Abstract
Objectives: Biofilm infections of intravascular catheters caused by Staphylococcus aureus may be treated with catheter lock solutions (CLSs). Here we investigated the antibacterial activity, cytotoxicity and CLS potential of 5-hydroxyethyl-3-tetradecanoyltetramic acid (5HE-C14-TMA) compared with the related compounds 3-tetradecanoyltetronic (C14-TOA) and 3-tetradecanoylthiotetronic (C14-TTA), which are variants of quorum sensing signalling molecules produced by Pseudomonas aeruginosa.
Methods: Antibacterial activity and mechanism of action of 5HE-C14-TMA, C14-TOA and C14-TTA were determined via MIC, bacterial killing, membrane potential and permeability assays. Susceptibility of S. aureus biofilms formed in the presence of plasma in vitro was investigated, MTT cytotoxicity testing was undertaken and cytokine release in human blood upon exposure to 5HE-C14-TMA and/or S. aureus biofilms was quantified. The effectiveness of 5HE-C14-TMA as CLS therapy in vivo was assessed using a rat intravascular catheter biofilm infection model.
Results: MICs of 5HE-C14-TMA, C14-TOA and C14-TTA ranged from 2 to 4 mg/L. 5HE-C14-TMA and C14-TTA were bactericidal; all three compounds perturbed the staphylococcal membrane by increasing membrane permeability, depolarized the transmembrane potential and caused ATP leakage. Cytotoxicity and haemolytic activity were compound and target cell type-dependent. 5HE-C14-TMA reduced S. aureus biofilm viability in a dose-dependent manner in vitro and in vivo and did not trigger release of cytokines in human blood, but inhibited the high levels of IL-8 and TNF-α induced by S. aureus biofilms.
Conclusions: 5HE-C14-TMA, C14-TOA and C14-TTA are membrane-active agents. 5HE-C14-TMA was the most potent, eradicating S. aureus biofilms at 512–1024 mg/L both in vitro and in vivo as a CLS.
Introduction
In routine healthcare, use of implantable medical devices, such as intravascular catheters (IVC), has increased significantly. However, colonization by surface-adhering bacteria is associated with biofilm formation and subsequent catheter-related bloodstream infection (CRBSI) resulting in significant patient morbidity and mortality, prolonged hospitalization and excess hospital-related costs.1,2 Staphylococcal biofilms are recognized as the most frequent cause of CRBSI.3,4 Such biofilms are highly refractory to both the innate immune system and antimicrobial therapy resulting in treatment failure and persistence of infection.
While the presence of a Staphylococcus aureus CRBSI usually necessitates systemic antimicrobial therapy along with IVC removal, there are common clinical situations that preclude IVC removal such as the lack of alternative vascular access, patient co-morbidities and bleeding disorders. An alternative treatment approach attempting to salvage the IVC and eradicate the biofilm, involves the combination of systemic antimicrobials and a catheter lock solution (CLS) filling the lumen of the IVC to deliver high concentrations of an antimicrobial at the site of infection. With respect to staphylococcal IVC infections, the Infectious Disease Society of America guidelines on CRBSI management recommend the use of CLSs for IVC salvage.5 However, there is no consensus on the most appropriate agent for use as a CLS. Several commonly used antibiotics and antiseptics are ineffective in the treatment of S. aureus biofilm infections.6
Given the increasing use of medical devices and the global threat posed by multi-antibiotic-resistant bacteria there is renewed interest in novel antimicrobials that inhibit bacterial growth or control infection through attenuation of virulence and biofilm formation.7 During a search for novel chemical scaffolds for such compounds, we discovered that the Pseudomonas aeruginosa quorum sensing signal molecule, N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL) inhibited S. aureus growth at high concentrations (MIC, 100 μM; 30 mg/L).8,9 At subinhibitory concentrations, 3-oxo-C12-HSL inhibited production of S. aureus exotoxin virulence determinants, including α-haemolysin and toxic shock syndrome toxin by antagonizing agr-dependent quorum sensing probably via an allosteric interaction with the sensor kinase AgrC.8,9 Under aqueous alkaline conditions, 3-oxo-C12-HSL can either ring open to form the corresponding homoserine compound10 or undergo a rearrangement reaction to form 5-hydroxyethyl-3-decanoyltetramic acid (5HE-C10-TMA).11 5HE-C10-TMA (denoted as C10-TA)12 retains both growth and quorum sensing inhibitory properties.9,12 It belongs to the tetramic acid (TMA) compound class that includes diverse natural products such as reutericyclin active against Gram-positive pathogens including staphylococci, clostridia and streptococci.13–15 The cellular targets of the antibacterial TMAs range from cell wall biosynthesis, DNA topoisomerase and RNA polymerase to membrane potential.15 5HE-C10-TMA in common with reutericyclin dissipates the cytoplasmic membrane potential and pH gradient, and is an iron(III) chelator.12 Serial passage of S. aureus in sub-growth inhibitory concentrations of 5HE-C10-TMA did not select for resistance.11,12 Although active against planktonic bacteria, 5HE-C10-TMA showed no efficacy against S. aureus biofilms.12
Extending the C10 acyl side chain of 5HE-C10-TMA increased potency such that the MIC and agr IC50 of 5-hydroxyethyl-3-tetradecanoyltetramic acid (5HE-C14-TMA) towards S. aureus were 12.5 and 14 μM, respectively.9 Removal of the 5-hydroxyethyl moiety of 5HE-C10-TMA to generate C10-TMA did not reduce antibacterial activity.9 By replacing the tetramic ring nitrogen with oxygen, a series of 3-acyltetronic acids were generated that lack the iron-chelating property of 5HE-C10-TMA. Of these, 3-tetradecanoyltetronic acid (C14-TOA) was the most potent (MIC 25 μM; agr IC50 3 μM). Both 5HE-C14-TMA and C14-TOA bound to the staphylococcal membrane with Kd values of 4 and 2 μM, respectively, and hyperbolic binding profiles consistent with a non-co-operative single site binding model.9 In a murine arthritis model neither C14-TOA nor 5HE-C10-TMA were toxic, but only C14-TOA reduced the frequency and severity of arthritis.9
Here, we build on our previous observations, by evaluating the antibacterial and anti-biofilm potential of 5HE-C14-TMA, C14-TOA and a novel sulphur-containing analogue (3-tetradecanoylthiotetronic acid; C14-TTA) towards MSSA and MRSA strains of S. aureus using a clinically relevant in vitro and in vivo model of IVC infection and examine their potential clinical use as CLSs.9,16
Materials and methods
Bacterial strains and growth conditions
Community-acquired MRSA (CA-MRSA) strain USA300, its isogenic agr mutant,17 the hospital-acquired MRSA (HA-MRSA) strain BH1CC,18 the HA-MSSA strain BH4819 and the MSSA strain SH100020 were cultured in Mueller–Hinton broth for susceptibility testing or in RPMI-1640 (Gibco) for biofilm formation. For the rat jugular vein catheter infection model, the S. aureus strain USA300 (LAC) lux21 was used.
Antibacterial agents and susceptibility testing
5HE-C14-TMA and C14-TOA were synthesized as described previously9 and C14-TTA as described in the Supplementary data available at JAC Online. Stock solutions of the compounds in DMSO at 10–50 mM were further diluted into tissue culture media or sodium chloride for the in vivo experiments. A DMSO solvent control was used in all in vitro experiments. The following antibiotics were used: vancomycin (Vancocin; Flynn Pharma Ltd., Republic of Ireland), daptomycin (Cubicin; Novartis, UK), gentamicin (Hospira, UK) and tigecycline (Tygacil; Pfizer Inc.). MICs were determined by broth microdilution in Mueller–Hinton broth, as recommended by the CLSI.
Bactericidal activity
S. aureus USA300 LAC was grown overnight in LB medium, diluted 250-fold and grown to an OD600 0.5. Cells were diluted to 1 × 106 per mL in challenge medium (LB plus 5HE-C14-TMA, C14-TOA or C14-TTA at 8 × MIC). Samples were removed and viable counts determined at 30 min intervals for the first hour followed by hourly sampling. The CLSI defines a compound as bactericidal if it reduces the starting inoculum colony count by at least 99.9% (3-logs).
Bacterial membrane permeabilization assays
Membrane permeabilization was evaluated by quantifying (i) ATP release,22 and (ii) the uptake of propidium iodide.22S. aureus strain USA300 LAC was grown overnight in CYGP medium,9 washed with sodium phosphate buffer (100 mM, pH 7), re-suspended to OD600 0.1 and incubated at 37 °C with a range of concentrations of each compound for 1 h. Nisin (0.6 μM) was used as a positive control. Cell-free supernatants were assayed for ATP using the Cell Titre-Glo kit (Promega). Staphylococcal cells were re-suspended in 100 mM sodium phosphate buffer (pH 7) containing propidium iodide (10 mg/L), incubated in the dark at room temperature for 10 min. After washing, propidium iodide fluorescence (excitation: 575 nm; emission 630 nm) was determined.
Dissipation of bacterial membrane potential
Transmembrane potential was determined as described by Breeuwer and Abee23 Staphylococcal cells were resuspended in potassium phosphate buffer (50 mM, pH 7) containing 5 μM of the cationic fluorescent dye DiSC3(5) (Invitrogen) and fluorescence determined (excitation 650 nm, emission 680 nM).
Comparative susceptibility of S. aureus biofilms to antimicrobials
Biofilms were formed as previously described to mimic in vivo conditions that promote formation of coagulase-mediated biofilms.16 Briefly, microtitre plates or glass-bottom culture dishes (MaTek Co., USA) were coated with either 20% (v/v) or 100% (v/v) human plasma, respectively, and inoculated with S. aureus at an OD600 ∼0.2. After 1 h, non-adherent bacteria were aspirated, replaced with fresh RPMI-1640 and biofilms allowed to form over 24 h at 37 °C. Pre-formed biofilms were treated for 24 h with either 5HE-C14-TMA, C14-TOA or C14-TTA or a positive control [ethanol at 40% (v/v)]. Biofilm viability was measured by (i) resazurin-conversion assay (in microtitre wells), or (ii) live/dead imaging (glass-bottom dishes). Conversion of the non-fluorescent redox dye, resazurin into the fluorescent resorufin was performed as described previously.16 A 44 μM resazurin (Sigma-Aldrich, UK) solution in RPMI-1640 was added to an equal biofilm volume and incubated for 1 h at 37ºC. Fluorescence (excitation 544 nm, emission 590 nm) was quantified using a Perkin Elmer 2030 Multilabel Reader VictorTM X3. Biofilms were stained with SYTO9 Green (at 13.36 μM) and propidium iodide (at 80 μM) (Molecular Probes®) for 1 h prior to washing. Four representative images at ×40 and ×100 were obtained per experimental group, per experiment using a Zeiss LSM 510 META confocal microscope. Each experiment was repeated at least three times. Fluorescence intensity was quantified using Image J1 software (http://imagej.net/Welcome).
MTT cell viability assay
Human keratinocyte (HaCaT) cells were cultured in DMEM supplemented with 10% (v/v) FBS. Primary human umbilical vein endothelial cells (HUVECs) were cultured in EGM™-2 Bulletkit (Lonza) medium. HaCaT and HUVECs were harvested using TrypLETM Express (Gibco). Cells were seeded in microtitre plates at 3 × 105 cells/mL and incubated for 24 h at 37 °C with an antimicrobial compound (up to 10 mg/L) or Triton X100 at 1% (v/v). After removing the medium, cells were incubated with 500 mg/L MTT (Sigma-Aldrich) for 4 h. The crystals formed were solubilized with DMSO. Absorbance was recorded at 560 nm and the CC50 was determined.
Haemolysis assay
Human blood containing EDTA (1.6 g/L) was centrifuged at 1000 g for 5 min. Red blood cells (RBC) were washed twice with PBS, diluted 2-fold and incubated with compounds or Triton X100 (positive control) at 0.5% (v/v). A570 was determined to quantify haemolysis. IC50s were derived from the corresponding dose–response curves.
Cytokine release assay
EDTA-treated human blood was incubated in microtitre wells containing either S. aureus USA300 biofilm, 5HE-C14-TMA (at 256 mg/L) only or biofilm with 5HE-C14-TMA (at 256 mg/L) and incubated for 2 h at 37 °C. Plasma samples were collected by centrifugation (1000 g) and assayed for human cytokines using a Bio-Plex 200 (Bio-Rad). A standard curve was employed to maximize sensitivity for samples containing low cytokine levels. At least three different donor blood samples (with three replicates for each) were used.
Rat IVC infection model
Rats (male, Sprague–Dawley) were pre-implanted with jugular vein polyurethane catheters (13.5 cm long, outside diameter 1.1 mm, inside diameter 0.6 mm) (Charles River, UK). Daily flushing was performed with 150 μL sodium chloride [0.1% (w/v)] following introduction of 40 μL heparin (100 IU), sodium chloride 0.1% (w/v) within the lumen. IVC-biofilm infection was developed as previously reported.24–26 Catheters were inoculated with 40 μL S. aureus USA300lux suspension at 1 × 104 cfu/mL ‘locked’ within the catheter for 1 day. Intravenous vancomycin (50 mg/kg) was administered twice daily at 12 h intervals to prevent systemic spread of infection. CLS consisting of 5HE-C14-TMA (40 μL) at 512 mg/L in sodium chloride 0.1% (w/v) was administered daily for 5 days upon development of infection. After 5 days of treatment, catheters were removed and luminescence imaged using a Perkin Elmer IVIS Spectrum instrument (exposure, 20 s; binning: 4, f1). Bacteria were harvested from the catheters by treatment with TrypLETM Express (Gibco) and washing prior to determining viable counts.
Ethics approval
Ethics approvals for blood collection and use (REC820 and REC951) and animal experiments (REC931) were granted by the Ethics Committee of the Royal College of Surgeons in Ireland. In vivo procedures were approved by Health Products Regulatory Authority and performed under Irish Government Department of Health guidelines.
Statistics
Statistical significance was assessed using one-way ANOVA except for Figure 6 where an unpaired t-test was applied. Statistical significance was indicated as *P < 0 .05, **P < 0.001 and ***P < 0.0001.
Results
Bactericidal activity of 5HE-C14-TMA, C14-TOA and C14-TTA
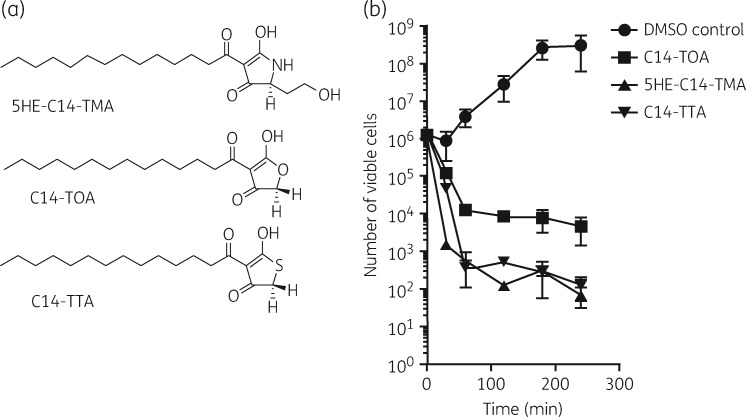
5HE-C14-TMA, C14-TTA and C14-TOA (Figure 1a) each inhibited growth of MRSA (USA300 JE2 and BH1CC) and MSSA (SH1000 and BH48) strains with MICs from 2 to 4 mg/L (Table 1). 5HE-C14-TMA and C14-TTA, but not C14-TOA, were bactericidal (Figure 1b); at 8 × MIC, 5HE-C14-TMA reduced the viability of USA300 by 3 logs over 30 min.
Figure 1.
(a) Chemical structures of 5HE-C14-TMA, C14-TOA and C14-TTA. (b) Planktonic S. aureus USA300 cells were treated with 8 × MIC of 5HE-C14-TMA, C14-TOA or C14-TTA, respectively, or a DMSO solvent control. Data presented are the mean viable counts (cfu) per mL from three independent experiments (±SD).
Table 1.
MICs of 5HE-C14-TMA, C14-TOA and C14-TTA for MRSA (USA300 and BH1CC) and MSSA (SH1000 and BH48) strains
| Compound | MIC [μM (mg/L)] for S. aureus strain |
|||
|---|---|---|---|---|
| USA300 | BH1CC | SH1000 | BH48 | |
| 5HE-C14-TMA | 6.25 (2) | 6.25 (2) | 6.25 (2) | 6.25 (2) |
| C14-TOA | 12.5 (4) | 12.5 (4) | 12.5 (4) | 12.5 (4) |
| C14-TTA | 12.5 (4) | 12.5 (4) | 12.5 (4) | 12.5 (4) |
5HE-C14-TMA, C14-TTA and C14-TOA perturb the S. aureus cytoplasmic membrane
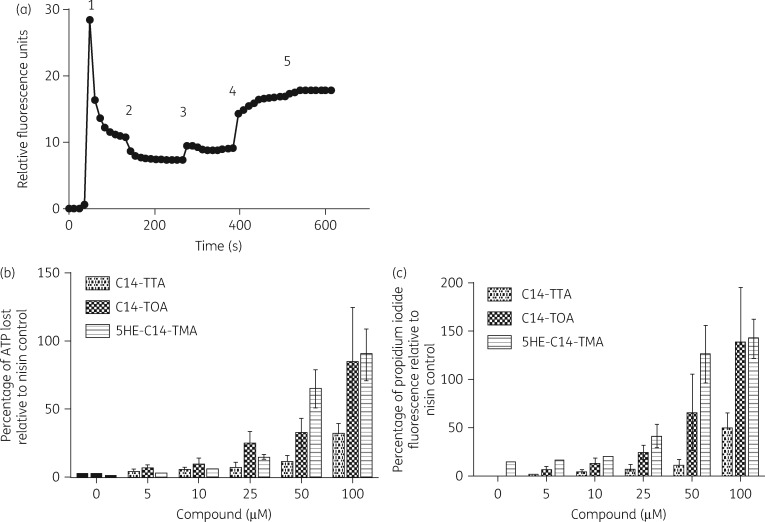
5HE-C14-TMA and C14-TOA (a tetronic acid analogue lacking the 5-hydroxyethyl substituent) both bind to S. aureus membranes with high affinity.9 Lowery et al. showed that 5HE-C10-TMA could dissipate the cytoplasmic transmembrane potential (ΔΨ).12 To determine whether 5HE-C14-TMA, C14-TTA and C14-TOA exhibit similar modes of action, the change in membrane potential following exposure to each compound was determined using the cationic fluorescent dye DiSC3(5). 5HE-C14-TMA (30 μM) caused an increase in fluorescence, indicative of membrane depolarization (Figure 2a). Similar results were obtained for C14-TTA and C14-TOA (Figure S1, available as Supplementary data at JAC Online). To investigate the extent of membrane perturbation caused by 5HE-C14TMA and related compounds, the uptake of propidium iodide in conjunction with the loss of the cytoplasmic ATP was assessed. Figure 2(b and c) shows that propidium iodide fluorescence and extracellular ATP levels increased with increasing concentrations of each compound, suggesting loss of membrane integrity is the likely cause of bacterial cell death. For both assays, C14-TTA was the least active.
Figure 2.
Perturbation of staphylococcal cytoplasmic membrane structure and function by 5HE-C14-TMA, C14-TTA and C14-TOA. (a) Depolarization of transmembrane potential by 5HE-C14-TMA. (1) Cationic fluorescent dye DiSC3(5) was added to cells followed (2) by glucose (10 mM), (3) nigericin (5 μM) to abolish the pH gradient and (4) 5HE-C14-TMA (30 μM). Complete depolarization of the membrane potential was achieved by the addition of 5 μM valinomycin (5). Experiments were carried out on three independent occasions with representative data presented. (b) Cytoplasmic ATP release relative to the positive control (nisin, 0.6 μM) of S. aureus cells treated with (from left to right) 0, 5, 10, 25, 50 and 100 μM 5HE-C14-TMA, C14-TTA or C14-TOA. (c) Membrane permeability. The percentage of propidium iodide uptake relative to the positive control (nisin, 0.6 μM) of S. aureus cells treated with (from left to right) 0, 5, 10, 25, 50 and 100 μM 5HE-C14-TMA, C14-TTA or C14-TOA. Data plotted are the mean values of three independent experiments; error bars represent standard deviations.
Biofilm killing activity
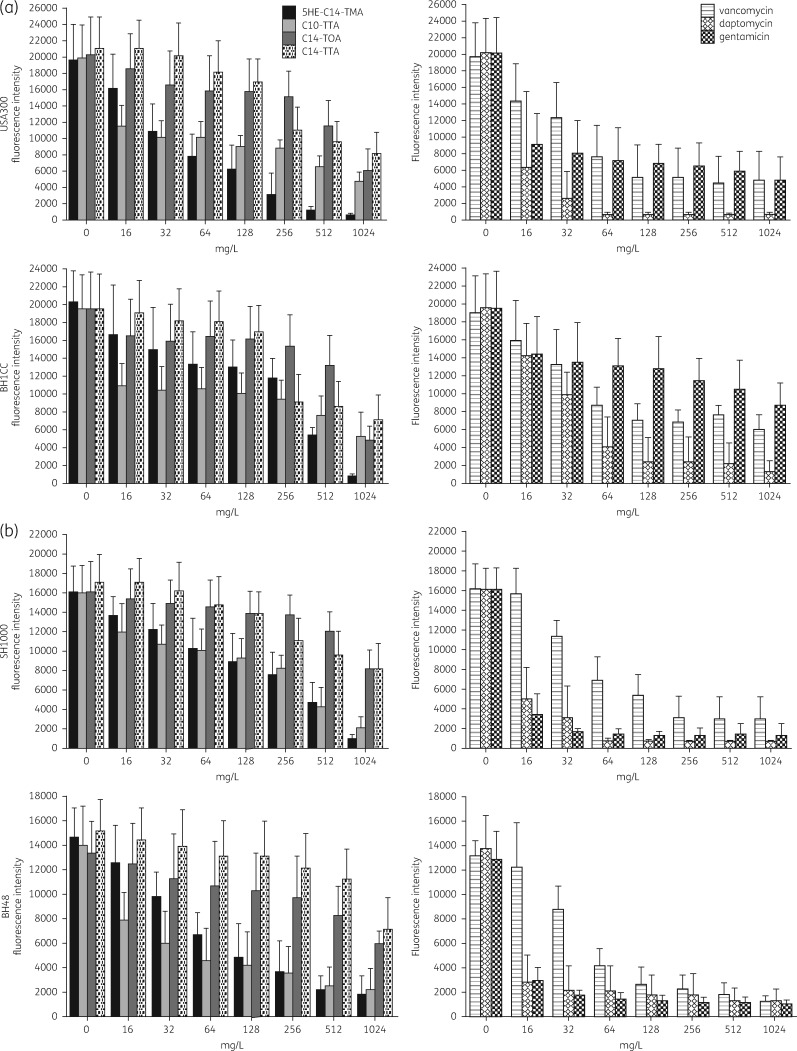
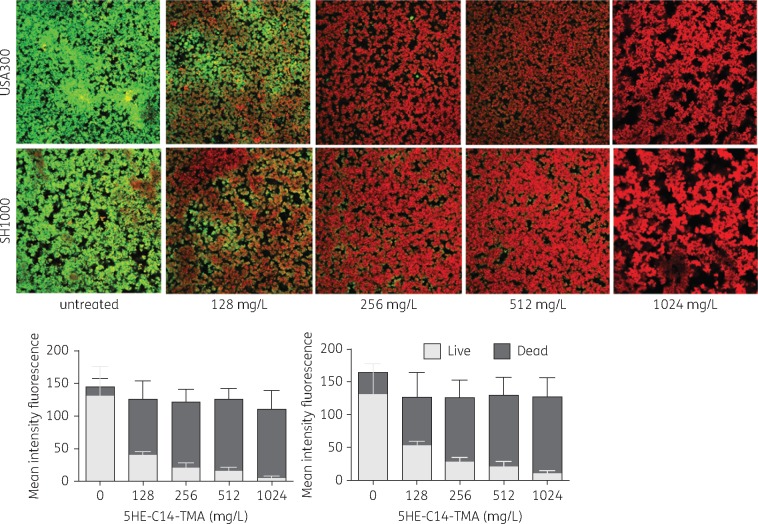
The biofilm-killing activity of 5HE-C14-TMA, C14-TOA and C14-TTA against in vivo-relevant biofilms of S. aureus was compared with clinically relevant antibiotics. Although all three compounds reduced biofilm viability, C14-TOA and C14-TTA were only active at the highest concentrations (512 and 1024 mg/L) reducing viability by ∼2.5–3-fold (Figure 3). In contrast, 5HE-C14-TMA (at 1024 mg/L) effectively eradicated biofilm reducing viability by ∼7–20-fold depending on the strain (Figure 3). This activity profile was similar to that of vancomycin although the latter only reduced MRSA biofilm viability by ∼3.5–4-fold at 1024 mg/mL (Figure 3). In contrast, daptomycin was the most active antimicrobial evaluated (Figure 3). The in vitro activity of 5HE-C14-TMA was further validated using live/dead staining. Figure 4 confirms the dose-dependent loss of viability of S. aureus USA300 and SH1000 biofilms and shows that substantial killing (>50%) occurs with as little as 128 mg/L for both strains.
Figure 3.
Comparative dose-dependent killing of biofilms formed by (a) MRSA strains USA300 Lac and BH1CC and (b) MSSA strains SH1000 and BH48 by 5HE-C14-TMA, C14-TTA, C14-TOA or the antibiotics vancomycin, daptomycin or gentamicin. S. aureus biofilms were grown in plasma-coated microtitre plate wells in RPMI-1640 for 24 h at 37 °C. 5HE-C14-TMA, C14-TOA or C14-TTA (or antibiotics) were incubated in the wells at a range of concentrations for 24 h. Resazurin-conversion assay was used to assess biofilm viability. Mean fluorescence intensities (±SD) of three independent experiments are shown.
Figure 4.
Dose-dependent killing of S. aureus USA300 and SH1000 biofilms by 5HE-C14-TMA. Bacterial biofilms were grown in plasma-coated glass-bottom MaTeKTM dishes in RPMI-1640 for 24 h at 37 °C. 5HE-C14-TMA was added at a range of concentrations and incubated for a further 24 h. Biofilm bacterial viability was evaluated using live/dead imaging (top panels), quantified using ImageJ and presented as mean fluorescence intensity (±SD) (left histogram, USA300; right histogram, SH1000).
Cytotoxicity and haemolytic activity
To obtain comparative cytotoxicity data for 5HE-C14-TMA, C14-TOA and C14-TTA, we examined the responses of HaCaT keratinocytes and primary HUVEC cells using the MTT viability assay. Table 2 shows that C14-TTA (CC50 26 μM) is more cytotoxic for HaCaT cells than 5HE-C14-TMA (CC50 181 μM) or C14-TOA (CC50 257 μM). For HUVECs, 5HE-C14-TMA and C14-TOA showed similar CC50s (Table 2). In contrast, C14-TOA was far less haemolytic towards human RBC (CC50 >2048 μM/>667 mg/L) than C14-TTA or 5HE-C14-TMA (Table 2). The ‘selectivity index’ (SI) of a given agent provides a measure of the preference for bacterial rather than mammalian cells and is defined as the ratio of mammalian cell cytotoxicity to MIC (CC50/MIC).27 Compounds with SI values >10 are usually considered suitable for in vivo evaluation. For HaCaT cells, SI values of 32, 20 and 2, respectively, were obtained for 5HE-C14-TMA, C14-TOA and C14-TTA suggesting that 5HE-C14-TMA shows the greatest selectivity. 5HE-C14-TMA and C14-TOA showed less selective toxicity towards HUVECs with SIs 3.5 and 2.3, respectively.
Table 2.
Cytotoxic and haemolytic activities of 5HE-C14-TMA, C14-TOA and C14-TTA
| Cell type | CC50 [μM (mg/L)] |
||
|---|---|---|---|
| 5HE-C14-TMA | C14-TOA | C14-TTA | |
| HaCaT | 181 (64) | 257 (80) | 26 (8.3) |
| HUVEC | 19 (7) | 28 (9) | not determined |
| RBC | 246 (87) | >2048 (>667) | 470 (153) |
5HE-C14-TMA treatment reduces release of pro-inflammatory cytokines
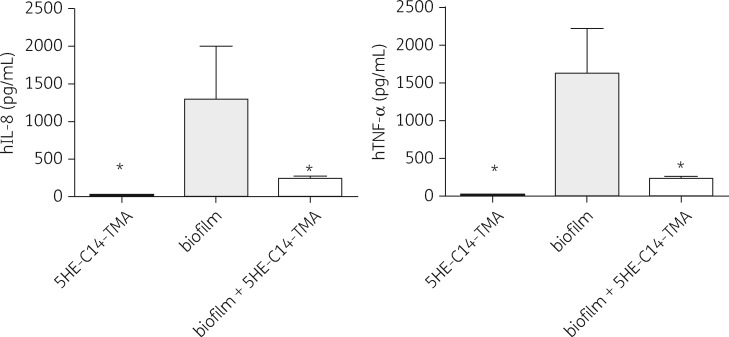
Cytokine levels in whole human blood were quantified upon exposure to S. aureus USA300 biofilm, 5HE-C14-TMA, or the biofilm with 5HE-C14-TMA.16 The levels of the pro-inflammatory IL-8 and TNF-α were significantly elevated in the blood exposed to biofilm while IL-2, IL-4, IL-6, IL-10, GM-CSF and IFN-γ remained unchanged (Figure 5 and data not shown). There was no increase in cytokine levels for the sample containing the agent only. However, exposure of the same biofilm to a sub-bactericidal dose (256 mg/L for 2 h) of 5HE-C14-TMA resulted in substantial reductions in IL-8 and TNF-α. These data suggest that 5HE-C14-TMA modifies the immunogenicity of S. aureus biofilms (Figure 5). Given that 5HE-C14-TMA inhibits the S. aureus agr quorum sensing system, we examined the cytokine levels produced in response to an S. aureus USA300 agr mutant biofilm treated with or without 5HE-C14-TMA. The data presented in Figure S2 show that, while both IL-8 and TNF-α are significantly reduced in response to the agr mutant biofilm, the same dose of 5HE-C14-TMA had no further effect on the levels of the two cytokines.
Figure 5.
Human IL-8 and TNF-α concentrations in whole human blood following exposure to 5HE-C14-TMA (256 mg/L), untreated USA300 biofilm or USA300 biofilm treated with 5HE-C14-TMA (256 mg/L) for 2 h. Supernatants were collected and the plasma fraction subjected to a Bio-Plex Precision Pro™ (magnetic) human cytokine immunoassay (Bio-Rad). Means of cytokine concentrations obtained from three donors (±SD) are shown. Statistical significance was determined using one-way ANOVA and is indicated (*P < 0.5). h, human.
In vivo activity of 5HE-C14-TMA against S. aureus biofilm
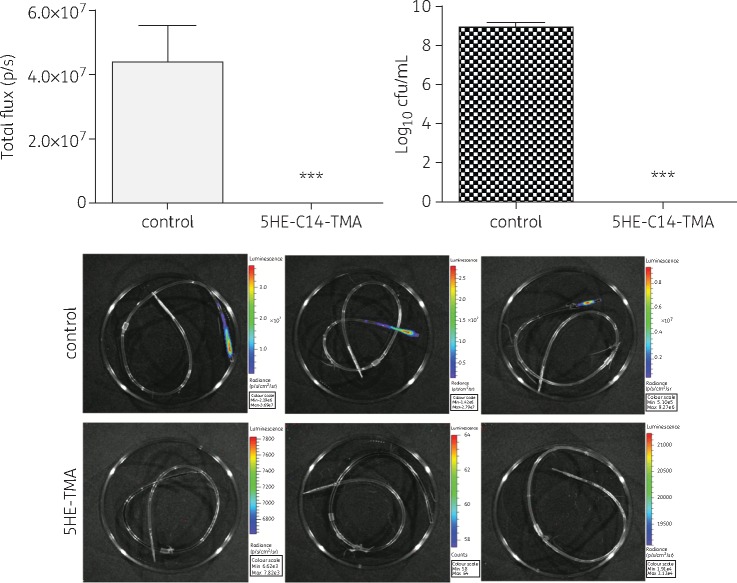
The efficacy of 5HE-C14-TMA as a CLS in an in vivo rat model of IVC infection using the MRSA strain USA300lux was investigated (Figure 6). Animals treated with CLS containing sodium chloride [0.1% (w/v)] were colonized by ∼109 cfu/mL at day 5 of the treatment regimen while in catheters treated with 5HE-C14-TMA the biofilm was effectively eradicated, suggesting in vivo efficacy of the compound.
Figure 6.
In vivo eradication of an MRSA biofilm with 5HE-C14-TMA. CLS consisting of 5HE-C14-TMA (512 mg/L) was instilled into a jugular vein catheter to treat IVC-associated S. aureus USA300lux biofilms. In the control group the CLS was replaced with 0.9% sodium chloride. CLS was renewed every 24 h for 5 days. The day after the final treatment, animals were sacrificed, catheters removed and subjected to quantification of bioluminescence using IVIS. Bacterial cells were harvested using TrypLETM reagent, serially diluted and plated on tryptic soy agar for cfu counting. Statistical significance is indicated (***P < 0.001). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
An important treatment option for the salvage of an IVC in patients with a CRBSI due to S. aureus is the use of a CLS in combination with systemic antibiotics.5 In choosing an antimicrobial agent for the CLS, consideration must be given to the likely effectiveness of the agent against S. aureus biofilms in the context of the environmental conditions in which such biofilm is formed.6 We recently developed an in vitro biofilm model of IVC infection employing physiologically relevant iron-deficient RPMI-1640 medium in conjunction with human plasma-conditioned surfaces which generate coagulase-mediated and fibrin-embedded biofilms that mimic those formed in vivo. These biofilms, unlike those formed in rich laboratory media under standard conditions demonstrated antibiotic susceptibilities similar to those found for in vivo infections established by S. aureus biofilms.6,16 Given the need for novel and effective agents for the treatment of device-related S. aureus infections that could be used as CLS or as potential treatment options for other device-related infections, we explored the mode of action and inhibitory activity of compounds that are related to a natural product (5HE-C10-TMA).11 This natural product is formed in vivo during chronic P. aeruginosa lung infections in individuals with cystic fibrosis and may well contribute to the eradication of competitor bacteria including S. aureus.12,28 Although 5HE-C10-TMA inhibits the growth of planktonic Gram-positive bacteria, it was reported to lack activity against S. aureus biofilms formed in rich laboratory media on polystyrene.9,12,29 Similarly, 5HE-C10-TMA was inactive in a murine arthritis infection model.9 Consequently we synthesized a series of TMAs and related compounds to provide a structure–function relationship (SAR) and to identify compounds with greater antibacterial and anti-biofilm activity.9
The most active TMA identified in our SAR study was the C14 analogue of 5HE-C10-TMA that exhibited an 8-fold increase in growth inhibitory activity against planktonic S. aureus.9 These data are also consistent with a reutericyclin SAR study that revealed that lipophilic analogues were generally more active against Gram-positive pathogens than those containing polar and charged substituents.15 Since ferric iron was reported to abolish the antibacterial activity of 5HE-C10-TMA towards Clostridium difficile29 we also synthesized variants of the TMAs in which we extended the acyl chain and replaced the ring nitrogen with oxygen or sulphur to generate the C14 tetronic (TOA)9 and thiotetronic (TTA; this paper) acids that in contrast to the TMAs, do not chelate iron (data not shown). The MICs of C14-TOA and C14-TTA at 4 mg/L were marginally higher than 5HE-C14-TMA (2 mg/L) for the MRSA and MSSA strains investigated. However, only 5HE-C14-TMA and C14-TTA were bactericidal in time-dependent killing assays at 8 × MIC against the CA-MRSA strain USA300 LAC. Consistent with their ability to bind to the S. aureus membrane with high affinity, 5HE-C14-TMA, C14-TOA and C14-TTA in common with 5HE-C10-TMA and antibiotics such as reutericyclin, daptomycin and telavancin dissipate the transmembrane potential, increase cellular permeability and promote the release of ATP.9,12,22,30 For 5HE-C10-TMA, dissipation of the transmembrane potential and pH gradient rather than membrane permeabilization were suggested to correlate most closely with bacterial cell death.
5HE-C10-TMA and 5HE-C12-TMA have been suggested to be non-toxic to human cells since neither reduced the viability of bone marrow-derived macrophages up to 100 μM.12 However, we observed that the cytotoxicity of 5HE-C14-TMA, C14-TOA and C14-TTA was cell type dependent. For example, 5HE-C14-TMA reduced the viability of HUVEC cells at much lower concentrations than for HaCaT cells. Nevertheless the SIs for 5HE-C14-TMA indicates that this compound is more selective for bacteria than mammalian cells.
Although 5HE-C10-TMA was inactive at 200 mg/L against S. aureus biofilms,12 we observed that the longer chain analogue, 5HE-C14-TMA killed over 50% of both MRSA and MSSA biofilms at 128 mg/L with complete killing achieved at 512–1024 mg/L. However, the differences observed may not only reflect the increased anti-biofilm activity of 5HE-C14-TMA compared with 5HE-C10-TMA, achieved via the four carbon acyl chain extension, but also the nature of the biofilm model used as a more in-vivo-like model of biofilm infection was used in this study. In contrast to 5HE-C14-TMA, and despite the similarities with respect to MIC and mechanism of action, C14-TOA and C14-TTA were far less effective at reducing the viability of either MSSA or MRSA biofilms. Since 5HE-C14-TMA, in contrast to C14-TOA and C14-TTA, is an iron chelator and as iron availability impacts on S. aureus biofilm development,31 the differential anti-biofilm activity observed may be due in part to this additional functionality.
The reduced anti-biofilm activity of C14-TOA compared with 5HE-C14-TMA is interesting given that C14-TOA significantly reduced the frequency and severity of arthritis and joint destruction in the same murine S. aureus infection model in which 5HE-C10-TMA was inactive.9 C14-TOA did not, however, reduce the numbers of viable staphylococci in the kidneys suggesting that the anti-virulence properties of the molecule as an agr inhibitor may account for the positive outcome on arthritis observed. Although 5HE-C14-TMA is a less effective agr inhibitor than C14-TOA,9S. aureus biofilms treated with sub-growth inhibitory 5HE-C14-TMA did not elicit production of IL-8 and TNF-α when exposed to human blood. This was also the case for agr mutant biofilms suggesting that 5HE-C14-TMA modifies the immunogenicity of S. aureus biofilms by inhibiting the production and release of agr-dependent pro-inflammatory toxins.32
Encouragingly, 5HE-C14-TMA was effective in the eradication of MRSA and MSSA biofilms formed in vitro, but under in vivo-mimicking conditions. To extend our in vitro findings a rat model of IVC infection assessed the effectiveness of 5HE-C14-TMA in vivo. 5HE-C14-TMA was highly effective in the eradication of MRSA biofilm formed in this model, whilst untreated catheters resulted in harvesting of 109 cfu/mL biofilm.
Taken together, the in vitro and in vivo efficacy of 5HE-C14-TMA towards S. aureus biofilms suggests that this compound scaffold has therapeutic potential for the treatment of S. aureus IVC infections within a CLS.
Supplementary Material
Acknowledgements
We thank Alex Truman (Centre for Biomolecular Sciences, University of Nottingham) for synthesizing 5HE-C14-TMA, C14-TOA and C14-TTA.
Funding
This work was supported by the Medical Research Council UK (Grant G9219778 to P. W. and W. C. C.) and a health research award from the Irish Health Research Board (Grant HRA_POR/2012/52/ to E. O. N.
Transparency declarations
None to declare.
Supplementary data
Synthesis details and Figures S1 and S2 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
References
- 1. O'Grady NP, Alexander M, Burns LA. et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011; 52: e162–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zapotoczna M, O'Neill E, O'Gara J. Untangling the diverse and redundant mechanisms of Staphylococcus aureus biofilm formation. PLoS Pathog 2016; 12: e1005671.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary from January 1992-June 2001. issued August 2001. Am J Infect Control 2001; 29: 404–21. [DOI] [PubMed] [Google Scholar]
- 4. Raad II, Hanna HA. Intravascular catheter-related infections: new horizons and recent advances. Arch Intern Med 2002; 162: 871.. [DOI] [PubMed] [Google Scholar]
- 5. Mermel LA, Allon M, Bouza E. et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hogan S, Zapotoczna M, Stevens NT. et al. In vitro approach for identification of the most effective agents for antimicrobial lock therapy in the treatment of intravascular catheter-related infections caused by Staphylococcus aureus. Antimicrob Agents Chemother 2016; 60: 2923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rampioni G, Leoni L, Williams P. The art of antibacterial warfare: deception through interference with quorum sensing-mediated communication. Bioorg Chem 2014; 55: 60–8. [DOI] [PubMed] [Google Scholar]
- 8. Qazi S, Middleton B, Muharram SH. et al. N-acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect Immun 2006; 74: 910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murray EJ, Crowley RC, Truman A. et al. Targeting Staphylococcus aureus quorum sensing with nonpeptidic small molecule inhibitors. J Med Chem 2014; 57: 2813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yates EA, Philipp B, Buckley C. et al. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect Immun 2002; 70: 5635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaufmann GF, Sartorio R, Lee SH. et al. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci USA 2005; 102: 309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowery CA, Park J, Gloeckner C. et al. Defining the mode of action of tetramic acid antibacterials derived from Pseudomonas aeruginosa quorum sensing signals. J Am Chem Soc 2009; 131: 14473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schobert R, Schlenk A. Tetramic and tetronic acids: an update on new derivatives and biological aspects. Bioorg Med Chem 2008; 16: 4203–21. [DOI] [PubMed] [Google Scholar]
- 14. Hurdle JG, Heathcott AE, Yang L. et al. Reutericyclin and related analogues kill stationary phase Clostridium difficile at achievable colonic concentrations. J Antimicrob Chemother 2011; 66: 1773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cherian PT, Wu X, Maddox MM. et al. Chemical modulation of the biological activity of reutericyclin: a membrane-active antibiotic from Lactobacillus reuteri. Sci Rep 2014; 4: 4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zapotoczna M, McCarthy H, Rudkin JK. et al. An essential role for coagulase in Staphylococcus aureus biofilm development reveals new therapeutic possibilities for device-related infections. J Infect Dis 2015; 212: 1883–93. [DOI] [PubMed] [Google Scholar]
- 17. Fey PD, Endres JL, Yajjala VK. et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio 2013; 4: e00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Neill E, Pozzi C, Houston P. et al. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol 2007; 45: 1379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Neill E, Pozzi C, Houston P. et al. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol 2008; 190: 3835–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horsburgh MJ, Aish JL, White IJ. et al. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 2002; 184: 5457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plaut RD, Mocca CP, Prabhakara R. et al. Stably luminescent Staphylococcus aureus clinical strains for use in bioluminescent imaging. PLoS One 2013; 8: e59232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins DL, Chang R, Debabov DV. et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2005; 49: 1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breeuwer P, Abee T. Assessment of viability of microorganisms employing fluorescence techniques. Int J Food Microbiol 2000; 55: 193–200. [DOI] [PubMed] [Google Scholar]
- 24. Van Praagh AD, Li T, Zhang S. et al. Daptomycin antibiotic lock therapy in a rat model of staphylococcal central venous catheter biofilm infections. Antimicrob Agents Chemother 2011; 55: 4081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ulphani JS, Rupp ME. Model of Staphylococcus aureus central venous catheter-associated infection in rats. Lab Anim Sci 1999; 49: 283–7. [PubMed] [Google Scholar]
- 26. Chauhan A, Lebeaux D, Ghigo JM. et al. Full and broad-spectrum in vivo eradication of catheter-associated biofilms using gentamicin-EDTA antibiotic lock therapy. Antimicrob Agents Chemother 2012; 56: 6310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panchal RG, Ulrich RL, Lane D. et al. Novel broad-spectrum bis-(imidazolinylindole) derivatives with potent antibacterial activities against antibiotic-resistant strains. Antimicrob Agents Chemother 2009; 53: 4283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Struss AK, Nunes A, Waalen J. et al. Toward implementation of quorum sensing autoinducers as biomarkers for infectious disease states. Anal Chem 2013; 85: 3355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ueda C, Tateda K, Horikawa M. et al. Anti-Clostridium difficile potential of tetramic acid derivatives from Pseudomonas aeruginosa quorum-sensing autoinducers. Antimicrob Agents Chemother 2010; 54: 683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silverman JA, Perlmutter NG, Shapiro HM. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 2003; 47: 2538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin MH, Shu JC, Huang HY. et al. Involvement of iron in biofilm formation by Staphylococcus aureus. PLoS One 2012; 7: e34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rath S, Ziesemer S, Witte A. et al. S. aureus haemolysin A-induced IL-8 and IL-6 release from human airway epithelial cells is mediated by activation of p38- and Erk-MAP kinases and additional, cell type-specific signalling mechanisms. Cell Microbiol 2013; 15: 1253–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.