Abstract
Severely injured burn patients receive multiple blood transfusions for anemia of critical illness despite the adverse consequences. One limiting factor to consider alternate treatment strategies is the lack of a reliable test platform to study molecular mechanisms of impaired erythropoiesis. This study illustrates how conditions resulting in a high catecholamine microenvironment such as burns can instigate myelo-erythroid reprioritization influenced by β-adrenergic stimulation leading to anemia. In a mouse model of scald burn injury, we observed, along with a threefold increase in bone marrow LSK cells (linneg Sca1+cKit+), that the myeloid shift is accompanied with a significant reduction in megakaryocyte erythrocyte progenitors (MEPs). β-Blocker administration (propranolol) for 6 days after burn, not only reduced the number of LSKs and MafB+ cells in multipotent progenitors, but also influenced myelo-erythroid bifurcation by increasing the MEPs and reducing the granulocyte monocyte progenitors in the bone marrow of burn mice. Furthermore, similar results were observed in burn patients’ peripheral blood mononuclear cell-derived ex vivo culture system, demonstrating that commitment stage of erythropoiesis is impaired in burn patients and intervention with propranolol (nonselective β1,2-adrenergic blocker) increases MEPs. Also, MafB+ cells that were significantly increased following standard burn care could be mitigated when propranolol was administered to burn patients, establishing the mechanistic regulation of erythroid commitment by myeloid regulatory transcription factor MafB. Overall, results demonstrate that β-adrenergic blockers following burn injury can redirect the hematopoietic commitment toward erythroid lineage by lowering MafB expression in multipotent progenitors and be of potential therapeutic value to increase erythropoietin responsiveness in burn patients.
Keywords: anemia, LSK cells, megakaryocyte erythrocyte progenitors, granulocyte monocyte progenitors, catecholamine, burns, erythropoiesis, erythro-myeloid, MafB, propranolol
in the united states alone, ~12 million units of packed red blood cells (pRBCs) are transfused each year (7). Many patients in the intensive care unit (ICU) receive blood transfusions during their ICU stay. A common laboratory finding in patients in the ICU is a significant decrease in hemoglobin and hematocrit levels, leading to anemia within 72 h of admission (47) that persists for at least 6 mo even after the ICU discharge (2). One of the major consequences of burn injury is the persistent anemia in patients with >10% total burn surface area (TBSA) (33, 51). Regardless of hemoglobin triggers (23), burn patients continue to receive multiple transfusions during their hospital stay. Over half of those transfusions are unrelated to surgical procedures (36), meaning red cell production is impaired in burn patients and cannot be explained by surgical blood loss alone. Of equal importance is that multiple transfusions lead to significant morbidity and mortality in burn patients (33). Despite the adverse consequences, treatment strategies to increase red cell production and to reduce transfusions are hampered by a lack of reliable test platforms to study the molecular mechanisms of impaired erythropoiesis and to assess the efficacy of treatments other than transfusion in critically ill burn patients. Here we report one such test platform that is both diagnostic and prognostic for anemia of critical illness in burn patients.
Bone marrow is the seat of hematopoiesis in mammalian biology wherein hematopoietic stem cells (HSCs) sequentially differentiate by a predefined program to form white blood cells, RBCs, and platelets and thus help regulate immunity, efficient oxygen delivery to tissues, and coagulation (38). Compromise in any one of the hematopoietic lineages of development can lead to severe health consequences. Bone marrow dysfunction has been observed in the erythroid fraction of thermally injured patients (46). In an electron microscope study of bone marrow fragments obtained by sternal biopsy, erythropoiesis was found to be depressed compared with granulopoiesis in burned patients (25). Because bone marrow is not a readily accessible compartment in burn patients, we have established a method to use peripheral blood mononuclear cells (PBMCs) to study the commitment stage of erythropoiesis. Previously, we utilized the hematopoietic cells residing in the PBMCs to study persistent anemia in burn patients and found that the erythropoietin (Epo)-dependent proliferation stage of erythropoiesis was intact in burn patients. More specifically, colony-forming unit-erythroid (CFU-E) production decreased within a week after burn and continued to decline for a study period of 1 mo (48). Given that rHu-Epo treatment fails to augment erythropoiesis both in burn patients (29) and animal models (37), and because Epo receptors begin to be expressed from the CFU-E stage (21, 50), it is likely that erythropoietic defects are initiated upstream of CFU-Es, which is at the commitment stage. In a mouse model of scald burn (15% TBSA), we have previously shown that bone marrow hematopoietic commitment is skewed away from erythroid and toward myeloid cells (37) and that monocytopoiesis is under the influence of norepinephrine (44). Additionally, functional β2-adrenergic receptors expressed by the hematopoietic progenitor cells at sequential ranks of lineage commitments suggest an essential role for catecholamines in dictating the bone marrow myeloid cells (32). Our recent work has shown that burn injury augments monocyte differentiation via high expression of MafB (16, 49) and that MafB is increased in common myeloid progenitors (CMPs) (18). Nonetheless, what causes the activation of myeloid-specific transcription factor MafB, which orchestrates lineage preferences and inhibits the erythropoietic potential following burn injury, is not known.
Our previous studies, and that of others, have led us to hypothesize that β-adrenergic stimulation following burn injury elicits a shift in the commitment of HSCs away from the megakaryocyte erythrocyte progenitors (MEPs) and toward nonerythroid progenitors with a possible involvement of myeloid-specific transcription factor MafB. We utilized burn patients’ PBMC-derived ex vivo culture system as well as a mouse model of burn injury to investigate the defects upstream of CFU-E production at the stage of erythroid commitment while focusing on the direct role of MafB. We further probed the effect of administering propranolol (nonselective β1- and β2-blocker) after burn injury and discovered the concomitant role of MafB-expressing multipotent progenitor (MPP) population on myelo-erythroid commitment. We established this myelo-erythroid commitment pattern in bone marrow progenitors of burn mice and also in burn patients using PBMC-derived ex vivo-generated MPPs. Results reveal that early-stage Epo-independent myelo-erythroid commitment is orchestrated via β1- and β2-adrenergic mechanisms following burn injury through MafB regulation.
MATERIALS AND METHODS
Human Blood Samples
Following institutional review board approval, we enrolled fifteen adult patients of both sexes, older than 18 yr, with >20% TBSA burn admitted between 2013 and 2016. Patients with chemical burns, electrical burns, anoxic brain injury, self-inflicted burn, pregnancy, and lactation were excluded. For those patients who received transfusions, we waited at least 12 h after the leuko-reduced pRBC transfusions before the blood samples were drawn for the study. Out of the fifteen enrolled, thirteen patients satisfied the inclusion criteria, and informed, written consent was received. Of the two patients who were excluded from the study, one did not follow the transfusion criteria (samples were drawn before 12 h following transfusion), and the other was already on some other prescription medication before and after admission.
Blood samples were collected and analyzed at four different time points, 1–3 days, 7–10 days, 30–35 days, and 42–48 days after burn. Results from first and second samples were grouped together to represent data at <2 wk; results from the third and fourth samples were grouped together to represent data at 4–7 wk. Blinded analysis revealed a clear demarcation of the results between patients after follow-up. Further stratification based on treatment module examination of each patient revealed that some patients happened to receive β-adrenergic blocker propranolol during the course of burn care as part of another clinical trial. Some (8/13) patients were given propranolol to reduce heart rate as part of a different prospective ongoing study. We then divided the burn patients into two cohorts, standard burn care patients (SBC) and those who received propranolol during their burn care. Patient demographics are shown in Tables 1 and 2. Propranolol dosage regimen in burn patients is provided in Table 2. For baseline values, blood samples from eight matched healthy volunteers (Table 3) were drawn and analyzed with informed, written consent.
Table 1.
Demographics of standard burn care patients
| Burn Patient | Sex | Age, yr | TBSA, % | Transfusions, pRBC Units |
|---|---|---|---|---|
| Patient 1 | M | 44 | 67 | 33.5 |
| Patient 2 | M | 43 | 23 | None |
| Patient 4 | M | 26 | 29 | None |
| Patient 8 (deceased) | F | 59 | 50 | 24 |
| Patient 15 | F | 26 | 35 | 18 |
| M = 3; F = 2 | Mean ± SE: 39.6 ± 6.2 | Mean ± SE: 40.8 ± 7.9 | Mean ± SE: 15.1 ± 6.6; Median = 18 |
TBSA, total burn surface area; pRBCs, packed red blood cells; M, male; F, female.
Table 2.
Demographics of propranolol-treated patients
| Burn Patient | Sex | Age, yr | TBSA, % | Transfusions, pRBC Units | Propranolol Dose |
|---|---|---|---|---|---|
| Patient 3 | M | 59 | 21 | 2 | PBD2–4: 10 mg 3×. PBD5–6: 20 mg 3×. PBD8–11: 20 mg 1–2×. Stopped after day11. |
| Patient 5 | M | 29 | 21 | 16 | PBD7–18: 10 mg 2–3×. PBD19–22: 10 mg 1–2×. PBD23–48: 10 mg 3–4×. |
| Patient 6 | M | 25 | 46 | 23 | PBD2–7: 10–30 mg 1–3×. PBD8–29: 40–70 mg 2–3×. 7th week sample not available. |
| Patient 7 | M | 38 | 36 | 11 | PBD4–7: 10 mg 3×. PBD8–10: 20 mg 2×. PBD11–12: 30 mg 3×. Stopped after day 12. 7th week sample not available. |
| Patient 9 | F | 37 | 67.6 | 30 | PBD2–30: 10 mg 1–2×. PBD37–48: 10–30 mg 1×. |
| Patient 10 | M | 50 | 21 | 6 | PBD2–4: 10 mg 1×. PBD9–25: 30 mg 1–3×. PBD26–29: 20 mg 1–3×. PBD30–33: 10 mg 2×. |
| Patient 12 | M | 26 | 30 | 5 | PBD1–30: 5–30 mg 1–3×. |
| Patient 14 | M | 55 | 32 | 9 | PBD2–4: 10–20 mg 1–2×. PBD5–9: 30–70 mg 1–2×. PBD10–28: 10–40 mg 1–2×. |
| PBD29–34: 40–60 mg 1–2×. PBD35–37: 8–30 mg 1–2×. | |||||
| M = 7; F = 1 | Mean ± SE: 39.8 ± 4.7 | Mean ± SE: 34.3 ± 5.7 | Mean ± SE: 12.75 ± 3.4; Median = 10 |
Six burn patients (3, 5, 7, 9, 10, and 12) received propranolol (PR) not exceeding 30-mg single dose (range = 5–30 mg), whereas 2 patients (6 and 14) received higher than 30-mg (range = 40–70 mg) single-dose PR during the study period. 7th week sample was not available for patients 6 and 7. PBD, post burn day; ×, times per day PR administration.
Table 3.
Control subjects
| Controls | Age, yr | Sex |
|---|---|---|
| Control 1 | 60 | F |
| Control 2 | 50 | F |
| Control 3 | 26 | M |
| Control 4 | 29 | M |
| Control 5 | 40 | M |
| Control 6 | 22 | M |
| Control 7 | 30 | F |
| Control 8 | 50 | F |
| Mean ± SE: 38.4 ± 4.8 | M = 4; F = 4 |
Isolation of Peripheral Blood Mononuclear Cells
Blood samples from patients and control subjects were collected in BD Vacutainer CPT cell preparation tubes (BD Biosciences, Franklin Lakes, NJ). PBMCs were isolated by Ficoll-Hypaque density-gradient centrifugation (11, 24). Samples of PBMCs (4 × 106 cells/ml) were stored at −80°C in fetal bovine serum with 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) until analysis.
Ex Vivo Erythroid Progenitor Differentiation From PBMCs
Ficoll separated PBMCs were placed in a growth factor cocktail conducive to preserve and proliferate the residing HSCs and differentiate into myelo- erythroid progenitors as described previously for phase 1 culture (48). Briefly, PBMCs (1 × 106/ml) were cultured in serum-free expansion medium (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with cyclosporine A (1 ng/ml; Sigma-Aldrich), granulocyte macrophage colony-stimulating factor (20 ng/ml; eBioscience, San Diego, CA), stem cell factor (45 ng/ml; Stem Cell Technologies), and interleukin-3 (5 ng/ml; Affymetrix, Santa Clara, CA). Cells were then incubated at 37°C with 5% CO2 for 5 days.
Enrichment of Lineage-Negative Cells by Magnetic Isolation and Flow Cytometric Sorting
On day 6, an aliquot of nonadherent cells was counted by Trypan blue exclusion, and the rest were washed in PBS containing 10% (vol/vol) FBS, and lineage-negative (linneg) cells were enriched using magnetic separation. Lineage-positive cells were excluded by staining with biotin-conjugated antibody cocktail specific for the lineage antigens (CD3, clone OKT3, cat. no. 13-0037-80; CD14, clone 61D3, cat. no. 13-0149-82; CD19, clone HIB19, cat. no. 13-0199-80; and CD20, clone 2H7, cat. no. 13-0209-80) (eBioscience) for 15 min at 4°C. After extensive washing with MACS buffer [PBS, 0.5% (wt/vol) BSA, 0.1% (wt/vol) d-glucose, 0.09% (wt/vol) sodium azide, and 2 mM EDTA], cells were incubated with anti-biotin magnetic microbeads (Miltenyi Biotec, Auburn, CA) for 15 min at 4°C. Cells were then washed and resuspended in 1 ml of MACS buffer. Magnetic cell separation was carried out using the AutoMACS separator (Miltenyi Biotec) by referring to the AutoMACS User Manual and applying the separation program “depletes,” which retains lin+ cells in the magnetic column.
The enriched linneg fraction (the eluate) was stained with cell surface antigens using anti-CD34-APC (clone-561, cat. no. 343608; BioLegend, San Diego, CA), anti-CD38-APC (clone HIT2, cat. no. 555462; BD PharMingen, San Jose, CA), anti-CD123-PE.Cy7 (clone 6H6, cat. no. 306010; BioLegend), and anti-CD45RA-V450 (clone HI100, cat. no. 560362; BD Biosciences) antibodies. An aliquot was analyzed using fluorescence-activated cell sorting (FACS) Canto II, and data were obtained by Flow Jo software (Tree Star, Ashland, OR). Detailed gating strategies are explained in Fig. 1A.
Fig. 1.
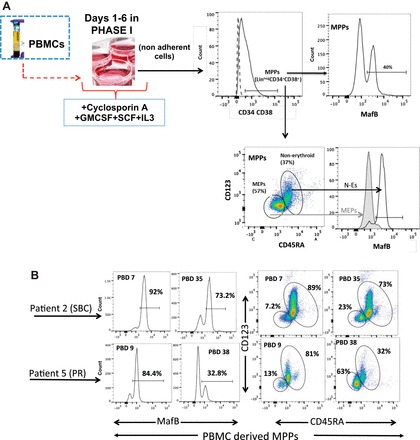
A: PBMCs were cultured in the specified growth factor cocktail for 6 days. Nonadherent cells collected from culture plates were classified as multipotent (MPP), erythroid (MEP), and nonerythroid (NE) progenitors based on a combination of cell surface antigens. First, lineage-negative (linneg), CD34+, and CD38+ cells (linnegCD34+CD38+) were selected for further characterization. The linnegCD34+CD38+ cells comprised both MafB+ and MafBneg cells, which confirms their multipotency (MPPs are the solid line in the histogram and the dashed line is Ab control). Furthermore, MPPs were subdivided according to differential expressions of CD123 (IL-3Rα) and CD45RA, such that MEPs are gated as CD123neg CD45RAneg and N-E cells as CD123+/− CD45RA+ and verified with MafB+ (N-E) and MafBneg (MEP) expressions. GMCSF, granulocyte macrophage colony-stimulating factor; SCF, stem cell factor. B: representative images of PBMC-derived MEPs and MafB-expressing cells in MPP population from standard burn care (SBC) patient (E02) at post burn day 7 (PBD7) (<2 wk) and PBD35 (4–7 wk); propranolol (PR)-treated patient (E05) at PBD9 (<2 wk) and PBD38 (4–7 wk).
Intracellular MafB Staining
The remaining linneg fraction was fixed using BD Cytofix/Cytoperm (BD Biosciences) for 20 min in the dark at room temperature (RT). Cells were permeabilized by adding perm buffer [PBS, 0.1% (wt/vol) d-glucose, 0.1% (wt/vol) BSA, 0.09% (wt/vol) sodium azide, and 0.1% (wt/vol) saponin] and incubated for an additional 10 min. Cells were then washed with perm wash [PBS, 0.1% (wt/vol) glucose, 0.09% (wt/vol) sodium azide, and 0.1% (wt/vol) saponin] and incubated with polyclonal anti-MafB-FITC (cat. no. 038061;US Biological Life Sciences, Salem, MA) and 0.4 µg/100 µl staining buffer for 24-h incubation until further analysis.
Mouse Model of Burn Injury
Mice.
Six- to eight-week-old B6D2F1 male mice weighing ~25 g were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in our Comparative Medicine Facility with a 12-h:12-h light/dark cycle and controlled temperature (20–22°C). The mice were allowed to acclimate to our facility for 7 days before use. The Institutional Animal Care and Use Committee at Loyola University Medical Center approved all the experimental protocols.
Burn injury.
B6D2F1 male mice were randomly divided into sham and burn groups. Mice were anesthetized using ketamine and xylazine (100 mg/kg, 2.5 mg/kg, respectively; intraperitoneal), and their dorsal hair was removed by shaving. Mice were subjected to a 15% TBSA full-thickness scald burn along the dorsum by immersion in a 100°C water bath for 9 s (45) and were resuscitated immediately with intraperitoneal injection of normal saline (2 ml). A sham group of mice was administered anesthesia, shaved, and resuscitated but were not subjected to burn injury.
Propranolol administration by injections and alzet pumps.
For short-term (post-burn day, PBD 7) administration, sham and burn groups were again reassigned to vehicle and propranolol groups 6 h after burn injury. Injections of vehicle (saline) or propranolol (cat. no. P0884;Sigma) were given subcutaneously at 1.2 mg/mouse once a day for 6 days until harvest on day 7 (27). Sham mice were subjected to propranolol and saline injections to eliminate any residual effect of β-blocker.
For long-term (PBD 14) administration, sham and burn groups were again reassigned to vehicle and propranolol groups 24 h after burn injury. Propranolol (5 mg/kg body wt per day) or saline-primed alzet mini osmotic pumps (model 1002; DURECT Corporation, Cupertino, CA) were implanted to each sham and burn mouse, resulting in four treatment groups, sham-saline (SS), burn-saline (BS), sham-propranolol (SP), and burn-propranolol (BP) mice, that were provided continuous infusion with either propranolol or vehicle via alzet pump (40) for over a period of 13 days. Six animals per group were used for each time point. No mortality or wound infection was associated with any experimental group during the 2-wk post-burn period.
Exogenous recombinant human Epo.
Following administration of propranolol or vehicle for 6 days, each burn group was reassigned to human recombinant Epo (12.5 U/day i.p.) or vehicle, which was administered subcutaneously for 2 days (37). Mice were allowed to rest for 3 days after the last injection before harvest.
Bone Marrow MPPs and Flow Cytometric Analysis
Total bone marrow (TBM) cells from the femurs of each mouse were eluted into McCoy’s medium (Invitrogen, Carlsbad, CA) and labeled with the following biotin-conjugated lineage-specific primary antibodies: anti-CD86 (clone GL1, cat. no. 553690), anti-CD11c (clone HL3, cat. no. 553800), anti-Ter119 (clone Ter119, cat. no. 553672), anti CD19 (clone1D3, cat. no. 553784), anti-B220 (clone RA3–6B2, cat. no. 553086), anti-CD11b (clone M1/70, cat. no. 553309), anti-CD90 (clone HIS51, cat. no. 554893), anti-CD8a (clone 53-6.7, cat. no. 553029), anti-Gr1 (clone RB6–8C5, cat. no. 553125), anti-CD127 (clone A7R34, cat. no. 13-1271-82), and anti-CD3e (clone 145-2C11, cat. no. 553060) (BD Biosciences) followed by incubation with anti-biotin magnetic beads (Miltenyi Biotec). Magnetic cell separation was carried out using the AutoMACS separator (Miltenyi Biotec) referring to the AutoMACS User Manual and applying the separation program “depletes.” The enriched lineage-negative (linneg) fraction was surface stained with PerCP-Cy5.5-Sca1 and clone D7 (cat. no. 108124; BD Biosciences), APC-CD117 (clone 2B8, cat. no. 553356, c-Kit receptor), efluor 450-CD34 (clone RAM34, cat. no. 48-0341-82), and Pe-Cy7-FcγR (clone 93, cat. no. 25-0161-82) (eBioscience) and analyzed by FACS analysis to either identify MEPs (linnegSca1negcKit+ CD34negFcγneg), granulocyte monocyte progenitors (GMPs) (linnegSca1negcKit+ CD34+FcγR+), LSK cells (linnegSca1+cKit+), or to sort MPPs (linneg cKit+). Sorted MPPs were then fixed and permeabilized as mentioned earlier for human samples. The cells were then incubated with anti-MafB-FITC (FITC was conjugated to Abcam’s anti-MafB polyclonal Ab; cat. no. ab66506, using FITC conjugation kit from Abcam, Cambridge, MA) in perm buffer and incubated at 4°C on shaker for 48 h. Cells were washed in perm wash and analyzed with FACS Canto II, and data were obtained using Flow Jo software (Tree Star).
Confocal Microscopy
An aliquot of MafB-stained MPPs was cytospun onto microscopic slides and preserved using Vectashield H-1500 mounting medium with DAPI (Vector Laboratories, Burlingame, CA). A Zeiss LSM 510 laser-scanning microscope (Carl Zeiss MicroImaging, Jena, Germany) was used to view with C-Apochromat 403 1.20 water immersion, and ×40 images were acquired using Zeiss LSM 510, version 4.2, SP1 software.
Bone Marrow Mature Myeloid and Erythroid Cells
An aliquot (1 × 106) of TBM cells was labeled with PerCP.Cy5.5-Ter119 (eBioscience; clone Ter119, cat. no. 45-5921-82) and Pe.Cy7-CD11b (BD Biosciences; clone M1/70, cat. no. 552850) antibodies for 30 min at 4°C in the dark. Cells were washed and resuspended in PBS and immediately analyzed with a FACS Canto II (BD Biosciences).
Total RNA Isolation, cDNA Synthesis, and Quantitative PCR
LinnegSca1+cKit+ (LSK) cells were sorted from TBM cells as described above. Total RNA was isolated from LSKs using RNeasy Mini Kit (Qiagen, Valencia, CA) as described by the manufacturer. First-strand cDNA was synthesized from 180 ng total RNA using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA), and reactions were run on a Veriti 96-well Fast Thermocycler (Life Technologies) per the manufacturer’s instructions. The expression levels of colony-stimulating factor 1 receptor (CSF1R) and colony-stimulating factor 2 receptor β common subunit (CSF2RB) were analyzed by real-time PCR using TaqMan primer probes and TaqMan Fast Advanced Master Mix (Life Technologies) in StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA). GAPDH was included as a normalization control for each analysis. Target gene Ct cycle values were normalized to GAPDH Ct values. Data were calculated using the ΔΔCt method, and fold gene expression in burn group is expressed relative to the sham group.
Immunohistochemical Analysis of Tyrosine Hydroxylase
Brains from sham and burn mice (PBD7) were freshly isolated and fixed in 10% formalin solution, embedded in optimal cutting temperature compound and stored at −80°C until analysis. Coronal sections (6 µm) of cryo-preserved brain were cut on a cryostat and mounted (3 sections per slide) onto commercially available charged slides (Fisher Scientific, Waltham, MA). After antigen retrieval (0.01 M citrate buffer, pH 6.0) by heating in boiling water, the tissue sections were then placed in 0.4% hydrogen peroxide for 30 min at RT to inactivate endogenous peroxidases and then blocked with 2.5% goat serum for 1 h at RT. Two of the sections were incubated with primary antibody anti-tyrosine hydroxylase (TH) (AB152, cat. no. 50-173-082; EMD Millipore, Billerica, MA) in 1:500 dilutions in PBS at 4°C overnight, and for negative controls the primary antibody was excluded. All sections were then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H+L) secondary antibody (cat. no. 31460) for 1 h at RT. A wash step with PBS for 10 min was carried out between each step of immunohistochemical staining. The end product was visualized by means of a 3, 3-diamino-benzidine tetrahydrochloride (DAB) and hydrogen peroxidase solution (Vector Laboratories) and then counterstained with hematoxylin for 10 s. The images of the mid brain region were captured in phase contrast using Evos microscope at ×10 magnification.
Western Blotting
Freshly isolated brains from sham and burn mice (PBD7) were snap frozen in liquid nitrogen and stored at −80°C until use. Tissue lysates were prepared by homogenization in RIPA buffer containing Halt protease inhibitor cocktail (Pierce, Rockford, IL) and sonication, and lysates were then obtained after centrifugation. Protein concentrations were determined using Pierce BCA-200 Protein Assay kit, and 20 µg protein was then resolved in 4–20% Mini-Protean TGX precast protein gel (Bio-Rad Laboratories, Hercules, CA) and transferred to Immun-Blot PVDF membrane using a semidry Trans-Blot system (Bio-Rad). Membranes were blocked with 5% BSA overnight at 4°C and incubated with primary antibodies against anti-TH (1:1,000, 6 h at 4°C; AB152; EMD Millipore). The membranes were washed three times with TBS supplemented with 0.05% Tween 20 (TBST) before incubation with secondary antibody conjugated with HRP (1:10,000; 1 h at RT). Membranes were washed five times with TBST, and protein detection was performed using ECL substrate (PerkinElmer, Hebron, KY). Image Laboratory 4.0.1 software was used to quantify the results. The membranes were stripped and reprobed with rabbit monoclonal antibody GAPDH (1:1,000, D16H11, cat. no. 5174; Cell Signaling Technology, Danvers, MA) for 2 h at RT followed by secondary Ab and imaging as above. The bands were quantified by densitometry and normalized with respect to endogenous control GAPDH.
Statistical Analysis
Results from all experiments are expressed as means ± SE. All experiments were repeated at least three times. The number of animals used per experiment is given in respective figure legends. Analysis of variance with Tukey’s post hoc test or Student-Newman-Keuls multiple- comparison test using KaleidaGraph statistical program (version 4.1.0, Synergy Software, Reading, PA) were carried out for multiple comparisons. Statistical significance was set at P < 0.05.
RESULTS
PBMC-Derived Hematopoietic Progenitors
PBMC resident HSCs were differentiated into myelo-erythroid progenitors as described previously (48). Nonadherent cells collected from culture plates were subjected to flow cytometric categorization based on lineagenegCD34+CD38+, which are MPPs. MPPs were subdivided according to differential expressions of intracellular MafB. MPPs were next further classified into nonerythroids and MEPs based on a combination of cell surface antigens. MEPs express neither CD123 nor CD45RA (CD123neg CD45RAneg), whereas nonerythroids are gated as CD123+/− CD45RA+ as described previously (30). Detailed gating strategies are shown in Fig. 1A. Representative FACS plots of patient 2 (SBC) and patient 5 (propranolol) as explained above for MafB-expressing cells in MPPs and percentages of MEPs and nonerythroids are shown in Fig. 1B.
Propranolol Treatment in Burn Patients Can Restore PBMC-Derived MEPs
We have previously shown that Epo-dependent erythropoiesis is not affected in burn patients and that CFU-E is probably the rate-limiting entity, affecting erythropoiesis (48). In this study, PBMC-derived MEPs were determined and expressed as percentages of MPPs. PBMC-derived MEPs of SBC patients and propranolol-treated patients are shown in Fig. 2, A and B, respectively. The percentage of MEPs was significantly decreased at <2 wk post burn in SBC patients compared with controls and continued to remain significantly low till 4–7 wk (P < 0.01 vs. controls). In patients who received propranolol, the decrease in PBMC-derived MEPs was not significantly different from controls but was significantly higher than SBC patients at <2 wk (P < 0.05 vs. SBC) and 4–7 wk post burn (P < 0.001 vs. SBC; Fig. 2C). Total MPP cell counts from cell culture plates are shown as bar graphs in Fig. 2D, which indicated robust proliferation of hematopoietic stem and progenitor cells.
Fig. 2.
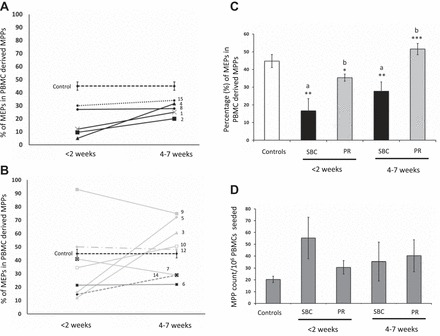
Ex vivo PBMC-derived MEPs. PBMC-derived linneg CD34+ CD38+cells are signified as MPPs. Line graph y-axes represent percentage of MEPs in PBMC-derived MPPs, and x-axis represents weeks post burn from week 1 to week 7. Each line in the graph represents data for each patient included in the study, and the dashed line represents baseline values (means ± SE) from control PBMCs. A: black lines represent percentage of MEPs in SBC-treated patients. B: gray lines represent PR-treated patients. The numbers next to the lines represent each patient matching those in Table 1. C: bar graph representing means ± SE of MEPs calculated as percentage of MPPs. D: bar graph representing proliferation of PBMC-derived MPPs in ex vivo culture condition in the presence of SCF, Hu-GMCSF, Hu-IL-3, and cyclosporin A. The y-axis represents total number of MPPs per million of PBMCs seeded. Control (n = 8), SBC (n = 5), PR (n = 8). a vs. controls, b vs. SBC. ***P < 0.001, **P < 0.01, *P < 0.05 by one-way ANOVA.
Propranolol Treatment in Burn Patients Decreases MafB Expression in PBMC-Derived MPPs
Our recent study has shown that burn injury-mediated increase in myeloid transcription factor MafB restricts the erythroid potential of bone marrow CMPs (18). To validate whether β-adrenergic signaling is the primary regulator of MafB controlling the myelo-erythroid commitment in burn patients, we quantified MafB-expressing cells in the ex vivo PBMC-derived MPPs. Percentages of MafB+ MPPs in SBC patients and propranolol-treated patients are shown in Fig. 3, A and B, respectively. Compared with controls, the percentages of MafB+ cells in the MPPs were significantly increased in patients with SBC at <2 wk (P < 0.01) and continued to remain significantly elevated till 4–7 wk post burn (P < 0.05). However, in propranolol-treated patients, the percentage of MafB expressing cells decreased considerably by 4–7 wk post burn compared with SBC patients (P < 0.05; Fig. 3C). The mean fluorescent intensity (MFI) of MafB expression in MPPs was also significantly increased in SBC patients compared with controls (P < 0.001) and was significantly reduced in propranolol -treated patients (P < 0.001 vs. SBC) at all time points studied (Fig. 3D). Overall, results indicate that MafB expression correlates reciprocally with the percentage of MEPs in PBMC-derived MPPs and is regulated by β-adrenergic mechanisms. More importantly, MafB appears to be a negative regulator averting MEP production in burn patients and thereby orchestrating the commitment stage of hematopoiesis through β-adrenergic mechanisms.
Fig. 3.
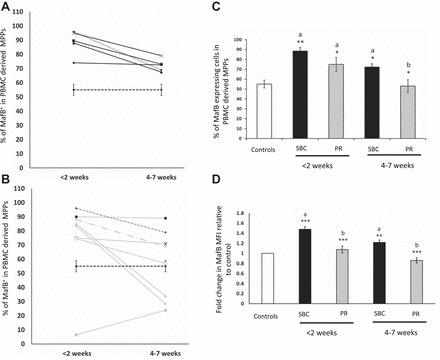
MafB+ cells in PBMC-derived MPPs (linneg CD34+ CD38+cells). In line graphs, y-axis represents the percentage of MafB-expressing cells in PBMC-derived MPPs, and x-axis represents weeks post burn from week 1 to week 7. Dashed black line represents means ± SE from control subjects. A: black lines represent percentage of MafB+ cells in SBC-treated patients. B: gray lines represent PR-treated patients. The numbers next to the lines represent each patient matching those in Table 1. C: bar graph shows means ± SE of MafB+ cells calculated as percentage of MPPs from controls, SBC, and PR patients from week 1 to week 7. D: fold change in mean fluorescent intensity of MafB in PBMC-derived MPPs from controls, SBC, and PR patients from <2 wk and 4–7 wk. Controls (n = 8), SBC (n = 5), PR (n = 8). ***P < 0.001, **P < 0.01, *P < 0.05. a vs. controls, b vs. SBC by one-way ANOVA.
Interestingly, patient 6 and patient 14 were on a regimen of propranolol, which exceeded 40–70 mg in a single dose, compared with others who received not more than 10–30 mg at one dose. These two patients did not respond as well, exhibiting lower MEPs (Fig. 2B) and higher MafB (Fig. 3B) levels. Detailed dose-response studies are warranted to draw more meaningful inferences. Nonetheless, all patients were included in the bar graphs shown as means ± SE in this study (Figs. 2C and 3C).
TBSA, Age, and Hemoglobin
Percentage of TBSA did not influence percentage of MEPs either in SBC or propranolol-treated patients at <2 wk and at 4–7 wk post burn (Fig. 4A). Similarly, age of the patients had no correlation with percentage of MEPs at both time points. Data shown are at 4–7 wk post burn (Fig. 4B). Blood hemoglobin levels were retrospectively recorded from the charts at the single time of sample collection for most burn patients, and the mean value (in the case of multiple recordings on a single day) was calculated and given at <2 wk and 4–7 wk post burn. Mean hemoglobin levels were below reference range (4) for the entire study period in both groups (Fig. 4C).
Fig. 4.
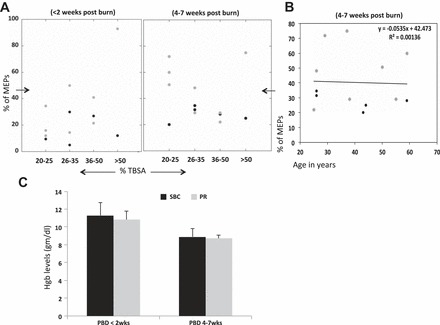
A: y-axis shows the percentage of MEPs in PBMC-derived MPPs at <2 wk and 4–7 wk post burn in SBC-treated (black points) and PR-treated (gray points) patients. The x-axis represents the percentage of total burn surface area (%TBSA). Percentage of MEPs and %TBSA are not correlated, as both groups have MEP levels distributed irrespective of %TBSA in both burn groups. B: y-axis shows the percentage of MEPs in PBMC-derived MPPs at 4–7 wk post burn in all burn patients, and x-axis represents the age distribution. Simple linear regression analysis shows no correlation between MEP levels and age parameter as the coefficient of determination (R2) = 0.0014. C: mean hemoglobin (Hgb) levels in SBC (black) and PR (gray) patients on <2 wk and 4–7 wk post burn. No differences in Hgb levels were observed in the two patient groups while significantly lower than reference range.
Sympathetic Activity Is Increased After Burn Injury
One way to evaluate the extent of sympathetic activity is to measure TH, which is the rate-limiting enzyme in catecholamine biosynthesis (8). Compared with sham, a 15% TBSA burn injury increased TH levels as detected by immunohistochemistry staining of brain sections at PBD7 (Fig. 5A) and the protein expression of TH by Western blot in brain tissue lysates (Fig. 5B). GAPDH served as a loading control. Bar graphs in Fig. 5C represent significantly higher TH expression relative to GAPDH following burn injury (P < 0.01 vs. sham).
Fig. 5.
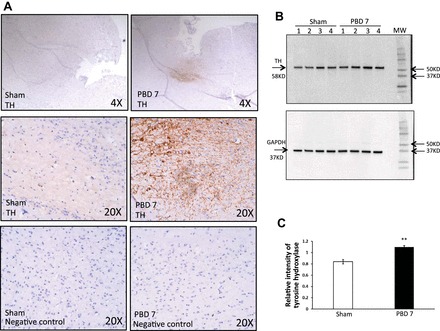
A: sympathetic activity is increased in brain after burn injury. Immunohistochemical detection of tyrosine hydroxylase (TH) in mouse brain after PBD7 is compared with sham. TH is represented as a dark brown reaction product of DAB oxidation in the presence of peroxidase and hydrogen peroxide at the site of enzymatic activity. All slides were counterstained with hematoxylin. Images were captured by light microscopy at ×4 and ×20 magnification using EVOS FL Cell Imaging System. Experiment was repeated 3 times with n = 4 mice per group. B: Western blot analysis of TH bands at 58 kDa and GAPDH bands at 37 kDa in brain of PBD7 mice compared with sham. MW, molecular weight. C: TH protein levels based on densitometry expressed as means ± SE relative to GAPDH as a loading control. Bar graph represents means ± SE, containing 7 mice/group showing increase in TH protein expression in the brains of PBD7 mice. **P < 0.01 vs. sham by one-way ANOVA.
Burn Injury Augments CSF1R and CSF2RB Gene Expression in LSKs
To get some insight whether the burn-induced microenvironment can influence the hematopoietic myeloid commitment, we performed gene expression analysis of myeloid-related genes, specifically CSF1R and CSF2RB in LSKs from sham and burn mice. Real-time PCR data demonstrate a significant increase (P < 0.001) in gene expression of CSF1R (4.9-fold) and CSF2RB (3.9-fold) in LSKs from burn mice on PBD7 compared with sham (Fig. 6, A and B, respectively). Although this observation supports the myeloid bias and a robust hematopoietic proliferation capacity after burn injury, with the assumption that the PBMC-resident HSCs are orchestrating lineage preference in burn patients, the true picture of the hematopoietic paradigm shift will emerge only from probing the bone marrow. Although bone marrow is the primary seat of erythropoiesis, obtaining bone marrow aspiration from burn patients is a highly invasive procedure. Therefore, we pursued a practical approach with a mouse model of burn injury such that the lineage-committed progenitors can be directly isolated from bone marrow with specific cell surface markers using the latest techniques.
Fig. 6.
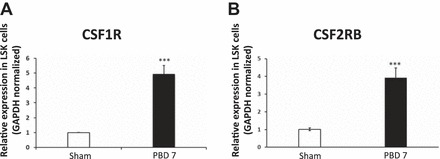
Real-time PCR analysis of bone marrow LSK cells indicates that burn injury significantly increases expression of myeloid-specific genes CSF1R (A) and CSF2RB (B). Total RNA was extracted from LSKs, which was sorted from the total bone marrow (TBM) of sham and PBD7 mice. cDNA was synthesized from the total RNA. Real-time PCR was performed according to manufacturer’s instructions. Bar graphs represent means ± SE of pooled LSKs isolated from 8 mice per group with three repetitions. ***P < 0.001 vs. sham by one-way ANOVA.
Propranolol Reverses Burn Injury-Mediated Increase in LSKs
We administered propranolol subcutaneously for 6 days after burn injury to evaluate the β-adrenergic influence on burn injury-mediated increase in the LSK cell population. As seen in the bar graph in Fig. 7A, the percentage of LSKs within the bone marrow begins to increase from as early as PBD3, and blocking with propranolol for 6 days post burn significantly lowered the proportion of LSKs expressed in linneg bone marrow fraction (P < 0.01 vs. burn).
Fig. 7.
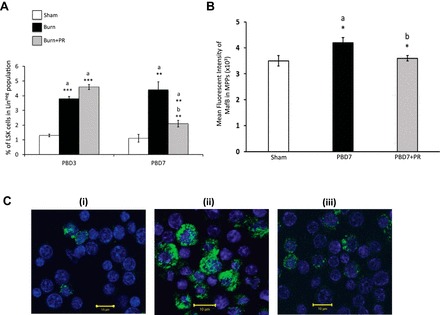
A: percentage of LSKs in linneg fraction of TBM cells from bilateral femurs in mice. Propranolol at 1.2 mg/mouse per day was delivered subcutaneously for PBD3 and PBD7. Bar graphs represent means ± SE of 8 mice per group with three repetitions. ***P < 0.001, **P < 0.01. a vs. sham, b vs. burn. B: bar graph represents changes in mean fluorescent intensity of MafB in the MPPs of three groups represented as means ± SE. MPPs were isolated from 8 mice (2 pooled in one)/group and repeated 3 times. *P < 0.05. a vs. sham, b vs. burn by one-way ANOVA. C: confocal images of MPPs (linneg c-Kit+) from mice bone marrow showing differential expression of intracellular MafB expression in sham (i), PBD7 (ii), PBD7 + propranolol (iii).
Propranolol Treatment Reduces MafB-Expressing Cells in the Bone Marrow Hematopoietic MPPs (Murine: linneg cKit+)
We next isolated MPPs residing within the bone marrow of mice and examined the intracellular MafB expression. Overall, the MFI of MafB expression was increased in MPPs from burn mice compared with sham and was mitigated with propranolol administration as determined by flow cytometry (Fig. 7B; P < 0.05).
With confocal imaging, we observed that, among bone marrow MPPs, MafB-expressing cells were particularly increased in burn compared with sham mice, which was significantly reduced with propranolol treatment, similar to propranolol-treated burn patient PBMC-derived MPPs. Representative confocal images of MPPs that display the differential expression of MafB+ cells between the three groups are shown in Fig. 7C.
We then evaluated the proportion of MafB+ cells in the MPPs from total bone marrow over the course of burn. The bar graphs in Fig. 8A represent the percentage of MafB+ cells (means ± SE) in all three groups harvested on PBD3, PBD7, and PBD14. We noticed a significant increase in the percentage of MafB+ cells in burn mice from PBD3 (P < 0.01 vs. sham). Propranolol treatment, on the other hand, significantly reduced the percentage of MafB+ cells at PBD7 (P < 0.01 vs. burn saline). No significant difference was seen between propranolol and saline administration in sham mice (results not shown). To confirm whether the bone marrow effect is a continuous response to burn injury, and to control the dose of propranolol to match clinical situations, we next placed alzet pumps under the skin to deliver either propranolol or placebo continuously for 13 days until harvest on PBD14 (3, 15). Results from all three groups with alzet implants also indicate that the bone marrow myeloid transcription factor MafB is under β-adrenergic regulation following burn injury (P < 0.05 vs. burn saline pump).
Fig. 8.
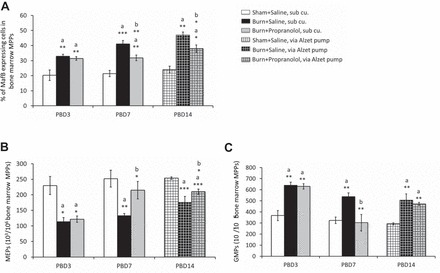
Percentage of MafB+ cells in MPPs, % of MEPs, and granulocyte monocyte progenitors (GMPs) in TBM cells isolated from bilateral femurs in mice. A: y-axis: % of MafB+ cells in MPPs; x-axis: time course after burn, PBD3, PBD7, and PBD14. B: y-axis: number of MEPs ×103 per million of MPPs; x-axis: time course after burn, PBD3, PBD7, and PBD14. C: number of GMPs ×103 per million of MPPs. Propranolol at 1.2 mg/mouse per day was delivered subcutaneously for PBD3 and PBD7. For longer duration (PBD14), alzet pumps were implanted subcutaneously in mice for continuous infusion of propranolol at 5 mg/kg body wt per day. Bar graphs represent means ± SE. Data were obtained from 8 mice (TBM from 2 mice pooled in one)/group and repeated 3 times. *P < 0.05, **P < 0.01, ***P < 0.001; a vs. sham; b vs. burn by one-way ANOVA.
Propranolol Treatment Influences Myelo-Erythroid Bifurcation in the Bone Marrow
We noticed a significant reduction in MEPs within the MPP population in the burn mice starting from PBD3, which remained low compared with sham until PBD14 (Fig. 8B). Propranolol-treated mice exhibited a significant increase in the MEP population from PBD7, which persisted until PBD14 (P < 0.05). In corroboration with our earlier results from burn patient PBMC-derived ex vivo culture, a similar trend in the commitment bias of MPPs away from MEPs was found in the burn mice that is counteracted with propranolol administration.
Unlike in the contrived ex vivo culture system explained previously, our in vivo mouse model will allow us to investigate the counterregulatory myeloid arm of hematopoietic progenitors, particularly the GMP population. As expected, bone marrow GMP fraction was significantly increased after burn injury from PBD3 and persisted until PBD14 (P < 0.01 vs. sham). Daily injections of propranolol for 6 days significantly reduced GMPs to sham levels (P < 0.01 vs. burn); however, a subcutaneous implant with lower-dose propranolol had an insignificant effect (Fig. 8C).
Bone Marrow Epo Responsiveness Is Improved After β-Adrenergic Blockade Following Burn Injury
To investigate whether or not the effect of propranolol is reflected on mature lineage-committed myeloid and erythroid cells, we next evaluated the percentage of terminal lineage markers CD11b and Ter119 in the total bone marrow cells. Although there was no change between the three groups on PBD3, we noticed a significant increase in CD11b+ cells and a corresponding decrease in Ter119+ cells in the burn group on PBD7 (Fig. 9A). However, in the propranolol group, we noticed a reciprocal reversal in the respective myeloid and erythroid cells compared with burn mice, indicating significant progress in erythroid maturation with propranolol treatment (P < 0.05). Given the influence of propranolol on erythro-myeloid commitment, these results proportionately reflect those of bone marrow progenitors (GMPs and MEPs) following burn injury. Nonetheless, Epo responsiveness was also improved after propranolol administration. Visual representation of pelleted total bone marrow cells (Fig. 9B) clearly depicts the red cell mass that is almost depleted in burn, improved with propranolol, and considerably enhanced with Epo administration. Quantitative analysis by flow cytometry using cell surface markers Ter119 and side scatter is represented in sample dot plots, and the results are enumerated (means ± SE) in Table 4. However, burn injury resulted in a concurrent increase in CD11b+ total bone marrow cells, which was reduced by propranolol and remained unchanged with Epo, indicating the specific response of Epo on Ter119-expressing erythroid cells. Therefore, a combined therapy with Epo to complement the action of propranolol can be effective in restoring bone marrow erythropoiesis in burn patients.
Fig. 9.
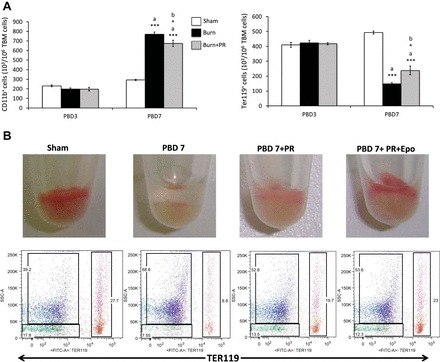
A: propranolol administration for 6 days after burn influences mature myeloid and erythroid cells in the bone marrow; y-axis: number of CD11b+ (×103) and Ter119+ (×103) cells per million TBM cells collected from bilateral femurs; x-axis: time course after burn, PBD3 and PBD7. Propranolol was dosed at 1.2 mg/mouse per day, delivered via subcutaneous injections. Bar graphs represent means ± SE, containing 6 mice/group and repeated 4 times. *P < 0.05, ***P < 0.001; a vs. sham; b vs. burn by one-way ANOVA. B: erythropoietin (Epo) responsiveness is improved with exogenous Epo in propranolol-treated burn mice. Pelleted total bone marrow cells from sham, PBD7, PBD7 + PR, and PBD7 + PR + Epo groups are shown along with representative dot plots showing Ter119+ cells in the far right quadrant (red) and CD11b+ cells in the upper left quadrant (blue).
Table 4.
Bone marrow myelo-erythroid cell distribution and erythropoietin responsiveness to propranolol administration after burn injury
| Total Bone Marrow Cell Distribution | Sham | PBD7 | PBD7 + PR | PBD7 + PR + Epo |
|---|---|---|---|---|
| Ter119+ (erythroid) | 33.0 ± 2.0% | 9.0 ± 1.0%* | 18.0 ± 0.8%*† | 24.0 ± 0.8%*†‡ |
| CD11b+ (myeloid) | 36.0 ± 2.0% | 66.0 ± 2.0%* | 55.0 ± 1.5%*† | 55.0 ± 1.2%* |
Values are means ± SE. Experiments repeated 3 times with n = 6 mice/group. Improved erythropoietin (Epo) responsiveness was observed with exogenous Epo in propranolol-treated burn mice.
P < 0.0001 vs. Sham;
P < 0.0001 vs. PBD7;
P < 0.008 vs. PBD7 + PR by one-way ANOVA.
DISCUSSION
In this study, we examined human PBMC-derived ex vivo cultures as a proxy to bone marrow hematopoiesis by evaluating the distribution of erythroid and nonerythroid progenitors and measuring intracellular MafB expression in MPPs. We report that impaired erythropoiesis in burn patients is primarily attributable to defective Epo-independent commitment stage. The possible molecular mechanism orchestrating the myeloid bias is the transcription factor MafB mediated by β1/β2-adrenergic stimulation following burn injury. Furthermore, we confirmed this observation using an in vivo mouse model of scald burn by investigating bone marrow stem and progenitor cell distribution with and without β1/β2-adrenergic blockade and showed that bone marrow Epo responsiveness can be improved. We also established that burn-induced sympathetic stimulation is evident in a 15% TBSA mouse model.
Given the multifactorial etiology of anemia in critically ill burn patients, the only treatment option is blood transfusion, which is often associated with adverse effects (23, 33). To consider alternate treatment strategies, we need a clearer understanding of the molecular mechanisms of anemia of critical illness in burn patients. Recent studies imply impaired bone marrow erythropoiesis as a plausible reason for anemia following burn injury (37) with a limitation in CFU-E production (48) explaining Epo resistance in burn patients (29) and the mouse model (37). To better understand the mechanism behind Epo-resistant anemia, it is logical to probe the stage upstream of CFU-Es. As burst-forming unit-erythroids (BFU-Es) cannot be morphologically identified, we chose to study the distribution of MEPs, the earliest identifiable erythroid progenitors at the bifurcation of CMPs to erythroid and myeloid lineages (1).
The reduction in PBMC-derived MEPs (31, 48) was consistent in all SBC patients, which can be explained by a reprioritization in the commitment of the progenitor cells or the HSCs, which is in line with our earlier studies on the mouse model of burn injury (18, 37). One of the limitations in ex vivo culture system is the likelihood of apoptosis and compromised proliferation of MPPs in burn patients. However, a concomitant increase in nonerythroid cells with no significant differences in MPPs supports reprioritization of lineage commitment over apoptosis. Moreover, these results were consistent with bone marrow MEP and GMP distribution in our mouse model.
It is known that myelopoietic commitment of bone marrow progenitors is modulated by norepinephrine following thermal injury with sepsis (44), and now we provide additional evidence that sympathetic activation is evident in our mouse model of 15% TBSA scald burn (Fig. 5). Therefore, bone marrow myeloid shift represented by increased GMPs and CD11b+ cells can be attributed to catecholamine action following burn injury. Because elevated catecholamine levels are evident in patients over 30% TBSA burn (22) and HSCs and progenitors express β-adrenergic receptors (32), it is reasonable to believe why burn patients who were treated with propranolol during burn care (10–30 mg) showed a reciprocal reversal in nonerythroid lineage commitment. The nonerythroid fraction should predominantly represent myeloid cells based on specified culture conditions.
For an uncommitted stem cell, the expression of receptors for the essential cytokines (growth factors) is an important requisite for commitment. Moreover, the predominance of one cytokine/receptor combination over the other can influence the lineage fate of the HSCs (52). CSF2RB is a colony-stimulating factor receptor sharing a common β-subunit for granulocyte macrophage colony-stimulating factor, IL-3, and IL-5. Together, all three growth factors upon binding to CSF2R can activate cell survival, proliferation and differentiation pathways (10, 35). Of significance, monocyte progenitors from thermally injured and septic mice revealed an increase in receptors for macrophage colony-stimulating factor (MCSF) with a consequent shift to monocytopoiesis (39). CSF1R is the receptor for MCSF, which is also closely associated with MafB (18). We have previously documented that increase in transcription factor MafB after burn injury was, not only responsible for monocytopoiesis (16, 49), but also accountable for hematopoietic reprioritization at the commitment stage of CMPs (18). Furthermore, retroviral expression of MafB cDNA in human CD34+ hematopoietic progenitors was found to induce monocyte commitment (12). Similarly, overexpression of MafB was found to inhibit erythropoiesis through downregulation of transferrin receptor gene in yeast and the erythroblast cell line (41). In the present study, burn patients who received propranolol showed a concomitant reduction both in proportion and intensity of MafB expression in PBMC-derived MPPs. These results strongly support the reciprocal relation between MafB expression and erythroid progenitors in burn patients and further confirm that erythro-myeloid commitment is orchestrated by β1/β2-mediated response following burn injury.
The quiescent HSCs along with their capacity to maintain “stemness,” also have the ability to respond to injury and initiate a program of rapid proliferation. Moreover, bone marrow is innervated by sympathoadrenergic efferent nerve fibers (6). Sympathoadrenergic signals and chronic stress exposure are known to regulate hematopoietic stem and progenitor cell mobilization from bone marrow (14, 19). Bible et al. (5) have shown that propranolol administration in trauma patients reduced hematopoietic progenitor cell mobilization in the peripheral blood. Catecholamines particularly elevated noradrenaline levels after stress has been associated with proliferation of HSCs in the bone marrow (14). In the present study, the increase in LSKs after burn injury can be attributed to the increased sympathetic activity as evidenced by increase in TH levels in the brain, influencing the HSC niche in the bone marrow (4, 6). A similar result on β-adrenergic blockade was found by Spiegel et al. (42), when epinephrine administration to mice increased LSK cells in bone marrow, whereas propranolol treatment reduced this population. Moreover, in a dose-response relationship study, increasing doses of exogenous norepinephrine administered to uninjured rats via alzet pumps was found to decrease the generation of CFU-E and BFU-E colonies from TBM cells (34), supporting our burn model that reinforces adrenergic influence on MEP generation in mice as well as in burn patients. Moreover, the requirement of a critical threshold of adrenergic activation for normal erythropoiesis observed in the same study may explain the differential responses in MEPs to low-dose (10–30 mg) and high-dose (40–70 mg) propranolol treatments in burn patients. Nonetheless, why chemical sympathectomy with 6-OHDA seemed to have the same effect as norepinephrine administration in rats was not clear from the study of Penn et al. (34). Unlike propranolol that blocks the action of catecholamines, 6-OHDA is known to ablate tissue norepinephrine content by preventing the release of norepinephrine from nerve endings (axotomy) (44). On the basis of our present observations, we can speculate that, if adrenergic stimulation increases bone marrow LSKs, then 6-OHDA will hamper the production of LSKs, which in turn can affect the downstream BFU-Es and CFU-Es.
Propranolol-mediated increase in MEPs after burn is positively reflected in erythroblast development as Ter119 begins to be expressed on the cell surface from proerythroblasts onward to mature erythrocytes (20). Epo binding to its receptors is crucial for definitive erythropoiesis (26); we and others (9, 28, 37, 43) have shown that burn injury results in hyporesponsiveness to Epo attributable to a reduction in Epo-responsive cells affecting RBC production. Our previous study using similar PBMC-derived culture technique indicated a restriction in CFU-E generation and not in Epo-dependent proliferation in burn patients (48). In line with these observations, we now show Epo-independent commitment to MEPs, which is upstream of the CFU-E stage of erythropoiesis and is impaired in burn patients. The ultimate read out to validate the efficacy of propranolol in improving erythropoiesis is peripheral blood hemoglobin levels and the associated transfusion requirements, which are based on hemoglobin trigger. In the present study, hemoglobin levels did not change between the two groups at the time points studied, which may not truly reflect improved erythropoiesis because burn patients were followed only for 7 wk, which is one of the limitations. Similarly, although transfusion requirements trend toward less pRBC units with propranolol treatment, further stratification based on the number of operative procedures and length of stay is warranted for conclusive interpretation of implications in patient care. Another limitation is that the megakaryocyte vs. erythrocyte potential of propranolol-generated MEPs has not been tested in the present study. However, this can be ruled out by the specific action of exogenous Epo resulting in increased Ter119 cells; this further strengthens the action of propranolol in improving erythroid lineage commitment of bipotential MEPs. The fact that exogenous Epo did not affect the CD11b fraction in bone marrow validates that myelo-erythroid bifurcation is orchestrated by β1/β2-adrenergic mechanisms through MafB. Other models of psychological stress with sympathetic activity resulting in elevated catecholamine also demonstrate similar effect with propranolol in lowering CD11b+ cells (13, 17). Together, these results imply the role of catecholamines in myelo-erythroid commitment consequent to stressful and traumatic pathological conditions.
β-Adrenergic stimulation following burn dampens bone marrow erythropoiesis, reducing MEP production by regulating MafB expression. β-Blockade in burn patients can be efficacious as a potential therapeutic tool to mitigate MafB and restore the myelo-erythroid balance. Therefore, sequential intervention with Epo could be used to supplement the effect of propranolol and boost erythroblast proliferation in efforts to reduce transfusion requirements and rescue burn-induced anemia in severely burned patients otherwise hyporesponsive to recombinant human Epo (9, 29). A schematic diagram representing the mechanistic alterations in myelo-erythroid commitment pattern leading to RBC deficits after burn injury and rescue efforts with β-blockade is shown in Fig. 10.
Fig. 10.
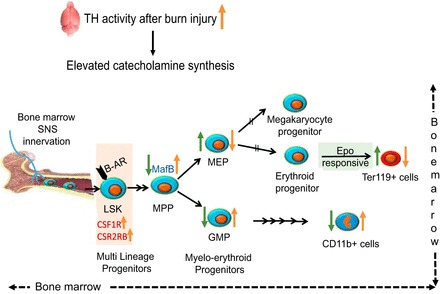
Schematic diagram representing the alterations in myelo-erythroid commitment pattern leading to red blood cell deficits after burn injury in the mouse model (trend shown in orange arrows). 15% TBSA in mouse scald burn model stimulates increased catecholamine synthesis as shown by increased TH levels in the brain, one of the most active organs of sympathetic nerve system. Bone marrow is highly innervated; LSKs in the bone marrow that express β-adrenergic receptor (β-AR) respond to increased catecholamines. Increased gene expression of CSF1R and CSF2RB in LSKs after burn likely gears them toward myeloid lineage. The MPPs through increased expression of MafB after burn injury differentiate away from erythroid progenitors (MEPs) and toward myeloid progenitors (GMPs). Administration of a β-AR blocker (PR) (trend shown in green arrows) reverses these effects by decreasing MafB expression, decreasing GMPs, and increasing MEP production. In line with progenitors, Ter119+ cells were increased after PR treatment, whereas mature myeloid cells represented by CD11b+ marker decreased. As MEPs are common progenitors to megakaryocytes and erythrocytes, their erythroid potential was verified by their responsiveness to Epo after PR treatment in burn mice.
Overall, our work provides important mechanistic clues and an alternative platform to study bone marrow erythropoiesis in burn patients. Obtaining bone marrow from burn patients is a barrier, but our innovative application of the culture system from PBMCs allows us, not only to evaluate the mechanisms that dictate erythroblast production in burn patients, but also to pave the way for translational research and the development of a test platform for future hematopoiesis-related drug efficacy studies. However, we acknowledge that it is a small sample size, and a larger patient population-based study is essential to further strengthen our results.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01DK097760-01 (to K. Muthumalaiappan) and NIH Training Grant T32 GM008750 (to R. Gamelli).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.H., N.B.J., P.C., and A.S. performed experiments; S.H., N.B.J., and K.M. analyzed data; S.H., N.B.J., and K.M. interpreted results of experiments; S.H. and K.M. prepared figures; S.H. drafted manuscript; M.J.M., R.S., R.L.G., and K.M. edited and revised manuscript; M.J.M., R.S., R.L.G., and K.M. approved final version of manuscript; K.M. conceived and designed the research.
REFERENCES
- 1.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197, 2000. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 2.Bateman AP, McArdle F, Walsh TS. Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med 37: 1906–1912, 2009. doi: 10.1097/CCM.0b013e3181a000cf. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, Kobilka BK. Differential cardioprotective/cardiotoxic effects mediated by β-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol 289: H2441–H2449, 2005. doi: 10.1152/ajpheart.00005.2005. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 107: 1747–1750, 2006. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bible LE, Pasupuleti LV, Alzate WD, Gore AV, Song KJ, Sifri ZC, Livingston DH, Mohr AM. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg 77: 54–60, 2014. doi: 10.1097/TA.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosentino M, Marino F, Maestroni GJ. Sympathoadrenergic modulation of hematopoiesis: A review of available evidence and of therapeutic perspectives. Front Cell Neurosci 9: 302, 2015. doi: 10.3389/fncel.2015.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curinga G, Jain A, Feldman M, Prosciak M, Phillips B, Milner S. Red blood cell transfusion following burn. Burns 37: 742–752, 2011. doi: 10.1016/j.burns.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508: 1–12, 2011. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deitch EA, Sittig KM. A serial study of the erythropoietic response to thermal injury. Ann Surg 217: 293–299, 1993. doi: 10.1097/00000658-199303000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle SE, Gasson JC. Characterization of the role of the human granulocyte-macrophage colony-stimulating factor receptor alpha subunit in the activation of JAK2 and STAT5. Blood 92: 867–876, 1998. [PubMed] [Google Scholar]
- 11.Feezor RJ, Baker HV, Mindrinos M, Hayden D, Tannahill CL, Brownstein BH, Fay A, MacMillan S, Laramie J, Xiao W, Moldawer LL, Cobb JP, Laudanski K, Miller-Graziano CL, Maier RV, Schoenfeld D, Davis RW, Tompkins RG; Inflammation and Host Response to Injury, Large-Scale Collaborative Research Program . Whole blood and leukocyte RNA isolation for gene expression analyses. Physiol Genomics 19: 247–254, 2004. doi: 10.1152/physiolgenomics.00020.2004. [DOI] [PubMed] [Google Scholar]
- 12.Gemelli C, Montanari M, Tenedini E, Zanocco Marani T, Vignudelli T, Siena M, Zini R, Salati S, Tagliafico E, Manfredini R, Grande A, Ferrari S. Virally mediated MafB transduction induces the monocyte commitment of human CD34+ hematopoietic stem/progenitor cells. Cell Death Differ 13: 1686–1696, 2006. doi: 10.1038/sj.cdd.4401860. [DOI] [PubMed] [Google Scholar]
- 13.Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun 26: 1150–1159, 2012. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med 20: 754–758, 2014. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon DN, Rodriguez NA, Diaz EC, Hegde S, Jennings K, Mlcak RP, Suri JS, Lee JO, Williams FN, Meyer W, Suman OE, Barrow RE, Jeschke MG, Finnerty CC. Long-term propranolol use in severely burned pediatric patients: A randomized controlled study. Ann Surg 256: 402–411, 2012. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell K, Posluszny J, He LK, Szilagyi A, Halerz J, Gamelli RL, Shankar R, Muthu K. High MafB expression following burn augments monocyte commitment and inhibits DC differentiation in hemopoietic progenitors. J Leukoc Biol 91: 69–81, 2012. doi: 10.1189/jlb.0711338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin J, Wang X, Wang Q, Guo X, Cao J, Zhang X, Zhu T, Zhang D, Wang W, Wang J, Shen B, Gao X, Shi Y, Zhang J. Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One 8: e74497, 2013. doi: 10.1371/journal.pone.0074497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson NB, Posluszny JA, He LK, Szilagyi A, Gamelli RL, Shankar R, Muthumalaiappan K. Perturbed MafB/GATA1 axis after burn trauma bares the potential mechanism for immune suppression and anemia of critical illness. J Leukoc Biol 100: 725–736, 2016. doi: 10.1189/jlb.1A0815-377R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124: 407–421, 2006. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol 109: 280–287, 2000. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 21.Koury MJ. Erythropoietin: The story of hypoxia and a finely regulated hematopoietic hormone. Exp Hematol 33: 1263–1270, 2005. doi: 10.1016/j.exphem.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock 33: 369–374, 2010. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwan P, Gomez M, Cartotto R. Safe and successful restriction of transfusion in burn patients. J Burn Care Res 27: 826–834, 2006. doi: 10.1097/01.BCR.0000245494.45125.3E. [DOI] [PubMed] [Google Scholar]
- 24.Laudanski K, Miller-Graziano C, Xiao W, Mindrinos MN, Richards DR, De A, Moldawer LL, Maier RV, Bankey P, Baker HV, Brownstein BH, Cobb JP, Calvano SE, Davis RW, Tompkins RG. Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways. Proc Natl Acad Sci USA 103: 15564–15569, 2006. doi: 10.1073/pnas.0607028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lelkes G, Bernát I. Electron microscopical study of the bone marrow of burned patients. Haematologia (Budap) 4: 295–301, 1970. [PubMed] [Google Scholar]
- 26.Lin CS, Lim SK, D’Agati V, Costantini F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev 10: 154–164, 1996. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 27.Lindenschmidt RC, Witschi HP. Propranolol-induced elevation of pulmonary collagen. J Pharmacol Exp Ther 232: 346–350, 1985. [PubMed] [Google Scholar]
- 28.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg 238: 748–753, 2003. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundy JB, Hetz K, Chung KK, Renz EM, White CE, King BT, Huzar T, Wolf SE, Blackbourne LH. Outcomes with the use of recombinant human erythropoietin in critically ill burn patients. Am Surg 76: 951–956, 2010. [PubMed] [Google Scholar]
- 30.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA 99: 11872–11877, 2002. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merryweather-Clarke AT, Atzberger A, Soneji S, Gray N, Clark K, Waugh C, McGowan SJ, Taylor S, Nandi AK, Wood WG, Roberts DJ, Higgs DR, Buckle VJ, Robson KJ. Global gene expression analysis of human erythroid progenitors. Blood 117: e96–e108, 2011. doi: 10.1182/blood-2010-07-290825. [DOI] [PubMed] [Google Scholar]
- 32.Muthu K, Iyer S, He LK, Szilagyi A, Gamelli RL, Shankar R, Jones SB. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol 186: 27–36, 2007. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmieri TL, Caruso DM, Foster KN, Cairns BA, Peck MD, Gamelli RL, Mozingo DW, Kagan RJ, Wahl W, Kemalyan NA, Fish JS, Gomez M, Sheridan RL, Faucher LD, Latenser BA, Gibran NS, Klein RL, Solem LD, Saffle JR, Morris SE, Jeng JC, Voigt D, Howard PA, Molitor F, Greenhalgh DG, American Burn Association Burn Multicenter Trials Group; American Burn Association Burn Multicenter Trials Group . Effect of blood transfusion on outcome after major burn injury: a multicenter study. Crit Care Med 34: 1602–1607, 2006. doi: 10.1097/01.CCM.0000217472.97524.0E. [DOI] [PubMed] [Google Scholar]
- 34.Penn A, Mohr AM, Shah SG, Sifri ZC, Kaiser VL, Rameshwar P, Livingston DH. Dose-response relationship between norepinephrine and erythropoiesis: Evidence for a critical threshold. J Surg Res 163: e85–e90, 2010. doi: 10.1016/j.jss.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perugini M, Brown AL, Salerno DG, Booker GW, Stojkoski C, Hercus TR, Lopez AF, Hibbs ML, Gonda TJ, D’Andrea RJ. Alternative modes of GM-CSF receptor activation revealed using activated mutants of the common beta-subunit. Blood 115: 3346–3353, 2010. doi: 10.1182/blood-2009-08-235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posluszny JA Jr, Conrad P, Halerz M, Shankar R, Gamelli RL. Classifying transfusions related to the anemia of critical illness in burn patients. J Trauma 71: 26–31, 2011. doi: 10.1097/TA.0b013e3181f2d9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posluszny JA Jr, Muthumalaiappan K, Kini AR, Szilagyi A, He LK, Li Y, Gamelli RL, Shankar R. Burn injury dampens erythroid cell production through reprioritizing bone marrow hematopoietic response. J Trauma 71: 1288–1296, 2011. doi: 10.1097/TA.0b013e31822e2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rieger MA, Schroeder T. Hematopoiesis. Cold Spring Harb Perspect Biol 4: a008250, 2012. doi: 10.1101/cshperspect.a008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santangelo S, Gamelli RL, Shankar R. Myeloid commitment shifts toward monocytopoiesis after thermal injury and sepsis. Ann Surg 233: 97–106, 2001. doi: 10.1097/00000658-200101000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahrara S, Sylvén C, Drvota V. Subtype specific downregulation of thyroid hormone receptor mRNA by beta-adrenoreceptor blockade in the myocardium. Biol Pharm Bull 23: 1303–1306, 2000. doi: 10.1248/bpb.23.1303. [DOI] [PubMed] [Google Scholar]
- 41.Sieweke MH, Tekotte H, Frampton J, Graf T. MafB represses erythroid genes and differentiation through direct interaction with c-Ets-1. Leukemia 11, Suppl 3: 486–488, 1997. [PubMed] [Google Scholar]
- 42.Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, Nagler A, Rubinstein M, Lapidot T. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol 8: 1123–1131, 2007. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 43.Still JM Jr, Belcher K, Law EJ, Thompson W, Jordan M, Lewis M, Saffle J, Hunt J, Purdue GF, Waymack JP, DeClement F, Kagan R, Chen A. A double-blinded prospective evaluation of recombinant human erythropoietin in acutely burned patients. J Trauma 38: 233–236, 1995. doi: 10.1097/00005373-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y, Shankar R, Gamboa M, Desai S, Gamelli RL, Jones SB. Norepinephrine modulates myelopoiesis after experimental thermal injury with sepsis. Ann Surg 233: 266–275, 2001. doi: 10.1097/00000658-200102000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker HL, Mason AD Jr. A standard animal burn. J Trauma 8: 1049–1051, 1968. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Wallner SF, Warren GH. The haematopoietic response to burning: An autopsy study. Burns 12: 22–27, 1985. doi: 10.1016/0305-4179(85)90179-2. [DOI] [PubMed] [Google Scholar]
- 47.Walsh TS, Saleh EE. Anaemia during critical illness. Br J Anaesth 97: 278–291, 2006. doi: 10.1093/bja/ael189. [DOI] [PubMed] [Google Scholar]
- 48.Williams KN, Szilagyi A, Conrad P, Halerz M, Kini AR, Li Y, Gamelli RL, Shankar R, Muthumalaiappan K. Peripheral blood mononuclear cell-derived erythroid progenitors and erythroblasts are decreased in burn patients. J Burn Care Res 34: 133–141, 2013. doi: 10.1097/BCR.0b013e3182642ccd. [DOI] [PubMed] [Google Scholar]
- 49.Williams KN, Szilagyi A, He LK, Conrad P, Halerz M, Gamelli RL, Shankar R, Muthumalaiappan K. Dendritic cell depletion in burn patients is regulated by MafB expression. J Burn Care Res 33: 747–758, 2012. doi: 10.1097/BCR.0b013e318250457f. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 83: 59–67, 1995. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 51.Yogore MG III, Boral L, Kowal-Vern A, Patel H, Brown S, Latenser BA. Use of blood bank services in a burn unit. J Burn Care Res 27: 835–841, 2006. doi: 10.1097/01.BCR.0000245418.73538.25. [DOI] [PubMed] [Google Scholar]
- 52.Zhang CC, Lodish HF. Cytokines regulating hematopoietic stem cell function. Curr Opin Hematol 15: 307–311, 2008. doi: 10.1097/MOH.0b013e3283007db5. [DOI] [PMC free article] [PubMed] [Google Scholar]


