Abstract
Background and aims
Despite advances in our knowledge of effective services for people who use drugs over the last decades globally, coverage remains poor in most countries, while quality is often unknown. This paper aims to discuss the historical development of successful epidemiological indicators and to present a framework for extending them with additional indicators of coverage and quality of harm reduction services, for monitoring and evaluation at international, national or subnational levels. The ultimate aim is to improve these services in order to reduce health and social problems among people who use drugs, such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infection, crime and legal problems, overdose (death) and other morbidity and mortality.
Methods and results
The framework was developed collaboratively using consensus methods involving nominal group meetings, review of existing quality standards, repeated email commenting rounds and qualitative analysis of opinions/experiences from a broad range of professionals/experts, including members of civil society and organisations representing people who use drugs. Twelve priority candidate indicators are proposed for opioid agonist therapy (OAT), needle and syringe programmes (NSP) and generic cross-cutting aspects of harm reduction (and potentially other drug) services. Under the specific OAT indicators, priority indicators included ‘coverage’, ‘waiting list time’, ‘dosage’ and ‘availability in prisons’. For the specific NSP indicators, the priority indicators included ‘coverage’, ‘number of needles/syringes distributed/collected’, ‘provision of other drug use paraphernalia’ and ‘availability in prisons’. Among the generic or cross-cutting indicators the priority indicators were ‘infectious diseases counselling and care’, ‘take away naloxone’, ‘information on safe use/sex’ and ‘condoms’. We discuss conditions for the successful development of the suggested indicators and constraints (e.g. funding, ideology). We propose conducting a pilot study to test the feasibility and applicability of the proposed indicators before their scaling up and routine implementation, to evaluate their effectiveness in comparing service coverage and quality across countries.
Conclusions
The establishment of an improved set of validated and internationally agreed upon best practice indicators for monitoring harm reduction service will provide a structural basis for public health and epidemiological studies and support evidence and human rights-based health policies, services and interventions.
Keywords: Substance abuse, People who use drugs/PWUD, People who inject drugs/PWID, Injecting drug users/IDU, Best practice, Harm reduction, Knowledge exchange, Interventions, Indicators, Coverage, Epidemiology, HIV, HCV, Monitoring, Evidence-based, Drug services
Background
Important advances in interventions for people who use drugs (PWUD), in particular those who use opioids and people who inject drugs (PWID), have occurred over recent decades. Harm reduction services such as needle and syringe programmes (NSP) and opioid agonist therapy (OAT) [1] have been increasingly established, with 90 countries having NSP to some degree and 80 at least one OAT programme operational by 2016 [2]. This has contributed to reductions in viral infections (e.g. human immunodeficiency virus (HIV), hepatitis C virus (HCV)) and bacterial infections (e.g. tuberculosis (TB), sexually transmissible infections, skin infections), crime, overdose and mortality among PWUD. Health cost savings are being achieved globally, where harm reduction is in place, especially where these services are combined with antiretroviral therapy (ART), allowing millions of people living with HIV to stay healthy [3–15]. The provision of naloxone, a drug to reverse overdose, has expanded from paramedics to drug workers and to PWUD themselves and their peers [16–18]. Treatments for infectious diseases (e.g. HIV, hepatitis B virus (HBV)) and new direct-acting antiviral (DAA) treatments for HCV, when available, are having large effects on survival and quality of life and have opened new avenues for effective prevention [19–22]. Evidence on intervention best practice is mounting and is increasingly based on larger and better designed studies [23, 24].
Drug policies have also started to shift, even if the translation of evidence into policy remains difficult [25–28]. In some countries, there is cooperation between judicial and health authorities to mitigate harms associated with the criminalisation of drug use [29] and explicit or de facto decriminalisation of drug use [30–32]—these may often go together [33]—or even legalisation, in the case of cannabis [34–37]. Human rights-based approaches to drug treatment, incorporating harm reduction and social integration, have been implemented in a number of countries despite universal, national and global drug prohibition policies [38–40]. Despite such positive progress, however, many countries still have very low implementation levels of evidence-based programmes, exposing PWUD and the wider society to unnecessary health risks [13, 15, 41]. Above all, interventions appear to be frequently lacking for some of the most socially deprived groups, such as homeless, migrants, sex workers and prisoners [42–52]. Harm reduction and drug policy more widely have not been high on the international political agenda, with the United Nations General Assembly Special Session on the World Drug Problem in 2016 being the first high-level meeting after many years with the aim to debate drug policy. Also, the global target to reduce new HIV infections by 50% by 2015 was missed, and the latest UNAIDS (The Joint United Nations Programme on HIV/AIDS) report suggests that HIV infections among this group actually increased by one third between 2011 and 2015 [53].
Critically, there are still continuous gaps in information on how effectively interventions are actually being provided; their coverage, quality, client characteristics and the degree to which they fulfil the needs of different populations of drug users [13, 15, 54–56]. While in many countries there are regular—often costly—epidemiological studies on the characteristics and behaviours of drug users, the collection of comparable and reliable monitoring data on the extent and quality of routine interventions (for example NSP) and service implementation remains rare. Epidemiological studies and routine analysis of health indicator data are key to evaluating drug service effectiveness, but they are infrequently extended to and combined with detailed information on intervention characteristics [15, 41, 57–59]. A tight nexus between indicators of quality and drug service provision and health outcomes has been documented [60–62]. Despite the wide range of quality standards and best practice guidelines for drug services on the national and international level [6, 23, 24, 56], research has shown that adherence to these guidelines should not be taken for granted, and there is a need for data that reflect the reality of actual practice ‘on the ground’ [63, 64]. There is increasing interest in the quality and coverage of harm reduction services for people who use drugs and in the development of methodologies for measuring these [6, 56, 65]. An understanding of what services are being provided, in what form and the extent to which they are provided to individual users, including their views on the provision (where possible extending to enumeration of costs and if possible—in separate studies by specialist researchers—modelling of cost-effectiveness) is critical to the analysis of public health needs and whether these are adequately addressed.
This paper aims to identify which standardised data are needed—and why—for monitoring both the coverage and quality of harm reduction services [56]. This is not the type of research question that can be readily addressed through standard epidemiological methods. Rather, useful approaches may include analysis of historical developments in the area, critical discussion of current best practices (i.e. indicators in use that have proved successful) and data gap analysis.
Methods
As a first step, we describe the historical development of established international monitoring systems and indicators in the field of drugs and health. We then propose a framework for further indicator development and evaluation in the area of harm reduction (and potentially other drug services, for examples see the footnotes below Table 3). This framework was developed using consensus methods, including nominal group meetings and email discussions [66, 67] reviewing existing quality standards [6, 56, 68], to capture and analyse the opinion and experience from a broad range of professionals/experts. The participating experts provided different perspectives and expertise (international and national monitoring system specialists, researchers, harm reduction professionals, government representatives) and included members of civil society organisations representing PWUD and people living with HIV/HCV. The framework lists candidate indicators for OAT, NSP and generic cross-cutting indicators for harm reduction (and potentially other drug) services. The framework with candidate indicators was developed in an iterative process of multiple commenting rounds until a stable consensus list of potential indicators (and areas for future indicator development) emerged. We discuss constraints (e.g. funding, ideology) and conditions for potential successful development of the suggested candidate indicators.
Table 3.
Framework for the development of indicators for quality monitoring of harm reduction services, with a focus on opioid agonist therapy (OAT) and needle and syringe programmes (NSP); priority indicators are in italics
| Specific OAT indicators may includea: |
| Coverage of estimated opioid user population (%, see Fig. 1 ) |
| Waiting time to first treatment admission (months) |
| Methadone/buprenorphine dosage (grams) |
| OAT available (including new initiation) in prisons (in all /in some /no) |
| OAT medicine covered by state /health insurance (yes /partly /no) |
| Illicit drug consumption tolerated (after dose induction phase) (yes /no) |
| Diagnosis or detailed assessment of current substance use, individualised treatment planning (yes /no) |
| Take home OAT available (yes /no) |
| Counselling required (yes /no) |
| Specific NSP indicators may includea: |
| Coverage of estimated PWID population (syringes /PWID /year, see Fig. 2 ) |
| Annual number of needles /syringes distributed and collected (administrative data, and /or estimated by weight) b |
| Provision of drug use equipment and injecting paraphernalia (including for non-injected use e.g. foils for heroin chasing, stems and filters for crack smoking) (in all /in some /no) |
| NSP available in prisons (in all /in some /no) |
| Coverage of all undertaken injections (syringes /100 injections) |
| Restrictions in numbers of syringes distributed per contact (yes /no) |
| Type of syringes (% low dead space, acceptance by users) |
| Modality (specialised NSP, outreach, pharmacy, other, e.g. drug treatment service) |
| Brief opportunistic motivational interventions provided (yes /no) |
| Generic cross-cutting indicators for harm reduction (and other drug services) may includea: |
| Infectious diseases counselling, testing, vaccination and referrals (e.g. HIV, HCV, HBV, TB) (in all /in some /no) |
| Take away naloxone provided (in all /in some /no) |
| Information provided on safer use, injecting and safer sex (in all /in some /no) |
| Condoms provided (in all /in some /no) |
| Accessibility: opening times and geographic coverage, outreach activities, costs to clients, no age limits, no parental consent requirements, targeted programmes for special populations (e.g. (pregnant) women, sex workers, underage users) (to construct overall index score: high /medium /low) |
| Integration /cooperation with other services and continuity of care: e.g. shared location /referrals to NSP, OAT, infectious diseases counselling and testing, antiviral and other medical treatment and care, overdose prevention, social support, housing, education, employment services (in all /in some /no) |
| Regular consultation with law enforcement /community /neighbourhood: avoiding nuisance and conflict, improving safety for both clients and community (index: high /medium /low) |
| Regular consultation with the users of the service: feedback, evaluation, client satisfaction (index: high /medium /low) |
| Assessment procedures: risk behaviours, needs, health status, informed consent, data confidentiality, written client records (index: high /medium /low) |
| Psycho-social interventions provided (with or without medication): (yes /no) |
| Frequency of contact with a counsellor /social worker (times per month) |
| Staff qualification, multidisciplinarity, education and (ongoing) training (index: high /medium /low) |
| Case /contact management follows protocol /guidelines (yes /no, specify which) |
| Type of funding source: private /public; national /international, etc.; and security of funding (per client, grant-based, etc.), utilisation monitoring (treatment slots used), peer support /aid (to construct an overall index score on funding continuity and reliability: high /medium /low) |
aThe quality indicators listed are mostly structural and procedural [56]. Outcome indicators are limited to OAT and NSP coverage estimates. Other outcome indicators may be considered (e.g. client retention and return rates, reductions in drug use, crime, improvements in health, etc.), but given their complexity, this may be more appropriate to assess in detailed service evaluation studies at national or local level [171] (although note [110]). Further work may be needed to link up more strongly with recently adopted EU quality standards [68]. Other harm reduction and drug interventions to be considered for monitoring may include antiviral and antibacterial therapy (e.g. HIV, HCV, HBV, TB), heroin-assisted treatment, drug consumption rooms/safer injecting facilities, testing drug content and handing out water at rave parties and similar events, police interactions with drug users affecting service utilisation, interventions in special settings (e.g. prisons, mobile or outreach interventions), social interventions, e.g. relating to children or family of PWUD, and monitoring and may even extend to drug policy indicators (e.g. minimum quantities of drugs allowed for personal use, sentencing practise, medical use of cannabis, decriminalisation/liberalisation of drug laws, drug treatment regulations, e.g. allowing opioid agonist therapy through primary caregivers), continuity of care following prison release or treatment discharge
bMeasuring infection rates in returned syringes may form an important and cost-effective method for monitoring prevalence and incidence of infection in the population [135, 136].
Measures of central tendency (e.g. mean, median) may be complemented by measures of variability (e.g. range, interquartile range) to better capture intra- and inter-national variation.
Results
Historical development of existing drug use monitoring systems
The global development of indicators in the drugs field was spearheaded in the area of HIV/AIDS. In 1989, one of the first common sets of indicators (behavioural) for people who inject drugs (PWID) was applied across countries by the World Health Organization (WHO) ‘13 cities study of drug injecting and HIV infection’ [69]. In 1998, the National Institute on Drug Abuse (NIDA), WHO and UNAIDS formed the ‘Global Research Network on HIV Prevention in Drug-Using Populations’ (GRN) to help control the HIV epidemic among PWID [70] by discussing best practice and exchanging national study methods and results in international meetings. The GRN was succeeded in 2004 by the ‘Reference Group to the United Nations on HIV and Injecting Drug Use’, a network funded by UNODC, WHO and UNAIDS, to estimate the global spread of HIV among PWID [71–73] and intervention coverage [13] using common methodology, which culminated in UN guidance for countries to set targets for intervention coverage [6, 74] and implementation [75]. Ongoing global monitoring has more recently been taken up by UN reporting systems [76, 77] and non-governmental and academic organisations [2, 78].
In Europe, comparable work on drug use started in 1982 with the ‘Multi-city study of drug misuse in Europe’ [79]. This expert network developed epidemiological indicators to interpret trends in drug use and their consequences from routine sources and studies across countries, leading to the first pan-European drug treatment data monitoring protocol [80, 81]. European multi-country impact studies on HIV/AIDS and PWID followed in 1989–1993 [82–87], leading to an increased interest in preventing HIV transmission in prisons [88–90]. The growing global attention paid to HIV/AIDS accelerated the urgency to improve responses for PWID, leading to the creation of a single agency for the European Union (EU) in the area of drugs. Since 1995, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) and its national partners (the ‘Reitox Network’ (Réseau Européen d ´Information sur les Drogues et les Toxicomanies) of National Focal Points, as well as multiple topic-specific expert networks, have collaborated to gather evidence on the situation of drugs and their consequences to support national policymaking [41, 91–97]. A central area of this work concerns the development of the five ‘key epidemiological indicators’ of drug use and its consequences (general population surveys, population size estimates of PWUD at high risk of (or already experiencing) negative consequences and that include hidden populations, infectious diseases—HIV and viral hepatitis, overdose deaths, treatment demand) (Table 1) [98–100]. Despite the difficulties of collecting reliable data at a pan-European level, [101–103] these are being relatively well reported (almost all countries reporting on most indicators, Table 1), and they have been followed at the global level [73, 104–106].
Table 1.
Epidemiological indicators for people who use drugs being used at European Union level
| Domain | Indicators | Countries, out of 30, reporting in 2011–2015b | Data type | Additional information |
|---|---|---|---|---|
| Prevalence of drug use in the general populationa | Prevalence of lifetime use, last year use, last month use | 25 | % | Representative (household) surveys with breakdowns by drug, age, gender, complemented by school surveys in 15-16 year old students (ESPAD) http://www.espad.org/ |
| High-risk drug use/problem drug usea,c | Population size estimates of high-risk PWUD including hidden populations (all, opioids, stimulants, PWID) | 25 | Rate/1000 | Confidence intervals, estimation methods |
| Treatment demanda | Clients entering treatment | 30 | Counts | Breakdowns by ever previously treated, treatment type, prison, main drug, sex, age at treatment, age at first use, referral source, living status, education, labour status, route of administration, frequency of use |
| Overdose deathsa | Number of deaths, average age | 30 | Counts | Breakdowns by gender, toxicology, ICD code |
| Infectious diseasesa | Notifications and prevalence of HIV/AIDS, HBV, HCV among PWID | Prevalence: HIV 29 HCV 25, HBV 18–16; notifications: HIV/AIDS 30/29 | Counts, % | Prevalence among young and new PWID |
| Seizures of drugs | Number, quantity in kg | 28, 30 | Counts, weights | Seizures by drug class, cannabis plants, tablets/doses |
| Price, purity/potency | Price, potency/purity | 29, 29 | Euro/g, % (%THC)e | Sample size, summary statistics, composition (% MDMAd/(meth)amphetamines) |
| Drug use in prison | Prevalence of lifetime use, last year use, last month use | 10 | % | Breakdowns by: before/in prison, drug class |
| Drug law offences | Number of: offences, offenders, either | 25, 21, 30 | Counts | Breakdowns by type (use, supply), drug class |
aFive ‘key epidemiological indicators’. Available at http://www.emcdda.europa.eu/data/stats2016
bYear of reporting data to EMCDDA–the actual study year (year of primary data collection) is mostly 1 year earlier
cThis key indicator has been renamed from ‘Problem Drug Use’ (definition: ‘injecting drug use or long duration/regular use of opiates, cocaine and/or amphetamines’) to ‘High Risk Drug Use’ (definition: ‘recurrent drug use that is causing actual harms (negative consequences) to the person (including dependence but also other health, psychological or social problems), or is placing the person at a high probability/risk of suffering such harms’). It attempts to define and estimate the population size of those PWUD that are likely to be in need of services due to having (a high risk of) negative consequences from their drug use, such as PWID or people who use opioids
d3,4-Methylenedioxymethamphetamine (‘ecstasy’)
eTetrahydrocannabinol
A smaller number of intervention indicators were also developed, in the areas of drug treatment and harm reduction (Table 2). These concern both the provision of services (counts of clients entering treatment or syringes and clients/contacts in NSP) as well as coverage indicators (provision divided by estimates of the population in need of the service) [15, 107–109]. In 2013, a majority of countries were able to provide most of the provision indicators. However, reporting of the coverage indicators was significantly weaker, mainly because they necessitate additional information, in the form of population size estimates for PWUD as their denominators (from Table 1) (Table 2). Although provision indicators are important, for example to follow trends over time, they have inherent limitations, and additional coverage indicators are essential.
Table 2.
Health and social intervention indicators for people who use opioids and people who inject drugs being used at European Union level
| Intervention | Indicators | Countries, out of 30, reporting in 2011–2015a | Data type | Additional information |
|---|---|---|---|---|
| Provision | ||||
| Drug treatment (total) | All clients | 30 | Counts | – |
| OAT | All clients, by OAT medication | 30, 30 | Counts | Legal framework/providers |
| NSP | Syringes provided, clients, contacts, fixed sites, outreach sites | 25, 19, 20, 28, 26 | Counts | Estimated reporting coverage (%), NUTS2/3 levelb |
| Coverage | ||||
| OAT | OAT clients divided by the estimated number of opioid users (Fig. 1) | 20 | % | Confidence intervals, estimation methods |
| NSP | Syringes provided divided by the estimated number of PWID (Fig. 2) | 14 | % | Confidence intervals, estimation methods |
Available at http://www.emcdda.europa.eu/data/stats2016
aYear of reporting data to EMCDDA–the actual study year (year of primary data collection) is mostly 1 year earlier
bNomenclature of Territorial Units for Statistics
Rates of drug use or drug injection differ strongly between countries, and thus, the comparability and interpretability of the simpler provision indicators (as counts, or rates per general population) may be seriously compromised with regard to the target populations of people who use opioids or PWID. Nevertheless, coverage indicators clearly also have limitations, for example uncertainty intervals around central estimates are often large and estimation methods not uniform, in addition to the lower reporting rates [41].
However, despite the significant drawbacks, they provide relatively comparable evidence (‘best available estimates’) across countries with regard to whether services meet the needs of the target population, with recent data suggesting that important differences in coverage may exist between countries in Europe (Figs. 1 and 2). These coverage indicators have been adopted at global level to assess policy implementation in the drug field [6, 13, 41, 74]. At the same time, it is clear that they are limited in terms of giving insight into modalities of provision and the perspectives of people using the service; thus, developing additional indicators of service quality is likely to improve the usefulness and interpretability of the intervention coverage indicators. Existing quality standards [6, 56, 68] provide an important basis for developing epidemiological indicators of service quality.
Fig. 1.
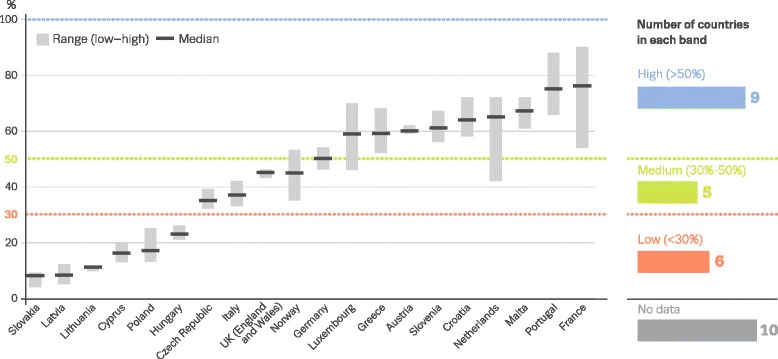
Estimated percentage of people who use opioids receiving opioid agonist therapy during 1 year (EMCDDA 2016) [41]. Note: data displayed as uncertainty intervals and point estimates. Estimates are based on latest data available on clients in opioid use treatment (2012–2014) combined with most recent estimates of opioid use prevalence (2007–2014). Below red dotted line, low (<30%); between red and green dotted lines, medium (30–50%); above green dotted line, high (>50%)
Fig. 2.
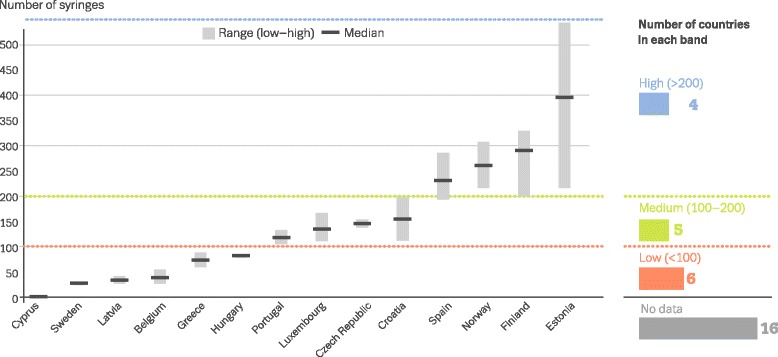
Estimated number of syringes provided annually through specialised programmes per person who injects drugs (EMCDDA 2016) [41]. Note: data displayed as uncertainty intervals and point estimates. Estimates are based on latest data available on syringe provision (2013–2014) combined with most recent estimates of PWID prevalence (2008–2014). Below red dotted line, low (<100); between red and green dotted lines, medium (100–200); above green dotted line, high (>200).
Results of the expert group consultation
During 2014 and 2015, an international expert network began discussions to advance the monitoring and evaluation of best practice in drug-related interventions in Europe. It recommended focusing on the monitoring of coverage and quality of harm reduction services, as a first step to improving best practice implementation of wider drug services. This could best be achieved by integrating a limited set of additional indicators into the existing intervention indicators as currently coordinated by the EMCDDA as well as strengthening the reporting of existing indicators. Any additional indicators would then benefit from the ongoing efforts by European countries to ensure the timeliness, quality and completeness of data. Candidate indicators should compare key aspects of intervention delivery across countries, should be relatively easy to collect, where possible be evidence-based and, if not, based on expert consensus, and represent quality and coverage of services [110]. It was decided to start in a pragmatic way by producing a ‘framework’, i.e. mapping a list of potentially suitable candidate indicators and areas for future indicators, building on existing quality standards [6, 56, 68], the available expert opinions and experience and using consensus methods, as described above. The candidate indicators were chosen on their potential to reflect the structural and procedural quality of harm reduction services and service coverage [6, 56]. In future work, similar indicators could be set up for other interventions for PWUD, e.g. antiviral therapy or infectious disease testing [5, 6, 111]. For the suggested framework with candidate indicators of harm reduction service quality and coverage (OAT, NSP and ‘generic cross-cutting’ indicators), see Table 3.
Framework of potential indicators and areas for consideration
As expected, two main interventions were indicated by the experts as central to harm reduction (mainly, prevention of infectious diseases such as HIV and viral hepatitis and of opiate-related overdose), namely, NSP and OAT. Other areas in harm reduction for further consideration of indicator development, but for practical reasons not included among the recommended indicators, included ART (both for HIV and viral hepatitis), consumption rooms and heroin-assisted treatment (Table 3). Under the specific OAT indicators, priority indicators included ‘coverage’, ‘waiting list time’, ‘dosage’ and ‘availability in prisons’. For the specific NSP indicators, the priority indicators included ‘coverage’, ‘number of needles/syringes distributed/collected’, ‘provision of other drug use paraphernalia’ and ‘availability in prisons’. Among the generic or cross-cutting indicators proposed for harm reduction services (and potentially other drug services), the priority indicators were ‘infectious diseases counselling and care’, ‘take home naloxone’, ‘information on safe use/sex’ and ‘condoms’ (for details, see Table 3).
Discussion
This consensus study provides a basis for the development and implementation of indicators of harm reduction quality and coverage and highlights further areas of potential monitoring of best practice intervention. Twelve priority candidate indicators were identified, on OAT, NSP and generic service quality aspects. Most of these seem relatively easy to monitor, consisting of simple ‘yes/no’ responses or a basic statistic. We propose conducting a pilot study to test the feasibility and applicability of the proposed indicators before their scaling up, to evaluate their effectiveness in comparing service quality across countries. From the experience in Europe, we suggest that this development should be collaborative (‘bottom-up’) making use of national and local experience and involving a broad range of experts and stakeholders (e.g. professionals, policymakers, representatives of people who use drugs and/or drug services, harm reduction organisations) across countries [56].
Important services were not included for monitoring, e.g. ART, mainly due to difficulties in finding a simple operationalisation or a key statistic from routine data that is readily available for all countries to be reported (such data may be obtained by special surveys; however, these are costly). While NSP and OAT are services that are specific for people who use opioids or PWID, respectively, and thus client numbers can be interpreted more easily, for ART this is not the case and in practice it is harder to come by reliable numbers for specific at-risk groups in treatment, e.g. PWID or men who have sex with men. Other services that are important but were not included are heroin-assisted treatment, drug consumption rooms/safer injection facilities, drug testing and water provision at rave parties, police interactions with drug users and interventions in special settings such as prisons. Again, their non-inclusion resulted not because they were considered unimportant but rather they were thought to be harder to monitor (e.g. police interactions) or to be partly overlapping with other indicators (e.g. safer injection rooms with NSP). However, indicators not included here might still be considered for implementation by individual countries depending on national context and priorities. For example, in many Latin American and Caribbean countries, stimulant use is more important than opioids, which might require adapting the indicators [2, 112]. Our approach might be extended to areas surrounding the actual implementation of drug services. For example, drug policy indicators could be considered for monitoring, e.g. sentencing practices and minimum quantities of drugs allowed for personal use, decriminalisation/liberalisation of drug laws or drug treatment regulations may have profound impact on health and well-being of PWUD. A recent study proposed a framework to classify countries by their models of ‘governance of addictions’ from an analysis of national drug strategies [33]. Monitoring both drug policies and their actual implementation and practice might reveal important discrepancies between the two, providing key policy relevant information [113, 114].
Indicators for the quality of drug services must be closely linked to epidemiological data and methods. The development of OAT and NSP coverage indicators (Figs. 1 and 2) was made possible by the increased availability of routine epidemiological monitoring data and the increased use of statistical modelling methods. The methods to estimate population sizes of PWUD/PWID originated in biology and continue to be improved for epidemiological application even if they have not essentially changed [97, 102, 105, 115–129]. Mathematical and statistical modelling has more generally been useful to improve our understanding of intervention effectiveness and cost-effectiveness as well as to give insight in potential epidemic courses and processes, thus providing some basis to evaluate interventions [95–97, 130–134]. Different types of intervention have been studied using mathematical models, such as impact of needle exchange programmes [135, 136], impact of behavioural changes [137] and impact of treatment on transmission [138, 139]. Recent studies suggest that molecular analyses of infectious diseases may also provide added value to epidemiological surveillance as a basis for evaluating interventions [48, 140–143]. Moreover, comprehensive reviews of epidemiological data (and intervention effectiveness and implementation) have been carried out to estimate the burden of disease and quality of life, providing a means to compare health and societal impact of interventions across different diseases including through cost-effectiveness analyses [144–148]. Indicators should not be limited to national-level data only. Having subnational breakdowns—by city or region—would be critical to understand within-country variation in epidemiological trends and intervention impact [149–152].
Apart from using the proposed indicators individually, they might be used for system-level evaluation to monitor and guide service integration and referral at national level. For example, it is important to use these indicators together to assess the comprehensiveness of harm reduction programming, given the evidence that harm reduction interventions are most effective when used in combination [138, 153]. Another example of a combined approach may be provided by a ‘harm reduction cascade’ model, similar to the recently proposed HIV or HCV care cascades [19, 154, 155], where the ‘flow’ of people who use drugs would be modelled through a tailored set of services, ranging from catering the needs of incidental or recreational users to those who inject drugs or are heavily dependent, and/or may have a range of health and social problems. The HIV and HCV cascade model enables the identification of gaps in health system performance by estimating the percentage of infected who know their status, percentage of those in care, percentage of those on ART and percentage of those with undetectable viral load/sustained virologic response. Care cascade indicators relate to the timely provision of ART for HIV and best medical practices for HBV, HCV and other diseases (endocarditis, methicillin-resistant staphylococcus aureus (MRSA), anthrax, TB, etc.) and might similarly be developed for drug prevention, treatment and harm reduction measures. Another example focuses on the interface between judicial and public health interventions. This includes the analysis of police interactions with drug users in the context of their service utilisation, policy indicators (e.g. minimum quantities of drugs allowed for personal use, sentencing practice, medical use of cannabis, decriminalisation/liberalisation of drug laws [37]) and the continuity of care following prison release [156, 157].
The feasibility of monitoring drug service implementation will depend on resources in countries and may therefore be more limited in low and middle income countries. However, where a country lacks the resources to implement and further develop these indicators, the proposed framework may be useful to document the absence of data in specific areas, even if in a rudimentary form (e.g. a binary ‘yes/no’ checklist). Monitoring performance should be evaluated only after several years of data collection using performance indicators such as the number of countries providing data and assessments of the credibility of the methods and sources behind the available data. In practice it may take many years to arrive at a high reporting rate with good quality data, and maintaining a long-term perspective is necessary. With respect to clinical services performance, which is evaluated by health insurance systems and/or national health authorities [158], monitoring drug services may pose specific difficulties due to their multi-disciplinary nature and as they may depend on different government and private entities and multiple funding sources. Service provision may thus depend on the type of service providers (public, private, non-governmental organizations including peer-driven initiatives, general medical practitioners), funding sources (central government, local and regional governments, social health insurance, private and other sources) and funding mechanisms (grants, treatment case, daily costs, fee for service or payment by result) [159]. Other aspects of funding might also impact on service performance, quality and outcomes—such as the way providers are chosen and the ways services are paid for, e.g. block grant, capitation, payment for activity or payment for outcome [160], although the evidence of how the funding provisions influence outcomes is mixed [161–163]. Additionally, disaggregated spending records could indicate whether programmes invest in adequate numbers of well-trained staff and procure quality commodities that meet the needs of the people accessing the service—all related to the quality of service provision. While we recommend monitoring harm reduction funding, this did not make it into the 12 priority indicators, as our focus has been on the service coverage and quality per se. While investment in itself would not denote quality, whether a programme is funded by government or an international donor can have implications for its sustainability that are important to monitor. There are several countries in Europe, as well as globally, facing issues with harm reduction sustainability and funding. It would be timely to consider a separate pilot study on the use of indicators relating to harm reduction spending.
There are several limitations to this analysis. While we were able to identify a set of priority candidate indicators using a consensus approach, we cannot at this stage present empirical evidence on the potential problems or advantages associated with implementation of these indicators. However, with the established, mostly epidemiological, indicators (Tables 1 and 2), this was a process of trial and error where a number of countries start jointly piloting such data collection using an agreed protocol, exchange experiences in regular working group meetings and improve quality and comparability of data collection practice, adjusting the protocol if necessary. A prior step could be to carry out specific literature reviews on each of the indicators; however, this was beyond the scope of our study. Also, we were unable to grade the information and suggestions obtained from our expert group by levels of evidence quality [164], again this was beyond the scope of our study, and given the broad area we cover would have not been feasible. If in a future step specific reviews are carried out on each indicator it would be important to attempt grading the evidence for each of them, although such evidence is likely to be scarce and in need of being generated. Our consensus approach was not a formal Delphi study and could as such be criticised. However, we did include various consensus methods (expert meetings, repeated email commenting rounds) [66, 67]. We believe it is unlikely the results would have differed much depending on the exact consensus approach, given that all participants agreed with the final version of framework and indicators. We have also not been able to identify clear candidate indicators for monitoring patient values and preferences regarding harm reduction services, although further work might well be able to define such indicators, as has been already attempted in drug treatment research [165–170]. Finally, the services here discussed and for which we propose to develop indicators are ‘services’ in the form of programmes that are established by governments or private professional organisations and run for the benefit of ‘society’ or, at least putatively, in the benefit of clients or patients. In organisational terms, these are top-down services. What is not discussed in this article is the array of self-financed or funded users’ groups and their activities both in helping each other and also in providing useful and needed critique of the top-down services and policies. There is clearly a need for further work on this area with strong involvement of the target populations and their organisational representatives that services are serving.
Conclusions
We propose a framework for the further development of indicators of coverage and quality of harm reduction services, as a first step to improving best practice implementation in the drug field. This is based on the successful development of established monitoring systems and indicators, and an international consensus exercise. This framework might be especially of use for professionals in charge of monitoring and/or funding service implementation and quality at higher (e.g. national, international) levels of aggregation, in addition to providing some guidance at the local and individual service levels. From the framework, 12 priority candidate indicators emerge that are conceptually simple, likely suitable to be collected on a routine basis, and should provide comparable key evidence on the quality and coverage of opioid agonist therapy, needle and syringe programmes and generic drug service aspects. We propose conducting a pilot study to test the feasibility and applicability of the proposed indicators before their scaling up and routine implementation, to evaluate their effectiveness in comparing service quality across countries. The implementation of a limited set of validated and internationally agreed indicators for monitoring harm reduction service best practice will provide a stronger basis for future public health and epidemiological studies, in order to advance evidence-based health policy.
Acknowledgements
The authors would like to acknowledge the additional collaborators in the EUBEST working group: Sabrina Molinaro, Michela Franchini, Valeria Siciliano, Elisa Benedetti (Institute of Clinical Physiology (CNR), Pisa, Italy); Marco Perduca (Associazione Luca Coscioni, Rome, Italy); Alban Ylli (Foundation Our Lady of Good Counsel–Catholic University Our Lady of Good Counsel, Tirana, Albania); Gregorio Barrio Anta (National School of Public Health; Carlos III Health Institute; Addictive Disorders Network (RTA); Madrid, Spain); Maria José Bravo Portela (National Centre of Epidemiology; Carlos III Health Institute; Consortium for Biomedical Research in Epidemiology and Public Health (CIBERESP); Madrid, Spain), Iciar Indave (National Centre of Epidemiology, Carlos III Health Institute, Madrid, Spain); József Rácz (Eötvös Loránd University, Institute of Psychology; Semmelweis University, Faculty of Health Sciences; Blue Point Drug Counselling and Outpatient Centre; Budapest, Hungary); Tomáš Zábranský (Department of Addictology, First Faculty of Medicine, Charles University and General University Hospital; ResAd–Research and Development; AdRes Institute; Prague, Czech Republic); Michaela Štefunková (Institute of Criminology and Social Prevention; AdRes Institute; Prague, Czech Republic); Percy Fernandez Dávila (Stop Sida, Barcelona, Spain); Maris Salekesin, Sigrid Vorobjov (National institute for health development, Infectious Diseases and Drug Monitoring Department, Tallinn, Estonia); Monica Dan, Cristina Fierbinteanu, Dan Popescu, Ludmila Verdes (ARAS - the Romanian Association Against AIDS, Bucharest, Romania); Adrian-Octavian Abagiu (National Institute for Infectious Diseases Prof. Dr. Matei Bals, Department for OMT–ARENA); Angelos Hatzakis (Dept. of Hygiene, Epidemiology and Medical Statistics, National and Kapodistrian University of Athens, Athens, Greece); Maria Moudatsou, Tzanetos Antypas (NGO Praksis, Athens, Greece); Agnes Cadet-Tairou (French Monitoring Centre for Drugs and Drug Addiction (OFDT), Saint-Denis, France); Anne Marie Collins (Consultant, Barcelona, Spain); David Liddell (Scottish Drug Forum, Glasgow, UK).
Funding
Viktor Mravčík was supported by the institutional support no. PRVOUK-P03/LF1/9 and the Project Nr. LO1611 with a financial support from the Czech Ministry of Youth and Sport under the NPU I program. Sam Friedman was supported by the National Institute on Drug Abuse Grants R01 DA13336 (Community Vulnerability and Response to IDU-Related HIV); DP1 DA034989 (HIV Transmission by Recently-Infected Drug Users); and P30 DA11041 (Center for Drug Use and HIV Research). Ana Sarasa-Renedo, Jeffrey V. Lazarus and Viktor Mravčík were supported by the joint action ‘677085/HA-REACT’ (‘The Joint Action on HIV and Co-infection Prevention and Harm Reduction’), which has received funding from the European Union’s Health Programme (2014–2020).
Availability of data and materials
Data and material are available from the EMCDDA.
Authors’ contributions
LW, MF, CC and CR conceived the paper. LW wrote all the versions. All co-authors provided comments on the draft and final versions and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ART
Antiretroviral therapy
- DAA
Direct-acting antivirals
- EMCDDA
European Monitoring Centre for Drugs and Drug Addiction
- EU
European Union
- GRN
Global Research Network
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- MDMA
3,4-Methylenedioxymethamphetamine (ecstasy)
- MRSA
Methicillin-resistant staphylococcus aureus
- NIDA
National Institute on Drug Abuse
- NSP
Needle and syringe programmes
- NUTS
Nomenclature of Territorial Units for Statistics
- OAT
Opioid agonist therapy
- PWID
People who inject drugs
- PWUD
People who use drugs
- Reitox
Réseau Européen d́Information sur les Drogues et les Toxicomanies
- TB
Tuberculosis
- THC
Tetrahydrocannabinol
- UNAIDS
The Joint United Nations Programme on HIV/AIDS
- WHO
World Health Organization
Contributor Information
Lucas Wiessing, Phone: +351-211210216, Email: Lucas.Wiessing@emcdda.europa.eu.
Marica Ferri, Email: Marica.Ferri@emcdda.europa.eu.
Vendula Běláčková, Email: vendulabelackova@gmail.com.
Patrizia Carrieri, Email: Pmcarrieri@aol.com.
Samuel R. Friedman, Email: friedman@ndri.org
Cinta Folch, Email: cfolch@iconcologia.net.
Kate Dolan, Email: K.Dolan@unsw.edu.au.
Brian Galvin, Email: bgalvin@hrb.ie.
Peter Vickerman, Email: peter.vickerman@bristol.ac.uk.
Jeffrey V. Lazarus, Email: jeffrey.lazarus@regionh.dk
Viktor Mravčík, Email: mravcik.viktor@vlada.cz.
Mirjam Kretzschmar, Email: mirjam.kretzschmar@rivm.nl.
Vana Sypsa, Email: vsipsa@med.uoa.gr.
Ana Sarasa-Renedo, Email: asrenedo@gmail.com.
Anneli Uusküla, Email: anneli.uuskula@ut.ee.
Dimitrios Paraskevis, Email: dparask@med.uoa.gr.
Luis Mendão, Email: gatluismendao@gmail.com.
Diana Rossi, Email: drossi@intercambios.org.ar.
Nadine van Gelder, Email: nadinevangelder@gmail.com.
Luke Mitcheson, Email: Luke.Mitcheson@slam.nhs.uk.
Letizia Paoli, Email: Letizia.Paoli@law.kuleuven.be.
Cristina Diaz Gomez, Email: Cristina.Diaz-Gomez@ofdt.fr.
Maitena Milhet, Email: Maitena.Milhet@ofdt.fr.
Nicoleta Dascalu, Email: nicoletele@gmail.com.
Jonathan Knight, Email: Jonathan.Knight@phe.gov.uk.
Gordon Hay, Email: g.hay@ljmu.ac.uk.
Eleni Kalamara, Email: Eleni.Kalamara@emcdda.europa.eu.
Roland Simon, Email: Roland.Simon@emcdda.europa.eu.
Catherine Comiskey, Email: CCOMISKE@tcd.ie.
Carla Rossi, Email: prof.carla.rossi@gmail.com.
Paul Griffiths, Email: Paul.Griffiths@emcdda.europa.eu.
EUBEST working group:
Sabrina Molinaro, Michela Franchini, Valeria Siciliano, Elisa Benedetti, Marco Perduca, Alban Ylli, Gregorio Barrio Anta, Maria José Bravo Portela, Iciar Indave, József Rácz, Tomáš Zábranský, Michaela Štefunková, Percy Fernandez Dávila, Maris Salekesin, Sigrid Vorobjov, Monica Dan, Cristina Fierbinteanu, Dan Popescu, Ludmila Verdes, Adrian-Octavian Abagiu, Angelos Hatzakis, Maria Moudatsou, Tzanetos Antypas, Agnes Cadet-Tairou, Anne Marie Collins, and David Liddell
References
- 1.Samet JH, Fiellin DA. Opioid substitution therapy-time to replace the term. Lancet. 2015;385:1508–9. doi: 10.1016/S0140-6736(15)60750-4. [DOI] [PubMed] [Google Scholar]
- 2.Harm Reduction International. The global state of harm reduction 2016. Edited by Stone K. London: Harm Reduction International; 2016. https://www.hri.global/files/2016/11/14/GSHR2016_14nov.pdf. Accessed 20 Mar 2017.
- 3.Committee on the Prevention of HIV Infection among Injecting Drug Users in High-Risk Countries. Preventing HIV infection among injecting drug users in high risk countries: an assessment of the evidence. Washington: Institute of Medicine of the National Academies; 2006.
- 4.WHO. Evidence for action series—technical papers and policy briefs on HIV/AIDS and injecting drug users. Geneva: WHO; 2015. http://www.who.int/hiv/pub/idu/evidence_for_action/en/. Accessed 20 Mar 2017.
- 5.ECDC, EMCDDA. Prevention and control of infectious diseases among people who inject drugs. Stockholm: ECDC/EMCDDA; 2011. https://goo.gl/2rQBzv. Accessed 20 Mar 2017.
- 6.WHO, UNODC, UNAIDS. WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users—2012 revision. Geneva: WHO; 2012. http://apps.who.int/iris/bitstream/10665/77969/1/9789241504379_eng.pdf?ua=1. Accessed 20 Mar 2017.
- 7.Palmateer NE, Taylor A, Goldberg DJ, Munro A, Aitken C, Shepherd SJ, et al. Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PLoS ONE. 2014;9:e104515. doi: 10.1371/journal.pone.0104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacArthur GJ, van Velzen E, Palmateer N, Kimber J, Pharris A, Hope V, et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25:34–52. doi: 10.1016/j.drugpo.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Palmateer N, Kimber J, Hickman M, Hutchinson S, Rhodes T, Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction. 2010;105:844–59. doi: 10.1111/j.1360-0443.2009.02888.x. [DOI] [PubMed] [Google Scholar]
- 10.Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy. 2014;25:53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:235–48. doi: 10.1093/ije/dyt243. [DOI] [PubMed] [Google Scholar]
- 12.Des Jarlais DC, Feelemyer JP, Modi SN, Abdul-Quader A, Hagan H. High coverage needle/syringe programs for people who inject drugs in low and middle income countries: a systematic review. BMC Public Health. 2013;13:53. doi: 10.1186/1471-2458-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375:1014–28. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 14.Hedrich D, Pirona A, Wiessing L. From margin to mainstream: the evolution of harm reduction responses to problem drug use in Europe. Drugs Educ Prev Policy. 2008;15:503–17. doi: 10.1080/09687630802227673. [DOI] [Google Scholar]
- 15.Wiessing L, Likatavicius G, Klempova D, Hedrich D, Nardone A, Griffiths P. Associations between availability and coverage of HIV-prevention measures and subsequent incidence of diagnosed HIV infection among injection drug users. Am J Public Health. 2009;99:1049–52. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2679784/. Accessed 20 Mar 2017. [DOI] [PMC free article] [PubMed]
- 16.Strang J, Manning V, Mayet S, Titherington E, Offor L, Semmler C, et al. Family carers and the prevention of heroin overdose deaths: unmet training need and overlooked intervention opportunity of resuscitation training and supply of naloxone. Drugs Educ Prev Policy. 2008;15:211–8. doi: 10.1080/09687630701731205. [DOI] [Google Scholar]
- 17.Williams AV, Strang J, Marsden J. Development of Opioid Overdose Knowledge (OOKS) and Attitudes (OOAS) Scales for take-home naloxone training evaluation. Drug Alcohol Depend. 2013;132:383–6. doi: 10.1016/j.drugalcdep.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Minozzi S, Amato L, Davoli M. Preventing fatal overdoses: a systematic review of the effectiveness of take-home naloxone. Office of the European Union: Luxembourg; 2015. http://www.emcdda.europa.eu/attachements.cfm/att_234376_EN_TDAU14009ENN.web_.pdf. Accessed 20 Mar 2017.
- 19.Grebely J, Bruggmann P, Treloar C, Byrne J, Rhodes T, Dore GJ. Expanding access to prevention, care and treatment for hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26:893–8. doi: 10.1016/j.drugpo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood E, Milloy MJ, Montaner JS. HIV treatment as prevention among injection drug users. Curr Opin HIV AIDS. 2012;7:151–6. doi: 10.1097/COH.0b013e32834f9927. [DOI] [PubMed] [Google Scholar]
- 22.Milloy MJ, Montaner J, Wood E. Barriers to HIV treatment among people who use injection drugs: implications for ‘treatment as prevention’. Curr Opin HIV AIDS. 2012;7:332–8. doi: 10.1097/COH.0b013e328354bcc8. [DOI] [PubMed] [Google Scholar]
- 23.EMCDDA. Best practice portal. Lisbon: EMCDDA; 2017. http://www.emcdda.europa.eu/best-practice. Accessed 20 Mar 2017.
- 24.Ferri M, Bo A. Best practice promotion in Europe: a web-based tool for the dissemination of evidence-based demand reduction interventions. Drugs Educ Prev Policy. 2013;20:331–7. doi: 10.3109/09687637.2012.745486. [DOI] [Google Scholar]
- 25.Maccoun RJ. The implicit rules of evidence-based policy analysis, updated. Addiction. 2010;105:1335–6. doi: 10.1111/j.1360-0443.2010.02936.x. [DOI] [PubMed] [Google Scholar]
- 26.MacCoun R, Reuter P. The implicit rules of evidence-based drug policy: a U.S. perspective. Int J Drug Policy. 2008;19:231–2. doi: 10.1016/j.drugpo.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Mravcik V. (De)criminalisation of possession of drugs for personal use—a view from the Czech Republic. Int J Drug Policy. 2015;26:705–7 [DOI] [PubMed]
- 28.Jolley E, Rhodes T, Platt L, Hope V, Latypov A, Donoghoe M et al. HIV among people who inject drugs in Central and Eastern Europe and Central Asia: a systematic review with implications for policy. BMJ Open. 2012;2:pii=e001465. http://bmjopen.bmj.com/content/bmjopen/2/5/e001465.full.pdf. Accessed 20 Mar 2017. [DOI] [PMC free article] [PubMed]
- 29.Buster M, Dorn T, Ceelen M, Das K. Detainees in Amsterdam, a target population of the Public Mental Health System? J Forensic Leg Med. 2014;25:55–9. doi: 10.1016/j.jflm.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Eastwood N, Fox E, Rosmarin A. A quiet revolution: drug decriminalisation across the globe. London: Release; 2016. [Google Scholar]
- 31.Goncalves R, Lourenco A, Silva SN. A social cost perspective in the wake of the Portuguese strategy for the fight against drugs. Int J Drug Policy. 2015;26:199–209. doi: 10.1016/j.drugpo.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Csete J. A balancing act: policymaking on illicit drugs in the Czech Republic. New York: Open Society Foundations; 2012. http://www.opensocietyfoundations.org/sites/default/files/A_Balancing_Act-03-14-2012.pdf. Accessed 20 Mar 2017.
- 33.Ysa T, Colom J, Albareda A, Ramon A, Segura L. Governance of addictions: European public policies. Oxford: OUP; 2014. [Google Scholar]
- 34.Hall W, Weier M. Assessing the public health impacts of legalizing recreational cannabis use in the USA. Clin Pharmacol Ther. 2015;97:607–15. doi: 10.1002/cpt.110. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson ST, Yarnell S, Radhakrishnan R, Ball SA, D’Souza DC. Marijuana legalization: impact on physicians and public health. Annu Rev Med. 2016;67:453–66. [DOI] [PMC free article] [PubMed]
- 36.Cressey D. The cannabis experiment. Nature. 2015;524:280–3. doi: 10.1038/524280a. [DOI] [PubMed] [Google Scholar]
- 37.Wiessing L, Des Jarlais D, Hughes B, Ferri M, Griffiths P. Cannabis: monitor policy changes. Nature. 2015;527:305. doi: 10.1038/527305d. [DOI] [PubMed] [Google Scholar]
- 38.Comiskey CM. We need to decriminalise small amounts of drugs and open safe heroin injecting centres. Int Bus Times. 2015. http://www.ibtimes.co.uk/we-need-decriminalise-small-amounts-drugs-open-safe-heroin-injecting-centres-1527421. Accessed 20 Mar 2017.
- 39.Duncan DF, Nicholson T, White JB, Ellis-Griffith G. A brief history of prohibition and treatment solutions for substance abusers. Int J Criminol Sociol. 2014;3:186–99.
- 40.Levine HG. The secret of worldwide drug prohibition. Indep Rev. 2002;7:165–80. http://www.independent.org/pdf/tir/tir_07_2_levine.pdf. Accessed 20 Mar 2017.
- 41.EMCDDA. European Drug Report 2016. Lisbon: EMCDDA; 2016. http://www.emcdda.europa.eu/system/files/publications/2637/TDAT16001ENN.pdf. Accessed 20 Mar 2017.
- 42.Strathdee SA, West BS, Reed E, Moazen B, Azim T, Dolan K. Substance use and HIV among female sex workers and female prisoners: risk environments and implications for prevention, treatment, and policies. J Acquir Immune Defic Syndr. 2015;69(Suppl 2):S110–7. doi: 10.1097/QAI.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larney S, Dolan K. A literature review of international implementation of opioid substitution treatment in prisons: equivalence of care? Eur Addict Res. 2009;15:107–12. doi: 10.1159/000199046. [DOI] [PubMed] [Google Scholar]
- 44.Dolan K, Kite B, Black E, Aceijas C, Stimson GV. HIV in prison in low-income and middle-income countries. Lancet Infect Dis. 2007;7:32–41. doi: 10.1016/S1473-3099(06)70685-5. [DOI] [PubMed] [Google Scholar]
- 45.UNODC, ILO, UNDP, WHO, UNAIDS. Prevention, treatment and care in prisons and other closed settings: a comprehensive package of interventions. Vienna: United Nations Office on Drugs and Crime; 2013. https://www.unodc.org/documents/hiv-aids/HIV_comprehensive_package_prison_2013_eBook.pdf. Accessed 20 Mar 2017.
- 46.Sypsa V, Paraskevis D, Malliori M, Nikolopoulos GK, Panopoulos A, Kantzanou M, et al. Homelessness and Other Risk Factors for HIV Infection in the Current Outbreak Among Injection Drug Users in Athens, Greece. Am J Public Health. 2015;105:196–204. [DOI] [PMC free article] [PubMed]
- 47.Folch C, Casabona J, Espelt A, Majo X, Merono M, Gonzalez V, et al. High prevalence and incidence of HIV and HCV among new injecting drug users with a large proportion of migrants—is prevention failing? Subst Use Misuse. 2016;51:250–60. doi: 10.3109/10826084.2015.1092991. [DOI] [PubMed] [Google Scholar]
- 48.Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Antoniadou A, Lazanas M, et al. HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Euro Surveill. 2011;16:pii=19962. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19962. Accessed 20 Mar 2017. [DOI] [PubMed]
- 49.Shannon K, Strathdee SA, Goldenberg SM, Duff P, Mwangi P, Rusakova M, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385:55–71. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiessing LG, van Roosmalen MS, Koedijk P, Bieleman B, Houweling H. Silicones, hormones and HIV in transgender street prostitutes. AIDS. 1999;13:2315–6. doi: 10.1097/00002030-199911120-00022. [DOI] [PubMed] [Google Scholar]
- 51.Miri L, Wakrim L, Kassar H, Hemminki K, Khyatti M. Impact of immigration on HIV-1 molecular epidemiology in West Africa. Maghreb and Southern Europe. AIDS Rev. 2014;16:109–16. [PubMed] [Google Scholar]
- 52.Blondell SJ, Kitter B, Griffin MP, Durham J. Barriers and Facilitators to HIV Testing in Migrants in High-Income Countries: A Systematic Review. AIDS Behav. 2015;19:2012–24. [DOI] [PubMed]
- 53.UNAIDS. Get on the fast-track—the life-cycle approach to HIV. Geneva: UNAIDS; 2016. http://www.unaids.org/sites/default/files/media_asset/Get-on-the-Fast-Track_en.pdf. Accessed 20 Mar 2017.
- 54.Ferri M, Bo A, Amato L, Correia Guedes I, Esteves CS, Wiessing L et al. What is needed in future drug treatment research? A systematic approach to identify gaps on effectiveness of drug treatment from the EMCDDA. Drugs Educ Prev Policy. 2014;22:86–92. http://www.tandfonline.com/doi/pdf/10.3109/09687637.2014.954988?needAccess=true. Accessed 20 Mar 2017.
- 55.Sanghani RM, Carlin AL, Moler AK. Assessing success—a commentary on the necessity of outcomes measures. Subst Abuse Treat Prev Policy. 2015;10:20. doi: 10.1186/s13011-015-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaub MP, Uchtenhagen A. Building a European consensus on minimum quality standards for drug treatment, rehabilitation and harm reduction. Eur Addict Res. 2013;19:314–24. doi: 10.1159/000350740. [DOI] [PubMed] [Google Scholar]
- 57.Nikolopoulos GK, Fotiou A, Kanavou E, Richardson C, Detsis M, Pharris A, et al. National income inequality and declining GDP growth rates are associated with increases in HIV diagnoses among people who inject drugs in Europe: a panel data analysis. PLoS ONE. 2015;10:e0122367. doi: 10.1371/journal.pone.0122367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman SR, Pouget ER, Chatterjee S, Cleland CM, Tempalski B, Brady JE, et al. Drug arrests and injection drug deterrence. Am J Public Health. 2011;101:344–9. doi: 10.2105/AJPH.2010.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pharris A, Wiessing L, Sfetcu O, Hedrich D, Botescu A, Fotiou A et al. Humanimmunodeficiency virus in injecting drug users in Europe following a reported increase of cases in Greece and Romania, 2011. Euro Surveill. 2011;16:pii=20032. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20032. Accessed 20 Mar 2017. [PubMed]
- 60.Nordt C, Stohler R. Incidence of heroin use in Zurich, Switzerland: a treatment case register analysis. Lancet. 2006;367:1830–4. doi: 10.1016/S0140-6736(06)68804-1. [DOI] [PubMed] [Google Scholar]
- 61.Abdul-Quader AS, Feelemyer J, Modi S, Stein ES, Briceno A, Semaan S, et al. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav. 2013;17:2878–92. doi: 10.1007/s10461-013-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emmanuelli J, Desenclos JC. Harm reduction interventions, behaviours and associated health outcomes in France, 1996–2003. Addiction. 2005;100:1690–700. doi: 10.1111/j.1360-0443.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- 63.Strike C, Watson TM, Lavigne P, Hopkins S, Shore R, Young D, et al. Guidelines for better harm reduction: evaluating implementation of best practice recommendations for needle and syringe programs (NSPs) Int J Drug Policy. 2011;22:34–40. doi: 10.1016/j.drugpo.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Haines A, Kuruvilla S, Borchert M. Bridging the implementation gap between knowledge and action for health. Bull World Health Organ. 2004;82:724–31. [PMC free article] [PubMed] [Google Scholar]
- 65.Eurasian Harm Reduction Network. Methodology to assess and monitor access to harm reduction services. Vilnius: EHRN; 2014. http://www.harm-reduction.org/sites/default/files/pdf/4_modules_glaossary_and_preface.zip. Accessed 20 Mar 2017.
- 66.Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74:979–83. doi: 10.2105/AJPH.74.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delbecq AL, Van de Ven AH, Gustafson DH. Group techniques for program planning: a guide to nominal group and Delphi processes. Glenview: Scott Foresman; 1975. [Google Scholar]
- 68.Council of the European Union. Council conclusions on the implementation of the EU Action Plan on Drugs 2013–2016 regarding minimum quality standards in drug demand reduction in the European Union. Brussels: Council of the European Union; 2015. https://goo.gl/l7NZK4. Accessed 20 Mar 2017.
- 69.Ball A, Des Jarlais DC, Donoghoe MC, Friedman SR, Goldberg D, Hunter GM, et al. Multi-city study on drug injecting and risk of HIV infection: a report prepared on behalf of the WHO International Collaborative Group. Geneva: World Health Organization; 1994. Programme on Substance Abuse. https://extranet.who.int/iris/restricted/bitstream/10665/62037/1/WHO_PSA_94.4.pdf. Accessed 20 Mar 2017.
- 70.Cire B. Global network will promote information exchange on HIV prevention in drug-using populations. NIDA Notes. National Institute on Drug Abuse; 1999. http://archives.drugabuse.gov/NIDA_Notes/NNVol13N5/Global.html. Accessed 20 Mar 2017.
- 71.Aceijas C, Stimson GV, Hickman M, Rhodes T. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS. 2004;18:2295–303. doi: 10.1097/00002030-200411190-00010. [DOI] [PubMed] [Google Scholar]
- 72.Aceijas C, Friedman SR, Cooper HL, Wiessing L, Stimson GV, Hickman M. Estimates of injecting drug users at the national and local level in developing and transitional countries, and gender and age distribution. Sex Transm Infect. 2006;82 Suppl 3:iii10–7. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2576733/pdf/iii10.pdf. Accessed 20 Mar 2017. [DOI] [PMC free article] [PubMed]
- 73.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 74.WHO, UNODC, UNAIDS. Technical Guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users. Geneva: WHO; 2009. http://www.who.int/hiv/pub/idu/idu_target_setting_guide.pdf. Accessed 20 Mar 2017.
- 75.WHO. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations, Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva: WHO; 2014. https://goo.gl/QxWY3m. Accessed 20 Mar 2017. [PubMed]
- 76.UNODC. World Drug Report 2015. Vienna: UNODC; 2015. http://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed 20 Mar 2017.
- 77.UNAIDS. Global AIDS Monitoring 2017—indicators for monitoring the 2016 United Nations political declaration on HIV and AIDS. Geneva: UNAIDS; 2017. http://www.unaids.org/sites/default/files/media_asset/2017-Global-AIDS-Monitoring_en.pdf. Accessed 20 Mar 2017.
- 78.ICPR. World prison brief. London: Institute for Criminal Policy Research (ICPR); 2015. http://www.prisonstudies.org/world-prison-brief. Accessed 20 Mar 2017.
- 79.Hartnoll R, Avico U, Ingold FR, Lange K, Lenke L, O’Hare A, et al. A multi-city study of drug misuse in Europe. Bull Narc. 1989;41:3–27. [PubMed] [Google Scholar]
- 80.Simon R, Donmall M, Hartnoll R, Kokkevi A, Ouwehand AW, Stauffacher M, et al. The EMCDDA/Pompidou Group treatment demand indicator protocol: a European core item set for treatment monitoring and reporting. Eur Addict Res. 1999;5:197–207. doi: 10.1159/000018994. [DOI] [PubMed] [Google Scholar]
- 81.Stauffacher M, Kokkevi A. The Pompidou Group treatment demand protocol: the first pan-European standard in the field. Eur Addict Res. 1999;5:191–6. doi: 10.1159/000018993. [DOI] [PubMed] [Google Scholar]
- 82.Richardson C, Ancelle-Park R, Papaevangelou G. Factors associated with HIV seropositivity in European injecting drug users. The European Community Study Group on HIV in injecting drug users. AIDS. 1993;7:1485–91. doi: 10.1097/00002030-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 83.Richardson SC, Papaevangelou G, Ancelle-Park R. Knowledge, attitudes and beliefs of European injecting drug users concerning preventive measures for HIV. Eur J Epidemiol. 1994;10:135–42. doi: 10.1007/BF01730362. [DOI] [PubMed] [Google Scholar]
- 84.Schlumberger MG, Desenclos JC, Papaevangelou G, Richardson SC, Ancelle-Park R. Knowledge of HIV serostatus and preventive behaviour among European injecting drug users: second study. European Community Study Group on HIV in injecting drug users. Eur J Epidemiol. 1999;15:207–15. doi: 10.1023/A:1007578402083. [DOI] [PubMed] [Google Scholar]
- 85.Wiessing LG, Toet J, Houweling H, Koedijk PM, van den Akker R, Sprenger MJW. Prevalentie en risicofactoren van HIV-infectie onder druggebruikers in Rotterdam. [Prevalence and risk factors of HIV infection among drug users in Rotterdam]. Bilthoven: RIVM; 1995. http://www.rivm.nl/bibliotheek/rapporten/213220001.pdf. Accessed 20 Mar 2017.
- 86.Houweling H, Wiessing LG, Hamers FF, Termorshuizen F, Gill ON, Sprenger MJ. An age-period-cohort analysis of 50,875 AIDS cases among injecting drug users in Europe. Int J Epidemiol. 1999;28:1141–8. doi: 10.1093/ije/28.6.1141. [DOI] [PubMed] [Google Scholar]
- 87.Jager JC, Achterberg PW, Postma MJ, Houweling H. Comparative impact assessment of AIDS: between doomsday and complacency. AIDS. 1996;10:238–40. doi: 10.1097/00002030-199602000-00024. [DOI] [PubMed] [Google Scholar]
- 88.Rotily M, Weilandt C, Bird SM, Kall K, Van Haastrecht HJ, Iandolo E, et al. Surveillance of HIV infection and related risk behaviour in European prisons. A multicentre pilot study. Eur J Public Health. 2001;11:243–50. doi: 10.1093/eurpub/11.3.243. [DOI] [PubMed] [Google Scholar]
- 89.Stover H, Nelles J. Ten years of experience with needle and syringe exchange programmes in European prisons. Int J Drug Policy. 2003;14:437–44. doi: 10.1016/j.drugpo.2003.08.001. [DOI] [Google Scholar]
- 90.Stover H, Casselman J, Hennebel L. Substitution treatment in European prisons: a study of policies and practices in 18 European countries. Int J Prison Health. 2006;2:3–12. http://www.drogenforschung.de/pdf/volltexte_pdf/nr18/subst_treatm_eupris.pdf. Accessed 20 Mar 2017.
- 91.Griffiths P, Mounteney J, Lopez D, Zobel F, Gotz W. Addiction research centres and the nurturing of creativity. Monitoring the European drug situation: the ongoing challenge for the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Addiction. 2012;107:254–8. doi: 10.1111/j.1360-0443.2011.03369.x. [DOI] [PubMed] [Google Scholar]
- 92.Mounteney J, Griffiths P, Sedefov R, Noor A, Vicente J, Simon R. The drug situation in Europe: an overview of data available on illicit drugs and new psychoactive substances from European monitoring in 2015. Addiction. 2016,111:34–48. [DOI] [PubMed]
- 93.Wiessing L, Ncube F, Hedrich D, Griffiths P, Hope V, Gill N et al. Surveillance of infectious diseases in IDUs across the EU: information from the EU expert network. Euro Surveill. 2004;8:pii=2368. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2368. Accessed 20 Mar 2017.
- 94.Wiessing L. New EMCDDA toolkit for monitoring infectious diseases. Drugnet Europe. 2014;85:2. http://www.emcdda.europa.eu/system/files/publications/785/Drugnet_85_weboptimised_461952.pdf. Accessed 20 Mar 2017.
- 95.Jager J, Limburg W, Kretzschmar M, Postma M, Wiessing L (eds). Hepatitis C and injecting drug use: impact, costs and policy options. EMCDDA: Lisbon; 2004. https://goo.gl/WgRoSB. Accessed 20 Mar 2017.
- 96.Wiessing L, Kraus L, Hay G, Rossi C, Frischer M, Jager J, et al. European network to develop policy relevant models and socio-economic analyses of drug use: consequences and interventions. Lisbon: EMCDDA; 2002. http://www.emcdda.europa.eu/html.cfm/index1376EN.html. Accessed 20 Mar 2017.
- 97.Godfrey C, Wiessing L, Hartnoll R (eds). Modelling drug use: methods to quantify and understand hidden processes. EMCDDA: Lisbon; 2000. http://www.emcdda.europa.eu/system/files/publications/922/Monograph6_159816.pdf. Accessed 20 Mar 2017.
- 98.Hartnoll RL. Drug epidemiology in the European institutions: historical background and key indicators. UN Bull Narc. 2003;55:53–72. http://www.unodc.org/pdf/bulletin/bulletin_2003_01_01_1.pdf#page=60. Accessed 20 Mar 2017.
- 99.Wiessing L, Hartnoll R, Rossi C. The epidemiology of drug use at the macro level: indicators, models and policy-making. UN Bull Narc. 2001;53:119–33. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_2001-01-01_1_page011.html. Accessed 20 Mar 2017.
- 100.Hartnoll R. Drug trends in the European Union. 1999. Epidemiologic trends in drug abuse. International epidemiology work group on drug abuse June 1999. Proceedings. p. 49–58. https://archives.drugabuse.gov/pdf/cewg/IEWG699.pdf. Accessed 20 Mar 2017.
- 101.Uhl A, Hunt G, van den Brink W, Stimson GV. How credible are international databases for understanding substance use and related problems? Int J Drug Policy. 2015;26:119–21. doi: 10.1016/j.drugpo.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 102.Jones HE, Hickman M, Welton NJ, De AD, Harris RJ, Ades AE. Recapture or precapture? Fallibility of standard capture-recapture methods in the presence of referrals between sources. Am J Epidemiol. 2014;179:1383–93. doi: 10.1093/aje/kwu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zabransky T. On a search for useful indicators… or not? Addiction. 2015;110:741–3. doi: 10.1111/add.12858. [DOI] [PubMed] [Google Scholar]
- 104.UNODC. Global Assessment Programme on Drug Abuse (GAP). 2003; UNODC. http://www.unodc.org/unodc/en/GAP/. Accessed 20 Mar 2017.
- 105.Hickman M, Taylor C, Chatterjee A, Degenhardt L, Frischer M, Hay G, et al. Estimating the prevalence of problematic drug use: a review of methods and their application. UN Bull Narc. 2002;54:15–32. https://www.unodc.org/pdf/bulletin/bulletin_2002_01_01_Art2.pdf. Accessed 20 Mar 2017.
- 106.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–83. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Habicht JP, Victora CG, Vaughan JP. Evaluation designs for adequacy, plausibility and probability of public health programme performance and impact. Int J Epidemiol. 1999;28:10–8. doi: 10.1093/ije/28.1.10. [DOI] [PubMed] [Google Scholar]
- 108.Wiessing LG, Denis B, Guttormsson U, Haas S, Hamouda O, Hariga F, et al. Estimating coverage of harm-reduction measures for injection drug users in Europe. 2001. Global Research Network On HIV Prevention In Drug-Using Populations - Third Annual Meeting.July 5-7, 2000, Durban. Bethesda: NIDA; 2001. https://goo.gl/BWHjSN. Accessed 20 Mar 2017.
- 109.EMCDDA. 2001 annual report on the state of the drugs problem in the European Union. Lisbon: EMCDDA; 2001. http://www.emcdda.europa.eu/attachements.cfm/att_37276_EN_ar01_en.pdf. Accessed 20 Mar 2017.
- 110.Humphreys K. Commentary on Gustafson et al. (2013): can we know that addiction treatment has been improved without evidence of better patient outcomes? Addiction. 2013;108:1158–9. doi: 10.1111/add.12144. [DOI] [PubMed] [Google Scholar]
- 111.Blystad H, Wiessing L. Guidelines for testing HIV, viral hepatitis and other infections in injecting drug users. EMCDDA manuals no 6. EMCDDA: Lisbon; 2010. http://www.emcdda.europa.eu/publications/manuals/testing-guidelines. Accessed 20 Mar 2017. [DOI] [PubMed]
- 112.Goltzman PM. Intervenciones desde la Reducción de Daños. Perspectivas y desafíos actuales. Memoria de encuentro, Buenos Aires 21-22 de junio 2016. Buenos Aires: Intercambios; 2016. http://intercambios.org.ar/wp-content/uploads/2016/10/RD-perspectivas-y-desafios-2016-Intercambios.pdf. Accessed 20 Mar 2017.
- 113.Ritter A, Livingston M, Chalmers J, Berends L, Reuter P. Comparative policy analysis for alcohol and drugs: current state of the field. Int J Drug Policy. 2016;31:39–50. doi: 10.1016/j.drugpo.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 114.Belackova V, Ritter A, Shanahan M, Hughes CE. Assessing the concordance between illicit drug laws on the books and drug law enforcement: comparison of three states on the continuum from “decriminalised” to “punitive”. Int J Drug Policy. 2017;41:148–57. [DOI] [PubMed]
- 115.Frischer M, Bloor M, Finlay A, Goldberg D, Green S, Haw S, et al. A new method of estimating prevalence of injecting drug use in an urban population: results from a Scottish city. Int J Epidemiol. 1991;20:997–1000. doi: 10.1093/ije/20.4.997. [DOI] [PubMed] [Google Scholar]
- 116.Jones HE, Welton NJ, Ades AE, Pierce M, Davies W, Coleman B et al. Problem drug use prevalence estimation revisited: heterogeneity in capture-recapture and the role of external evidence. Addiction. 2016;111:438–47. [DOI] [PMC free article] [PubMed]
- 117.Hay G, McKeganey N, Wiessing L, Bello PY, D’Ippoliti D, Domingo-Salvany A, et al. Methodological guidelines to estimate the prevalence of problem drug use on the local level. Lisbon: EMCDDA; 1999. https://goo.gl/9RK1hz. Accessed 20 Mar 2017.
- 118.Kraus L, Augustin R, Frischer M, Kummler P, Uhl A, Wiessing L. Estimating prevalence of problem drug use at national level in countries of the European Union and Norway. Addiction. 2003;98:471–85. doi: 10.1046/j.1360-0443.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- 119.Frischer M, Hickman M, Kraus L, Mariani F, Wiessing L. A comparison of different methods for estimating the prevalence of problematic drug misuse in Great Britain. Addiction. 2001;96:1465–76. doi: 10.1046/j.1360-0443.2001.9610146510.x. [DOI] [PubMed] [Google Scholar]
- 120.Chatterjee S, Tempalski B, Pouget ER, Cooper HL, Cleland CM, Friedman SR. Changes in the prevalence of injection drug use among adolescents and young adults in large U.S. metropolitan areas. AIDS Behav. 2011;15:1570–8. doi: 10.1007/s10461-011-9992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holmberg SD. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86:642–54. doi: 10.2105/AJPH.86.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cooper HL, Brady JE, Friedman SR, Tempalski B, Gostnell K, Flom PL. Estimating the prevalence of injection drug use among black and white adults in large U.S. metropolitan areas over time (1992–2002): estimation methods and prevalence trends. J Urban Health. 2008;85:826–56. doi: 10.1007/s11524-008-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the U.S. and in large U.S. metropolitan areas from 1992 to 2002. J Urban Health. 2008;85:323–51. doi: 10.1007/s11524-007-9248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pouget ER, Friedman SR, Cleland CM, Tempalski B, Cooper HL. Estimates of the population prevalence of injection drug users among Hispanic residents of large US metropolitan areas. J Urban Health. 2012;89:527–64. doi: 10.1007/s11524-012-9670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hickman M, De AD, Jones H, Harris R, Welton N, Ades AE. Multiple parameter evidence synthesis—a potential solution for when information on drug use and harm is in conflict. Addiction. 2013;108:1529–31. doi: 10.1111/add.12185. [DOI] [PubMed] [Google Scholar]
- 126.Tempalski B, Pouget ER, Cleland CM, Brady JE, Cooper HL, Hall HI, et al. Trends in the population prevalence of people who inject drugs in US metropolitan areas 1992–2007. PLoS ONE. 2013;8:e64789. doi: 10.1371/journal.pone.0064789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pierce M, Bird SM, Hickman M, Millar T. National record linkage study of mortality for a large cohort of opioid users ascertained by drug treatment or criminal justice sources in England, 2005–2009. Drug Alcohol Depend. 2015;146:17–23. doi: 10.1016/j.drugalcdep.2014.09.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tempalski B, Cleland CM, Pouget ER, Chatterjee S, Friedman SR. Persistence of low drug treatment coverage for injection drug users in large US metropolitan areas. Subst Abuse Treat Prev Policy. 2010;5:23. doi: 10.1186/1747-597X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stimson GV, Hickman M, Quirk A, Frischer M, Taylor C (eds.). Estimating the prevalence of problem drug use in Europe. Lisbon: EMCDDA/Pompidou Group; 1997. http://old.ntakd.lt/files/leidiniai/emcdda/6-Monographs/5-Estimating.pdf. Accessed 20 Mar 2017.
- 130.Vickerman P, Martin NK, Hickman M. Understanding the trends in HIV and hepatitis C prevalence amongst injecting drug users in different settings—implications for intervention impact. Drug Alcohol Depend. 2012;123:122–31. doi: 10.1016/j.drugalcdep.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 131.Martin NK, Vickerman P, Miners A, Foster GR, Hutchinson SJ, Goldberg DJ, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55:49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 132.Kretzschmar M, Wiessing LG. Modelling the spread of HIV in social networks of injecting drug users. AIDS. 1998;12:801–11. doi: 10.1097/00002030-199807000-00017. [DOI] [PubMed] [Google Scholar]
- 133.Kretzschmar M, Wiessing L. New challenges for mathematical and statistical modelling of HIV and HCV in IDUs. AIDS. 2008;22:1527–37. [DOI] [PubMed]
- 134.Cousien A, Tran VC, Deuffic-Burban S, Jauffret-Roustide M, Dhersin JS, Yazdanpanah Y. Dynamic modelling of hepatitis C virus transmission among people who inject drugs: a methodological review. J Viral Hepat. 2015;22:213–29. doi: 10.1111/jvh.12337. [DOI] [PubMed] [Google Scholar]
- 135.Kaplan EH, O’Keefe E. Let the needles do the talking! Evaluating the New Haven needle exchange. Interfaces. 1993;23:7–26. https://goo.gl/50mafW. Accessed 20 Mar 2017.
- 136.de Vos AS, Prins M, Kretzschmar ME. Hepatitis C virus treatment as prevention among injecting drug users: who should we cure first? Addiction. 2015;110:975–83. doi: 10.1111/add.12842. [DOI] [PubMed] [Google Scholar]
- 137.de Vos AS, Kretzschmar ME. The efficiency of targeted intervention in limiting the spread of HIV and hepatitis C virus among injecting drug users. J Theor Biol. 2013;333:126–34. doi: 10.1016/j.jtbi.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 138.Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598–609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Vos AS, Prins M, Coutinho RA, van der Helm JJ, Kretzschmar ME. Treatment as prevention among injecting drug users; extrapolating from the Amsterdam cohort study. AIDS. 2014;28:911–8. doi: 10.1097/QAD.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 140.Paraskevis D, Paraschiv S, Sypsa V, Nikolopoulos G, Tsiara C, Magiorkinis G, et al. Enhanced HIV-1 surveillance using molecular epidemiology to study and monitor HIV-1 outbreaks among intravenous drug users (IDUs) in Athens and Bucharest. Infect Genet Evol. 2015;35:109–21. doi: 10.1016/j.meegid.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 141.Niculescu I, Paraschiv S, Paraskevis D, Abagiu A, Batan I, Banica L, et al. Recent HIV-1 outbreak among intravenous drug users in Romania: evidence for cocirculation of CRF14_BG and subtype F1 strains. AIDS Res Hum Retroviruses. 2015;31:488–95. doi: 10.1089/aid.2014.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paraskevis D, Nikolopoulos G, Fotiou A, Tsiara C, Paraskeva D, Sypsa V, et al. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS ONE. 2013;8:e78941. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0078941. Accessed 20 Mar 2017. [DOI] [PMC free article] [PubMed]
- 143.Balode D, Skar H, Mild M, Kolupajeva T, Ferdats A, Rozentale B, et al. Phylogenetic analysis of the Latvian HIV-1 epidemic. AIDS Res Hum Retroviruses. 2012;28:928–32. doi: 10.1089/aid.2011.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 145.Hser YI, Evans E, Grella C. Commentary on Degenhardt et al. (2014): Regional variation in the global burden of disease attributable to opioid dependence-where do the data come from and does population size matter? Addiction. 2014;109:1334–5. doi: 10.1111/add.12597. [DOI] [PubMed] [Google Scholar]
- 146.Degenhardt L, Mathers B, Hall WD. Response to Hser et al. (2014): the necessity for more and better data on the global epidemiology of opioid dependence. Addiction. 2014;109:1335–7. doi: 10.1111/add.12612. [DOI] [PubMed] [Google Scholar]
- 147.Wiessing L, Ferri M, Grady B, Kantzanou M, Sperle I, Cullen KJ, et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS ONE. 2014;9:e103345. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0103345. Accessed 20 Mar 2017. [DOI] [PMC free article] [PubMed]
- 148.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–79. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 149.Friedman SR, Tempalski B, Brady JE, Friedman JJ, Cooper HL, Flom PL, et al. Predictors of the degree of drug treatment coverage for injection drug users in 94 metropolitan areas in the United States of America. Int J Drug Policy. 2007;18:475–85. doi: 10.1016/j.drugpo.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 150.Tempalski B, Flom PL, Friedman SR, Des Jarlais DC, Friedman JJ, McKnight C, et al. Social and political factors predicting the presence of syringe exchange programs in 96 US metropolitan areas. Am J Public Health. 2007;97:437–47. doi: 10.2105/AJPH.2005.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Friedman SR, Cooper HL, Tempalski B, Keem M, Friedman R, Flom PL, et al. Relationships of deterrence and law enforcement to drug-related harms among drug injectors in US metropolitan areas. AIDS. 2006;20:93–9. doi: 10.1097/01.aids.0000196176.65551.a3. [DOI] [PubMed] [Google Scholar]
- 152.Wiessing L, Likatavicius G, Hedrich D, Guarita B, van de Laar MJ, Vicente J. Trends in HIV and hepatitis C virus infections among injecting drug users in Europe, 2005 to 2010. Euro Surveill. 2011;16:pii=20031 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20031. Accessed 20 Mar 2017. [PubMed]
- 153.van den Berg C, Smit C, van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102:1454–62. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hull MW, Wu Z, Montaner JS. Optimizing the engagement of care cascade: a critical step to maximize the impact of HIV treatment as prevention. Curr Opin HIV AIDS. 2012;7:579–86. doi: 10.1097/COH.0b013e3283590617. [DOI] [PubMed] [Google Scholar]
- 155.Yehia BR, Schranz AJ, Umscheid CA, Lo Re III V. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS ONE. 2014;9:e101554. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0101554. Accessed 20 Mar 2017. [DOI] [PMC free article] [PubMed]
- 156.Strathdee SA, Beletsky L, Kerr T. HIV, drugs and the legal environment. Int J Drug Policy. 2015;26(Suppl 1):S27–32. doi: 10.1016/j.drugpo.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bird SM, Hutchinson SJ. Male drugs-related deaths in the fortnight after release from prison: Scotland, 1996–99. Addiction. 2003;98:185–90. doi: 10.1046/j.1360-0443.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 158.OECD. OECD health statistics 2015—definitions, sources and methods. Paris: OECD; 2015. http://stats.oecd.org/wbos/fileview2.aspx?IDFile=587d7574-6ed6-4408-9c32-481d322936e6. Accessed 20 Mar 2017.
- 159.EMCDDA. Cost and financing of drug treatment services in Europe: an exploratory study. Lisbon: EMCDDA; 2011. http://www.emcdda.europa.eu/attachements.cfm/att_143682_EN_TDSI11001ENC.pdf. Accessed 20 Mar 2017.
- 160.Ritter A, Hull P, Berends L, Chalmers J, Lancaster K. A conceptual schema for government purchasing arrangements for Australian alcohol and other drug treatment. Addict Behav. 2016;60:228–34. doi: 10.1016/j.addbeh.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 161.Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care? Ann Intern Med. 2006;145:265–72. doi: 10.7326/0003-4819-145-4-200608150-00006. [DOI] [PubMed] [Google Scholar]
- 162.Mason T, Sutton M, Whittaker W, McSweeney T, Millar T, Donmall M, et al. The impact of paying treatment providers for outcomes: difference-in-differences analysis of the ‘payment by results for drugs recovery’ pilot. Addiction. 2015;110:1120–8. doi: 10.1111/add.12920. [DOI] [PubMed] [Google Scholar]
- 163.McLellan AT, Kemp J, Brooks A, Carise D. Improving public addiction treatment through performance contracting: the Delaware experiment. Health Policy. 2008;87:296–308. doi: 10.1016/j.healthpol.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Broome KM, Simpson DD, Joe GW. Patient and program attributes related to treatment process indicators in DATOS. Drug Alcohol Depend. 1999;57:127–35. doi: 10.1016/S0376-8716(99)00080-0. [DOI] [PubMed] [Google Scholar]
- 166.Gossop M, Marsden J, Stewart D. NTORS After Five Years. The National Treatment Outcome Research Study - Changes in substance use, health and criminal behaviour during the five years after intake. London: National Addiction Centre; 2001.
- 167.Godfrey C, Stewart D, Gossop M. Economic analysis of costs and consequences of the treatment of drug misuse: 2-year outcome data from the National Treatment Outcome Research Study (NTORS) Addiction. 2004;99:697–707. doi: 10.1111/j.1360-0443.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 168.McKeganey N, Bloor M, McIntosh J, Neale J. Key findings from the Drug Outcome Research in Scotland (DORIS) study. Glasgow: University of Glasgow - Centre for Drug Misuse Research; 2008. http://substanceuseresearch.org/wp-content/uploads/2016/02/DORIS-Key.pdf. Accessed 20 Mar 2017.
- 169.Donmall M, Jones A, Davies L, Barnard M. Summary of key findings from the Drug Treatment Outcomes Research Study (DTORS). Research Report 23 (summary). UK: Home Office; 2009. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/116599/horr23.pdf. Accessed 20 Mar 2017.
- 170.Jones A, Donmall M, Millar T, Moody A, Weston S, Anderson T, et al. The drug treatment outcomes research study (DTORS): final outcomes report - 3rd edition. UK: Home Office; 2009. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.604.904&rep=rep1&type=pdf. Accessed 20 Mar 2017.
- 171.Mant J. Process versus outcome indicators in the assessment of quality of health care. Int J Qual Health Care. 2001;13:475–80. http://intqhc.oxfordjournals.org/content/13/6/475.full-text.pdf. Accessed 20 Mar 2017. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material are available from the EMCDDA.


