Abstract
Background
Biological mechanisms underlying symptom and prognostic heterogeneity in Attention-Deficit/Hyperactivity Disorder (ADHD) are unclear. Sleep impacts neurocognition and daytime functioning and is disrupted in ADHD, yet little is known about sleep in ADHD during adolescence, a period characterized by alterations in sleep, brain structure, and environmental demands as well as diverging ADHD trajectories.
Methods
A systematic review identified studies published prior to August 2016 assessing sleep in adolescents (aged 10–19 years) with ADHD or participating in population-based studies measuring ADHD symptoms.
Results
Twenty-five studies were identified (19 subjective report, 6 using actigraphy/polysomnography). Findings are mixed but overall suggest associations between sleep disturbances and 1) ADHD symptoms in the population and 2) poorer clinical, neurocognitive, and functional outcomes among adolescents with ADHD. Common limitations of studies included small or non-representative samples, non-standardized sleep measures, and cross-sectional methodology.
Conclusions
Current data on sleep in adolescent ADHD are sparse and limited by methodological concerns. Future studies are critical for clarifying a potential role of sleep in contributing to heterogeneity of ADHD presentation and prognosis. Potential mechanisms by which sleep disturbances during adolescence may contribute to worsened symptom severity and persistence of ADHD into adulthood and an agenda to guide future research are discussed.
Keywords: ADHD, sleep disturbances, adolescence, adolescents, development, neurocognition
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD)1 is a common neuro-developmental disorder impacting approximately 7% of youth worldwide (Thomas, Sanders, Doust, Beller, & Glasziou, 2015). Despite its prevalence and impact on public health, the underlying biological mechanisms contributing to the development, variable clinical presentation, and course of ADHD across the lifespan are poorly understood (Tarver, Daley, & Sayal, 2014). Specifically, within ADHD samples, youth vary considerably in symptom manifestation, neurocognitive capability, and academic and social functioning (Heidbreder, 2015). In addition, the developmental trajectory and prognosis of ADHD is heterogeneous, with some individuals exhibiting persistence of ADHD into adulthood while others experience reduced severity or remission of symptoms over time (Faraone, Biederman, & Mick, 2006; Langley et al., 2010).
Sleep is a complex biological process with widespread effects on physical and mental health (Fernandez-Mendoza & Vgontzas, 2013; Sutton, 2014), neurocognitive performance (Durmer & Dinges, 2005; Fortier-Brochu, Beaulieu-Bonneau, Ivers, & Morin, 2012), and psychosocial functioning (Shochat, Cohen-Zion, & Tzischinsky, 2014) for individuals of all ages. Prior research has revealed that both self-reported and objectively-measured sleep disturbances are common and distressing phenomena experienced by individuals with ADHD (Cortese, Faraone, Konofal, & Lecendreux, 2009; Hvolby, 2015) and may increase ADHD symptom severity (Dahl, 1996). However, it is currently unclear how poor sleep may contribute to variable clinical, neurocognitive, and psychosocial presentations in ADHD, or to the developmental course and persistence of ADHD symptoms over the lifespan.
Importantly, relatively little is known about sleep among adolescents with ADHD. This is a significant gap in the literature, since adolescence is a developmental period characterized by significant normative changes in sleep and circadian rhythms and the brain structures and endocrine systems supporting their maintenance (Colrain & Baker, 2011; Darchia & Cervena, 2014). Adolescence is also associated with substantial increases in academic and social demands (Crockett & Crouter, 2014). The ability to undertake new challenges and succeed in these arenas is highly impacted by neurocognitive performance, which is also closely associated with sleep quality in adolescence (Carskadon, 2011). Finally, disparate developmental trajectories of ADHD may begin to diverge during adolescence (Halperin & Schulz, 2006), suggesting that deviations in biological processes occurring during this period may contribute to variable prognosis and clinical outcomes within the ADHD population. Given the significant alterations in sleep occurring during puberty, abnormalities in the normative developmental course of sleep changes in adolescence may have a profound impact on ADHD symptom trajectories.
Thus, it is plausible that disrupted sleep in adolescence is a biological mechanism contributing to the diverse clinical and neurocognitive presentations within ADHD as well as divergent developmental symptom trajectories in adolescence and adulthood. This review includes 1) an overview of sleep in ADHD and normative developmental changes in sleep, brain composition, and environmental demands during adolescence, 2) a systematic review of literature pertaining to sleep and ADHD in adolescent-specific samples, 3) a discussion of potential mechanisms by which sleep disturbances may contribute to ADHD symptom presentation and progression, and 4) a guide to further research in this important area.
Sleep Disturbances in ADHD across the Lifespan
Sleep problems occur in 25–55% of ADHD children (Corkum, Tannock, & Moldofsky, 1998; Hodgkins et al., 2013; Hvolby, 2015), including longer sleep onset latency (SOL), frequent awakenings, non-restorative sleep, greater bedtime resistance, poorer sleep efficiency (SE), decreased total sleep time (TST), and greater symptoms of sleep disordered breathing (SDB) and periodic limb movements (PLM) (Cortese, Faraone, Konofal, & Lecendreux, 2009; Sadeh, Pergamin, & Bar-Haim, 2006). ADHD youth are also more likely to display sleep disorders (Herman, 2015), disrupted circadian rhythms (Imeraj et al., 2012), and daytime sleepiness (Cortese et al., 2009; Golan, Shahar, Ravid, & Pillar, 2004; Lecendreux, Konofal, Bouvard, Falissard, & Mouren-Simeoni, 2000) than peers. Importantly, sleep difficulties may exacerbate ADHD symptom severity (Dahl, 1996) and neurocognitive deficits (Sawyer et al., 2009).
Emerging evidence suggests a similarly high prevalence of both subjectively- and objectively-measured sleep problems in adults with ADHD (see Snitselaar, Smits, van der Heijden, & Spijker, 2013 for a review), including reduced sleep quality, increased SOL, more restless sleep and frequent nighttime awakenings, shorter sleep duration, greater difficulty awakening, and non-restorative sleep. Adults with ADHD also experience specific sleep disorders and daytime sleepiness more often than healthy peers. Of note, disturbed sleep is related to poorer collegiate academic functioning (Langberg, Dvorsky, Becker, & Molitor, 2014) and driving performance (Bioulac et al., 2015) among adults with ADHD.
Stimulant Medication and its Relationship to Sleep Functioning in ADHD
While researchers agree that stimulants likely impact sleep in ADHD, whether the effect is harmful or beneficial is disputed. In child samples, some studies suggest stimulants disrupt sleep (e.g., increased SOL and insomnia, reduced TST and efficiency; Corkum, Panton, Ironside, Macpherson, & Williams, 2008; Ironside, Davidson, & Corkum, 2010), while others indicate that stimulants improve sleep by reducing ADHD symptoms and bedtime resistance (Chatoor, Wells, Conners, Seidel, & Shaw, 1983; Coghill et al., 2013; Findling et al., 2011). Notably, stimulants’ impact on sleep may depend on gender, dose frequency, and length of time on stimulants (Kidwell, Van Dyk, Lundahl, & Nelson, 2015). In adults, results are mixed but generally support a positive impact of stimulant medications on sleep (i.e, reduced SOL and awakenings, more restorative sleep; Snitselaar et al., 2013).
Adolescence: A Critical Developmental Period for Sleep and Circadian Rhythm Changes, Brain Development, and Expanding Environmental Demands
Comparatively less is known about how sleep manifests during adolescence for ADHD youth. Normatively, adolescence is associated with significant alterations in sleep/circadian rhythms, brain and hormonal systems supporting these developmental changes, and environmental demands (Colrain & Baker, 2011). Thus, disruptions to normative brain development and sleep in this critical period may have a particularly deleterious impact on symptom presentations and prognosis for ADHD youth.
Developmental Changes in Sleep and Circadian Rhythms in Adolescence
Adolescence is associated with dramatic shifts in sleep architecture, behaviors, and circadian rhythms (Carskadon, 2002a; Colrain & Baker, 2011). Specifically, adolescent sleep is characterized by a circadian phase shift toward eveningness, such that adolescents stay up later and sleep later than school age children (Jenni & Carskadon, 2005). This chronotype shift appears to be intrinsic, occurs irrespective of environmental influences (Roenneberg et al., 2004), and may be associated with delayed melatonin-secretion offset in adolescence (Carskadon, Acebo, Richardson, Tate, & Seifer, 1997). Sleep behavior also changes significantly, including SOL increases (Short, Gradisar, Lack, Wright, & Dohnt, 2013) and TST reductions (Olds, Maher, Blunden, & Matricciani, 2010), which contribute to increased daytime sleepiness (Sivertsen, Harvey, Pallesen, & Hysing, 2015). Because adolescents are also subjected to early rising demands through early school start times, these patterns of sleep behavior often result in chronic patterns of insufficient sleep for typically developing teens (Jenni & Carskadon, 2005).
Sleep architecture alterations also occur during adolescence (Colrain & Baker, 2011). EEG studies have shown that time spent in slow wave sleep (SWS; deep, non-rapid eye movement (NREM) sleep) declines by 40% (Carskadon, Acebo, & Jenni, 2004). In addition, typically developing teens display reduced slow delta wave count (number of delta waves during sleep period), amplitude, and slope (Feinberg, Higgins, Khaw, & Campbell, 2006; Jenni & Carskadon, 2004; Kurth et al., 2010), suggesting they experience a slower accumulation of homeostatic sleep drive (Gaudreau, Carrier, & Montplaisir, 2001; Jenni, Achermann, & Carskadon, 2005). Specifically, delta activity (EEG activity between 0.5 and 4.5 Hz) steeply drops (by 66%) beginning at age 12, while theta (6–7 Hz) declines onset earlier (age 6) and decline steadily across adolescence (Feinberg & Campbell, 2010). Rapid eye movement (REM) sleep also undergoes alterations during adolescence, including declines in total amount (but not percentage) of REM (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). As described in the next section, normative alterations in sleep architecture may be directly tied to developmental changes in brain structure.
Neural Development and its Relationship to Sleep Changes in Adolescence
Significant neuro-maturational alterations in the brain’s composition and organization occur during adolescence (Shaw et al., 2008). Specifically, magnetic resonance imagining (MRI) studies have revealed normative gray matter loss indicative of pruning beginning in dorsal sensorimotor areas in early puberty and spreading rostrally into “higher-order” frontal cortical regions during late adolescence (Gogtay et al., 2004). Concurrent with reductions in synaptic density, typically developing teens exhibit significant increases in cerebral white matter, including increases in myelination, myelin integrity, and axonal thickness (Bartzokis et al., 2001; Sowell et al., 2003). This pattern of white matter growth begins in inferior, posterior areas of the brain and spreads into superior, anterior areas over the course of adolescence, with significant increases in the corpus collosum, prefrontal cortex, and internal capsule (Barnea-Goraly et al., 2005). Notably, these neuromaturational processes are likely influenced by hormonal changes occurring in the pubertal period (Giedd et al., 2006), including increased development and excretion of sex steroids in adolescence (Peper et al., 2009; Schulz & Sisk, 2006).
Interestingly, converging evidence suggests that neuromaturational changes in the adolescent brain may be closely tied to the normative developmental alterations in sleep architecture observed during this period (Feinberg & Campbell, 2010). For example, developmental declines in SWS, delta activity, and delta power appear to correspond with the pattern and timing of cortical thinning exhibited by adolescents, primarily in the frontal cortex (Giedd et al., 2010). Similar to the “back to front” synaptic pruning pattern observed in adolescence, longitudinal EEG studies have shown that declines in delta power originate in occipital regions and extend into frontal areas over the course of puberty (Feinberg, de Bie, Davis, & Campbell, 2011). Kurth and colleagues (2010) have similarly shown that maximal SWS activity undergoes a forward shift from posterior regions in childhood to anterior areas in adolescence. Evidence for a relationship between developmental changes in brain structure and sleep architecture is further supported by cross sectional work indicating reduced delta power in mature teens compared to pre-pubertal adolescents in frontal and posterior regions (Jenni, van Reen, & Carskadon, 2005).
Thus, changes in brain structure across adolescence may impact sleep; however, this relationship is likely bidirectional. Healthy sleep is believed to support optimal development and organization of brain circuitry by reversing the negative effects of waking on plastic neurons and reestablishing synaptic homeostasis (Tononi & Cirelli, 2012). For example, animal studies have shown that NREM sleep enhances cortical plasticity during critical developmental periods characterized by synaptogenesis and synaptic plasticity, while sleep loss blocks cortical plasticity enhancement (Frank, Issa, & Stryker, 2001). Thus, sleep disturbances in adolescence may impede, delay, or prevent healthy brain development trajectories (e.g., excessive or insufficient synaptic pruning; reduced white matter growth). Thus, it is possible that teens with neurodevelopmental disorders such as ADHD may be vulnerable to a vicious cycle in which neurodevelopmental abnormalities lead to sleep deficits, which in turn, may further hinder brain development.
Environmental Changes and Associations with Sleep Behavior in Adolescence
Sleep behaviors are also highly influenced by alterations in the environment as youth transition from childhood into adolescence (Owens et al., 2014). Specifically, during middle school, control over sleep habits (e.g., setting bedtime, wake time) shifts from parents to the teens themselves (Russo, Bruni, Lucidi, Ferri, & Violani, 2007). As adolescents age, they experience competing psychosocial demands on their evening time, and frequently stay up late to complete homework, socialize, watch television, use electronic media devices, and participate in after-school activities or jobs (Carskadon, 2002a). Reduced parental monitoring paired with increased engagement in these age-appropriate activities contributes to later bedtimes, sleep loss, and increased daytime sleepiness and naps (Carskadon, 1990; Short et al., 2011). Resulting in part from daytime fatigue, teens also display increased caffeine consumption, which likely exacerbates poor sleep patterns (Owens et al., 2014). Notably, both high caffeine intake (Martin et al., 2008) and overuse of electronics/internet (Seyrek, Cop, Sinir, Ugurlu, & Senel, 2016) have been associated with ADHD symptoms in adolescents, suggesting that investigation of the links between these factors and sleep may be particularly informative for understanding functional impairments in ADHD.
Early school start times observed in the U.S. also contribute to sleep loss during adolescence (Minges & Redeker, 2015). Specifically, school days begin progressively earlier as youth move from elementary to middle to high school (Allen, 1991, 1992). Because adolescents experience concurrent shifts in their biological circadian rhythm and increased social and academic demands resulting in later bedtimes, the requirement to rise earlier during high school results in premature termination of sleep and sleep debt as well as associated impairments in social and academic function as a result of chronic fatigue (Carskadon, Wolfson, Acebo, Tzischinsky, & Seifer, 1998). In contrast, in cultures with later school start times, such as Australia, reductions in sleep duration during adolescence are not as prominent (Olds et al., 2010). Environmental factors restricting sleep in adolescence may be particularly detrimental among youth at increased risk for adverse psychosocial or mental health outcomes (Matamura et al., 2014).
Summary
Significant biological and environmental shifts occurring during adolescence suggest that this period is critical for healthy sleep development, psychosocial adjustment, and mental health. As sleep impairments are observed in many, but not all, children and adults with ADHD, disruptions to sleep development during this critical period may contribute to the heterogeneity of symptom presentation, neurocognitive and psychosocial performance, and developmental course and prognosis within the ADHD population. However, as relatively few studies have examined sleep among ADHD youth during puberty, there is little information to elucidate the role of sleep development in ADHD outcomes. Although some studies of sleep in children with ADHD include teens in their sample (e.g.,Golan et al., 2004; Kirov, Banaschewski, Uebel, Kinkelbur, & Rothenberger, 2007; Kirov et al., 2004), this ascertainment strategy is limited in its utility to shed light on sleep during adolescence and may mask important differences in sleep as a function of age and pubertal status. In the next section, we review the data available regarding sleep and ADHD in adolescent-specific samples. Although limited in the number of studies and methodology used, these investigations represent an important first step in clarifying a potential role of sleep disturbances in the phenomenology and course of ADHD over the lifespan.
Sleep Disturbances in Adolescents with ADHD: A Systematic Review
Method
A systematic literature search using PsychINFO, PubMed, EMBASE, Web of Science, and Scopus databases was conducted to identify peer-reviewed articles published prior to August, 2016 including sleep data on adolescents with ADHD diagnoses or symptoms. Search terms included: sleep, sleep disturbances, or sleep disorders; ADHD, attention deficit/hyperactivity disorder, or attention deficit disorder (ADD); and adolescents, teenagers, or adolescence. Inclusion criteria were studies reporting on original data regarding sleep in adolescent-specific human samples (age range between 10 and 19, inclusive) 1) with an ADHD diagnosis or 2) participating in population-based or cohort studies that inquired about ADHD diagnosis or symptoms. Population-based studies were included due to 1) the relatively few number of studies assessing sleep in adolescent-specific samples with formally-diagnosed ADHD and 2) the provision of important information that may guide future studies. However, these studies are subject to limitations (e.g., non-standardized sleep and ADHD measures) discussed below.
The relatively broad age range was selected based on literature suggesting that puberty-related sleep changes emerge around age 10 (Laberge et al., 2001) and continue through later adolescence (age 18–19; Carskadon et al., 2004; Ohayon, Roberts, Zulley, Smirne, & Priest, 2000). However, sleep changes in adolescence are likely tied to shifts in pubertal development (e.g., shifts in hormonal secretions; Carskadon et al., 2004) rather than age per se, and puberty and age are often confounded in sleep studies in adolescent populations (Colrain & Baker, 2011). While current studies have typically used age for inclusion/exclusion criteria, it will be critical for future studies to include standardized puberty measures.
Exclusion criteria included 1) inclusion of children (age < 10) or adults (age > 20), 2) animal models or case report methodology, 3) examination of sleep and attention symptoms in specific medical (e.g., seizure disorder, diabetes), non-ADHD psychiatric (e.g., autism spectrum disorder), or intellectually disabled samples, 4) absence of original data (e.g., theoretical papers), and/or 5) non-peer reviewed sources (e.g., dissertation abstracts). Two of the authors (JRLA and SHK) independently 1) screened titles and abstracts from the initial search to identify articles for full-text retrieval and 2) reviewed full-text articles for inclusion in the review. References of identified articles were examined for additional sources.
Sleep Functioning and ADHD in Adolescent-Specific Samples
The database search yield 3381 titles and/or abstracts with 553 selected for full-text retrieval. Twenty-five studies met inclusion criteria; 6 were population-based or cohort studies, 1 was based on chart review, 1 was a descriptive study, and 17 were empirical studies. The most common reasons for exclusion included: inclusion of children or adults, examination of sleep and ADHD symptoms within other psychiatric- or medical-disordered samples, and absence of original data. Figure A.1 in Appendix A depicts a flowchart of the article search and selection process and Table B.1 in Appendix B summarizes the included studies; indentions signify data obtained within a single research group and italics denote studies referenced in multiple sections of the table.
Sleep Disturbances in Adolescence and ADHD Symptoms in the Population
Findings from population-based studies revealed that self-reported sleep disturbances, including reduced duration (Hysing, Lundervold, Posserud, & Sivertsen, 2015; Lam & Yang, 2008) and increased SOL, time in bed (TIB), and nocturnal awake time (Hysing et al., 2015) were associated with increased ADHD symptoms among teens. In addition, ADHD symptoms during adolescence increased the odds of having a sleep disorder, including insomnia (Hysing et al., 2015), delayed sleep phase syndrome (Hysing et al., 2015; Sivertsen et al., 2015), and symptoms of SDB (Johnson & Roth, 2006) and restless leg syndrome (RLS) (Turkdogan, Bekiroglu, & Zaimoglu, 2011). One study did not find an association between “inadequate sleep” and ADHD among adolescents (Smaldone, Honig, & Byrne, 2007a).
All of these investigations used large community samples with representative demographics (e.g., females and males, wide age range, racial diversity), enhancing generalizability of findings. In addition, these studies controlled for potential confounds, including demographics (e.g., age, gender, race, parental education; all studies), comorbid anxiety and depression (Hysing et al., 2015; Johnson & Roth, 2006; Sivertsen et al., 2015; Smaldone, Honig, & Byrne, 2007b), medications (Hysing et al., 2015; Johnson & Roth, 2006; Lam & Yang, 2008), school performance (Johnson & Roth, 2006), health problems (obesity; Lam & Yang, 2008), day of the week sleep was assessed (weekend vs. weekday; Hysing et al., 2015), and electronic device use (Hysing et al., 2015). Strengths of individual studies included multiple informants regarding sleep (parent and adolescent; Johnson & Roth, 2006; Turkdogan et al., 2011) and visual inspection of sleep data to ensure validity (Hysing et al., 2015; Sivertsen et al., 2015).
Conclusions based on these studies are limited, however, by several methodological concerns. Five of 6 studies relied on parent- or adolescent-report to establish ADHD symptoms rather than clinical interview and non-standardized sleep assessments (i.e., developing sleep queries for the individual study) (Johnson & Roth, 2006 is the exception). In addition, no studies utilized actigraphy or polysomnography (PSG), despite assessing syndromes that are typically diagnosed by these measures (e.g., SDB, Delayed Sleep Phase Syndrome). Finally, all 6 studies were cross-sectional, such that the direction of causation between sleep problems and ADHD symptoms could not be evaluated.
It is further notable that the only study that did not find a relationship between problematic sleep and ADHD symptoms in teens (Smaldone et al., 2007b) assessed sleep using only one question (“During the past week, on how many nights did your child get enough sleep?”) and coded any number of nights endorsed (1–7) as a dichotomous variable (“inadequate sleep”). It is likely that this strategy masked important differences across subjects and reduced power to detect a relationship. It also relied on parent-report of sleep difficulties, which is noteworthy in that adolescents are more accurate reporters of their sleep problems than their parents (Meltzer et al., 2014; Stoleru, Nottelmann, Belmont, & Ronsaville, 1997; Waters, Stewart-Brown, & Fitzpatrick, 2003). Finally, they did not assess whether sleep problems were acute or chronic (i.e., only queried about the past week) or contributors to the sleep deficiency (e.g., choosing to stay up late to use electronics versus trying to sleep but failing to do so).
Prevalence of Sleep Disturbances in ADHD-Diagnosed Samples
Regarding ADHD-specific samples, Brook and Boaz (2005) administered clinical interviews and self-report questionnaires to assess sociobehavioral functioning in teens with ADHD, many of whom displayed comorbid learning disabilities (94%). Thirty-seven percent of their sample described daytime fatigue and the need for more sleep. More recently, Fisher and colleagues (2014) employed chart review methodology to examine sleep problems in ADHD across the lifespan. They found that sleep problems were prevalent among ADHD adolescents (74% of their sample), with the most commonly endorsed difficulties including initial insomnia, nocturnal awakenings, and non-restorative or restless sleep (shortened duration and hypersomnia were less frequently endorsed). Interestingly, males reported a fewer number of sleep problems (1–3), while females reported a greater number (4–7), suggesting a gender difference in reported sleep problems among ADHD teens. Based on these 2 studies, prevalence rates of sleep problems in adolescent ADHD appear to be similar to or somewhat higher than in children.
While these studies have several strengths including relatively large sample sizes (including both males and females) with clinician-provided ADHD diagnoses, conclusions are limited by cross-sectional and non-empirical methodology (i.e., descriptive study, chart review), absence of healthy control (HC) comparison groups, potentially non-representative samples (i.e., clinic-referred patients or students in a special education school), and use of non-standardized sleep measures (i.e., questions developed for the individual study). In addition, in the Brook and Boaz (2005) investigation, a single item assessed the need for additional hours of sleep and daytime fatigue. Failure to assess for a wide range of potential sleep difficulties (e.g., nocturnal awakenings, initial insomnia) may explain the relatively lower prevalence of sleep problems reported in their sample compared to that of Fisher and colleagues (2014).
Subjective Sleep Disturbances in ADHD-Diagnosed Teens compared to Healthy Controls
Empirical studies suggest subjectively-measured sleep deficits in teens with ADHD compared to healthy peers. Five studies examined sleep in a single sample of adolescents with ADHD, who were followed into adolescence subsequent to childhood diagnosis. Using the K-SADS diagnostic interview, researchers within this group found a higher incidence of sleep disorders in adolescents with ADHD compared to HC peers (Gau & Chiang, 2009; Gau, Lin, et al., 2010) and their unaffected siblings (Yang, Shang, & Gau, 2011). Specific sleep disorders endorsed by ADHD teens varied by subtype, such that circadian rhythm, sleep-talking, and nightmare disorders were related to ADHD-Combined (ADHD-C), while hypersomnia was associated with ADHD-Inattentive (ADHD-I). In addition, teens with ADHD-C and ADHD-Hyperactive/Impulsive (ADHD-H/I) slept longer than ADHD-I and HC on school days, and ADHD-C and ADHD-I adolescents engaged in more napping behaviors than HC youth. Across subtypes, sleep problems were associated with increased ADHD symptom severity (Chiang et al., 2010).
As this was a longitudinal study, researchers were able to compare sleep among adolescents with “persistent” (present since childhood) versus “non-persistent” (childhood diagnosis, current subthreshold symptoms) ADHD. Current and lifetime sleep disorders were more prevalent among adolescents with both persistent and subthreshold ADHD compared to healthy peers (Gau, Ni, et al., 2010), including insomnia, sleep walking, sleep terrors, nightmares, bruxism, and snoring. Additionally, adolescents with persistent ADHD displayed longer duration and more sleep talking than subthreshold ADHD and HC groups, and teens with subthreshold ADHD symptoms displayed hypersomnia and nightmares at a higher rate than HC youth. Circadian rhythm disorder, sleep-wake schedule, and napping behavior did not differ among the 3 groups. Across ADHD groups, specific sleep disorders (insomnia, nightmares) were associated with increased severity of parent- and teacher-rated ADHD symptoms (Gau & Chiang, 2009).
While the findings of these 5 studies were strengthened by their longitudinal method, relatively large sample sizes, clinical diagnosis of ADHD based on multiple informants, and inclusion of covariates (i.e., demographics, comorbidities, and medications) in primary analyses, the sample was potentially non-representative (81–86% male, clinic-referred) and only 2 studies incorporated standardized sleep measures (i.e., Sleep Disturbance Questionnaire) in addition to the K-SADS clinical interview of sleep disorders. Continued assessment into adulthood with this sample has not yet been reported, so it is currently unclear how sleep problems during adolescence may contribute to adult symptom presentation and prognosis.
Several cross–sectional studies in adolescent ADHD have utilized validated subjective sleep scales. Using the Children Sleep Behavior scale, Mick and colleagues (2000) found that teens with ADHD exhibited greater nocturnal waking, restless sleep, initial insomnia, and sleep talking and were less likely to go to bed willingly and fall asleep/wake easily than HC. They found no group differences in sleep disorders, napping, sleepiness, duration, nightmares, or walking, sitting up, or grinding teeth during sleep. Importantly, significant group differences disappeared after covarying for comorbid anxiety and stimulant use. Results of this study should be interpreted with caution, however, as the Children Sleep Behavior Scale is normed in younger children and is based on parent rather than adolescent report. Use of a non-representative (all-male, Caucasian) sample may also reduce the generalizability of these findings.
Using a relatively small sample (30 ADHD, 28 HC), Lufi and Tzischinsky (2014) used a standardized self-report measure (Sleep Schedules Questionnaire) and found greater initial insomnia and difficulty awakening among ADHD teens compared to healthy peers, but no significant differences in daytime sleepiness. However, this study did not control for comorbidity and relied on parent-reported history of ADHD diagnosis rather than a current clinical assessment. Finally, Weinstein and colleagues (2015) found that adolescent males with ADHD were more likely to report later bedtimes (after 12 am) than HC. Both of these studies were comprised of medicated (currently or past) and largely or entirely male samples and completed data collection in school settings, and neither controlled for important covariates, such as interventions (behavioral or pharmacological), intelligence, and demographics.
Objective Sleep Disturbances in ADHD-Diagnosed Teens compared to Healthy Controls
While studies utilizing subjective measures provide important information about sleep behaviors in adolescent ADHD, they are limited by potential recall bias and/or cognitive distortions. To date, 3 studies have utilized an objective measure to characterize sleep patterns and physiology among teens with ADHD. As part of a larger community cohort study, Moore and colleagues (2011) used actigraphy to evaluate associations between the amount and variability of TST and various health outcomes. They did not find an association between amount/variability of TST and ADHD diagnosis, but noted limited power due to the nature of their sample (i.e., community based, stable health without significant disease, parent-reported diagnosis, oversampling of African American, low income, and preterm children, 41% overweight) and low base rate of ADHD diagnosis. In addition, the study did not examine the full range of sleep variables of interest typically available from actigraphy (awakenings, SE, circadian rhythms).
Mullin and colleagues (2011) conducted a pilot study comparing sleep among ADHD-C (non-medicated), bipolar (medicated), and HC adolescents using 4 nights of actigraphy. Controlling for gender, they detected no differences in sleep between ADHD and HC adolescents, but noted low power due to small sample size. In addition, authors did not control for whether sleep data were collected on weekdays (restricted by environmental demands such as school start time) versus weeknights (unrestricted sleep). Importantly, the ADHD group displayed non-significant reductions in TST compared to HC and bipolar groups via sleep diary and actigraphy, despite having more weekend nights of recording compared to the other groups (when sleep periods should be longer as they are unrestricted). Researchers hypothesized that if the study was sufficiently powered to covary for weekday/weekend assessment, the ADHD group would have exhibited greater TST reductions relative to other groups. Finally, the study did not include ADHD-I or ADHD-H/I subtypes, and thus was unable to examine sleep across the ADHD spectrum.
A second pilot study conducted by Prehn-Kristensen et al. (2011) utilized PSG and found that non-medicated adolescents with ADHD displayed shorter SWS latency, increased SOL, reduced SE, and more REM than HC. No differences were observed in TIB, TST, number of awakenings or arousals, REM latency or density, time in sleep stages, non-REM amount, number of eye movements, or spindle density. As the only PSG study comparing ADHD and HC teens, this study provides important initial information about sleep physiology in this population, and the validity of its findings is strengthened by clinical diagnosis of ADHD and exclusion of teens with comorbidities. However, it was comprised of a small (12 ADHD, 12 HC), entirely male sample.
Sleep Disturbances and Comorbidity in Adolescents with ADHD
Several of the studies described above also investigated relationships between poor sleep and comorbid disorders in adolescent ADHD samples. Specifically, increased sleep disturbances and daytime sleepiness have been shown to be correlated with more sensory problems (i.e., movement, vision, touch, sensory sensitivity, and sensation seeking) in adolescents with ADHD but not controls (Lufi & Tzischinsky, 2014). In addition, associations between subjective sleep problems and internalizing symptoms, such as depression and anxiety, have been observed in adolescents with ADHD (Mick et al., 2000; Stein et al., 2002). Finally, later bedtimes and higher rates of internet addiction were observed in the same sample of ADHD teens compared HC (Weinstein et al., 2015). The relative strengths and weaknesses of these studies are detailed above.
Sleep Disturbances and Neurocognition in Adolescents with ADHD
Two of the reviewed studies have demonstrated links between sleep disturbances and impaired neurocognition. Using chart review (described above), Fisher and colleagues (2014) found associations between self-reported sleep disturbances and deficits in sequencing, cognitive flexibility, slow- and fast-paced input, divided attention, and whole brain functioning; however, they collapsed across adolescent and adult subgroups of their sample for this analysis. In their PSG study, Prehn-Kristensen et al. (2011) assessed declarative memory consolidation in non-medicated (48 hour washout) ADHD versus HC across sleep (learning performed in the evening, memory tested after sleep) and wake (learning conducted in the morning, memory tested after a day of wakefulness) conditions. They found that NREM duration and slow oscillation power during NREM correlated with improved memory in HC but not ADHD youth, suggesting a breakdown in biological processes supporting sleep-dependent memory consolidation in adolescents with ADHD. Notably, in follow-up studies from this group, application of slow oscillating transcranial direct current stimulation resulted in increased slow oscillations during NREM sleep among ADHD adolescents, improving declarative memory to a level observed in HC (Prehn-Kristensen et al., 2014) and improved behavioral inhibition (Munz et al., 2015). Both studies used small, male, younger adolescent (10–14 years old) samples and one (Munz et al., 2015) did not include a HC group.
Sleep Disturbances and Functional Outcomes in Adolescents with ADHD
Only 1 study has assessed sleep difficulties and functional impairment in adolescents with ADHD. Langberg and colleagues (2013) found that self-reported sleep duration was not associated with academic functioning in middle schoolers (ages 10–14). However, higher Pediatric Daytime Sleepiness Scale scores were associated with greater parent-rated academic and homework impairment and reduced teacher-rated academic competence after controlling for ADHD symptom severity. ADHD subtype and comorbidity (anxiety/depression) were not associated with sleep duration or daytime sleepiness. Importantly, this study did not include a HC group, so it is unclear whether relationships between sleepiness and academic functioning are specific to ADHD. Additionally, sleep disturbances other than duration (e.g., nocturnal awakenings) were not examined. It is also unclear whether “daytime sleepiness” stems from a specific sleep disturbance (e.g., insomnia) or a response to environment demands (e.g., early school start times). Notably, longitudinal research examining specific sleep problems in childhood ADHD and risk for poor high school performance is underway (Zendarski, Sciberras, Mensah, & Hiscock, 2016).
Impact of Sleep Disturbances on Prognosis among Adolescents with ADHD
There is little information to inform how sleep problems during adolescence may contribute to prognosis in ADHD. In their chart review, Fisher and et al. (2014) split their sample into cohorts (child, adolescent, and adult) and found that sleep problems became more prevalent across the lifespan (i.e., more common in adolescents than children, more common in adults than teens). While this suggests that sleep disturbance may amplify over the lifespan among individuals with ADHD, due to its cross-sectional cohort design, the study was unable to shed light on how problematic sleep may confer risk for continued symptoms into adulthood for affected teens. To date only one longitudinal study has examined relationships between sleep difficulties during the adolescent period and clinical outcomes over time among ADHD teens (Becker, Langberg, & Evans, 2015). This study administered the Children’s Sleep Habits Questionnaire to parents of ADHD youth aged 10–14. Researchers found that sleep problems endorsed at clinical intake predicted greater Oppositional Defiant Disorder (ODD)/externalizing behaviors and depression a year later after controlling for demographics, comorbidity, ADHD symptom severity, and baseline internalizing/ODD/externalizing symptoms. Sleep problems at intake were not predictive of anxiety symptoms at follow-up among adolescents with ADHD. It is notable that this study utilized a young, largely male sample and a parent-report sleep measure, did not differentiate among types of sleep problems endorsed, and did not include a HC comparison group.
Impact of Stimulant Medications on Sleep Functioning among Adolescents with ADHD
Several of the aforementioned studies provided information about the effect of stimulants on self- or parent-reported sleep among ADHD teens, with 2 studies finding no relationship between medications and self-reported sleep problems (Becker, Langberg, & Evans, 2015; Gau & Chiang, 2009) or disorders (with the exception of Bruxism; Gau & Chiang, 2009) while another study indicated an adverse effect of stimulants on parent-reported risk of restless sleep, nocturnal awakenings, sleep talking, fear of the dark, and nighttime bathroom use (Mick et al., 2000). Methodological differences between these studies (parent versus adolescent report, differing sleep measures) may contribute to this discrepancy in findings.
Stein and colleagues (2002) also examined associations between stimulant use and sleep problems experienced among adolescents with ADHD. They found greater sleep disturbances (i.e., total score on the Mini Sleep Questionnaire) among medicated adolescents with ADHD symptoms compared to non-medicated youth, who did not differ from HC. They did not observe specific differences in sleep duration between non-medicated and medicated groups on weekends or weekdays. Although ADHD was well diagnosed (multiple informants) in early childhood, it is notable that a significant portion of their teens did not exhibit “full blown” ADHD at the time of study. Individuals displaying subthreshold ADHD were less likely to be in the medicated group, suggesting a potentially significant confound.
These first 4 studies utilized correlational or quasi-experimental designs wherein youth either reported on medication use (Becker, Langberg, & Evans, 2015; Gau & Chiang, 2009; Mick et al., 2000) or were prescribed medication based on clinical judgment rather than random assignment (Stein et al., 2002). A fourth study randomly assigned adolescents with ADHD (aged 10 – 17) to receive mixed amphetamine salts or dexmethylphenidate in a double-blind, randomized, placebo-controlled, crossover, dose-response study (Santisteban, Stein, Bergmame, & Gruber, 2014). Higher doses of both medications resulted in longer actigraphic-measured SOL and shorter TST; there were no differences between medications on sleep parameters. In addition, no difference was observed between medications and placebo regarding nocturnal awakenings. It is notable that this study was relatively brief in duration and excluded adolescents with comorbid sleep disorders. Thus it is possible that sleep would have stabilized with ongoing treatment, and that the impact on medications on sleep may differ among subgroups of teens within the ADHD population (i.e., those with/without a prior history of sleep disturbances).
All of the studies reviewed in this section utilized standardized subjective or objective (actigraphy) measures of sleep and formally assessed for ADHD diagnosis (during childhood or currently) and most excluded or controlled for demographics and/or comorbidity (Santisteban et al., 2014 is the exception). However, the relatively small samples were all or mostly male and clinic- or school-referred, limiting generalizability. No studies have used PSG to examine the relationship between medication use and sleep physiology in adolescent ADHD.
Summary
Studies investigating sleep and ADHD in adolescent-specific samples suggest that specific sleep disturbances (e.g., greater SOL, TIB, nocturnal waking) and disorders (e.g., RLS, SDB, delayed sleep phase, insomnia) are associated with ADHD symptoms in the population. In addition, teens diagnosed with ADHD may exhibit greater self-reported sleep problems (e.g., difficulties falling asleep/waking) and disorders (e.g., insomnia, hypersomnia, circadian rhythm disorder) than healthy peers, which appear to be associated with poorer mental health, cognitive and functional performance, and prognosis. Results from objective sleep studies in adolescents are sparse and mixed, with 1 PSG study suggesting abnormalities in SOL, SWS latency, REM amount, and SE in adolescent ADHD and 2 actigraphy studies finding no associations between sleep and ADHD in teens. The impact of stimulants on sleep in adolescent ADHD is also unclear, with some studies finding an adverse impact (at least in the short-term) and others finding no effect. Taken together, research to date suggests careful clinical assessment of specific sleep difficulties (e.g., sleep latency, nocturnal awakenings) and sleep-based intervention may have the potential to improve functional outcomes in ADHD youth; however, additional study is warranted (described below).
While the reviewed studies provide important information about sleep and ADHD during adolescence, the reported results should be considered in the context of several significant limitations. Specifically, population-based studies typically relied on non-standardized measures of sleep and ADHD and cross-sectional methodology. Limitations of empirical studies frequently included non-representative (e.g., males only) and/or small samples, excluded potentially confounding covariates from analyses, and did not incorporate a HC group. In addition, these studies largely relied on subjective measurements of sleep. Subjective sleep assessments provide important information not captured by objective measures. For example, among ADHD children, self- and parent-reports have revealed difficulties with sleep quality and behaviors not captured by actigraphy or PSG (Owens et al., 2009). However, these measurements are vulnerable to cognitive and memory biases (Lockley, Skene, & Arendt, 1999), do not assess disturbances occurring during sleep (e.g., brief awakenings outside of an individual’s awareness), and cannot detect breathing-related sleep disorders.
Studies using objective measures are critical for enhancing understanding of sleep in adolescent ADHD, including circadian rhythms (best measured by actigraphy), sleep architecture (e.g., REM versus NREM; PSG-measured), and breathing-related sleep disorders (requires PSG). Two of the 3 objective studies comparing sleep in teens with ADHD versus HC to date were limited by extremely small sample size, which resulted in low power and/or limited capacity to control for confounds (e.g., weekday versus weeknight). The third study drew from a non-representative population and only measured one aspect of sleep (i.e., duration). In general, limitations of current research on this topic render any conclusions about sleep in adolescent ADHD preliminary. Future research using complimentary measures (subjective and objective) and incorporating actigraphy and/or PSG with large, representative samples is essential to characterize sleep in adolescent ADHD and associations with symptom manifestation and prognosis.
It further notable that relationships between sleep disturbances and ADHD symptoms in adolescence are likely complex (Becker, Langberg, & Byars, 2015). For example, there is substantial symptom overlap among adolescents who are sleep deprived and those with ADHD (e.g., inattention; Owens et al., 2013), sleep problems are associated with other psychiatric disorders often comorbid with ADHD (e.g., anxiety, depression; Becker, Langberg, & Byars, 2015), and some sleep disturbances in ADHD may result from stimulant intervention (Stein, Weiss, & Hlavaty, 2012). Studies to date, many of which did not control for comorbidity and medication use, do not provide sufficient information to disentangle these complex associations. Additionally, population-based studies, which relied on parent- or self-report of ADHD symptoms rather than careful clinical diagnosis, are particularly vulnerable to conflation of adolescents who suffer from sleep deprivation, ADHD, other mental illnesses, or a combination. Additional longitudinal studies that carefully diagnose adolescents with ADHD and consider confounding factors are critical for clarifying the nature of sleep-ADHD relationships in teens.
Potential Relationships of Disturbed Sleep to ADHD Symptom Presentation and Prognosis in Teens
As sleep disturbances may occur in higher rates in teens with ADHD compared to their healthy peers, it is possible that problematic sleep patterns and disruptions to normative developmental changes in sleep may contribute to greater functional impairments and worsened prognosis for at least a subset of adolescents with ADHD. In particular, disrupted sleep may contribute to neuromaturational abnormalities, neurocognitive deficits, and increased stress during a critical developmental period, which in turn may worsen ADHD core symptoms and support the persistence of ADHD into adulthood.
Delayed Neural Development in ADHD and Potential Associations with Disrupted Sleep
Longitudinal studies of individuals with ADHD have provided evidence for neuromaturational delays across adolescence (Rubia, Alegria, & Brinson, 2014). Specifically, peak cortical thickness and surface area is delayed by 2 to 5 years, particularly in frontal, temporal, and parietal areas (Shaw et al., 2007; Shaw et al., 2013). Less is known about white matter tract development in adolescents with ADHD, although emerging evidence supports a global delay in the development of white matter integrity and connectivity (Nagel et al., 2011; Pavuluri et al., 2009) as well as slower maturation of specific fiber tracts (e.g., caudate nucleus; Silk, Vance, Rinehart, Bradshaw, & Cunnington, 2009).
As noted above, normative neuromaturational processes such as synaptic pruning and myelination may be tied to SWS declines occurring in adolescence (Feinberg & Campbell, 2010). Among adolescents with ADHD, neurodevelopmental delays may contribute to abnormal trajectories of the SWS declines expected to occur during puberty. Supporting this hypothesis, a preliminary EEG study examining ADHD children on the cusp of adolescence (mean age: 11.9) revealed higher SWS power over central areas compared to HC, suggesting that a neuromaturational delay in ADHD youth may disrupt normative developmental SWS declines (Ringli et al., 2013). Perhaps concurrently, SWS abnormalities may also actively interfere with normative neurodevelopment in ADHD. Given its role in supporting cortical plasticity and homeostasis (Tononi & Cirelli, 2012), SWS abnormalities may drive a vicious cycle in which optimal neurodevelopment is further impeded by disrupted SWS developmental processes.
Magnetic resonance imaging (MRI) studies focusing on specific regions of interest (ROI) of the brain lend additional support for a potential link between neural development and sleep problems in ADHD. For example, studies have shown abnormalities in sleep-dependent areas of the brain (e.g., thalamus) in ADHD youth (Batty et al., 2015; Ivanov et al., 2010; Xia et al., 2012), as well as brain areas commonly impacted in insomnia, including the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC; Cubillo, Halari, Smith, Taylor, & Rubia, 2012). These brain differences are similarly present in adults with ADHD (Proal et al., 2011), suggesting persistence of abnormalities in brain areas supporting sleep across development. Diffusion tensor imaging (DTI) studies focusing on white matter integrity have also shown decreased fractional anisotropy (FA) in adults with childhood-onset ADHD in the posterior thalamic radiation, independent of remission status (Cortese et al., 2013). Additionally, abnormalities in the anterior thalamic radiation are present in healthy siblings of youth with ADHD (Lawrence et al., 2013), suggesting a potential biomarker for the disorder. Future longitudinal research with adolescents with ADHD may shed light on the potentially bidirectional relationships between these sleep phenomena (e.g., SWS) and brain development and their potential impact on ADHD core symptoms.
Sleep Disturbances and Impaired Neurocognitive Performance
In typically developing teens, sleep disturbance is associated with impaired neurocognition, including deficits in attention, memory, and motivation (Carskadon, 2011). Further, insufficient sleep is commonly correlated with impaired executive functioning (Dahl, 1996; Nilsson et al., 2005), emotional regulation (van der Helm, Gujar, & Walker, 2010), reward-based decision making (Dijk, 2011), and memory (Stickgold, 2013) in individuals of all ages. As mentioned above, there is significant overlap in the neurocognitive deficits associated with poor sleep and those observed in ADHD. Specifically, deficits in attention, vigilance, working memory, memory consolidation, emotion regulation, reward/risk-based decision making, and executive function are common in both ADHD and sleep-disordered populations, and may be tied to brain-based abnormalities observed in ADHD, including altered structure and/or function of regions of the cortex, striatum, and limbic system (Owens et al., 2013).
Neurocognitive impairment associated with poor sleep in typical adolescents may be amplified in youth with ADHD. For example, children with ADHD and poor sleep display greater distractibility than youth with either disorder alone (Sawyer et al., 2009), and self-reported sleep problems among children with ADHD are related to working memory deficits (Sciberras, DePetro, Mensah, & Hiscock, 2015). Specific sleep deficits are also related to impaired neurocognition in youth with ADHD. For example, REM abnormalities are associated with language, visual spatial, attention/executive functioning, and memory deficits (O’Brien et al., 2003) in children with ADHD, and as reviewed above, reduced functionality of slow oscillations in NREM interfere with consolidation of declarative memory (Prehn-Kristensen et al., 2011) in adolescents with ADHD. However, the direction of effect for associations between sleep and neurocognitive performance in ADHD is currently unknown. Poor sleep may disrupt neurocognition through its impact on brain plasticity; in turn, neurocognitive deficits may contribute to poor sleep habits by increasing environmental demands restricting sleep (e.g., requiring more time to complete homework may result in later bedtimes). Alternatively, abnormalities in biological systems (e.g., neural circuity) may underlie both sleep and neurocognitive deficits in ADHD (Owens et al., 2013).
Sleep Disturbances, Psychosocial Stress, and Emotional/Behavioral Outcomes
Sleep disturbance during adolescence is regularly associated with broader functional impairment, including poorer academic and social performance (Shochat et al., 2014). For example, later bedtimes and reduced duration are associated with lower grades, decreased concentration during lectures, dozing in class, and lower social competence (Acebo & Carskadon, 2002; Giannotti & Cortesi, 2002). Disrupted sleep may also result in increased risky behaviors (Shochat et al., 2014), such as more frequent use of legal and illegal substances (e.g., caffeine, cigarettes, alcohol, cannabis; Pasch, Latimer, Cance, Moe, & Lytle, 2012) and unsafe driving behaviors (Carskadon, 2002b). Importantly, these relationships between poor sleep and impaired functioning among typically developing adolescents, particularly in the academic arena, may be mediated by the adverse impact of sleep loss on neurocognition and learning (Carskadon, 2011). Sleep disturbances are also associated with worsened emotional and behavioral outcomes for typically developing adolescents, including increased stress (Sadeh & Gruber, 2002), interference with the development of coping skills, increased anxiety and depression, and poorer self-esteem (Acebo & Carskadon, 2002; Giannotti & Cortesi, 2002; Shochat et al., 2014).
Although increased stress is typical in adolescence, largely due to the pressure of enhanced environmental demands, the association between poor sleep and stress is likely exacerbated in adolescents with ADHD. Indeed, because executive functioning problems are associated with increases in social and academic impairment among ADHD youth (Langberg et al., 2013; Marshall, Evans, Eiraldi, Becker, & Power, 2014), increased difficulties in these areas as a result of sleep loss may contribute to even greater stress and poorer mental health outcomes in teens with ADHD than for healthy peers. This hypothesis is supported by the literature reviewed above, which revealed relationships between sleep and circadian rhythm problems, greater neurocognitive impairment (Fisher et al., 2014; Prehn-Kristensen et al., 2011), and greater severity of both core ADHD symptoms (Chiang et al., 2010; Gau & Chiang, 2009) and depression and anxiety (Mick et al., 2000; Stein et al., 2002) in adolescents with ADHD. Again, it is important to note that it is not yet possible to draw causal inferences. Sleep difficulties may result in increased stress and worsened clinical outcomes or vice versa, or perhaps more likely, this relationship may be bidirectional. Future research is needed to clarify these relationships.
Summary
ADHD is characterized by neuromaturational delay, neurocognitive deficits, and psychosocial stress, and these factors likely interact to result in functional impairment for affected youth. Evidence from typically developing adolescents has shown that unhealthy sleep is associated with similar brain-based and functional deficits, suggesting a potential role of sleep disturbances in exacerbating these processes in ADHD. The reviewed literature suggests greater sleep disturbances in ADHD adolescents compared to healthy peers and provides preliminary evidence for associations between sleep and neurocognitive and psychiatric health in this population. However, it is notable that sleep disturbances are not universal in ADHD, and the mechanisms and extent to which disrupted sleep and circadian patterns contribute to heterogeneous clinical presentations and symptom trajectories are currently unclear.
Conclusions and Agenda to Guide Future Research
Adolescents with ADHD endorse greater sleep disturbance than typically developing peers. Although puberty is characterized by changes in brain organization, sleep patterns, and environmental demands for all youth, the adverse impact of sleep disturbances on neurocognitive and psychosocial functioning may be particularly pronounced among ADHD teens, who already experience deficits in these areas. While prevalent, sleep disturbances do not affect all youth with ADHD, and it is currently unclear which adolescents are at risk for sleep difficulties and the mechanisms by which disrupted sleep and circadian rhythms may contribute to symptom and functional heterogeneity during puberty and over time as teens transition into adulthood. Future research should clarify specific sleep deficits occurring during adolescence for youth with ADHD, mechanisms by which abnormal sleep may contribute to worsened clinical presentations and prognosis, and potential tools for early identification, prevention, and intervention to target sleep problems as well as core ADHD symptoms in this population. Based on review of existing literature, below are presented 5 priority areas to help guide future research in this area.
Question 1: Does objectively-measured sleep behavior, sleep physiology, and circadian functioning differ between ADHD youth and healthy controls during the adolescent period?
Despite ample research to support the presence of sleep disturbances in school-aged children and adults with ADHD, relatively few studies have characterized sleep and circadian rhythms of affected adolescents. The studies that have addressed this question have relied primarily on subjective measures, which are subject to cognitive biases and memory recall issues. In addition, actigraphy and PSG studies with ADHD teens to date have been limited by small or non-representative samples. Future studies should expand on prior work using gold-standard objective measures (PSG and actigraphy) to characterize the sleep behavior, physiology, and circadian rhythms of adolescents with ADHD. Given normative changes in sleep and circadian rhythms during adolescence, studies using these designs have the potential to clarify how sleep problems manifest in ADHD within a developmental context.
Both PSG and actigraphy provide important information about TST, SOL, efficiency, nocturnal awakenings, sleep movements, and circadian rhythms. In addition, due to its EEG technology, PSG may shed light on potential abnormalities in sleep physiology, such as REM, NREM/SWS, sleep spindles (i.e., rapid bursts of rhythmic brain wave activity during stage 2 NREM), and sleep EEG frequency content. Importantly, PSG has also been critical to identifying phenotypes that differ in etiology, severity, adverse health outcomes, and treatment response among adults with insomnia. Specifically, evidence suggests that adults with insomnia with PSG-measured TST less than 6 hours are at significant risk for hypertension (Bathgate, Edinger, Wyatt, & Krystal, 2016) and mortality (Sivertsen et al., 2014; Vgontzas et al., 2010), while this is not the case for insomnia patients with self-reported TST under 6 hours (Bathgate et al., 2016) or those with PSG-measured TST greater than or equal to 6 hours (Vgontzas & Fernandez-Mendoza, 2013). Further, this shortened PSG-measured TST phenotype is less responsive to cognitive behavioral therapy for insomnia (CBT-I) compared with longer sleeping adults with insomnia (Bathgate, Edinger, & Krystal, under review), suggesting that shortened PSG-measured TST may represent the most severe insomnia phenotype (Vgontzas, Fernandez-Mendoza, Liao, & Bixler, 2013).
PSG studies may be particularly beneficial for clarifying sleep abnormalities in ADHD. Given the normative developmental changes in SWS typically observed in teens, investigation of EEG variables such as delta power and delta slope overnight (i.e., physiological markers of homeostatic sleep drive; Merica, Blois, & Gaillard, 1998; Perlis et al., 2001) may be particularly vital for elucidating divergent developmental trajectories of sleep in adolescents with ADHD compared to healthy peers. Sleep spindle activity may represent another area for future research in ADHD, as sleep spindle deficits are associated with reduced neurocognitive capacity in healthy children (Chatburn et al., 2013; Hoedlmoser et al., 2014) and adults (Lustenberger, Maric, Durr, Achermann, & Huber, 2012; Schabus et al., 2006). Finally, the adult insomnia literature suggests that PSG studies may be particularly well suited to identifying sleep-related phenotypes among adolescents with ADHD, and future studies should examine this possibility.
Question 2: What is the relationship between sleep and neurocognitive performance among adolescents with ADHD? Does sleep disturbance represent a biomarker for subtypes of ADHD?
The substantial overlap in neurocognitive deficits seen in ADHD and sleep-disordered children is well-documented, and sleep-related impairments in neurocognition appear to be exacerbated in ADHD. In a recent review, Owens and colleagues (2013) highlighted neurocognitive features common to ADHD and sleep disturbances and suggested that investigation of this overlap may elucidate biological mechanisms contributing to difficulties with focus, vigilance, and arousal/alertness. It is possible that specific disruptions in neural circuitry and neurotransmitters supporting sleep may contribute to the severity of core symptoms and neurocognitive deficits in individuals with ADHD, but this has yet to be examined. As highlighted by these researchers, future studies investigating the following areas may be particularly informative for clarifying biological mechanisms underlying ADHD: 1) alterations in neural circuitry and neurotransmitter function supporting sleep and wakefulness in ADHD, 2) phenotypic similarities between neurocognitive, emotional, and behavioral manifestations of ADHD and sleep loss, and 3) interrelationships between sleep/circadian disturbance and genetic/environmental factors in ADHD (e.g., genetic vulnerability to sleep dysregulation, differential response to external sleep-wake signals).
These research avenues may be particularly relevant to understanding clinical heterogeneity and identifying phenotypic subgroups within adolescent ADHD. Neurocognitive deficits in children and teens with ADHD are not universal (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005) and researchers have suggested that impaired executive functioning may represent an endophenotype for ADHD subgroups (Bidwell, Willcutt, Defries, & Pennington, 2007; Gau & Shang, 2010). Similarly, poor sleep is common to many, but not all, children with ADHD (Corkum, Davidson, & Macpherson, 2011). As noted above, biological mechanisms contributing to sleep difficulty and impaired neurocognition in some individuals with ADHD but not others are poorly understood. Sleep disturbance may represent one biological process contributing to neurocognitive and functional impairments observed in phenotypic ADHD subgroups. Future studies should examine associations between disrupted sleep and variable clinical and cognitive outcomes in ADHD teens to evaluate 1) whether sleep disturbances during adolescence characterize a phenotypic subgroup within ADHD, and 2) genetic and environmental influences conferring risk for sleep disturbances and related neurocognitive and academic/social deficits among adolescents with ADHD.
Question 3: Do adolescents with ADHD exhibit delays in normative sleep development trajectories (e.g., SWS declines)? If so, are these lags associated with neuromaturational delays in ADHD? Do hormonal abnormalities contribute to anomalies in sleep, neuromaturation, and their interaction in ADHD?
Normative reductions in SWS occur during adolescence and appear to reflect developmental brain changes (synaptic pruning; Feinberg & Campbell, 2010). A preliminary study in children with ADHD (age 11) suggested that SWS declines may be delayed (Ringli et al., 2013); however, this study was limited by small sample size and cross-sectional design. Future longitudinal studies should examine SWS developmental trajectories across adolescence and associations with neuromaturational processes in teens with ADHD. These studies may clarify whether adolescents with ADHD exhibit abnormalities in SWS developmental trajectory, and if so, whether this disruption may 1) result from neuromaturational delays characteristic of ADHD, 2) actively interfere with brain development in ADHD, or 3) contribute to a bidirectional cycle in which SWS and neuromaturational delays interact to exacerbate ADHD core symptoms. As developmental trajectories of sleep and brain functioning may be more closely related to pubertal development rather than age, future studies should include a measure of pubertal status at each time point. If SWS abnormalities are found to contribute to or interact with delays in brain development across adolescence for individuals with ADHD, this specific sleep disturbance may represent a target for early identification and prevention strategies in this population (see Question 5 below).
Relatedly, future longitudinal studies of sleep and circadian functioning and neuromaturation in adolescent ADHD would be strengthened through inclusion of hormonal measures. Specifically, developmental shifts in hormonal secretions, such as melatonin and cortisol, have been linked to both shifts in sleep/circadian functioning (e.g., Carskadon et al., 2004; Rea, Figueiro, Sharkey, & Carskadon, 2012) and brain development during puberty (Giedd et al., 2006). Both melatonin (Novakova et al., 2011) and cortisol (Isaksson, Nilsson, Nyberg, Hogmark, & Lindblad, 2012) abnormalities are observed in children and teens with ADHD. Elucidation of abnormal patterns of hormonal secretion across development in ADHD adolescents would significantly enhance our understanding of the interplay between sleep functioning, neuromaturation, and symptom manifestation in this population.
Question 4: Are sleep disturbances predictive of persistence of ADHD into adulthood?
ADHD persists into adulthood for some, but not all, individuals and continues to impair functioning (Faraone et al., 2006; Langley et al., 2010). At present, mechanisms contributing to disparate symptom trajectories and the persistence or remission of ADHD over the lifespan are poorly understood. Although factors such as severity of childhood ADHD, childhood treatment, and neurocognition have preliminary support as predictors (Biederman, Petty, Clarke, Lomedico, & Faraone, 2011; Kessler et al., 2005; van Lieshout, Luman, Buitelaar, Rommelse, & Oosterlaan, 2013), causal mechanisms underlying these associations are unclear. Identifying those at risk for persistent ADHD is a high priority, as it may allow for targeted prevention and intervention efforts and more positive outcomes for affected youth.
Sleep disturbance in adolescence is one biological process than may contribute to persistence of ADHD. First, developmental changes in sleep are characteristic of adolescence (Colrain & Baker, 2011; Darchia & Cervena, 2014), a time in which developmental trajectories of ADHD symptoms also begin to diverge (Halperin & Schulz, 2006). Second, among typically developing teens, sleep dysfunction is associated with neuromaturational abnormalities, neurocognitive deficits (e.g., attention, vigilance), and difficulty meeting increased environmental demands (Carskadon, 2011). Although not yet fully examined (see Question 2), this process may be amplified in at least a subset of ADHD youth (e.g., those with sleep deficits), who are already experiencing impairments in these areas. It is plausible that disrupted sleep occurring in a subset of adolescents with ADHD may lead to a vicious cycle, in which syndrome-related neural developmental, cognitive, and psychosocial impairments are compounded by poor sleep in adolescence to result in worsened clinical trajectories and maintenance of core symptoms into adulthood.
Question 5: If sleep and circadian functioning is disrupted in ADHD adolescents compared to healthy peers, do behavioral and/or pharmacological treatments targeting sleep reduce 1) sleep disturbances and/or 2) core symptoms of ADHD in this population?
Clarification of sleep problems in adolescent ADHD may be particularly relevant to developing effective behavioral and/or pharmacological interventions. Several interventions have shown to be effective in targeting sleep deficits and disorders (e.g., insomnia) in adolescents, including sleep hygiene, behavioral therapies, and pharmacology (Nunes & Bruni, 2015). Depending on the specific sleep deficits identified in adolescents with ADHD, these treatments may be adapted for ADHD youth and serve as either stand-alone or supplemental therapies implemented in addition to standard care for ADHD. For example, sleep hygiene strategies focus on implementing consistent bed- and wake times/routines, reducing caffeine intake, and limiting activating activities such as electronic use before bed (Mindell & Owens, 2015). As ADHD in adolescence is associated with inconsistent routines, high caffeine intake (Martin et al., 2008), and overuse of electronic media (Weiss, Baer, Allan, Saran, & Schibuk, 2011), these techniques may be particularly potent in reducing sleep problems in teens with ADHD. Indeed, a recent randomized control trial with school-aged children with ADHD (5–12 years old) found that enhancing sleep hygiene and implementing behavioral strategies (behavioral sleep management, bedtime fading) improved sleep behaviors, reduced ADHD symptom severity, and improved social and family functioning, classroom behavior, quality of life, and working memory performance (Hiscock et al., 2015).
Regarding behavioral therapy, CBT-I increases sleep efficiency and TST, reduces SOL and nocturnal awakenings, and results in a more rapid decline in delta power overnight in adults (Krystal & Edinger, 2010; Trauer, Qian, Doyle, Rajaratnam, & Cunnington, 2015). With adolescents, emerging literature has suggested that CBT-I improves TST, SOL, nocturnal awakenings, and efficiency (de Bruin, Oort, Bogels, & Meijer, 2014) as well as psychiatric outcomes (Clarke et al., 2015). If adolescents with ADHD evidence deficits in these specific areas of sleep, CBT-I may be effective for reducing both sleep disturbances and ADHD symptoms. Finally, pharmacological treatments used in clinical settings target sleep disorders and disturbances in children and adolescents (with or without neurodevelopmental disorders such as ADHD), including antihistaminic agents, alpha-2 adrenergic agonists, melatonin, iron supplements, benzodiazepines, and tricyclic anti-depressants (Barrett, Tracy, & Giaroli, 2013; Bruni et al., 2015; Hollway & Aman, 2011). These agents may have utility in reducing severe sleep problems in teens with ADHD; however, widespread use in pediatric populations is not recommended due to lack of empirical investigations and side effects (Nunes & Bruni, 2015).
Future investigations may examine sleep-related interventions in reducing sleep disturbances in adolescents with ADHD. Given the overlap between attentional and neurocognitive deficits observed in sleep disorders and ADHD, future studies may also investigate the benefit of these therapies in targeting core ADHD symptoms. Importantly, intervention studies may also elucidate cause and effect relationships between sleep problems, ADHD symptoms, and neurocognition. If sleep-specific interventions improve ADHD symptoms and neurocognition in adolescents in the absence of other ADHD-specific treatments, this would support a causal role of sleep disturbances in worsened clinical and cognitive outcomes. Finally, stimulant medications appear to improve sleep in adults with ADHD. The impact of stimulant medication on sleep in adolescents is currently unclear and may represent a target for future research.
Highlights.
Sleep disturbances in adolescent-specific samples with ADHD symptoms are reviewed.
Self-reported sleep disturbances are associated with ADHD symptoms in adolescence.
Mechanisms by which sleep may impact ADHD symptoms and prognosis are outlined.
A guide to inform future research of sleep in adolescents with ADHD is proposed.
Appendix A
Figure A1.
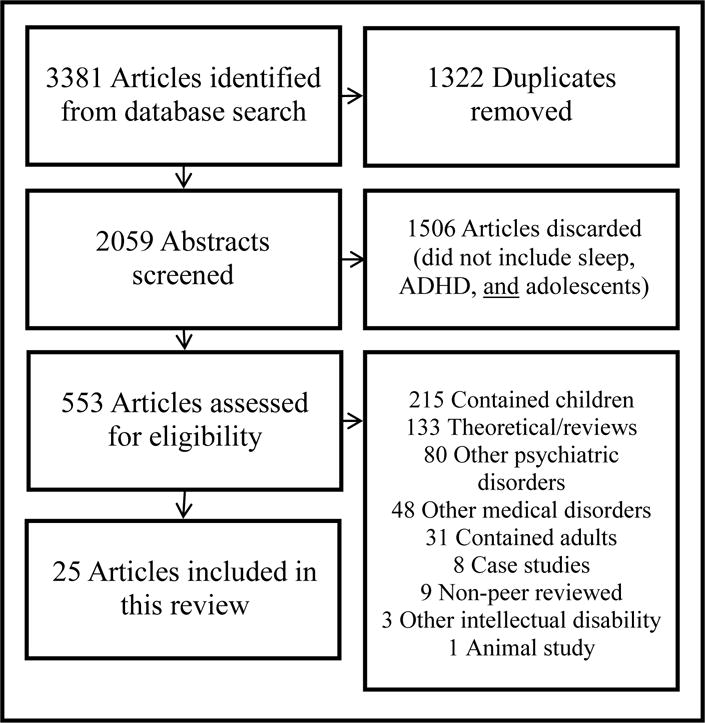
Flowchart of selection process
Appendix B
Table B1.
Studies examining sleep and ADHD in adolescent-specific samples
| Study | Study Type | Timeframe | Sample Size | Sleep Measurement | ADHD Dx (Y/N) | Age Range |
|---|---|---|---|---|---|---|
| Associations of Sleep Disturbances and ADHD Symptoms in the Population | ||||||
|
| ||||||
| Hysing et al., 2015 | Population-Based | Cross-sectional | 9,846 | Self-Report | N | 16–19 |
| Sivertsen et al., 2015 | Population-Based | Cross-sectional | 9,338 (306 Delayed Sleep Phase) | Self-Report | N | 16–19 |
| Turkdogan et al., 2011 | Population-Based | Cross-sectional | 4,346 (119 RLS) | Self-Report; Clinical Interview | N | 10–19 |
| Lam & Yang, 2008 | Population-Based | Cross-sectional | 1,429 | Self-Report | N | 13–17 |
| Smaldone et al., 2007 | Population-Based | Cross-sectional | 34,120 | Parent-Report | N | 12–17 |
| Johnson & Roth, 2006 | Population-Based | Cross-sectional | 1,014 | Sleep Wake Activity Inventory (Self-Report); Clinical Interview | Y | 13–16 |
|
| ||||||
| Prevalence of Sleep Disturbances in ADHD Samples | ||||||
|
| ||||||
| Fisher et al., 2014 | Chart Review | Cross-sectional | 218 | Physical Complaints Checklist (Self-Report) | Y | 15–17 |
| Brook & Boaz, 2005 | Descriptive | Cross-sectional | 308 Comorbid ADHD/LD | Self-Report | Y | 12–18 |
|
| ||||||
| Subjective Sleep Disturbances in ADHD-Diagnosed Teens compared to Healthy Controls | ||||||
|
| ||||||
| Weinstein et al., 2015 | Empirical | Cross-sectional | 50 ADHD; 50 HC (Males) | Self-Report | Y | 13–15 |
| Lufi & Tzischinsky, 2014 | Empirical | Cross-sectional | 30 ADHD (90% Male); 28 HC (50% Male) | Sleep Schedules Questionnaire (Self-Report) | Y (Past diagnosis) | 13–17 |
| Yang et al., 2011 | Empirical | Longitudinal | 136 ADHD (85% Male); 136 HC (85% Male); 47 Aff. Sib (58% Male); 89 Unaff. Sib (37% Male) | KSADS | Y | Not reported; mean = 12.8 (ADHD) |
| Gau, Lin, et al., 2010 | Empirical | Longitudinal | 46 Persistent ADHD/47 Subthreshold ADHD (83% Male); 93 HC (gender matched) | K-SADS | Y | 11–16 |
| Gau, Ni, et al., 2010 | Empirical | Longitudinal | 186 Persistent ADHD/110 Subthreshold ADHD (86% Male); 185 HC (72% Male) | K-SADS | Y | 11–17 |
| Chiang et al., 2010 | Empirical | Longitudinal | 325 (81.5% Male); 257 HC (60% Male) | K-SADS; Sleep Disturbance Questionnaire; Sleep/Nap Schedule (Self-Report) | Y | 10–17 |
| Gau & Chiang, 2009 | Empirical | Longitudinal | 145 Persistent ADHD/136 Subthreshold ADHD (86% Male); 185 HC (73% Male) | K-SADS; Sleep Disturbance Questionnaire; Sleep/Nap Schedule (Self-Report) | Y | 11–17 |
| Mick et al., 2000 | Empirical | Longitudinal | 122 ADHD; 105 HC (Males) | Children Sleep Behavior Scale (Parent-Report); DICA | Y (Past Diagnosis) | 12–17 |
|
| ||||||
| Objective Sleep Disturbances in ADHD-Diagnosed Teens compared to Healthy Controls | ||||||
|
| ||||||
| Prehn-Kristensen et al., 2011 | Empirical | Cross-sectional | 12 ADHD; 12 HC (Males) | Polysomnography | Y | 10–16 |
| Mullin et al., 2011 | Empirical | Cross-sectional | 14 ADHD; 13 BD; 17 HC | Actigraphy; Sleep Diary | Y | 11–17 |
| Moore et al, 2011 | Community Cohort | Cross-sectional | 247 | Actigraphy; Sleep Diary | Y (Per Parent Report) | 13–16 |
|
| ||||||
| Associations between Sleep Disturbances and Comorbidity, Neurocognition, and/or Daytime Functioning in ADHD-Diagnosed Adolescent Samples | ||||||
|
| ||||||
| Weinstein et al., 2015 | Empirical | Cross-sectional | 50 ADHD; 50 HC (Males) | Self-Report | Y | 13–15 |
| Lufi & Tzischinsky, 2014 | Empirical | Cross-sectional | 30 ADHD (90%Male); 28 HC (50%Male) | Sleep Schedules Questionnaire (Self-Report) | Y (Past Diagnosis) | 13–17 |
| Fisher et al., 2014 | Chart Review | Cross-sectional | 218 | Physical Complaints Checklist (Self-Report) | Y | 15–17 |
| Langberg et al., 2013 | Empirical | Cross-sectional | 100 | Pediatric Daytime Sleepiness Scale (Self-Report) | Y | 10–14 |
| Prehn-Kristensen et al., 2011 | Empirical | Cross-sectional | 12 ADHD; 12 HC (Males) | Polysomnography | Y | 10–16 |
| Prehn-Kristensen et al., 2014 | Empirical | Cross-sectional | 12 ADHD; 12 HC (Males) | Polysomnography | Y | 10–14 |
| Munz et al., 2015 | Empirical | Cross-sectional | 14 ADHD (Males) | Polysomnography | Y | 10–14 |
| Stein et al., 2002 | Empirical | Cross-sectional | 32 Non-medicated ADHD (15% “Full Blown” ADHD); 35 Medicated ADHD (60% “Full Blown” ADHD); 77 HC (Males) | Mini Sleep Questionnaire (Self-Report) | Y (Past Diagnosis) | 13–16 |
| Mick et al., 2000 | Empirical | Longitudinal | 122 ADHD; 105 HC (Males) | Children Sleep Behavior Scale (Parent-Report); DICA | Y (Past Diagnosis) | 12–17 |
|
| ||||||
| Contribution of Sleep Disturbances to Prognosis in ADHD-Diagnosed Samples | ||||||
|
| ||||||
| Becker et al., 2015 | Empirical | Longitudinal | 81 (75% Male) | Child Behavior Checklist (Parent-Report) | Y | 10–14 |
| Fisher et al., 2014 | Chart Review | Cross-sectional | 218 | Physical Complaints Checklist (Self-Report) | Y | 15–17 |
|
| ||||||
| Impact of Interventions on Sleep with ADHD-Diagnosed Adolescents | ||||||
|
| ||||||
| Becker et al., 2015 | Empirical | Longitudinal | 81 (75% Male) | Child Behavior Checklist (Parent-Report) | Y | 10–14 |
| Santisteban et al., 2014 | Empirical | Cross-sectional | 37 (73% Male) | Actigraphy; Sleep Diary | Y | 10–17 |
| Gau & Chiang, 2009 | Empirical | Longitudinal | 145 Persistent ADHD/136 Subthreshold ADHD (86% Male); 185 HC (73% Male) | K-SADS; Sleep Disturbance Questionnaire; Sleep/Nap Schedule (Self-Report) | Y | 11–17 |
| Stein et al., 2002 | Empirical | Cross-sectional | 32 Non-medicated ADHD (15% “Full Blown” ADHD); 35 Medicated ADHD (60% “Full Blown” ADHD); 77 HC (Males) | Mini Sleep Questionnaire (Self-Report) | Y (Past Diagnosis) | 13–16 |
| Mick et al., 2000 | Empirical | Longitudinal | 122 ADHD; 105 HC (Males) | Children Sleep Behavior Scale (Parent-Report); DICA | Y (Past Diagnosis) | 12–17 |
Abbreviations: ADHD (Attention-Deficit/Hyperactivity Disorder); ODD (Oppositional Defiant Disorder); LD (Specific Learning Disorder); KSADS (Kiddie Schedule for Affective Disorders and Schizophrenia); DICA (Diagnostic Interview for Children and Adolescents); RLS (Restless Leg Syndrome); Aff. Sib (Affected Sibling); Non-aff. Sib (Non-Affected Sibling)
Note: Indentions represent data collected within a research group. Italics denote studies previously referenced in an above section of the table.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations
ADHD: Attention-Deficit/Hyperactivity Disorder (-C = Combined, -I = Inattentive, -H/I = Hyperactive/Impulsive presentations)
HC: Healthy Controls
NREM: Non Rapid Eye Movement Sleep
REM: Rapid Eye Movement Sleep
PLM: Periodic Leg Movements
PSG: Polysomnography
RLS: Restless Leg Syndrome
SDB: Sleep Disordered Breathing
SE: Sleep Efficiency (Percentage of time in bed spent asleep)
SOL: Sleep Onset Latency (Length of time to sleep onset)
SWS: Slow Wave Sleep
TIB: Time in Bed
TST: Total Sleep Time (Duration of sleep period)
References
- Acebo C, Carskadon MA. Influence of irregular sleep patterns on waking behavior. In: Carskadon MA, editor. Adolescent sleep patterns: biological, social, and psychological influences. New York, NY: Cambridge University Press; 2002. pp. 220–235. [Google Scholar]
- Allen R. School-week sleep lag: sleep problems with earlier starting of senior high schools. Sleep Research. 1991;20:198. [Google Scholar]
- Allen R. Social factors associated with the amount of school week sleep lag for seniors in an early starting suburban high school. Sleep Research. 1992;21:114. [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Barrett JR, Tracy DK, Giaroli G. To sleep or not to sleep: a systematic review of the literature of pharmacological treatments of insomnia in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23(10):640–647. doi: 10.1089/cap.2013.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bathgate CJ, Edinger JD, Krystal AD. Does objective short sleep duration predict a poor response to cognitive behavioral therapy for insomnia treatment? doi: 10.1093/sleep/zsw012. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016 doi: 10.5665/sleep.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty MJ, Palaniyappan L, Scerif G, Groom MJ, Liddle EB, Liddle PF, Hollis C. Morphological abnormalities in prefrontal surface area and thalamic volume in attention deficit/hyperactivity disorder. Psychiatry Res. 2015;233(2):225–232. doi: 10.1016/j.pscychresns.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Langberg JM, Byars KC. Advancing a biopsychosocial and contextual model of sleep in adolescence: a review and introduction to the special issue. J Youth Adolesc. 2015;44(2):239–270. doi: 10.1007/s10964-014-0248-y. [DOI] [PubMed] [Google Scholar]
- Becker SP, Langberg JM, Evans SW. Sleep problems predict comorbid externalizing behaviors and depression in young adolescents with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2015;24(8):897–907. doi: 10.1007/s00787-014-0636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, Defries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;62(9):991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Clarke A, Lomedico A, Faraone SV. Predictors of persistent ADHD: an 11-year follow-up study. J Psychiatr Res. 2011;45(2):150–155. doi: 10.1016/j.jpsychires.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioulac S, Chaufton C, Taillard J, Claret A, Sagaspe P, Fabrigoule C, Philip P. Excessive daytime sleepiness in adult patients with ADHD as measured by the Maintenance of Wakefulness Test, an electrophysiologic measure. J Clin Psychiatry. 2015;76(7):943–948. doi: 10.4088/JCP.14m09087. [DOI] [PubMed] [Google Scholar]
- Brook U, Boaz M. Attention deficit and hyperactivity disorder (ADHD) and learning disabilities (LD): adolescents perspective. Patient Educ Couns. 2005;58(2):187–191. doi: 10.1016/j.pec.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Bruni O, Alonso-Alconada D, Besag F, Biran V, Braam W, Cortese S, Curatolo P. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122–133. doi: 10.1016/j.ejpn.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Adolescent sleepiness: Increased risk in a high-risk population. Alcohol, Drugs & Driving. 1990;5/6:317–328. [Google Scholar]
- Carskadon MA. Factors influencing sleep patterns of adolescents. In: Carskadon MA, editor. Adolescent sleep patterns: biological, social, and psychological influences. New York, NY: Cambridge University Press; 2002a. pp. 4–26. [Google Scholar]
- Carskadon MA. Risks of driving while sleepy in adolescents and young adults. In: Carskadon MA, editor. Adolescent sleep patterns: biological, social, and psychological influences. New York, NY: Cambridge University Press; 2002b. pp. 148–158. [Google Scholar]
- Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3):637–647. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12(3):278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21(8):871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Chatburn A, Coussens S, Lushington K, Kennedy D, Baumert M, Kohler M. Sleep spindle activity and cognitive performance in healthy children. Sleep. 2013;36(2):237–243. doi: 10.5665/sleep.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatoor I, Wells KC, Conners CK, Seidel WT, Shaw D. The effects of nocturnally administered stimulant medication on EEG sleep and behavior in hyperactive children. J Am Acad Child Psychiatry. 1983;22(4):337–342. doi: 10.1016/s0002-7138(09)60668-3. [DOI] [PubMed] [Google Scholar]
- Chiang HL, Gau SS, Ni HC, Chiu YN, Shang CY, Wu YY, Soong WT. Association between symptoms and subtypes of attention-deficit hyperactivity disorder and sleep problems/disorders. J Sleep Res. 2010;19(4):535–545. doi: 10.1111/j.1365-2869.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- Clarke G, McGlinchey EL, Hein K, Gullion CM, Dickerson JF, Leo MC, Harvey AG. Cognitive-behavioral treatment of insomnia and depression in adolescents: A pilot randomized trial. Behav Res Ther. 2015;69:111–118. doi: 10.1016/j.brat.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, Squires L. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23(10):1208–1218. doi: 10.1016/j.euroneuro.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychol Rev. 2011;21(1):5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum P, Davidson F, Macpherson M. A framework for the assessment and treatment of sleep problems in children with attention-deficit/hyperactivity disorder. Pediatr Clin North Am. 2011;58(3):667–683. doi: 10.1016/j.pcl.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Corkum P, Panton R, Ironside S, Macpherson M, Williams T. Acute impact of immediate release methylphenidate administered three times a day on sleep in children with attention-deficit/hyperactivity disorder. J Pediatr Psychol. 2008;33(4):368–379. doi: 10.1093/jpepsy/jsm106. [DOI] [PubMed] [Google Scholar]
- Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(6):637–646. doi: 10.1097/00004583-199806000-00014. [DOI] [PubMed] [Google Scholar]
- Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(9):894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009;48(9):894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- Cortese S, Imperati D, Zhou J, Proal E, Klein RG, Mannuzza S, Castellanos FX. White matter alterations at 33-year follow-up in adults with childhood attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(8):591–598. doi: 10.1016/j.biopsych.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett LJ, Crouter AC. Pathways through adolescence: Individual development in relation to social contexts. New York, NY: Psychology Press; 2014. [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48(2):194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: development and psychopathology. Development and psychopathology. 1996;8(1):3–27. [Google Scholar]
- Darchia N, Cervena K. The journey through the world of adolescent sleep. Rev Neurosci. 2014;25(4):585–604. doi: 10.1515/revneuro-2013-0065. [DOI] [PubMed] [Google Scholar]
- de Bruin EJ, Oort FJ, Bogels SM, Meijer AM. Efficacy of internet and group-administered cognitive behavioral therapy for insomnia in adolescents: a pilot study. Behav Sleep Med. 2014;12(3):235–254. doi: 10.1080/15402002.2013.784703. [DOI] [PubMed] [Google Scholar]
- Dijk DJ. Risk-taking and other effects of sleep loss on brain function and behaviour. J Sleep Res. 2011;20(3):375–376. doi: 10.1111/j.1365-2869.2011.00941.x. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72(1):56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Feinberg I, de Bie E, Davis NM, Campbell IG. Topographic differences in the adolescent maturation of the slow wave EEG during NREM sleep. Sleep. 2011;34(3):325–333. doi: 10.1093/sleep/34.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1724–1729. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN. Insomnia and its impact on physical and mental health. Curr Psychiatry Rep. 2013;15(12):418. doi: 10.1007/s11920-013-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira-Cornwell MC, Squires L. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(4):395–405. doi: 10.1016/j.jaac.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Fisher BC, Garges DM, Yoon SY, Maguire K, Zipay D, Gambino M, Shapiro CM. Sex differences and the interaction of age and sleep issues in neuropsychological testing performance across the lifespan in an ADD/ADHD sample from the years 1989 to 2009. Psychol Rep. 2014;114(2):404–438. doi: 10.2466/15.10.PR0.114k23w0. [DOI] [PubMed] [Google Scholar]
- Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30(1):275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Gau SS, Chiang HL. Sleep problems and disorders among adolescents with persistent and subthreshold attention-deficit/hyperactivity disorders. Sleep. 2009;32(5):671–679. doi: 10.1093/sleep/32.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS, Lin YJ, Cheng AT, Chiu YN, Tsai WC, Soong WT. Psychopathology and symptom remission at adolescence among children with attention-deficit-hyperactivity disorder. Aust N Z J Psychiatry. 2010;44(4):323–332. doi: 10.3109/00048670903487233. [DOI] [PubMed] [Google Scholar]
- Gau SS, Ni HC, Shang CY, Soong WT, Wu YY, Lin LY, Chiu YN. Psychiatric comorbidity among children and adolescents with and without persistent attention-deficit hyperactivity disorder. Aust N Z J Psychiatry. 2010;44(2):135–143. doi: 10.3109/00048670903282733. [DOI] [PubMed] [Google Scholar]
- Gau SS, Shang CY. Executive functions as endophenotypes in ADHD: evidence from the Cambridge Neuropsychological Test Battery (CANTAB) J Child Psychol Psychiatry. 2010;51(7):838–849. doi: 10.1111/j.1469-7610.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J Sleep Res. 2001;10(3):165–172. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F. Sleep patterns and daytime function in adolescence: an epidemiological survey of an Italian high school student sample. In: Carskadon MA, editor. Adolescent sleep patterns: biological, social, and psychological influences. New York, NY: Cambridge University Press; 2002. pp. 132–147. [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Chrousos GP. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Stockman M, Weddle C, Liverpool M, Alexander-Bloch A, Wallace GL, Lenroot RK. Anatomic magnetic resonance imaging of the developing child and adolescent brain and effects of genetic variation. Neuropsychol Rev. 2010;20(4):349–361. doi: 10.1007/s11065-010-9151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan N, Shahar E, Ravid S, Pillar G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep. 2004;27(2):261–266. doi: 10.1093/sleep/27.2.261. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Heidbreder R. ADHD symptomatology is best conceptualized as a spectrum: a dimensional versus unitary approach to diagnosis. Atten Defic Hyperact Disord. 2015;7(4):249–269. doi: 10.1007/s12402-015-0171-4. [DOI] [PubMed] [Google Scholar]
- Herman JH. Attention Deficit/Hyperactivity Disorder and Sleep in Children. Sleep Med Clin. 2015;10(2):143–149. doi: 10.1016/j.jsmc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Hiscock H, Sciberras E, Mensah F, Gerner B, Efron D, Khano S, Oberklaid F. Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: randomised controlled trial. BMJ. 2015;350:h68. doi: 10.1136/bmj.h68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkins P, Setyawan J, Mitra D, Davis K, Quintero J, Fridman M, Harpin V. Management of ADHD in children across Europe: patient demographics, physician characteristics and treatment patterns. Eur J Pediatr. 2013;172(7):895–906. doi: 10.1007/s00431-013-1969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoedlmoser K, Heib DP, Roell J, Peigneux P, Sadeh A, Gruber G, Schabus M. Slow sleep spindle activity, declarative memory, and general cognitive abilities in children. Sleep. 2014;37(9):1501–1512. doi: 10.5665/sleep.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollway JA, Aman MG. Pharmacological treatment of sleep disturbance in developmental disabilities: a review of the literature. Res Dev Disabil. 2011;32(3):939–962. doi: 10.1016/j.ridd.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Hvolby A. Associations of sleep disturbance with ADHD: implications for treatment. Atten Defic Hyperact Disord. 2015;7(1):1–18. doi: 10.1007/s12402-014-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysing M, Lundervold AJ, Posserud MB, Sivertsen B. Association Between Sleep Problems and Symptoms of Attention Deficit Hyperactivity Disorder in Adolescence: Results From a Large Population-Based Study. Behav Sleep Med. 2015:1–15. doi: 10.1080/15402002.2015.1048448. [DOI] [PubMed] [Google Scholar]
- Imeraj L, Sonuga-Barke E, Antrop I, Roeyers H, Wiersema R, Bal S, Deboutte D. Altered circadian profiles in attention-deficit/hyperactivity disorder: an integrative review and theoretical framework for future studies. Neurosci Biobehav Rev. 2012;36(8):1897–1919. doi: 10.1016/j.neubiorev.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Ironside S, Davidson F, Corkum P. Circadian motor activity affected by stimulant medication in children with attention-deficit/hyperactivity disorder. J Sleep Res. 2010;19(4):546–551. doi: 10.1111/j.1365-2869.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- Isaksson J, Nilsson KW, Nyberg F, Hogmark A, Lindblad F. Cortisol levels in children with attention-deficit/hyperactivity disorder. J Psychiatr Res. 2012;46(11):1398–1405. doi: 10.1016/j.jpsychires.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, Peterson BS. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167(4):397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28(11):1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27(4):774–783. [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Normal human sleep at different ages: infants to adolescents. SRS basics of sleep guide. 2005:11–19. [Google Scholar]
- Jenni OG, van Reen E, Carskadon MA. Regional differences of the sleep electroencephalogram in adolescents. J Sleep Res. 2005;14(2):141–147. doi: 10.1111/j.1365-2869.2005.00449.x. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roth T. An epidemiologic study of sleep-disordered breathing symptoms among adolescents. Sleep. 2006;29(9):1135–1142. doi: 10.1093/sleep/29.9.1135. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV, Zaslavsky AM. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the national comorbidity survey replication. Biol Psychiatry. 2005;57(11):1442–1451. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell KM, Van Dyk TR, Lundahl A, Nelson TD. Stimulant Medications and Sleep for Youth With ADHD: A Meta-analysis. Pediatrics. 2015;136(6):1144–1153. doi: 10.1542/peds.2015-1708. [DOI] [PubMed] [Google Scholar]
- Kirov R, Banaschewski T, Uebel H, Kinkelbur J, Rothenberger A. REM-sleep alterations in children with co-existence of tic disorders and attention-deficit/hyperactivity disorder: impact of hypermotor symptoms. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):45–50. doi: 10.1007/s00787-007-1006-4. [DOI] [PubMed] [Google Scholar]
- Kirov R, Kinkelbur J, Heipke S, Kostanecka-Endress T, Westhoff M, Cohrs S, Rothenberger A. Is there a specific polysomnographic sleep pattern in children with attention deficit/hyperactivity disorder? J Sleep Res. 2004;13(1):87–93. doi: 10.1111/j.1365-2869.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD. Sleep EEG predictors and correlates of the response to cognitive behavioral therapy for insomnia. Sleep. 2010;33(5):669–677. doi: 10.1093/sleep/33.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, Huber R. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33(4):475–480. doi: 10.1093/sleep/33.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. J Sleep Res. 2001;10(1):59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Lam LT, Yang L. Duration of sleep and ADHD tendency among adolescents in China. J Atten Disord. 2008;11(4):437–444. doi: 10.1177/1087054707299403. [DOI] [PubMed] [Google Scholar]
- Langberg JM, Dvorsky MR, Becker SP, Molitor SJ. The impact of daytime sleepiness on the school performance of college students with attention deficit hyperactivity disorder (ADHD): a prospective longitudinal study. J Sleep Res. 2014;23(3):318–325. doi: 10.1111/jsr.12121. [DOI] [PubMed] [Google Scholar]
- Langberg JM, Dvorsky MR, Marshall S, Evans SW. Clinical implications of daytime sleepiness for the academic performance of middle school-aged adolescents with attention deficit hyperactivity disorder. J Sleep Res. 2013;22(5):542–548. doi: 10.1111/jsr.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Fowler T, Ford T, Thapar AK, van den Bree M, Harold G, Thapar A. Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2010;196(3):235–240. doi: 10.1192/bjp.bp.109.066274. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Levitt JG, Loo SK, Ly R, Yee V, O’Neill J, Narr KL. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. J Am Acad Child Adolesc Psychiatry. 2013;52(4):431–440 e434. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecendreux M, Konofal E, Bouvard M, Falissard B, Mouren-Simeoni MC. Sleep and alertness in children with ADHD. J Child Psychol Psychiatry. 2000;41(6):803–812. [PubMed] [Google Scholar]
- Lufi D, Tzischinsky O. The relationships between sensory modulation and sleep among adolescents with ADHD. J Atten Disord. 2014;18(8):646–653. doi: 10.1177/1087054712457036. [DOI] [PubMed] [Google Scholar]
- Lustenberger C, Maric A, Durr R, Achermann P, Huber R. Triangular relationship between sleep spindle activity, general cognitive ability and the efficiency of declarative learning. PLoS One. 2012;7(11):e49561. doi: 10.1371/journal.pone.0049561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Evans SW, Eiraldi RB, Becker SP, Power TJ. Social and academic impairment in youth with ADHD, predominately inattentive type and sluggish cognitive tempo. J Abnorm Child Psychol. 2014;42(1):77–90. doi: 10.1007/s10802-013-9758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Cook C, Woodring JH, Burkhardt G, Guenthner G, Omar HA, Kelly TH. Caffeine use: association with nicotine use, aggression, and other psychopathology in psychiatric and pediatric outpatient adolescents. Scientific World Journal. 2008;8:512–516. doi: 10.1100/tsw.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamura M, Tochigi M, Usami S, Yonehara H, Fukushima M, Nishida A, Sasaki T. Associations between sleep habits and mental health status and suicidality in a longitudinal survey of monozygotic twin adolescents. J Sleep Res. 2014;23(3):290–294. doi: 10.1111/jsr.12127. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Brimeyer C, Russell K, Avis KT, Biggs S, Reynolds AC, Crabtree VM. The Children’s Report of Sleep Patterns: validity and reliability of the Sleep Hygiene Index and Sleep Disturbance Scale in adolescents. Sleep Med. 2014;15(12):1500–1507. doi: 10.1016/j.sleep.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10(5):1826–1834. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Jetton J, Faraone SV. Sleep disturbances associated with attention deficit hyperactivity disorder: the impact of psychiatric comorbidity and pharmacotherapy. J Child Adolesc Psychopharmacol. 2000;10(3):223–231. doi: 10.1089/10445460050167331. [DOI] [PubMed] [Google Scholar]
- Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. Philadelphia, PA: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- Minges KE, Redeker NS. Delayed school start times and adolescent sleep: A systematic review of the experimental evidence. Sleep Med Rev. 2015;28:82–91. doi: 10.1016/j.smrv.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med. 2011;12(3):239–245. doi: 10.1016/j.sleep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin BC, Harvey AG, Hinshaw SP. A preliminary study of sleep in adolescents with bipolar disorder, ADHD, and non-patient controls. Bipolar Disord. 2011;13(4):425–432. doi: 10.1111/j.1399-5618.2011.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz MT, Prehn-Kristensen A, Thielking F, Molle M, Goder R, Baving L. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front Cell Neurosci. 2015 Aug;9:307. doi: 10.3389/fncel.2015.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, Nigg JT. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(3):283–292. doi: 10.1016/j.jaac.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson JP, Soderstrom M, Karlsson AU, Lekander M, Akerstedt T, Lindroth NE, Axelsson J. Less effective executive functioning after one night’s sleep deprivation. J Sleep Res. 2005;14(1):1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- Novakova M, Paclt I, Ptacek R, Kuzelova H, Hajek I, Sumova A. Salivary melatonin rhythm as a marker of the circadian system in healthy children and those with attention-deficit/hyperactivity disorder. Chronobiol Int. 2011;28(7):630–637. doi: 10.3109/07420528.2011.596983. [DOI] [PubMed] [Google Scholar]
- Nunes ML, Bruni O. Insomnia in childhood and adolescence: clinical aspects, diagnosis, and therapeutic approach. J Pediatr (Rio J) 2015;91(6 Suppl 1):S26–35. doi: 10.1016/j.jped.2015.08.006. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Holbrook CR, Mervis CB, Klaus CJ, Bruner JL, Raffield TJ, Gozal D. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111(3):554–563. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Roberts RE, Zulley J, Smirne S, Priest RG. Prevalence and patterns of problematic sleep among older adolescents. J Am Acad Child Adolesc Psychiatry. 2000;39(12):1549–1556. doi: 10.1097/00004583-200012000-00019. [DOI] [PubMed] [Google Scholar]
- Olds T, Maher C, Blunden S, Matricciani L. Normative data on the sleep habits of Australian children and adolescents. Sleep. 2010;33(10):1381–1388. doi: 10.1093/sleep/33.10.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, Au R, Carskadon M, Millman R, Wolfson A, Braverman PK, Murray PJ. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–932. doi: 10.1542/peds.2014-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, Gruber R, Brown T, Corkum P, Cortese S, O’Brien L, Weiss M. Future research directions in sleep and ADHD: report of a consensus working group. J Atten Disord. 2013;17(7):550–564. doi: 10.1177/1087054712457992. [DOI] [PubMed] [Google Scholar]
- Pasch KE, Latimer LA, Cance JD, Moe SG, Lytle LA. Longitudinal bi-directional relationships between sleep and youth substance use. J Youth Adolesc. 2012;41(9):1184–1196. doi: 10.1007/s10964-012-9784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, Zhou XJ. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65(7):586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Hulshoff Pol HE. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34(3):332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Kehr EL, Smith MT, Andrews PJ, Orff H, Giles DE. Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J Sleep Res. 2001;10(2):93–104. doi: 10.1046/j.1365-2869.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Goder R, Fischer J, Wilhelm I, Seeck-Hirschner M, Aldenhoff J, Baving L. Reduced sleep-associated consolidation of declarative memory in attention-deficit/hyperactivity disorder. Sleep Med. 2011;12(7):672–679. doi: 10.1016/j.sleep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Munz M, Goder R, Wilhelm I, Korr K, Vahl W, Baving L. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014;7(6):793–799. doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, Castellanos FX. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry. 2011;68(11):1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MS, Figueiro MG, Sharkey KM, Carskadon MA. Relationship of morning cortisol to circadian phase and rising time in young adults with delayed sleep times. Int J Endocrinol. 2012;2012:749460. doi: 10.1155/2012/749460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli M, Souissi S, Kurth S, Brandeis D, Jenni OG, Huber R. Topography of sleep slow wave activity in children with attention-deficit/hyperactivity disorder. Cortex. 2013;49(1):340–347. doi: 10.1016/j.cortex.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Rubia K, Alegria AA, Brinson H. Brain abnormalities in attention-deficit hyperactivity disorder: a review. Rev Neurol. 2014;58(Suppl 1):S3–16. [PubMed] [Google Scholar]
- Russo PM, Bruni O, Lucidi F, Ferri R, Violani C. Sleep habits and circadian preference in Italian children and adolescents. J Sleep Res. 2007;16(2):163–169. doi: 10.1111/j.1365-2869.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R. Stress and sleep in adolescence: A clinical-developmental perspective. In: Carskadon MA, editor. Adolescent sleep patterns: biological, social, and psychological influences. New York, NY: Cambridge University Press; 2002. [Google Scholar]
- Sadeh A, Pergamin L, Bar-Haim Y. Sleep in children with attention-deficit hyperactivity disorder: A meta-analysis of polysomnographic studies. Sleep Medicine Reviews. 2006;10(6):381–398. doi: 10.1016/j.smrv.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Santisteban JA, Stein MA, Bergmame L, Gruber R. Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: a double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder. CNS Drugs. 2014;28(9):825–833. doi: 10.1007/s40263-014-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer AC, Clark CR, Keage HA, Moores KA, Clarke S, Kohn MR, Gordon E. Cognitive and electroencephalographic disturbances in children with attention-deficit/hyperactivity disorder and sleep problems: new insights. Psychiatry Res. 2009;170(2–3):183–191. doi: 10.1016/j.psychres.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Schabus M, Hodlmoser K, Gruber G, Sauter C, Anderer P, Klosch G, Zeitlhofer J. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006;23(7):1738–1746. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Sciberras E, DePetro A, Mensah F, Hiscock H. Association between sleep and working memory in children with ADHD: a cross-sectional study. Sleep Med. 2015;16(10):1192–1197. doi: 10.1016/j.sleep.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Seyrek S, Cop E, Sinir H, Ugurlu M, Senel S. Factors associated with internet addiction: A cross-sectional study among Turkish adolescents. Pediatr Int. 2016 doi: 10.1111/ped.13117. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(8):599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev. 2014;18(1):75–87. doi: 10.1016/j.smrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright HR, Dohnt H. The sleep patterns and well-being of Australian adolescents. J Adolesc. 2013;36(1):103–110. doi: 10.1016/j.adolescence.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Wright H, Lack LC, Dohnt H, Carskadon MA. Time for bed: parent-set bedtimes associated with improved sleep and daytime functioning in adolescents. Sleep. 2011;34(6):797–800. doi: 10.5665/SLEEP.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009;30(9):2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B, Harvey AG, Pallesen S, Hysing M. Mental health problems in adolescents with delayed sleep phase: results from a large population-based study in Norway. J Sleep Res. 2015;24(1):11–18. doi: 10.1111/jsr.12254. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Pallesen S, Glozier N, Bjorvatn B, Salo P, Tell GS, Overland S. Midlife insomnia and subsequent mortality: the Hordaland health study. BMC Public Health. 2014;14:720. doi: 10.1186/1471-2458-14-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007a;119(Suppl 1):S29–37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007b;119(Suppl 1):S29–37. doi: 10.1542/peds.2006-2089F. (SUPPL. 1) [DOI] [PubMed] [Google Scholar]
- Snitselaar MA, Smits MG, van der Heijden KB, Spijker J. Sleep and Circadian Rhythmicity in Adult ADHD and the Effect of Stimulants : A Review of the Current Literature. Atten Disord. 2013 doi: 10.1177/1087054713479663. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stein D, Pat-Horenczyk R, Blank S, Dagan Y, Barak Y, Gumpel TP. Sleep disturbances in adolescents with symptoms of attention-deficit/hyperactivity disorder. J Learn Disabil. 2002;35(3):268–275. doi: 10.1177/002221940203500308. [DOI] [PubMed] [Google Scholar]
- Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509–517. doi: 10.1007/s13311-012-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. Parsing the role of sleep in memory processing. Curr Opin Neurobiol. 2013;23(5):847–853. doi: 10.1016/j.conb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru S, Nottelmann ED, Belmont B, Ronsaville D. Sleep problems in children of affectively ill mothers. J Child Psychol Psychiatry. 1997;38(7):831–841. doi: 10.1111/j.1469-7610.1997.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Sutton EL. Psychiatric disorders and sleep issues. Med Clin North Am. 2014;98(5):1123–1143. doi: 10.1016/j.mcna.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Tarver J, Daley D, Sayal K. Attention-deficit hyperactivity disorder (ADHD): an updated review of the essential facts. Child Care Health Dev. 2014;40(6):762–774. doi: 10.1111/cch.12139. [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994–1001. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Time to be SHY? Some comments on sleep and synaptic homeostasis. Neural Plast. 2012;2012:415250. doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- Turkdogan D, Bekiroglu N, Zaimoglu S. A prevalence study of restless legs syndrome in Turkish children and adolescents. Sleep Med. 2011;12(4):315–321. doi: 10.1016/j.sleep.2010.08.013. [DOI] [PubMed] [Google Scholar]
- van der Helm E, Gujar N, Walker MP. Sleep deprivation impairs the accurate recognition of human emotions. Sleep. 2010;33(3):335–342. doi: 10.1093/sleep/33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lieshout M, Luman M, Buitelaar J, Rommelse NN, Oosterlaan J. Does neurocognitive functioning predict future or persistence of ADHD? A systematic review. Clin Psychol Rev. 2013;33(4):539–560. doi: 10.1016/j.cpr.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J. Insomnia with Short Sleep Duration: Nosological, Diagnostic, and Treatment Implications. Sleep Med Clin. 2013;8(3):309–322. doi: 10.1016/j.jsmc.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, Bixler EO. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E, Stewart-Brown S, Fitzpatrick R. Agreement between adolescent self-report and parent reports of health and well-being: results of an epidemiological study. Child Care Health Dev. 2003;29(6):501–509. doi: 10.1046/j.1365-2214.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Yaacov Y, Manning M, Danon P, Weizman A. Internet Addiction and Attention Deficit Hyperactivity Disorder Among Schoolchildren. Isr Med Assoc J. 2015;17(12):731–734. [PubMed] [Google Scholar]
- Weiss MD, Baer S, Allan BA, Saran K, Schibuk H. The screens culture: impact on ADHD. Atten Defic Hyperact Disord. 2011;3(4):327–334. doi: 10.1007/s12402-011-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Xia S, Li X, Kimball AE, Kelly MS, Lesser I, Branch C. Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2012;204(2–3):161–167. doi: 10.1016/j.pscychresns.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LK, Shang CY, Gau SS. Psychiatric comorbidities in adolescents with attention-deficit hyperactivity disorder and their siblings. Can J Psychiatry. 2011;56(5):281–292. doi: 10.1177/070674371105600507. [DOI] [PubMed] [Google Scholar]
- Zendarski N, Sciberras E, Mensah F, Hiscock H. A longitudinal study of risk and protective factors associated with successful transition to secondary school in youth with ADHD: prospective cohort study protocol. BMC Pediatr. 2016;16:20. doi: 10.1186/s12887-016-0555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


