | 2.6 (15.7) | −1.4 | 0 .2897 | 2.1 | 0.4468 | |
| 1.25 mg | 96 | 56.0 (22.5) | 82 | 11.4 (19.1) | 81 | 3.4 (15.8) | 1.3 | 0 .5802 | 2.9 | 0.2445 | |
| Placebo | 92 | 54.1 (23.0) | 78 | 10.2 (20.0) | 79 | 0.5 (14.1) | |||||
SD, standard deviation.
Non-parametric Wilcoxon rank-sum test.
Linear regression with dose group as explanatory variable.
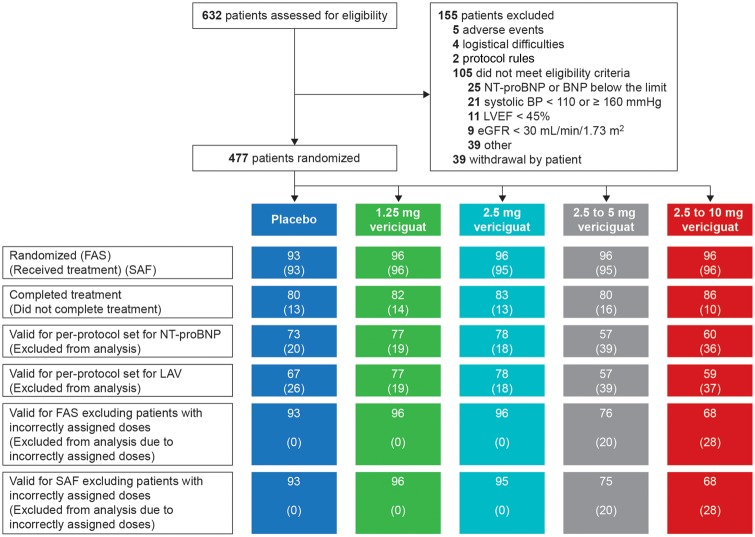
Echocardiographic parameters of cardiac function and structure at rest were pre-specified as exploratory outcomes. Descriptive analysis of more than 80 directly measured or derived variables was considered for hypothesis generation. Variables were selected in line with previous publications of HFpEF study results and are presented in Figure 2 and Supplementary material online, Table S6. General trends observed included increased transmitral diastolic left ventricular (LV) inflow (mitral E velocity) and increased LV relaxation (mitral e′ velocity) from baseline to 12 weeks with treatment.
Figure 2.
Echocardiography at rest. Full analysis set excluding patients with incorrectly assigned doses. Data are displayed as mean ± standard error. (A) E wave peak velocity antegrade flow (mitral valve early diastolic filling, Doppler echo of LV diastolic inflow pattern),*1 difference relative to placebo: 9 cm/s (95% CI 2 to 17 cm/s); (B) medial e′ (mitral valve medial annular peak early diastolic tissue velocity, tissue Doppler imaging of LV relaxation),*2 baseline-adjusted linear regression slope 0.08 cm/s (95% CI 0.02 to 0.14 cm/s); (C) lateral e′ (mitral valve lateral annular peak early diastolic tissue velocity). CI, confidence interval; LV, left ventricular.
No trends were observed at 12 weeks in the biomarkers osteopontin, TIMP, cGMP, Procollagen III N-terminal propetide, GDF-15, sST2 or Gal-3 (see Supplementary material online, Table S7).
In the FAS, the incidence of the composite of HF hospitalization and cardiovascular (CV) death at 12 weeks was 7.5% in the placebo arm, and 6.3% in the vericiguat 1.25 mg, 11.5% in 2.5 mg, 7.3% in the 5 mg, and 5.2% in the 10 mg target dose arms. CV-specific and all-cause mortality were 1.5 and 2.3%, respectively, for the period from baseline to the end of follow-up at 16 weeks in the FAS. Deaths in all treated patients are presented in Table 4. In the SAF, there were two deaths reported due to ‘worsening heart failure’. One was in the placebo group and the other was in the 2.5–10 mg arm. In the 10 mg group, a death due to ‘pneumonia’ was also reported, and no cause of death was reported more than once in the vericiguat arms: no death in the 1.25 mg arm, one death in the 2.5 mg arm (‘multi-organ failure’), occurring 35 days after discontinuation of study drug, and seven deaths in the 5 mg arm with different causes (‘myocardial infarction’, ‘cardiogenic shock’, ‘sudden death’, ‘acute kidney injury’, ‘respiratory failure’, ‘circulatory collapse’, and ‘suicide’). In all but one patient (‘sudden death’), death occurred after previous cessation of study drug for 2–35 days. Considering the similar death rates in the placebo and vericiguat dose groups, and the overall low number of deaths, there is no trend towards an increase in mortality compared with placebo.
Table 4.
Adverse events (safety analysis set and #, safety analysis set excluding patients with incorrectly assigned doses)
| Placebo |
Vericiguat |
||||||
|---|---|---|---|---|---|---|---|
| 1.25 mg | 2.5 mg | 2.5–5 mg | 2.5–5 mg | 2.5–10 mg | 2.5–10 mg | ||
| N = 93 (100%) | N = 96 (100%) | N = 95 (100%) | N = 95 (100%) | N = 75# (100%) | N = 96 (100%) | N = 68# (100%) | |
| Number of patients with adverse events | |||||||
| Any AE | 68 (73.1%) | 67 (69.8%) | 65 (68.4%) | 73 (76.8%) | 61 (81.3%) | 67 (69.8%) | 46 (67.6%) |
| Any study drug-related AE | 13 (14.0%) | 20 (20.8%) | 10 (10.5%) | 20 (21.1%) | 17 (22.7%) | 13 (13.5%) | 9 (13.2%) |
| AE with outcome death | 1 (1.1%) | 0 | 1 (1.1%) | 7 (7.4%) | 7 (9.3%) | 2 (2.1%) | 1 (1.5%) |
| Any SAE | 26 (28.0%) | 23 (24.0%) | 29 (30.5%) | 24 (25.3%) | 22 (29.3%) | 24 (25.0%) | 17 (25.0%) |
| Any study drug-related SAE | 2 (2.2%) | 1 (1.0%) | 1 (1.1%) | 1 (1.1%) | 1 (1.3%) | 1 (1.0%) | 1 (1.5%) |
| Discontinuation of study drug due to AE | 3 (3.2%) | 4 (4.2%) | 8 (8.4%) | 6 (6.3%) | 5 (6.7%) | 5 (5.2%) | 4 (5.9%) |
| Discontinuation of study drug due to SAE | 2 (2.2%) | 2 (2.1%) | 3 (3.2%) | 1 (1.1%) | 1 (1.3%) | 4 (4.2%) | 3 (4.4%) |
| Number of patients with treatment-emergent protocol-specified AEs of special interest, investigator-assessed | |||||||
| Cardiac failurea | 10 (10.8%) | 9 (9.4%) | 12 (12.6%) | 11 (11.6%) | 10 (13.3%) | 6 (6.3%) | 5 (7.4%) |
| Acute kidney injuryb | 1 (1.1%) | 1 (1.0%) | 1 (1.1%) | 2 (2.1%) | 2 (2.7%) | 3 (3.1%) | 3 (4.4%) |
| Number of patients with treatment-emergent AEs of special safety interest | |||||||
| Hypotensionc | 3 (3.2%) | 5 (5.2%) | 4 (4.2%) | 5 (5.3%) | 3 (4.0%) | 4 (4.2%) | 1 (1.5%) |
| Asymptomaticd | 0 | 2 (2.1%) | 2 (2.1%) | 2 (2.1%) | 0 | 2 (2.1%) | 0 |
| Symptomaticd | 3 (3.2%) | 3 (3.1%) | 2 (2.1%) | 3 (3.2%) | 3 (4.0%) | 2 (2.1%) | 1 (1.5%) |
| Presyncopeb | 0 | 0 | 0 | 0 | 0 | 1 (1.0%) | 0 |
| Syncopeb | 2 (2.2%) | 1 (1.0%) | 1 (1.1%) | 0 | 0 | 2 (2.1%) | 0 |
| Renal and urinary disorderse | 7 (7.5%) | 11 (11.5%) | 1 (1.1%) | 11 (11.6%) | 11 (14.7%) | 5 (5.2%) | 4 (5.9%) |
AEs, adverse events; SAEs, serious adverse events.
System organ class (SOC) cardiac disorders, includes preferred terms (PT) cardiac failure (acute, chronic, congestive) and right ventricular failure.
Preferred term.
System organ class vascular disorders.
Asymptomatic = lower level terms (LLT) hypotension asymptomatic and low blood pressure, Symptomatic = LLTs hypotension, hypotension orthostatic symptomatic, and hypotension symptomatic.
SOC from standardized MedDRA query (SMQ) acute renal failure, includes PTs acute kidney injury, prerenal failure, renal failure, and renal impairment.
Safety
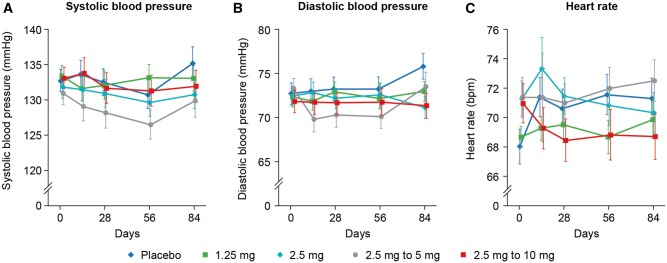
There was no change in blood pressure in the highest target dose arm and no dose–response relationship for blood pressure was seen. The largest difference in diastolic blood pressure at 12 weeks was between the placebo and 2.5 mg vericiguat groups (-4.1 mmHg, 95% CI -7.6 to -0.6 mmHg; Figure3). Resting heart rate decreased in the 10 mg group at 12 weeks by −2.6 ± 1.2 bpm (mean ± SE) compared with an increase in the placebo group of 3.3 ± 1.5 bpm [difference relative to placebo −5.9 bpm (95% CI −10.0 to −1.9)]. Although the nominal P-value for this difference is P = 0.0045, it may reflect the play of chance or the prevention of an increase in resting heart rate by vericiguat, and was driven mainly by patients in sinus rhythm (see Supplementary material online, Table S5). Notably, cardiac output at rest in the 10 mg arm was unchanged at 12 weeks (3.4 ± 1.0 L/min at baseline and 3.4 ± 1.1 L/min at 12 weeks). No changes from baseline and no differences from placebo were found for eGFR or high-sensitivity troponin (data not shown). Rates of adverse events in the SAF (69.8% in the 10 mg arm, compared with 73.1% in the placebo arm) and serious adverse events (25.0% in the 10 mg arm, compared with 28.0% in the placebo arm) were evenly distributed across all randomized groups (Table 4).
Figure 3.
Blood pressure and heart rate at given time points. Safety analysis set excluding patients with incorrectly assigned doses. Data are displayed as mean ± standard error. (A) systolic blood pressure; (B) diastolic blood pressure; (C) heart rate, difference in heart rate between the placebo and 2.5 mg vericiguat groups at 12 weeks was −3.7 bpm (95% CI −7.32 to 0.00 bpm), and between the 10 mg and placebo groups −5.9 bpm (95% CI −10.0 to −1.9 bpm). Baseline-adjusted linear regression slope −0.34 bpm (95% CI −0.66 to −0.02 bpm). bpm, beats per minute; CI, confidence interval.
Discussion
The Phase 2 SOCRATES-PRESERVED study showed that, in patients with a clear diagnosis of HFpEF, elevated NT-proBNP levels, and left atrial enlargement, 12 weeks of treatment with vericiguat in the studied dose range did not reduce either of the primary endpoints, log-transformed NT-proBNP and LAV, compared with placebo. Despite lack of an effect on these surrogate markers of disease severity, patient reported symptoms and physical limitations, as assessed by the KCCQ, were improved in patients receiving the two higher doses of vericiguat compared with patients receiving placebo.12 In NEAT-HF, quality of life assessed by the KCCQ did not improve in patients receiving isosorbide mononitrate over 6 weeks, and physical activity decreased.14 Likewise, KCCQ and Minnesota Living with Heart Failure scores did not improve in response to sildenafil,15,16 whereas vericiguat may have led to a significant dose-dependent improvement in quality of life, with a clinically meaningful effect size in the vericiguat 10 mg arm despite no change in the primary endpoints. These observations, if confirmed, support the hypothesis that cGMP elevation in response to NO donors or PDE5 inhibition differs from the direct sGC stimulation capacity in the absence of NO by vericiguat. Since during the 4 weeks following randomization patients were both in a heterogeneous period of natural recovery after a recent HF event and a period of dose titration, the additional post hoc analysis of Weeks 4–12 allows for separate consideration of the period following the final dose titration step for the 10 mg arm and gives an important insight into the potential difference between the 5 mg and 10 mg doses.
In comparison with previous HFpEF trials, patients enrolled in SOCRATES-PRESERVED had higher median NT-proBNP at baseline as well as more severely enlarged left atria with echocardiographic characteristics consistent with the presence of pronounced concentric hypertrophy. In line with this, 40% of patients had atrial fibrillation at baseline.
Several factors deserve consideration in examining the primary endpoints of change in NT-proBNP and LAV. First, the time frame in which NT-proBNP is expected to respond to treatment following worsening chronic HFpEF is uncertain, and the 12-week time point for primary analysis in SOCRATES-PRESERVED may have been too early to observe a reduction. In previous studies of patients with HFrEF, metoprolol17 and carvedilol treatment18 caused a transient increase in NT-proBNP before decreases became manifest at time points > 3 months. However, in SOCRATES-REDUCED, vericiguat dose-dependently reduced NTproBNP as early as 12 weeks after treatment initiation in patients with HFrEF,10 and LCZ696 in the PARAMOUNT study led to an early decrease from week 4, after a 2-week, single-blind, placebo run-in period, with a peak difference from placebo at 12 weeks.19 Longer duration studies will be needed to clarify whether vericiguat has a potential to decrease NT-proBNP in patients with HFpEF. Similarly, longer treatment duration may be required to observe a potential effect on left atrial (LA) size. In PARAMOUNT, which had less severely elevated LA sizes at baseline, a trend towards decreasing LA size was seen at 12 weeks that became significant only at 36 weeks.19
Second, it is possible that, in contrast to patients with HFrEF, the tested doses in SOCRATES-PRESERVED were too low to reduce effectively NT-proBNP,10 and that the pharmacodynamic response to vericiguat in patients with HFpEF could differ from that in patients with HFrEF.
Third, the studied population had a more severe phenotype than populations in previous HFpEF trials and, thus, may have been more refractory to treatment. However, underpowered subgroup analyses did not unmask any responder phenotypes.
Analysis of change in echocardiographic measurements apart from LAV was exploratory. Nonetheless, the resting echocardiographic data suggest increased early LV filling with improved early diastolic relaxation (thus without increasing the E/e′ ratio in spite of increased early mitral inflow) with vericiguat. Importantly, the improvement in e′ velocity was not related to a reduction in afterload, since effective arterial elastance and peripheral blood pressure remained unchanged with vericiguat. In contrast, the improvement in e′ velocity with valsartan in patients with hypertension was related to reduction in afterload.20 This suggests that vericiguat potentially has a direct myocardial effect in patients with HFpEF. It should also be noted that echocardiography was only conducted at rest in SOCRATES-PRESERVED; thus, these data do not address whether echocardiographic measurements under exercise stress conditions may improve to a greater extent with vericiguat. This warrants further study of potential mechanisms underlying the improved patient-reported outcomes with vericiguat. Potential effects of sGC stimulation on cardiac as well as non-cardiac tissues such as skeletal muscle, carotid bodies, or visceral fat could contribute to the observed improvement in patient-reported outcomes.
Resting heart rate decreased in the vericiguat treatment arms, in contrast to an increase in heart rate in placebo-treated patients, even in the context of 80% of patients receiving β-blockers, and in the absence of changes in systolic and diastolic blood pressure in the 5 mg and 10 mg target dose arms. The observed difference in resting heart rate coincided with improvement in patient-reported outcomes with vericiguat, in contrast with the deterioration in peak VO2 with ivabradine21 and the lack of an impact on quality of life with nebivolol.22 Our data do not resolve whether this is a direct cardiac effect, an indirect heart rate reduction secondary to improved diastolic filling, or a non-cardiac effect reflective of improved health status (e.g. lower sympathetic drive).
Limitations of this study include the short treatment duration, assessment of exploratory echocardiography and vital signs only at rest, and the non-confirmatory nature of reported exploratory outcomes in view of the neutral findings for the primary endpoints. Additional limitations apply to the interpretation of echocardiographic and heart rate changes, which may be chance findings in the absence of correction for multiplicity. There was no linear dose-relationship in E wave, and analysis of the A wave was limited to the subgroup of patients in sinus rhythm (60% of the study population). As in previous HFpEF trials, echocardiographic findings are not confirmatory but hypothesis generating, most variables did not show consistent trends across dose arms, and no trend was consistently seen across a full domain. Further analyses including strain measures, and longer treatment in future studies might be required. There was loss of information in 48 out of 477 randomized patients affected by the erroneous software update of the drug dispensation system.
In conclusion, vericiguat did not change the primary endpoints NT-proBNP and LAV at 12 weeks compared with placebo in patients with HFpEF after recent HF decompensation. Vericiguat was well tolerated, and exploratory analyses of pre-defined patient-reported outcomes suggest the potential for vericiguat to improve quality of life in patients with HFpEF requiring confirmatory studies. Further studies with vericiguat considering longer follow-up, higher doses, and additional prognostic endpoints should be conducted to clarify its potential impact on clinically important outcomes in HFpEF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We would like to thank Sabine Broeker-Zweering and Saila Jahangir for their contribution to project and study management of SOCRATES-PRESERVED.
Funding
This work was supported by Bayer Pharma AG, Berlin, and Merck Sharp & Dohme, a subsidiary of Merck & Co, Inc., Kenilworth, New Jersey.
Conflicts of interest: A-V.S., K.M. and L.R. are employees of Bayer AG. B.P. reports personal fees from Abbott Vascular, AstraZeneca, Bayer Healthcare, Daiichi-Sankyo, Novartis, Servier, and Stealth Peptides. A.P.M. has received grants from Cardiorentis and Novartis. C.S.P.L. has received grants from Bayer, Boston Scientific, Medtronic, Thermofisher, and Vifor Pharma, and reports personal fees from AstraZeneca, Janssen Research & Development, Menarini, Merck, Novartis, and Takeda. E.P-K. reports consultancy fees from Bayer Healthcare and Stealth Peptides. G.F. reports consultancy fees from Bayer, Novartis, and Servier for committee work. J.B. reports consultancy fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, CardioCell, CVRx, Janssen, Merck, Medtronic, Novartis, Relypsa, Stealth Peptide, Trevena, and Z-Pharma. P.P. has received grants from Cordea, Respicardia, Servier, Singulex, and Vifor Pharma, and reports personal fees from Abbott Vascular, Amgen, Cardiorentis, Celladon, Coridea, Novartis, Respicardia, Servier, Singulex, and Vifor Pharma. S.J.S. has received grants from Actelion, AstraZeneca, Corvia, and Novartis, and reports personal fees from Actelion, AstraZeneca, Bayer, Ironwood, Merck, Novartis, and Sanofi. S.D.S. has received grants from Amgen and Novartis, and reports personal fees from Amgen, Bayer, Ironwood, and Novartis. M.G. reports consultancy fees from Bayer, Cardiocell, Johnson & Johnson, Novartis, Stealth BioTherapeutics, and Takeda.
References
- 1. van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ.. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- 2. Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC Jr., Roessig L, Stasch JP, Solomon SD, Paulus WJ, Butler J.. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc 2013;2:e000536.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schroder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M.. NO-independent regulatory site on soluble guanylate cyclase. Nature 2001;410:212–215. [DOI] [PubMed] [Google Scholar]
- 4. Butler J, Braunwald E, Gheorghiade M.. Recognizing worsening chronic heart failure as an entity and an end point in clinical trials. JAMA 2014;312:789–790. [DOI] [PubMed] [Google Scholar]
- 5. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'connor CM, Sun JL, Yancy CW, Young JB.. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 6. Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA.. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–3744. [DOI] [PubMed] [Google Scholar]
- 7. Kristensen SL, Jhund PS, Kober L, McKelvie RS, Zile MR, Anand IS, Komajda M, Cleland JG, Carson PE, McMurray JJ.. Relative importance of history of heart failure hospitalization and N-terminal Pro-B-type natriuretic peptide level as predictors of outcomes in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2015;3:478–486. [DOI] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P.. Authors/Task Force M, Document R. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 9. Pieske B, Butler J, Filippatos G, Lam C, Maggioni AP, Ponikowski P, Shah S, Solomon S, Kraigher-Krainer E, Samano ET, Scalise AV, Muller K, Roessig L, Gheorghiade M.. Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES). Eur J Heart Fail 2014;16:1026–1038. [DOI] [PubMed] [Google Scholar]
- 10. Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Muller K, Roessig L, Pieske B.. Effect of Vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA 2015;314:2251–2262. [DOI] [PubMed] [Google Scholar]
- 11. Ferrari R, Bohm M, Cleland JG, Paulus WJ, Pieske B, Rapezzi C, Tavazzi L.. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail 2015;17:665–671. [DOI] [PubMed] [Google Scholar]
- 12. Filippatos G, Maggioni AP, Lam CSP, Pieske-Kraigher E, Butler J, Spertus J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Bamber L, Gheorghiade M, Pieske B. Patient-reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES-PRESERVED) study. Eur J Heart Fail 2017;doi:10.1002/ejhf.800. [DOI] [PubMed] [Google Scholar]
- 13. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS.. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 14. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E.. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R.. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA.. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J 2015;36:2565–2573. [DOI] [PubMed] [Google Scholar]
- 17. Davis ME, Richards AM, Nicholls MG, Yandle TG, Frampton CM, Troughton RW.. Introduction of metoprolol increases plasma B-type cardiac natriuretic peptides in mild, stable heart failure. Circulation 2006;113:977–985. [DOI] [PubMed] [Google Scholar]
- 18. Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Trawinski J, Amann-Zalan I, Hoersch S, Katus HA.. NT-proBNP in severe chronic heart failure: rationale, design and preliminary results of the COPERNICUS NT-proBNP substudy. Eur J Heart Fail 2004;6:343–350. [DOI] [PubMed] [Google Scholar]
- 19. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ.. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–1395. [DOI] [PubMed] [Google Scholar]
- 20. Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP.. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet 2007;369:2079–2087. [DOI] [PubMed] [Google Scholar]
- 21. Pal N, Sivaswamy N, Mahmod M, Yavari A, Rudd A, Singh S, Dawson DK, Francis JM, Dwight JS, Watkins H, Neubauer S, Frenneaux M, Ashrafian H.. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation 2015;132:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei Cas L.. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail 2012;14:219–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.