Abstract
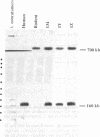
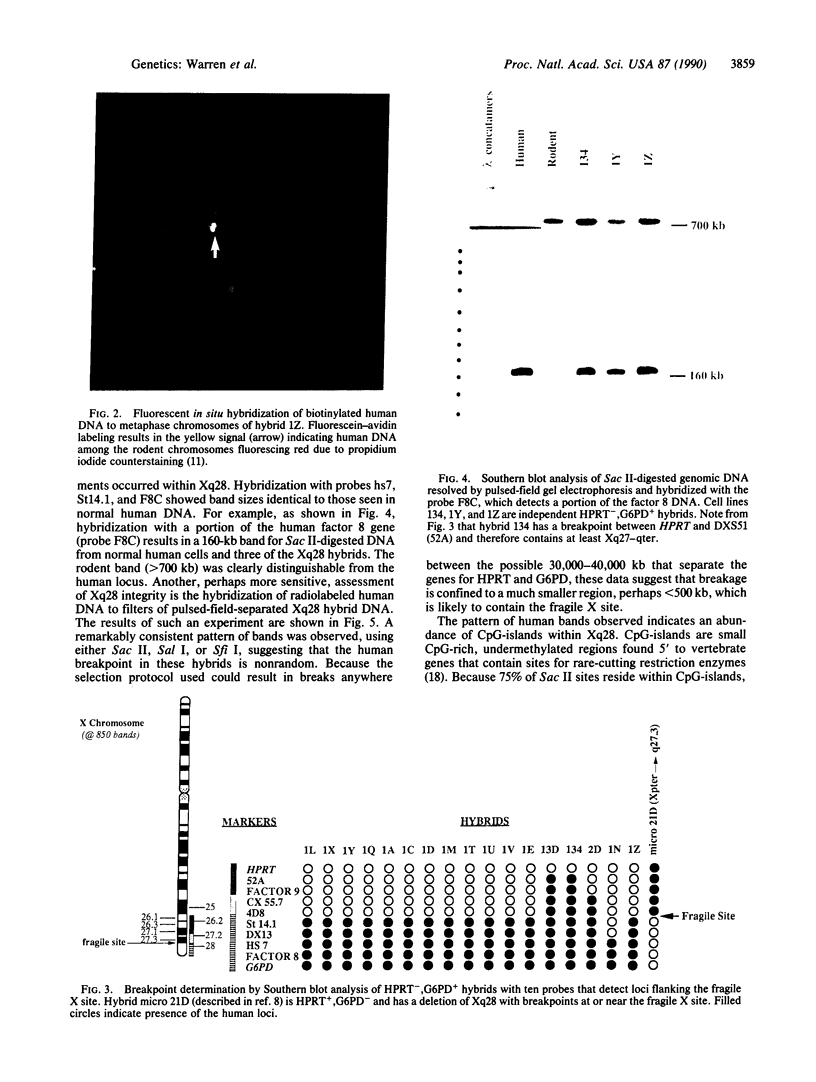
The chromosomal fragile-site mapping to Xq27.3 is associated with a frequent form of mental retardation and is prone to breakage after induced deoxyribonucleotide pool perturbation. The human hypoxanthine phosphoribosyltransferase (HPRT) and glucose-6-phosphate dehydrogenase (G6PD) genes flank the fragile X chromosome site and can be used to monitor integrity of the site in human-hamster somatic cell hybrids deficient in the rodent forms of these activities. After induction of the fragile X site, negative selection for HPRT and positive enrichment for G6PD resulted in 31 independent colonies of HPRT-,G6PD+ phenotype. Southern blot analysis demonstrated the loss of all tested markers proximal to the fragile X site with retention of all tested human Xq28 loci in a majority of the hybrids. In situ hybridization with a human-specific probe demonstrated the translocation of a small amount of human DNA to rodent chromosomes in these hybrids, suggesting chromosome breakage at the fragile X site and the subsequent translocation of Xq28. Southern blot hybridization of hybrid-cell DNA, resolved by pulsed-field gel electrophoresis, for human-specific repetitive sequences revealed abundant CpG-islands within Xq28, consistent with its known gene density. The electrophoretic banding patterns of human DNA among the hybrids were remarkably consistent, suggesting that fragile X site breakage is limited to a relatively small region in Xq27-28. These somatic cell hybrids, containing Xq27.3-qter as the sole human DNA, will aid the search for DNA associated with the fragile X site and will augment the high resolution genomic analysis of Xq28, including the identification of candidate genes for genetic-disease loci mapping to this region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dana S., Wasmuth J. J. Linkage of the leuS, emtB, and chr genes on chromosome 5 in humans and expression of human genes encoding protein synthetic components in human--Chinese hamster hybrids. Somatic Cell Genet. 1982 Mar;8(2):245–264. doi: 10.1007/BF01538680. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Ledbetter S. A., Nelson D. L., Warren S. T., Ledbetter D. H. Rapid isolation of DNA probes within specific chromosome regions by interspersed repetitive sequence polymerase chain reaction. Genomics. 1990 Mar;6(3):475–481. doi: 10.1016/0888-7543(90)90477-c. [DOI] [PubMed] [Google Scholar]
- Lindsay S., Bird A. P. Use of restriction enzymes to detect potential gene sequences in mammalian DNA. 1987 May 28-Jun 3Nature. 327(6120):336–338. doi: 10.1038/327336a0. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstraus M. J., Chasin L. A. Mutants of Chinese hamster ovary cells with altered glucose-6-phosphate dehydrogenase activity. Somatic Cell Genet. 1977 May;3(3):323–333. doi: 10.1007/BF01538750. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M., Chasin L. A. Isolation of mammalian cell mutants deficient in glucose-6-phosphate dehydrogenase activity: linkage to hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):493–497. doi: 10.1073/pnas.72.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. L., Morton N. E., Jacobs P. A., Turner G. The marker (X) syndrome: a cytogenetic and genetic analysis. Ann Hum Genet. 1984 Jan;48(Pt 1):21–37. doi: 10.1111/j.1469-1809.1984.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Approaches to physical mapping of the human genome. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):115–122. doi: 10.1101/sqb.1986.051.01.014. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Davidson R. L. Expression of fragile X chromosome in human-rodent somatic cell hybrids. Somat Cell Mol Genet. 1984 Jul;10(4):409–413. doi: 10.1007/BF01535636. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Zhang F. P., Sutcliffe J. S., Peters J. F. Strategy for molecular cloning of the fragile X site DNA. Am J Med Genet. 1988 May-Jun;30(1-2):613–623. doi: 10.1002/ajmg.1320300162. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Zhang F., Licameli G. R., Peters J. F. The fragile X site in somatic cell hybrids: an approach for molecular cloning of fragile sites. Science. 1987 Jul 24;237(4813):420–423. doi: 10.1126/science.3603029. [DOI] [PubMed] [Google Scholar]
- Webb T. P., Bundey S. E., Thake A. I., Todd J. Population incidence and segregation ratios in the Martin-Bell syndrome. Am J Med Genet. 1986 Jan-Feb;23(1-2):573–580. doi: 10.1002/ajmg.1320230151. [DOI] [PubMed] [Google Scholar]