Abstract
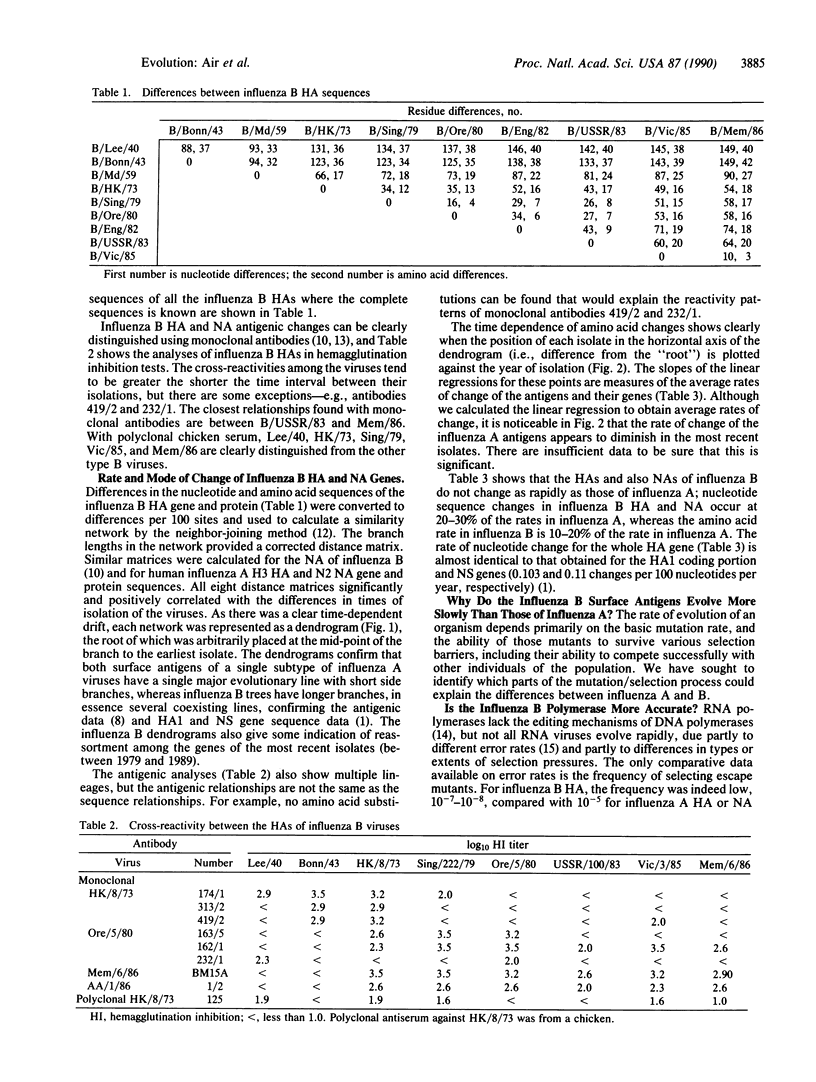
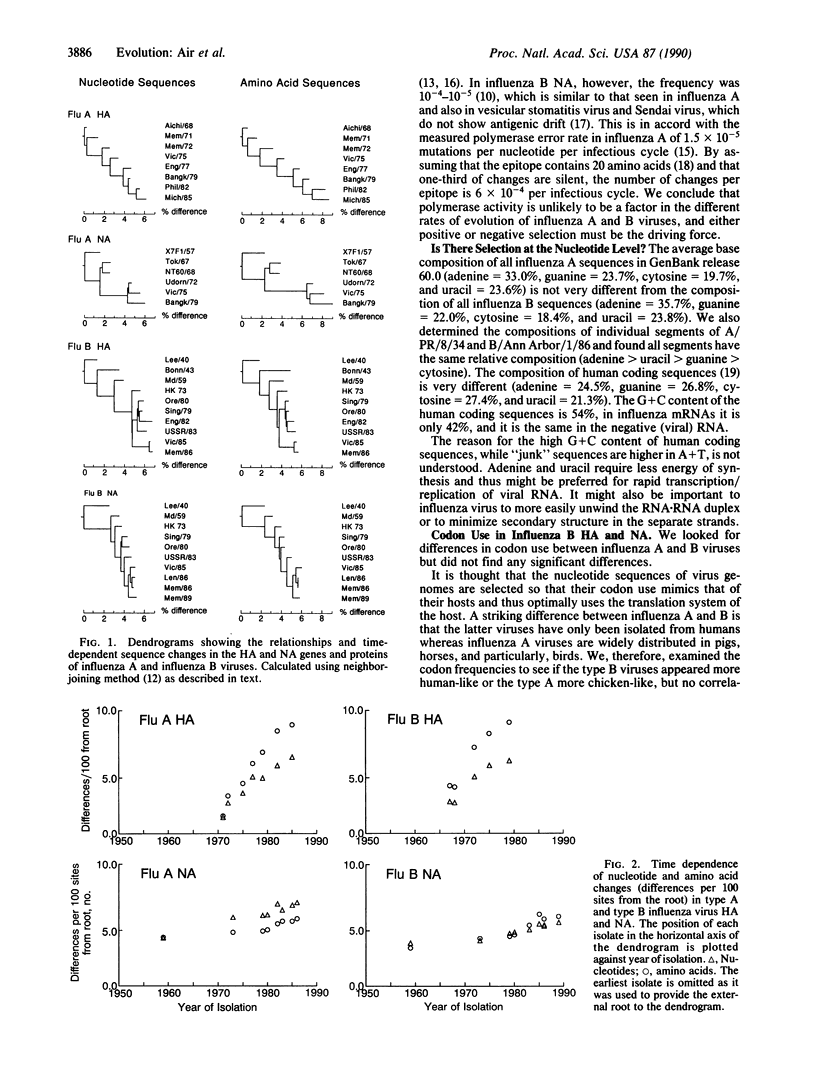
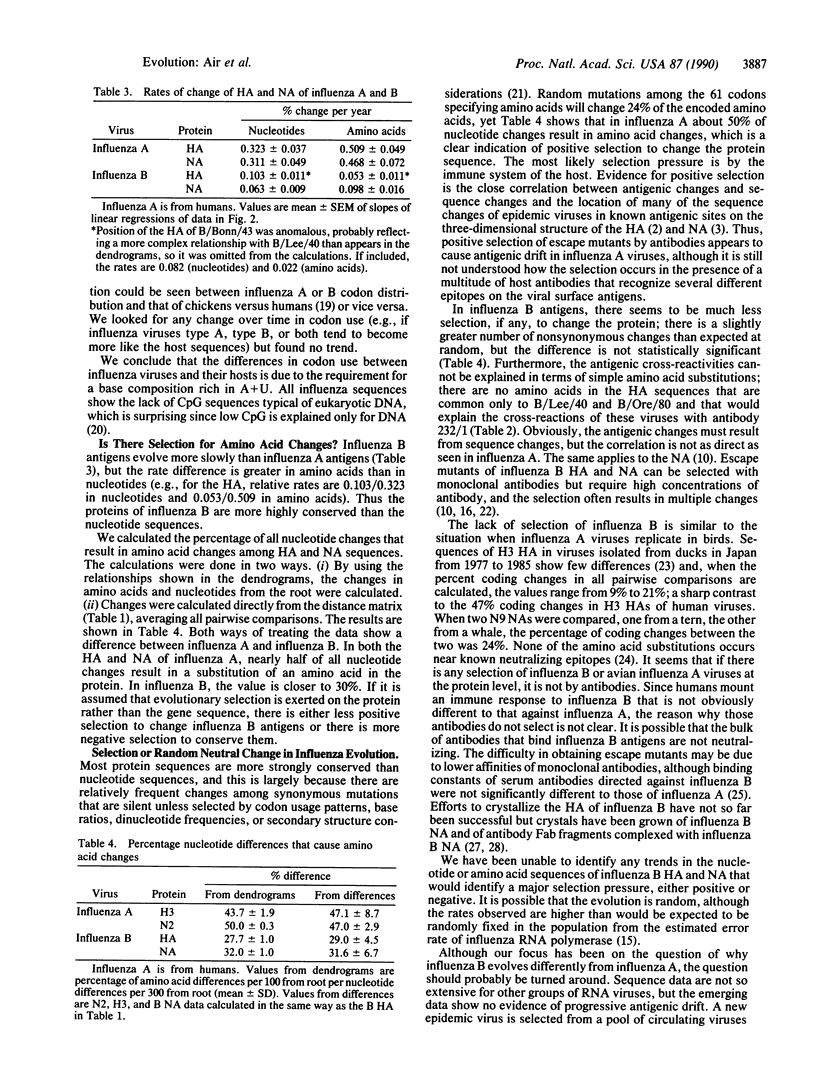
Influenza B viruses evolve more slowly than human influenza A, but no reasons for the difference have been established. We have analyzed sequence changes in the hemagglutinin and neuraminidase of influenza B viruses (and have determined four hemagglutinin sequences, of B/Bonn/43, B/USSR/100/83, B/Victoria/3/85, and B/Memphis/6/86) in relation to antigenic properties and compared these with similar analyses of variation in influenza A antigens. Independent of the slower rate of change in influenza B antigens, only approximately 30% of nucleotide changes in either the hemagglutinin or neuraminidase gene sequence result in amino acid changes in the protein, whereas in influenza A 50% of nucleotide changes result in altered amino acids. Thus, there is less selection for change, or less tolerance to change, in the influenza B antigens. This is similar to findings with influenza C and findings with influenza A viruses that replicate in lower animals and birds and is closer to the type of variation found in other RNA viruses. We propose that human influenza A is unique in that it is the only virus group in which antibody selection dominates evolutionary change.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Webster R. G., Colman P. M., Laver W. G. Distribution of sequence differences in influenza N9 neuraminidase of tern and whale viruses and crystallization of the whale neuraminidase complexed with antibodies. Virology. 1987 Oct;160(2):346–354. doi: 10.1016/0042-6822(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Bao-Lan L., Webster R. G., Brown L. E., Nerome K. Heterogeneity of influenza B viruses. Bull World Health Organ. 1983;61(4):681–687. [PMC free article] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Berton M. T., Webster R. G. The antigenic structure of the influenza B virus hemagglutinin: operational and topological mapping with monoclonal antibodies. Virology. 1985 Jun;143(2):583–594. doi: 10.1016/0042-6822(85)90396-4. [DOI] [PubMed] [Google Scholar]
- Bossart P. J., Babu Y. S., Cook W. J., Air G. M., Laver W. G. Crystallization and preliminary X-ray analyses of two neuraminidases from influenza B virus strains B/Hong Kong/8/73 and B/Lee/40. J Biol Chem. 1988 May 5;263(13):6421–6423. [PubMed] [Google Scholar]
- Both G. W., Sleigh M. J., Cox N. J., Kendal A. P. Antigenic drift in influenza virus H3 hemagglutinin from 1968 to 1980: multiple evolutionary pathways and sequential amino acid changes at key antigenic sites. J Virol. 1983 Oct;48(1):52–60. doi: 10.1128/jvi.48.1.52-60.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Fitch W. M., Palese P. Epidemiology of influenza C virus in man: multiple evolutionary lineages and low rate of change. Virology. 1986 Aug;153(1):12–21. doi: 10.1016/0042-6822(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Hovanec D. L., Air G. M. Antigenic structure of the hemagglutinin of influenza virus B/Hong Kong/8/73 as determined from gene sequence analysis of variants selected with monoclonal antibodies. Virology. 1984 Dec;139(2):384–392. doi: 10.1016/0042-6822(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Kida H., Kawaoka Y., Naeve C. W., Webster R. G. Antigenic and genetic conservation of H3 influenza virus in wild ducks. Virology. 1987 Jul;159(1):109–119. doi: 10.1016/0042-6822(87)90353-9. [DOI] [PubMed] [Google Scholar]
- King J. L., Jukes T. H. Non-Darwinian evolution. Science. 1969 May 16;164(3881):788–798. doi: 10.1126/science.164.3881.788. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Luo M., Bossart P. J., Babu Y. S., Smith C., Accavitti M. A., Tulloch P. A., Air G. M. Crystallization and preliminary X-ray analysis of type B influenza virus neuraminidase complexed with antibody Fab fragments. Virology. 1988 Dec;167(2):621–624. [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Studies on the origin of pandemic influenza. 3. Evidence implicating duck and equine influenza viruses as possible progenitors of the Hong Kong strain of human influenza. Virology. 1973 Feb;51(2):383–391. doi: 10.1016/0042-6822(73)90437-6. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Takeuchi Y., Nakajima K. Location on the evolutionary tree of influenza H3 haemagglutinin genes of Japanese strains isolated during 1985-6 season. Epidemiol Infect. 1988 Apr;100(2):301–310. doi: 10.1017/s0950268800067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford J. S., Klimov A. I., Corcoran T., Ghendon Y. Z., Schild G. C. Biochemical and serological studies of influenza B viruses: comparisons of historical and recent isolates. Virus Res. 1984;1(3):241–258. doi: 10.1016/0168-1702(84)90042-x. [DOI] [PubMed] [Google Scholar]
- Parvin J. D., Moscona A., Pan W. T., Leider J. M., Palese P. Measurement of the mutation rates of animal viruses: influenza A virus and poliovirus type 1. J Virol. 1986 Aug;59(2):377–383. doi: 10.1128/jvi.59.2.377-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Webster R. G., Bean W. J. Similar frequencies of antigenic variants in Sendai, vesicular stomatitis, and influenza A viruses. Virology. 1980 Jul 15;104(1):235–238. doi: 10.1016/0042-6822(80)90382-7. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Pereira M. S., Chakraverty P., Coleman M. T., Dowdle W. R., Chang W. K. Antigenic variants of influenza B virus. Br Med J. 1973 Oct 20;4(5885):127–131. doi: 10.1136/bmj.4.5885.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulip W. R., Varghese J. N., Webster R. G., Air G. M., Laver W. G., Colman P. M. Crystal structures of neuraminidase-antibody complexes. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):257–263. doi: 10.1101/sqb.1989.054.01.032. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Berton M. T. Analysis of antigenic drift in the haemagglutinin molecule of influenza B virus with monoclonal antibodies. J Gen Virol. 1981 Jun;54(Pt 2):243–251. doi: 10.1099/0022-1317-54-2-243. [DOI] [PubMed] [Google Scholar]
- Webster R. G. The immune response to influenza virus. II. Effect of the route and schedule of vaccination on the quantity and avidity of antibodies. Immunology. 1968 Jan;14(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Krystal M., Fitch W. M., Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology. 1988 Mar;163(1):112–122. doi: 10.1016/0042-6822(88)90238-3. [DOI] [PubMed] [Google Scholar]


