Abstract
Cortisol and inflammatory proteins are released into the blood in response to stressors and chronic elevations of blood cortisol and inflammatory proteins may contribute to ongoing disease processes and could be useful biomarkers of disease. How chronic circadian misalignment influences cortisol and inflammatory proteins, however, is largely unknown and this was the focus of the current study. Specifically, we examined the influence of weeks of chronic circadian misalignment on cortisol, stress ratings, and pro- and anti- inflammatory proteins in humans. We also compared the effects of acute total sleep deprivation and chronic circadian misalignment on cortisol levels. Healthy, drug free females and males (N=17) aged 20-41 participated. After three weeks of maintaining consistent sleep-wake schedules at home, six laboratory baseline days and nights, a 40-h constant routine (CR, total sleep deprivation) to examine circadian rhythms for melatonin and cortisol, participants were scheduled to a 25-day laboratory entrainment protocol that resulted in sleep and circadian disruption for eight of the participants. A second constant routine was conducted to reassess melatonin and cortisol rhythms on days 34-35. Plasma cortisol levels were also measured during sampling windows every week and trapezoidal area under the curve (AUC) was used to estimate 24-h cortisol levels. Inflammatory proteins were assessed at baseline and near the end of the entrainment protocol. Acute total sleep deprivation significantly increased cortisol levels (p<0.0001), whereas chronic circadian misalignment significantly reduced cortisol levels (p<0.05). Participants who exhibited normal circadian phase relationships with the wakefulness-sleep schedule showed little change in cortisol levels. Stress ratings increased during acute sleep deprivation (p<0.0001), whereas stress ratings remained low across weeks of study for both the misaligned and synchronized control group. Circadian misalignment significantly increased plasma tumor necrosis factor-alpha (TNF-α), interleukin 10 (IL-10) and C-reactive protein (CRP) (p<0.05). Little change was observed for the TNF-α/IL-10 ratio during circadian misalignment, whereas the TNF-α/IL-10 ratio and CRP levels decreased in the synchronized control group across weeks of circadian entrainment. The current findings demonstrate that total sleep deprivation and chronic circadian misalignment modulate cortisol levels and that chronic circadian misalignment increases plasma concentrations of pro- and antiinflammatory proteins.
Keywords: Circadian Clock, Cytokines, Inflammation, Sleep loss, Tumor Necrosis Factor Alpha, Interleukin-10, C-Reactive Protein, Cortisol
1. Introduction
The internal circadian clock and sleep-wakefulness physiology modulate daily patterns in most behavioral and physiological systems (Bass and Takahashi, 2010; Czeisler and Klerman, 1999; Davies et al., 2014; Wright et al., 2012). Insufficient sleep and circadian misalignment have negative impacts on endocrine, metabolic, cardiovascular, immune, bone, stress, cognition, and neurological health and function (Depner et al., 2014; Dimitrov et al., 2004; Everson et al., 2012; Everson and Szabo, 2011; Haack et al., 2004; Lekander et al., 2013; Markwald et al., 2013; Scheer et al., 2009; Spiegel et al., 1999; Thompson et al., 2014; Weil et al., 2013; Wright et al., 2006; Yu et al., 2013). Sleep deprivation is considered a physiological stressor and a metabolic challenge that is often associated with increased cortisol levels and stress ratings (Chapotot et al., 2001; Dinges et al., 1997; Leproult et al., 1997; Minkel et al., 2012; Parry et al., 2000; Spiegel et al., 1999; von Treuer et al., 1996; Weibel et al., 1995; Weitzman et al., 1983). Sleep loss is also reported to elevate blood concentrations of inflammatory proteins and may be reflective of impaired physiological function and disease processes (Irwin et al., 2010; Mullington et al., 2010). While much is known about the influence of insufficient sleep on stress, cortisol, inflammation and the risk of impaired heath and disease in humans, less is known about the influence of chronic circadian misalignment on cortisol and inflammatory proteins. Circadian misalignment results when sleep and wakefulness occur at inappropriate circadian times; i.e., when wakefulness occurs at a time the internal circadian clock is promoting sleep and/or when sleep occurs at a time when the internal clock is promoting wakefulness (Baron and Reid, 2014; Gronfier et al., 2007; Wright et al., 2006). Circadian misalignment can be acute such as during total sleep deprivation (Frey et al., 2004; McHill et al., 2014), intermittent as during shift work and jet lag (Sack et al., 2007a; Wright et al., 2013; Zee et al., 2010), or chronic as in circadian rhythm sleep-wake disorders (Sack et al., 2007a, b).
The daily pattern of the endocrine hormone cortisol is strongly driven by the master circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Moore and Eichler, 1972). The circadian clock modulates the near-24-hour rhythm in cortisol via the hypothalamic-pituitary-adrenal (HPA) axis and via neural innervation through a polysynaptic pathway from the SCN to the autonomic area of the paraventricular nucleus of the hypothalamus and the spinal cord (Buijs et al., 1999) providing sympathetic innervation (Buijs et al., 2003). The circadian rhythm in cortisol shows high levels in the morning near habitual waketime in humans, declines across the biological day, shows low levels in the early evening and increases across the biological night (Czeisler and Klerman, 1999; Desir et al., 1980; Van Cauter et al., 1994). The cortisol rhythm can thus be used as a phase marker of the circadian clock (Desir et al., 1980; Van Cauter and Refetoff, 1985). Factors such as stress (Morgan et al., 2001; Stratakis and Chrousos, 1995), meals (Follenius et al., 1982; Ishizuka et al., 1983), exercise (Brandenberger and Follenius, 1975), and awakening from sleep (Gribbin et al., 2012) induce acute increases in cortisol levels and factors such as sleep (Gronfier et al., 1998; Gronfier et al., 1997; Gronfier et al., 1999; Weibel et al., 1995) and bright light exposure (Jung et al., 2010) can induce acute decreases of cortisol levels.
Daily patterns of immune factors and responses to immune challenge are modulated by sleep and circadian phase (Curtis et al., 2014; Fonken et al., 2013; Gibbs et al., 2012; Keller et al., 2009; Moller-Levet et al., 2013; Morrow and Opp, 2005; Narasimamurthy et al., 2012; Pollmacher et al., 1996; Rahman et al., 2014). Immune factors contribute to the natural sleep process (Imeri and Opp, 2009; Krueger et al., 2011; Marshall and Born, 2002) and sleep and circadian disruption are reported to alter inflammatory proteins (Axelsson et al., 2013; Chennaoui et al., 2011; Fondell et al., 2011; Frey et al., 2007; Haack et al., 2007; Meier-Ewert et al., 2004; Mullington et al., 2010; Redwine et al., 2000; Shearer et al., 2001). Most prior studies of how circadian disruption in humans influences inflammation however, are limited methodologically by infrequent sampling rates, typically sampling at only one or a few time points across the 24-h day (Copertaro et al., 2011; Khosro et al., 2011; Puttonen et al., 2011; Sookoian et al., 2007) and limited inflammatory protein assessment. One notable exception regarding sampling rate is a study in which C-reactive protein (CRP) was examined every 4h over 24-h at baseline and on day 8 of sleep restriction during which days 2-3 and 5-6 the participants were also circadian misaligned by scheduling sleep during the daytime (Leproult et al., 2014). As sleep-wakefulness state and circadian phase modulate immune function, additional studies with frequent sampling of multiple inflammatory proteins and concurrent assessment of other biological factors that influence inflammation, such as endogenous cortisol (Yeager et al., 2011), are needed to improve our understanding of immune changes associated with circadian disruption. How cortisol and inflammatory proteins are influenced by chronic circadian misalignment is largely unknown. Therefore, the focus of the current analyses was to determine the influence of chronic circadian misalignment on cortisol and frequently sampled inflammatory proteins including the pro-inflammatory proteins tumor necrosis factor alpha (TNF-α) and CRP and the anti-inflammatory cytokine interleukin-10 (IL-10). The current analysis also compared the influence of chronic circadian misalignment to the influence of acute total sleep deprivation on cortisol levels. As noted, because stress increases cortisol levels (Morgan et al., 2001; Stratakis and Chrousos, 1995), the current study also examined changes in stress ratings across total sleep deprivation and chronic circadian misalignment.
2. Methods
Detailed methods and circadian melatonin phase, sleep, leptin, and performance findings from the studies presented here have been published (Nguyen and Wright, 2010; Wright et al., 2001; Wright et al., 2006). The current manuscript represents planned analyses for cortisol, inflammatory proteins and stress ratings.
2.1. Participant Screening and Pre-laboratory Conditions
We studied healthy females and males (N=17 [3 females]) aged 31.7 ± 6.1 (Mean ± SD). Participants gave written informed consent and the Partners Health Care (Boston, MA) and the University of Colorado Boulder Institutional Review Boards approved the procedures and/or analyses for the protocol. Data collection was conducted at the Brigham and Women's Hospital. All participants were determined to be healthy after passing a rigorous health screening, including medical history, physical exam, electrocardiogram, blood and urine chemistries, a toxicology screen for drug use, psychological tests and an interview with a clinical psychologist. None reported regular night work or rotating shift work within the past three years or crossing more than one time zone in the previous three months. Participants maintained a regular routine of 8-h scheduled sleep and 16-h scheduled wakefulness for a minimum of three weeks while living at home before the in-laboratory protocol, as verified by sleep logs, call-in times to a time stamped voice recorder and wrist actigraphy recordings for at least one week prior to laboratory admission (Philips Respironics, Mini Mitter, Bend OR).
2.2. In-Laboratory Conditions
Participants were tested individually in an environment free of time cues. Ambient light, room temperature, sleep-wakefulness opportunities, activity, and nutrition intake (breakfast, lunch, dinner and a snack; 150 mEq Na++, 100 mEq K+ ± 20%, 1500 to 2500 cc fluids, isocaloric) were strictly controlled. Exercise and napping were proscribed. Participants were initially scheduled to a 16-h wakefulness 8-h sleep schedule for 6 days at their habitual wakefulness-sleep times (Fig. 1). Habitual bedtime was calculated by subtracting four hours from the average midpoint of the participants' self-selected wakefulness-sleep schedule during the week prior to laboratory admission. Following the 6 baseline days, an initial constant routine protocol (CR, Days 7-8) was used to examine the circadian rhythms of melatonin and cortisol and to assess the influence of 40-h of total sleep deprivation on cortisol levels. During the CR, participants were maintained in constant sedentary bedrest conditions with the head of the bed raised to ∼35 degrees; wakefulness was maintained by research assistants interacting with the participant and monitored via continuous EEG recordings; caloric intake was increased to account for increased energy need during the extended duration of wakefulness (Jung et al., 2011) and was equally distributed across the constant routine in hourly snacks (i.e., isocaloric); ambient light levels were equivalent to dim candle light (∼1.5 lux in the angle of gaze). Following CR1, individuals were scheduled to a 24.0-h or 24.6-h day length for 25 days (Days 9-33, Fig. 1; circadian entrainment portion of the protocol). This was followed by CR2 (Days 34-35) that was used to reassess the circadian rhythms of melatonin and cortisol after exposure to these scheduled day lengths. Females, with consistent regular menstrual cycles of 25-32 days in length, began the study during the week of menses so that CR1 and CR2 would occur during the follicular phase of their menstrual cycle.
Fig. 1. Protocol figures.
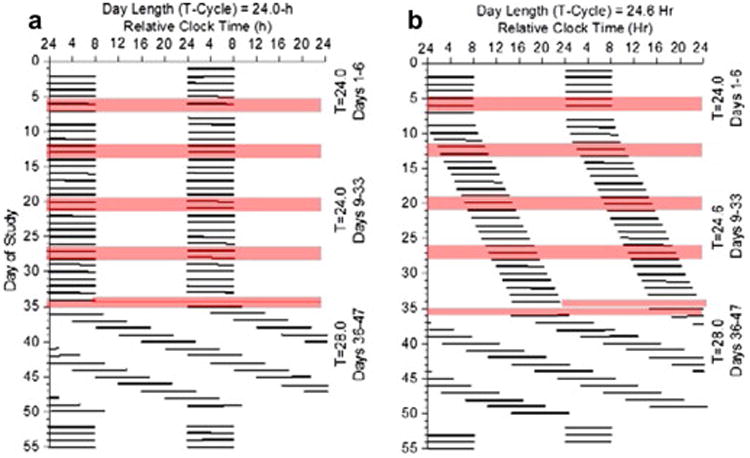
Data are plotted to a relative clock hour with wake time arbitrarily assigned a value of 0800h on baseline Day 1 and all other times referenced to this value. Black bars represent scheduled sleep. Day 1-6 are baseline days with scheduled 8-h sleep episodes at the participants' habitual bedtime. Days 7-8 is a 40-h constant routine 1 (CR1) with 8-h scheduled recovery sleep. Days 9-33 are experimental conditions and Days 34-35 is CR 2. (a) imposed maintenance of a 24.0-h day for 25 days, and (b) imposed 24.6-h day for 25 days with lights out and lights on delayed by 36 min each day. Blood sampling segments denoted by shading on Days 5-8, 12-14, 19-21, 26-28 and 34-35. Cortisol assessed at each blood sampling segment and inflammatory proteins assessed at baseline Days 5-6 and experimental Days 26-27. Circadian period findings from 28-h forced desynchrony Days 36-49 previously reported in (Wright et al., 2001, 2006).
Blood was sampled through an indwelling 18-gauge intravenous catheter throughout each weekly blood sampling window. Heparinized saline (0.45% sodium chloride, 10 U of heparin/ml) was infused at a rate of 5–10 ml/h between samples. Blood samples were processed immediately and centrifuged in a refrigerated centrifuge and then frozen at -80 degrees Celsius until assayed. Cortisol was assessed from samples collected every 30 min during the sleep episode before and throughout CR1 to assess the effect of sleep deprivation on cortisol levels. Blood samples collected on baseline Days 5-6, on Days 7-8 (CR1), weekly during the 25 day circadian entrainment portion of the protocol (Days 12-14, 19-21, 26-28) and on Day 33 (CR2) were used to assess the influence of weeks of circadian misalignment on cortisol levels. Insufficient samples were available to assess inflammatory proteins during total sleep deprivation. Remaining blood samples analyzed every 60 min on baseline Days 5-6 and near the end of the 25 day circadian entrainment portion of the protocol, Days 26-28, were used to assess the influence of weeks of circadian misalignment on TNF-α, IL-10 and CRP levels. The blood sample volume at each time point was small due to a limit on the amount of blood permitted to be taken from each participant during the long duration 55-day inpatient study with frequent blood sampling windows and multiple blood parameters analyzed for each sample, as well as blood measures reported previously (Nguyen and Wright, 2010; Wright et al., 2001; Wright et al., 2006). This limited the number of pro- and anti- inflammatory proteins analyzed. Visual analog scales consisting of 100 mm horizontal lines on a computer screen were used to assess subjective stress ratings (endpoints of the line were “relaxed” and “stressed”) each day beginning 2h after scheduled awakening and every 2h thereafter until 2h prior to bedtime. Hours of light and darkness were 16:8 h for the 24.0-h day and 16.4:8.2 h for the 24.6-h day. As reported previously (Nguyen and Wright, 2010; Wright et al., 2001; Wright et al., 2006), nine of the participants studied were classified as being synchronized based on their melatonin onset (DLMO25%) consistently occurring near habitual bedtime across the 25 day entrainment protocol (nine of seventeen participants, 1 female, 8 males). The remaining eight participants were classified as being not-synchronized as their melatonin onset occurred at an abnormal time relative to the sleep-wakefulness schedule (2 females and 6 males).
2.3. Cortisol and Inflammatory Protein Analyses
Plasma cortisol was measured by chemiluminescent assay (Beckman Coulter, Chaska, MN); sensitivity, 0.4 μg/dL; intra- and interassay coefficients of variation, 6.4% and 7.9%, respectively. Plasma melatonin levels were assayed using radioimmunoassay 125I (Elias USA, Inc., Osceola,WI); sensitivity, 2.5 pg/ml; intra- and interassay coefficients of variation, 5.9% and 9.8%, respectively. Plasma TNF-α and IL-10 levels were assayed using ELISA high-sensitivity assays using the Quantikine® Kits (R&D Systems, Minneapolis, MN, USA) TNF-α sensitivity, 0.12 pg/ml; intra- and interassay coefficients of variation, 6.6% and 13.4% respectively; and IL-10 sensitivity, 0.5 pg/ml; intra- and interassay coefficients of variation, 7.6% and 11.3% respectively. Plasma CRP levels were assayed using Elisa high-sensitivity CRP (Alpco Diagnostics, Windham, NH) (Aziz et al., 2003; Mahmud and Feely, 2005); sensitivity, 0.00124 mg/L; intra and interassay coefficients of variation, 9.6% and 84% respectively. The current samples went through one previous freeze thaw cycle prior to the analysis of pro- and anti-inflammatory proteins. Even though we used a high-sensitivity IL-10 assay, the assay is insufficient to detect very low values in healthy participants and thus 17% percent of IL-10 samples were undetectable. Undetectable values were assigned the lower sensitivity limit of the assay.
2.4. Sleep Deprivation
Sleep deprivation analyses were performed on available cortisol data across 48-h that included a standard 8-h sleep 16-h wakefulness day and sleep deprivation (hours awake 17-40 during CR1, Days 7-8).
2.5. Chronic Circadian Misalignment
The effects of chronic circadian misalignment on the cortisol rhythm during constant wakefulness were analyzed using data from CR1 and CR2 (Days 7-8 and 34-35). Cortisol data from the constant routines were aligned by melatonin phase to permit averaging of participant cortisol curves since the phase angle between internal circadian time and scheduled sleep-wakefulness was not similar across participants during circadian misalignment (Nguyen and Wright, 2010; Wright et al., 2001; Wright et al., 2006). Data were assigned to 7.5 degree (0.5h) bins with the phase of the dim light melatonin onset (Wright et al., 2001) assigned to 0°. Data were also linearly resampled/interpolated to provide equidistant sampling times. Individual participant cortisol curves during the sleep-wakefulness schedule during baseline days 5-6 and entrainment days 26-28 are provided for descriptive purposes. Trapezoidal area under the curve (AUC) analyses are used to estimate twenty-four hour cortisol levels at baseline days 5-6, CR1 and CR2, and during exposure to the 25 day protocol. Cortisol AUC was analyzed as a change from baseline (CR2 minus CR1 and days 12-14, 19-21, and 26-28 minus baseline days 5-6). Bi-hourly averages across sleep deprivation and daily averages across the protocol were computed for visual analog stress ratings. The effects of chronic circadian misalignment on pro- and anti- inflammatory proteins were analyzed using data from baseline days 5-6 and near the end of the entrainment protocol on days 26-28. We also calculated the TNF-α/IL-10 ratio as a measure of cytokine balance.
2.6. Statistical Analysis
Some participants studied are not included in analyses if samples were unavailable due to blood sampling difficulties or due to insufficient sample volume available for analysis (see figure legends). Changes in cortisol, inflammatory proteins and stress levels were examined with mixed model ANOVA with group, sample time, and/or day as fixed factors and planned dependent t-tests within group for individual time points. Modified Bonferroni correction factors were used to correct for planned comparisons. Independent t-tests were used to compare synchronized and not-synchronized cortisol AUC and single sample t-tests were used to test for reductions in cortisol AUC from a zero baseline within group. F-tests and Pitman-Morgan tests were used to compare the variability in the timing of the dual-harmonic fitted cortisol maximum during constant routines between and within groups, respectively.
3. Results
3.1. Effects of Sleep Deprivation on Plasma Cortisol and Stress Ratings
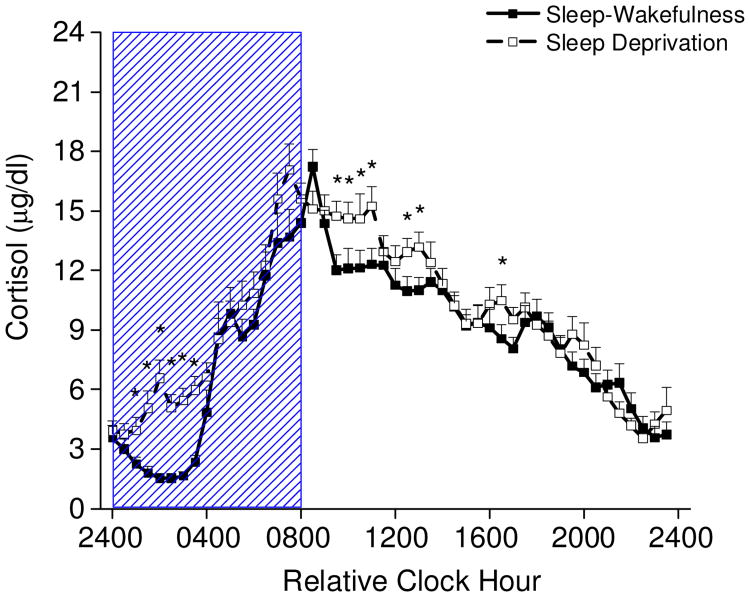
One night of total sleep deprivation increased cortisol levels compared to baseline (main effect of day, baseline 8.4±0.18 μg/dL ±SEM, versus sleep deprivation 9.6±0.18, p < 0.0001; day by time of day interaction, p < 0.05). Cortisol levels were higher during the first half of the night when participants were kept awake compared to the corresponding time of night 24-h earlier when participants were permitted to sleep (Fig. 2). In addition, cortisol levels were higher after the night of sleep deprivation for some time points during the daytime, especially in the morning hours, compared to a typical 16-h day of wakefulness. Subjective stress ratings (Fig. 3a) were also higher during the daytime after the night of sleep deprivation compared to a typical 16-h day of wakefulness (main effect of hours awake, p < 0.0001).
Fig. 2. Plasma cortisol levels every 30 min across a standard 8-h sleep – 16-h wakefulness day and sleep deprivation (n=17).
Two consecutive 24-h episodes of plasma cortisol plotted overlying each other beginning with the sleep episode on Day 6 and ending after 40-h of wakefulness of the constant routine on Days 7-8. Scheduled waketime arbitrarily assigned a value of 0800h (relative clock hour). Box represents scheduled sleep during baseline and nighttime sleep deprivation 24-h later. * denotes p< 0.049
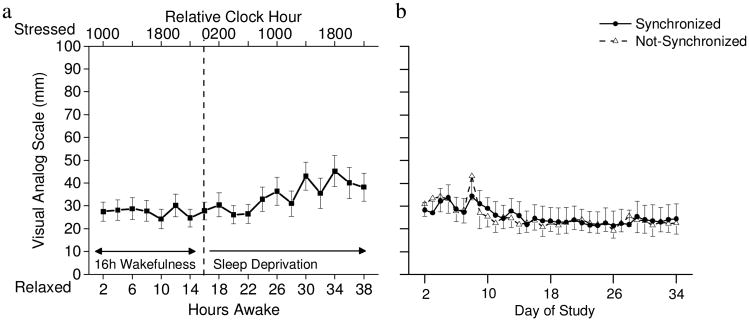
Fig 3. Stress ratings (a) bi-hourly during total sleep deprivation (n=17) and (b) average daily for synchronized (n=9) and not-synchronized (n=8) participants.
(a) Stress ratings were relatively stable across the first 24-h of wakefulness and were higher during the day of sleep deprivation. Dashed line indicates end of standard 16-h waking day and beginning of sleep deprivation. (b) Average daily stress ratings were similar for the synchronized and not-synchronize participants across the protocol. Note that day 8 shows average stress ratings during sleep deprivation for hours awake 25-40 on constant routine 1.
3.2. Effects of Circadian Misalignment on Cortisol, Melatonin, and Stress Levels
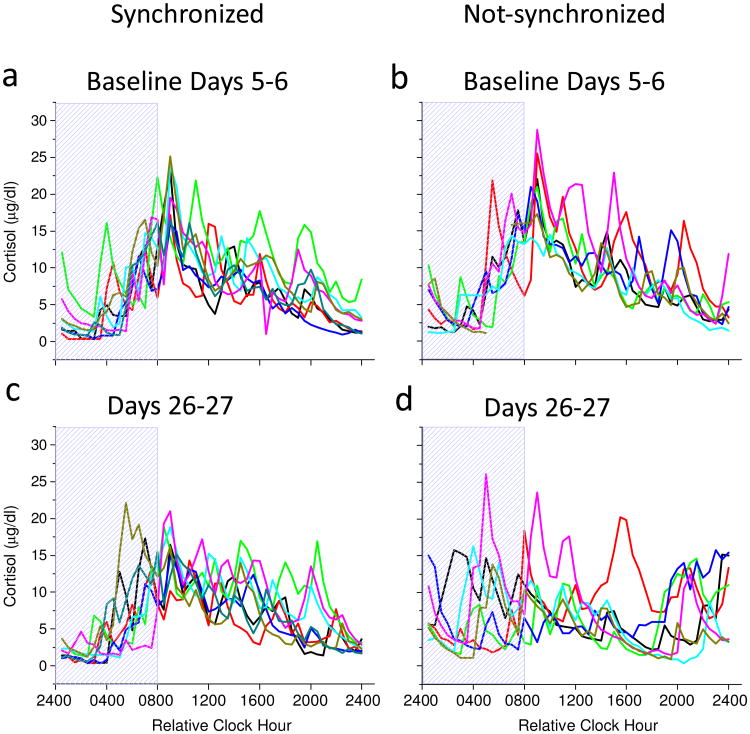
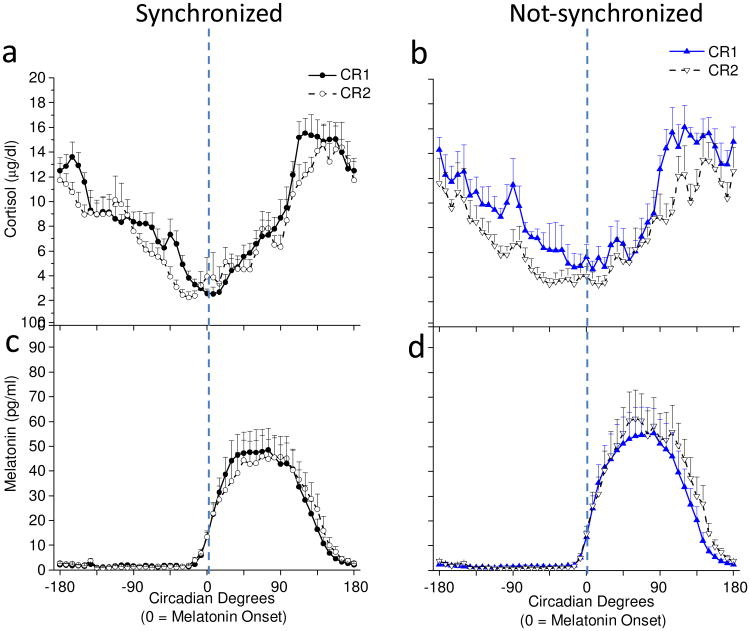
Fig. 4 shows individual participant cortisol rhythm plots aligned to scheduled sleep-wakefulness time on baseline Days 5-6 (Fig. 4a & 4b) and near the end of the entrainment protocol on Days 26-27 (Fig. 4c & 4d). On baseline days 5-6, cortisol levels were consistently low during the first half of the scheduled sleep episode and peaked near habitual wake time for baseline days when all participants were entrained to the 24.0-h day (Fig. 4a & 4b). On days 26-27, cortisol levels remained consistently low during the first half of the scheduled sleep episode and peaked near habitual wake time for participants who were synchronized (Fig. 4c), whereas cortisol levels were high at different times of day for participants who were not-synchronized (Fig. 4d) being dependent upon the degree of circadian misalignment as reported previously for melatonin levels (Nguyen and Wright, 2010; Wright et al., 2001; Wright et al., 2006). The individual participant data in Fig. 4 were not averaged for participant groups as this would make the not-synchronized group average appear flat as their circadian melatonin (Nguyen and Wright, 2010; Wright et al., 2001; Wright et al., 2006) and cortisol rhythms occur at different phase angles relative to sleep. F-tests showed that variability in the timing of the cortisol maximum between synchronized and not-synchronized groups were similar at baseline (synchronized standard deviation(SD)=0.69 hours versus not-synchronized SD=0.53 hours; p=0.23), but variability was significantly greater in the not-synchronized group for days 26-27 (synchronized SD=1.44 hours versus not-synchronized SD=5.98 hours; p<0.001). Pitman-Morgan tests further showed that variability in the timing of the cortisol maximum within groups were similar across baseline and day 26-27 assessments for the synchronized group (p=0.10), but was different across assessments for the not-synchronized group (p<0.05).Fig. 5 shows average cortisol rhythms aligned to the circadian phase of melatonin onset assessed during the constant routines. Average cortisol levels in synchronized participants were similar during the first and second constant routines (Fig. 5a), whereas cortisol levels in not-synchronized participants were lower during the second constant routine following weeks of circadian misalignment (Fig. 5b; group x day interaction, p < 0.0001). In both groups, the circadian rhythm of cortisol was maintained with a circadian trough near melatonin onset. Melatonin levels were similar within groups during the first and second constant routines (Fig. 5c & 5d).). Fig. 6 further shows that regardless of whether cortisol 24-h AUC levels were assessed during scheduled sleep-wakefulness or during continuous wakefulness of the constant routine, change from baseline of 24-h cortisol AUC levels were reduced in the not-synchronized compared to synchronized group (main effect of group, p < 0.0001). Fig. 6 further shows that cortisol 24-h AUC levels were reduced as compared to baseline for the not-synchronized group.
Fig. 4. Individual participant cortisol rhythm plots aligned to scheduled sleep-wakefulness time on baseline Days 5-6 (a & b) and on entrainment protocol Days 26-27 (c & d).
Scheduled waketime arbitrarily assigned a value of 0800h (relative clock hour). Box represents scheduled sleep.
Fig. 5. Cortisol (a & b) and melatonin (c & d) levels every 30 min during constant routines and aligned to melatonin onset (n=14).
Average cortisol levels in (a) synchronized (n=8) and (b) non-synchronized (n=6). Average melatonin levels in (c) synchronized and (d) not-synchronized participants. Dashed lines represent timing of the melatonin onset.
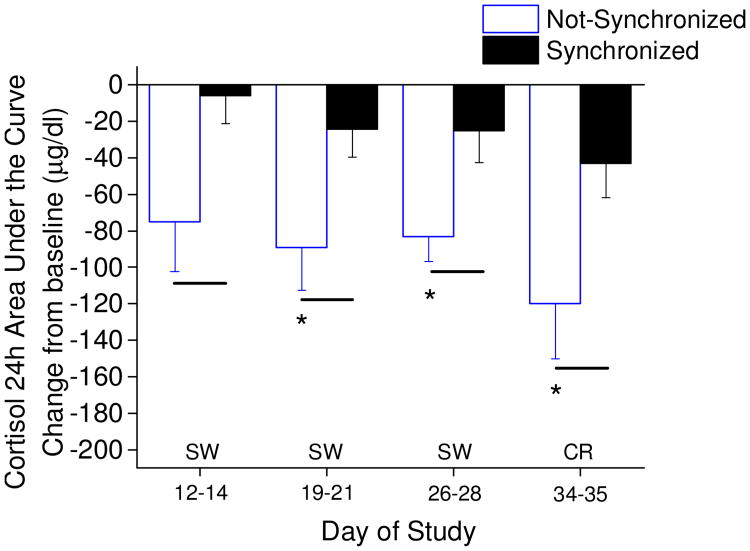
Fig. 6. Twenty-four hour cortisol area under the curve assessments.
Not-synchronized (n=5, 7, 7, 6; respectively for days of study shown) compared to the synchronized participants (n=7, 7, 6, 8; respectively for days of study shown). Lines denote significant differences in cortisol AUC between synchronized and not-synchronized groups (independent t-test; p<0.05); * denote significant reduction in cortisol AUC for the not-synchronized participants (single sample t-test difference from zero change; p< 0.05). Not-synchronized group difference from zero change on day 12-14 showed non-significant trend p=0.052, and synchronized group difference from zero change on day 34-35 showed non-significant trend p=0.053.
Fig. 3b shows that average daily stress ratings across the entrainment protocol were similar for the synchronized and not-synchronized participants. When groups were combined, stress levels were statistically higher on “day 8” which represents hours awake 25-40 of the first constant routine, as compared to most other days (p < 0.05; also see Fig. 2b). When synchronized and non-synchronized groups were compared, stress ratings were similar and stable across the days examined.
3.3. Effects of Circadian Misalignment on Inflammatory Proteins
TNF-α levels were significantly higher in the synchronized versus the non-synchronized group (main effect of group; average log TNF-α pg/mL for synchronized=0.267±0.009, ±SEM, versus not-synchronized=0.177±0.001, p < 0.00001). Figures 7a & 7b shows TNF-α levels were similar across the protocol for the synchronized group whereas log TNF-α levels were significantly higher during circadian misalignment in the non-synchronized group compared to baseline (main effect of day for not-synchronized group; average log TNF-α at baseline=0.161 ±0.006 versus not-synchronized experimental day average=0.193±0.005, p < 0.001).
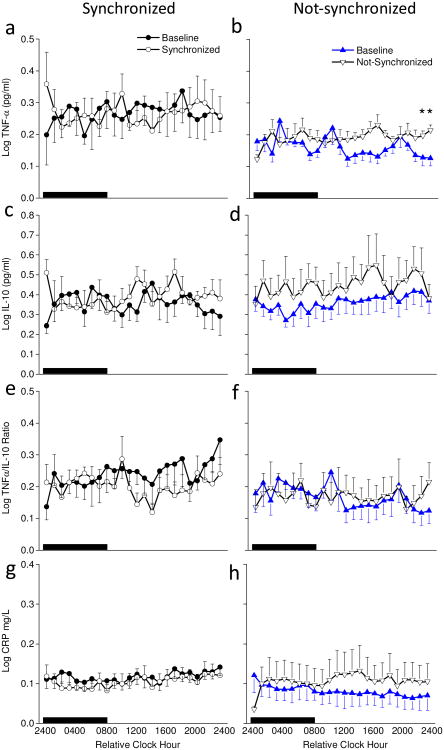
Fig. 7. Hourly pro- and anti- inflammatory proteins on baseline days 5-6 and experimental days 26-27.
Average samples during scheduled sleep (black box) and scheduled wakefulness for baseline days (filled symbols) and entrainment protocol days (open symbols) for synchronized (left panels) and not-synchronized (right panels) groups. Sample sizes were n=6, 5, 5, and 6 for synchronized, and n=7, 7, 7, and 6 for not-synchronized groups for TNF, IL-10, TNF/IL-10 ration and CRP, respectively. * denote significant differences between days within group for that time of day (p<0.0239).
IL-10 levels were significantly higher at the end of the entrainment protocol (main effect of day; average log IL-10 pg/mL at baseline=0.36±0.01 versus experimental day=0.43±0.01, p < 0.001). Fig. 7d shows that IL-10 levels increased in the not-synchronized group during circadian misalignment (main effect of day for not-synchronized group; average log IL-10 at baseline=0.36±0.01 versus not-synchronized experimental day=0.45±0.02, p < 0.001). IL-10 levels remained similar to baseline across the protocol in the synchronized group (Fig. 7c).
The ratio of pro-inflammatory TNF-α to anti-inflammatory IL-10 was significantly higher in the synchronized versus not-synchronized group (main effect of group; average log TNF-α/IL-10 ratio for synchronized=0.22±0.01 versus not-synchronized=0.17±0.01, p < 0.00001). Fig 7e shows the TNF-α/IL-10 ratio decreased in the synchronized group during circadian entrainment (main effect of day for the synchronized group; average log TNF-α/IL-10 ratio at baseline=0.24±0.01 versus synchronized experimental day=0.21±0.01, p < 0.05) whereas, Fig 7f shows the TNF-α/IL-10 ratio was similar across the protocol in the not-synchronized group.
CRP levels significantly decreased in the synchronized group and increased in the not-synchronized group (day by group interaction; p < 0.01). Fig. 7h shows that hourly CRP levels were increased predominantly during scheduled wakefulness of circadian misalignment in the non-synchronized group (main effect of day for the not-synchronized group; average log CRP at baseline=0.080 ±0.006 mg/L versus not-synchronized experimental day=0.108±0.009, p < 0.05), whereas Fig. 7g shows that Average CRP levels were decreased in the synchronized group after weeks of entrainment compared to baseline (main effect of day for the synchronized group, baseline day average log CRP=0.117 ±0.004 versus synchronized experimental day=0.104±0.004, p < 0.05).
4. Discussion
Acute sleep deprivation and circadian misalignment are common in modern work environments and in circadian sleep-wake disorders. The current findings show that acute total sleep deprivation and chronic circadian misalignment have opposite effects on cortisol levels and that chronic circadian misalignment increases plasma concentrations of pro- and antiinflammatory proteins. Specifically, one night of total sleep deprivation increased cortisol levels, especially in the early evening and early morning hours, whereas weeks of circadian misalignment decreased cortisol levels across the 24-h day. Stress ratings increased during the day after one night of sleep deprivation, whereas stress ratings were not significantly altered by chronic circadian misalignment as compared to stress ratings of a synchronized control group. Weeks of circadian misalignment increased levels of the anti-inflammatory cytokine IL-10 and the pro-inflammatory proteins TNF-α and CRP, especially during scheduled wakefulness. Little change was observed in the TNF-α/IL-10 cytokine balance ratio during circadian misalignment. Taken together, these findings suggest that acute sleep deprivation and associated circadian misalignment represents a different physiological challenge than chronic circadian misalignment. Acute sleep deprivation appears to be associated with a physiological stress/metabolic response of higher cortisol levels, especially at night, whereas chronic circadian misalignment appears to result in a physiological adaptation that reduces 24-h cortisol levels with associated increases in pro- and anti- inflammatory proteins. Because chronic circadian misalignment increased TNF-α and CRP levels, yet also increased IL-10, a powerful anti-inflammatory protein, and had little impact on the TNF-α/IL-10 ratio, acute circadian misalignment in the healthy participants tested did not appear to result in a prevailing pro-inflammatory state as least given the inflammatory proteins measured. Future studies should use multiplex technologies of pro- and antiinflammatory proteins and soluble receptors with frequent sampling rates to examine the complexity of inflammatory responses to chronic circadian misalignment.Our current finding of an increase in plasma cortisol levels during total sleep deprivation is consistent with prior research (Chapotot et al., 2001; Leproult et al., 1997; von Treuer et al., 1996; Weibel et al., 1995; Weitzman et al., 1983). Most studies, including ours that have shown that sleep deprivation increases cortisol levels, show increases near the trough of the circadian rhythm of cortisol (Leproult et al., 1997; von Treuer et al., 1996; Weibel et al., 1995; Weitzman et al., 1983). One previous study has also reported increased cortisol levels during the daytime following sleep deprivation (Chapotot et al., 2001), although few studies have examined daytime cortisol levels during total sleep deprivation. Most studies that have reported no effect of sleep deprivation on plasma cortisol levels have been limited by infrequent sampling rates (e.g., one or two samples per 24-h) (Dinges et al., 1994; Gary et al., 1996; Gonzalez-Ortiz et al., 2000; Ozturk et al., 1999). Findings from two studies in which salivary cortisol levels were examined suggest that free cortisol levels during the daytime were not significantly increased by one night of sleep deprivation (Frey et al., 2007; Heiser et al., 2000). Thus, there is relative consistency in findings of increased plasma cortisol levels in studies with frequently sampled levels (e.g., every 30 min), which is not surprising given that cortisol release is pulsatile and studies with infrequent sampling rates may have missed cortisol pulses. It has been hypothesized that the increase in cortisol during sleep deprivation is associated with arousal (von Treuer et al., 1996). Our findings indicate that increased cortisol levels during the circadian trough are not associated with increased stress ratings at night. During the daytime after one night of total sleep deprivation, increased cortisol and stress ratings were observed. However, increased cortisol levels during the daytime were relatively small. Acute total sleep deprivation likely increases cortisol levels during the circadian trough of the cortisol rhythm because the absence of sleep is permissive of cortisol pulsatility (Thorsley et al., 2012), whereas sleep, especially slow wave sleep, is associated with decreased cortisol pulses and levels (Gronfier et al., 1998; Gronfier et al., 1997); i.e., an inhibitory effect of sleep on cortisol levels. During sleep, the responsiveness to corticotrophin releasing hormone (CRH) and vasopressin are reduced (e.g., Antonijevic et al. 1999; Bierwolf et al., 1997) resulting in reduced ACTH and cortisol levels. Sleep deprivation can also lead to increased CRH and subsequent corticosterone levels (e.g., Opp 1995).
The individual participant cortisol data showing peaks at various times of the sleep-wakefulness cycle during circadian misalignment are consistent with circadian melatonin rhythm data reported previously for these participants (Nguyen and Wright, 2010; Wright et al., 2006), as the timing of the cortisol and melatonin rhythms are both markers of the SCN circadian clock in humans. During circadian misalignment, sleep occurred at circadian phases when cortisol levels would be high and this could contribute to the lower cortisol levels observed in some of the individuals in Fig 4. However, a sleep-induced reduction of cortisol cannot completely explain the reduction in 24-h cortisol AUC levels we observed as 24-h cortisol AUC levels were also reduced during continuous wakefulness of the constant routine. Thus, chronic circadian misalignment appears to induce lower total cortisol levels regardless of sleep-wakefulness state. The time course of the reduction in 24-h cortisol levels during circadian misalignment appears to be on the order of days to weeks as a non-significant trend for a difference from baseline was observed within the first week of circadian misalignment and was significant in the second week of misalignment. Whether such changes in 24-h cortisol levels are associated with changes in, CRH, ACTH, glucocorticoid receptor sensitivity and/or down regulation of receptor number require follow-up non-human animal models and additional human studies of chronic circadian misalignment. Our findings of lower 24-h cortisol levels and altered timing of the cortisol rhythm relative to sleep-wakefulness timing may have implications for metabolic function under conditions of intermittent recurring circadian misalignment (e.g., shift work and jet lag) and for chronic circadian misalignment (e.g., circadian rhythm sleep-wake disorders). Others have reported alterations in cortisol levels in actual shift workers. For example, cortisol levels were higher during sleep and lower during the nightshift compared to daytime workers (Weibel and Brandenberger, 1998), consistent with rapid and acute changes in sleep-wakefulness timing relative to the work schedule with little change in circadian phase. Further, when examining cortisol levels at home after circadian entrainment to two weeks of shift work under highly controlled light-dark conditions of working on offshore oil platforms, the cortisol awakening response was reported to be lower and the pre-bedtime evening cortisol levels to be higher, suggesting that the cortisol rhythm had yet to resynchronize to the home schedule (Harris et al., 2010). In such studies, the influence of sleep and circadian phase on cortisol levels was not controlled in the analyses and thus it is unknown whether 24-h plasma cortisol levels are reduced in shift workers. Further research is thus needed to assess shift workers under constant routine conditions to determine whether there are changes in overall cortisol levels in response to shift work and to determine the physiological implications of such changes. Related, hair cortisol levels are reported to be higher in shift workers and thus shift work may not result in a decrease in 24-h cortisol levels (Manenschijn et al., 2011). Further research is also needed to determine the influence of chronic circadian misalignment in circadian rhythm sleep-wake disorders as our model of chronic circadian misalignment perhaps bests mimics such conditions (e.g., non-24 hour).
As glucocorticoids can be both pro- and anti-inflammatory (Yeager et al., 2011), one implication of reduced 24-h cortisol levels and altered cortisol timing relative to sleep-wakefulness rest-activity is altered inflammatory proteins. Higher median CRP levels have been shown to occur after 8 days of sleep restriction combined with circadian misalignment on days 2-3 and 5-6 due to scheduling sleep during the daytime (Leproult et al., 2014). Higher morning CRP levels were also reported in 3-shift but not 2-shift working men, but not for shift working women (Puttonen et al., 2011). We found evidence of increased plasma concentrations of IL-10, TNF-α, and CRP, and little change in TNF-α/IL-10 ratio after weeks of circadian misalignment. The net impact of these changes on inflammation is unclear, but our results suggest that circadian misalignment in healthy participants impacts circulating pro- and anti- inflammatory proteins, but does not clearly increase pro-inflammatory processes, although the increased IL-10 may represent a counter-regulatory response to increased TNF-α levels. Also, the pro- and antiinflammatory proteins we examined remained at levels considered in the healthy range. Our current findings are consistent with prior findings from our laboratory regarding changes in inflammatory proteins during total sleep deprivation in healthy participants (Frey et al., 2007); although total sleep deprivation reduced CRP in our prior study also suggesting that there are differences in the CRP response between total sleep deprivation and chronic circadian misalignment in healthy participants. Together, these findings highlight the need to assess both pro- and anti- inflammatory proteins and their soluble receptors, otherwise inaccurate interpretation may be made that sleep and circadian disruption always leads to a proinflammatory state.
Future studies are needed to assess the influence of sleep and circadian disruption in healthy individuals and in those with pre-existing or existing disease to determine if individuals who are perhaps primed for an inflammatory response due to ongoing disease processes respond with a shift away from inflammatory balance to a pro-inflammatory state. As discussed in detail previously (Wright et al., 2006), we find a relatively small reduction in total sleep time during circadian misalignment and this well-established effect that sleep loss is a part of circadian misalignment could contribute to the current findings. The increase in stress ratings we observed with total sleep deprivation is consistent with prior research (Minkel et al., 2012), whereas the relatively low stress ratings in both synchronized and not-synchronized participants across the protocol suggests that participants were not stressed while living in the laboratory. Future studies should also assess other models of circadian misalignment, include other inflammatory markers, and examine other populations with equal numbers of males and females. Although we controlled for menstrual cycle phase and had females begin the study during the week of menses, we only studied three females and thus any sex differences could not be examined. Also, the sources of the circulating pro- and anti- inflammatory proteins we measured are unknown. As we examined inflammatory proteins only at baseline and after weeks of circadian misalignment, future studies are needed to determine the time course of such changes.
In conclusion, our results show that total sleep deprivation and chronic circadian misalignment differentially influence cortisol levels and that chronic circadian misalignment increase both pro- and anti- inflammatory proteins in healthy young adults. The acute total sleep deprivation increase in cortisol is likely due to the absence of the sleep induced decrease in cortisol, whereas the decrease in cortisol during circadian misalignment is likely to be a physiological adaption of unknown mechanism. We found increases in both pro- and anti- inflammatory proteins during circadian misalignment, which may reflect inflammatory balance rather than a switch to an overall pro-inflammatory state for the healthy participants studied.
Highlights.
Sleep deprivation and circadian misalignment have opposite effects on cortisol
Acute total sleep deprivation increases cortisol levels
Chronic circadian misalignment reduces 24-hour cortisol levels
Chronic circadian misalignment increases TNF-α, CRP and IL-10 levels
Acknowledgments
Supported by NASA Cooperative Agreement NCC9-58 with the National Space Biomedical Research Institute and NASA, by NIH RO1 HL081761 and R21 DK092624. Data collection carried out in the Brigham and Women's Hospital Center for Clinical Investigation (formally supported by NIH Grant M01 RR02635 currently part of Harvard Clinical and Translational Science Center supported by NIH UL1 RR025758).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonijevic IA, Murck H, Frieboes R, Holsboer TT, Steiger A. Hyporesponsiveness of the pituitary to CRH during slow wave sleep is not mimicked by systemic GHRH. Neuroendocrinol. 1999;69:88–96. doi: 10.1159/000054406. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Rehman JU, Akerstedt T, Ekman R, Miller GE, Hoglund CO, Lekander M. Effects of sustained sleep restriction on mitogen-stimulated cytokines, chemokines and T helper 1/T helper 2 balance in humans. PLoS One. 2013;8:e82291. doi: 10.1371/journal.pone.0082291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26:139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierwolf C, Struve K, Marshall L, Born J, Fehm HL. Slow wave sleep drives inhibition of pituitary-adrenal secretion in humans. J Neuroendocrinol. 1997;9:479–484. doi: 10.1046/j.1365-2826.1997.00605.x. [DOI] [PubMed] [Google Scholar]
- Brandenberger G, Follenius M. Influence of timing and intensity of musclar exercise on temporal patterns of plasma cortisol levels. J Clin Endocrinol Metab. 1975;40:845–849. doi: 10.1210/jcem-40-5-845. [DOI] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Chapotot F, Buguet A, Gronfier C, Brandenberger G. Hypothalamo-pituitary- adrenal axis activity is related to the level of central arousal: effect of sleep deprivation on the association of high-frequency waking electroencephalogram with cortisol release. Neuroendocrinology. 2001;73:312–321. doi: 10.1159/000054648. [DOI] [PubMed] [Google Scholar]
- Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrilhon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine. 2011;56:318–324. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Copertaro A, Bracci M, Gesuita R, Carle F, Amati M, Baldassari M, Mocchegiani E, Santarelli L. Influence of shift-work on selected immune variables in nurses. Ind Health. 2011;49:597–604. doi: 10.2486/indhealth.ms1210. [DOI] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O'Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130. discussion 130-132. [PubMed] [Google Scholar]
- Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, Cui N, Middleton B, Ackermann K, Kayser M, Thumser AE, Raynaud FI, Skene DJ. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A. 2014;111:10761–10766. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desir D, van Cauter E, Golstein J, Fang VS, Leclercq R, Refetoff S, Copinschi G. Circadian and ultradian variations of ACTH and cortisol secretion. Horm Res. 1980;13:302–316. doi: 10.1159/000179297. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun. 2004;18:341–348. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Everson CA, Folley AE, Toth JM. Chronically inadequate sleep results in abnormal bone formation and abnormal bone marrow in rats. Exp Biol Med (Maywood) 2012;237:1101–1109. doi: 10.1258/ebm.2012.012043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson CA, Szabo A. Repeated exposure to severely limited sleep results in distinctive and persistent physiological imbalances in rats. PLoS One. 2011;6:e22987. doi: 10.1371/journal.pone.0022987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenius M, Brandenberger G, Hietter B. Diurnal cortisol peaks and their relationships to meals. J Clin Endocrinol Metab. 1982;55:757–761. doi: 10.1210/jcem-55-4-757. [DOI] [PubMed] [Google Scholar]
- Fondell E, Axelsson J, Franck K, Ploner A, Lekander M, Balter K, Gaines H. Short natural sleep is associated with higher T cell and lower NK cell activities. Brain Behav Immun. 2011;25:1367–1375. doi: 10.1016/j.bbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Weil ZM, Nelson RJ. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav Immun. 2013;34:159–163. doi: 10.1016/j.bbi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Badia P, Wright KP., Jr Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13:305–315. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gary KA, Winokur A, Douglas SD, Kapoor S, Zaugg L, Dinges DF. Total sleep deprivation and the thyroid axis: effects of sleep and waking activity. Aviat Space Environ Med. 1996;67:513–519. [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Ortiz M, Martinez-Abundis E, Balcazar-Munoz BR, Pascoe-Gonzalez S. Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes Nutr Metab. 2000;13:80–83. [PubMed] [Google Scholar]
- Gribbin CE, Watamura SE, Cairns A, Harsh JR, Lebourgeois MK. The cortisol awakening response (CAR) in 2- to 4-year-old children: effects of acute nighttime sleep restriction, wake time, and daytime napping. Dev Psychobiol. 2012;54:412–422. doi: 10.1002/dev.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Chapotot F, Weibel L, Jouny C, Piquard F, Brandenberger G. Pulsatile cortisol secretion and EEG delta waves are controlled by two independent but synchronized generators. Am J Physiol. 1998;275:E94–100. doi: 10.1152/ajpendo.1998.275.1.E94. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G. Temporal relationships between pulsatile cortisol secretion and electroencephalographic activity during sleep in man. Electroencephalogr Clin Neurophysiol. 1997;103:405–408. doi: 10.1016/s0013-4694(97)00013-1. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Simon C, Piquard F, Ehrhart J, Brandenberger G. Neuroendocrine processes underlying ultradian sleep regulation in man. J Clin Endocrinol Metab. 1999;84:2686–2690. doi: 10.1210/jcem.84.8.5893. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci U S A. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Pollmacher T, Mullington JM. Diurnal and sleep-wake dependent variations of soluble TNF- and IL-2 receptors in healthy volunteers. Brain Behav Immun. 2004;18:361–367. doi: 10.1016/j.bbi.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Waage S, Ursin H, Hansen AM, Bjorvatn B, Eriksen HR. Cortisol, reaction time test and health among offshore shift workers. Psychoneuroendocrinology. 2010;35:1339–1347. doi: 10.1016/j.psyneuen.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Heiser P, Dickhaus B, Schreiber W, Clement HW, Hasse C, Hennig J, Remschmidt H, Krieg JC, Wesemann W, Opper C. White blood cells and cortisol after sleep deprivation and recovery sleep in humans. Eur Arch Psychiatry Clin Neurosci. 2000;250:16–23. doi: 10.1007/pl00007534. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka B, Quigley ME, Yen SS. Pituitary hormone release in response to food ingestion: evidence for neuroendocrine signals from gut to brain. J Clin Endocrinol Metab. 1983;57:1111–1116. doi: 10.1210/jcem-57-6-1111. [DOI] [PubMed] [Google Scholar]
- Jung CM, Khalsa SB, Scheer FA, Cajochen C, Lockley SW, Czeisler CA, Wright KP., Jr Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 2010;25:208–216. doi: 10.1177/0748730410368413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosro S, Alireza S, Omid A, Forough S. Night work and inflammatory markers. Indian J Occup Environ Med. 2011;15:38–41. doi: 10.4103/0019-5278.82996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Majde JA, Rector DM. Cytokines in immune function and sleep regulation. Handb Clin Neurol. 2011;98:229–240. doi: 10.1016/B978-0-444-52006-7.00015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekander M, Andreasson AN, Kecklund G, Ekman R, Ingre M, Akerstedt T, Axelsson J. Subjective health perception in healthy young men changes in response to experimentally restricted sleep and subsequent recovery sleep. Brain Behav Immun. 2013;34:43–46. doi: 10.1016/j.bbi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenschijn L, van Kruysbergen RG, de Jong FH, Koper JW, van Rossum EF. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab. 2011;96:E1862–1865. doi: 10.1210/jc.2011-1551. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Born J. Brain-immune interactions in sleep. Int Rev Neurobiol. 2002;52:93–131. doi: 10.1016/s0074-7742(02)52007-9. [DOI] [PubMed] [Google Scholar]
- McHill AW, Smith BJ, Wright KP., Jr Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythms. 2014;29:131–143. doi: 10.1177/0748730414523078. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, Simpson NS, Dinges DF. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12:1015–1020. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, Lo JC, Santhi N, von Schantz M, Smith CP, Dijk DJ. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013;110:E1132–1141. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Wang S, Rasmusson A, Hazlett G, Anderson G, Charney DS. Relationship among plasma cortisol, catecholamines, neuropeptide Y, and human performance during exposure to uncontrollable stress. Psychosom Med. 2001;63:412–422. doi: 10.1097/00006842-200105000-00010. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav Immun. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J, Wright KP., Jr Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep. 2010;2:9–18. doi: 10.2147/NSS.S7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp MR. Corticotropin-releasing hormone involvement in stressor-induced alterations in sleep and in the regulation of waking. Adv Neuroimmunol. 1995;5:127–143. doi: 10.1016/0960-5428(95)00004-l. [DOI] [PubMed] [Google Scholar]
- Ozturk L, Pelin Z, Karadeniz D, Kaynak H, Cakar L, Gozukirmizi E. Effects of 48 hours sleep deprivation on human immune profile. Sleep Res Online. 1999;2:107–111. [PubMed] [Google Scholar]
- Parry BL, Javeed S, Laughlin GA, Hauger R, Clopton P. Cortisol circadian rhythms during the menstrual cycle and with sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Biol Psychiatry. 2000;48:920–931. doi: 10.1016/s0006-3223(00)00876-3. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Mullington J, Korth C, Schreiber W, Hermann D, Orth A, Galanos C, Holsboer F. Diurnal variations in the human host response to endotoxin. J Infect Dis. 1996;174:1040–1045. doi: 10.1093/infdis/174.5.1040. [DOI] [PubMed] [Google Scholar]
- Puttonen S, Viitasalo K, Harma M. Effect of shiftwork on systemic markers of inflammation. Chronobiol Int. 2011;28:528–535. doi: 10.3109/07420528.2011.580869. [DOI] [PubMed] [Google Scholar]
- Rahman SA, Castanon-Cervantes O, Scheer FA, Shea SA, Czeisler CA, Davidson AJ, Lockley SW. Endogenous circadian regulation of proinflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.11.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An american Academy of Sleep Medicine review. Sleep. 2007a;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007b;30:1484–1501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Fernandez Gianotti T, Burgueno A, Alvarez A, Gonzalez CD, Pirola CJ. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261:285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Ann N Y Acad Sci. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Thompson RS, Strong PV, Clark PJ, Maslanik TM, Wright KP, Jr, Greenwood BN, Fleshner M. Repeated fear-induced diurnal rhythm disruptions predict PTSD-like sensitized physiological acute stress responses in F344 rats. Acta Physiol (Oxf) 2014;211:447–465. doi: 10.1111/apha.12239. [DOI] [PubMed] [Google Scholar]
- Thorsley D, Leproult R, Spiegel K, Reifman J. A phenomenological model for circadian and sleep allostatic modulation of plasma cortisol concentration. Am J Physiol. 2012;303:E1190–1201. doi: 10.1152/ajpendo.00271.2012. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Refetoff S. Multifactorial control of the 24-hour secretory profiles of pituitary hormones. J Endocrinol Invest. 1985;8:381–391. doi: 10.1007/BF03348519. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L'Hermite-Baleriaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol. 1994;266:E953–963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- von Treuer K, Norman TR, Armstrong SM. Overnight human plasma melatonin, cortisol, prolactin, TSH, under conditions of normal sleep, sleep deprivation, and sleep recovery. J Pineal Res. 1996;20:7–14. doi: 10.1111/j.1600-079x.1996.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Weibel L, Brandenberger G. Disturbances in hormonal profiles of night workers during their usual sleep and work times. J Biol Rhythms. 1998;13:202–208. doi: 10.1177/074873098129000048. [DOI] [PubMed] [Google Scholar]
- Weibel L, Follenius M, Spiegel K, Ehrhart J, Brandenberger G. Comparative effect of night and daytime sleep on the 24-hour cortisol secretory profile. Sleep. 1995;18:549–556. [PubMed] [Google Scholar]
- Weil BR, Greiner JJ, Stauffer BL, Desouza CA. Self-reported habitual short sleep duration is associated with endothelial fibrinolytic dysfunction in men: a preliminary report. Sleep. 2013;36:183–188. doi: 10.5665/sleep.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab. 1983;56:352–358. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci U S A. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18:508–521. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager MP, Pioli PA, Guyre PM. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose Response. 2011;9:332–347. doi: 10.2203/dose-response.10-013.Yeager. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, Rothman PB, Takahashi JS, Hooper LV. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee PC, Wang-Weigand S, Wright KP, Jr, Peng X, Roth T. Effects of ramelteon on insomnia symptoms induced by rapid, eastward travel. Sleep Med. 2010;11:525–533. doi: 10.1016/j.sleep.2010.03.010. [DOI] [PubMed] [Google Scholar]