Abstract
Exercise results in the rapid remodeling of skeletal muscle. This process is underpinned by acute and chronic changes in both gene and protein synthesis. In this short review we provide a brief summary of our current understanding regarding how exercise influences these processes as well as the subsequent impact on muscle protein turnover and resultant shift in muscle phenotype. We explore concepts of ribosomal biogenesis and the potential role of increased translational capacity vs. translational efficiency in contributing to muscular hypertrophy. We also examine whether high-intensity sprinting-type exercise promotes changes in protein turnover that lead to hypertrophy or merely a change in mitochondrial content. Finally, we propose novel areas for future study that will fill existing knowledge gaps in the fields of translational research and exercise science.
Keywords: resistance exercise training, high-intensity intermittent exercise, muscle protein synthesis, ribosomal biogenesis
the study of how exercise impacts human skeletal muscle protein turnover is a rapidly evolving area of scientific research, which is testament to the critical role that skeletal muscle plays in the development and maintenance of human health (72, 94). Here, we provide a brief overview of our current understanding of exercise-induced skeletal muscle adaptation. Specifically, we focus on the time-course change in the transcriptional response of skeletal muscle to exercise and how changes in gene expression relate to the synthesis of new muscle proteins and subsequent phenotypic adaptation. We refer primarily to human studies, but when appropriate, other experimental models are cited to substantiate our points.
Skeletal muscle is a highly plastic tissue, capable of adapting to fine changes in nutritional intake and contractile activity. For instance, resistance exercise results in a mild stimulation in rates of muscle protein breakdown (MPB) but a greater stimulation of the rates of muscle protein synthesis (MPS) (4, 62, 71). When resistance exercise is performed before protein ingestion, there is a synergistic combination of the two stimuli such that rates of MPS are stimulated over and above those of MPB (11, 12, 39). Thus repeated bouts of resistance exercise when coupled with protein ingestion result in the accretion of skeletal muscle protein referred to as hypertrophy (17). Importantly, by changing the nature of the exercise stimulus, it is possible to redirect the focus of the type of skeletal muscle proteins that are being synthesized during the recovery period. For example, we know that prolonged and repeated lower-load dynamic stimulation of skeletal muscle (i.e., endurance exercise training) results in an increase in the expression of mitochondrial genes (65), proteins (30, 44, 65), and ultimately enhanced mitochondrial content (35), leading to a shift toward an oxidative phenotype and improved fatigue resistance (31). Resistance exercise training also stimulates the transcription of genes and accrual of new muscle proteins (60, 63), but these genes and proteins are largely associated with the myofibrillar protein fraction (16, 92). However, during the early stages of exercise training, particularly in training naïve participants, there is a significant increase in the expression of genes common to both modalities of exercise (34, 88). It is only with sustained exercise training that there is a "fine tuning" of the transcriptome, the protein synthetic response, and then the proteome that gives rise to divergent hypertrophic and oxidative phenotypes (34, 56).
Transcriptional Responses to Exercise Training
The biological basis of exercise-induced phenotypic changes in skeletal muscle is that exercise stimulates repeated increases in mRNA expression resulting in enhanced translation of the protein and ultimately adaptive changes in muscle protein content (31, 56). The temporal pattern of these changes with respect to endurance and resistance exercise training is now becoming clearer. Exercise-induced mitochondrial biogenesis, for example, is a characteristic feature of endurance exercise training (30, 31, 85) and is underpinned by the coordinated upregulation of both mitochondrial and nuclear transcripts that encode for proteins involved in the electron transport chain (76–78) and lipid metabolism (36). These transcripts include peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α, nuclear respiratory factors (NRFs), and the mitochondrial transcription factor A (TFAM) (46, 47, 69). An acute bout of endurance exercise activates sensors of cellular stress (65, 95), decreases methylation of promoter regions (6), stimulates phosphorylation of mediators of translation initiation (92), and increases the expression of the aforementioned transcripts (PGC-1α, NRFs, and TFAM) (31, 65). The transient increase in the expression of genes in the hours following an acute endurance exercise bout provides the gene template that precedes the respective increase in protein content observed during exercise training (30, 65). Taking exercise-induced changes in PGC-1α as an example: a seminal study showed that an acute bout of endurance exercise in humans stimulated an increase in PGC-1α mRNA peaking ~10-fold above baseline at 4 h postexercise but returned to baseline levels the following day (65). Further endurance exercise sessions continued to result in increases in PGC-1α mRNA expression, but the magnitude of the increase progressively declined. Corresponding changes in PGC-1α protein content were not detectable until 24 h after the initial exercise bout. There was also a rapid upregulation in both citrate synthase and β-hydroxyacyl CoA dehydrogenase mRNA following the first exercise session; however, there was no significant change in their protein expression until 1 wk of training (65). Others have also demonstrated changes in PGC-1α mRNA coupled with delayed increases in mitochondrial content with short-term (2 wk) endurance exercise training (30). One caveat of these studies is that no direct measurements of mitochondrial protein synthesis were made; nevertheless, it is quite clear that, at least with endurance exercise training, mitochondrial adaptations occur in a tightly coordinated (mRNA to protein) and time (hours to days)-dependent manner (30, 65, 69).
Changes in Translational Efficiency and Capacity with Resistance Exercise
Compared with our understanding of transcriptional responses to endurance training, the corresponding picture with respect to resistance exercise is relatively less complete. What is known is that resistance exercise results in a phosphorylation/activation of the mechanistic target of rapamycin complex 1 (mTORC1) (5, 29, 41, 59). mTORC1 activation serves to enhance MPS by activating downstream protein kinases such as the ribosomal protein of 70-kDa S6 kinase 1 (p70S6K1) and 4E-binding protein-1 (4EBP1) that subsequently promote ribosomal binding to mRNA to initiate protein synthesis (38, 45, 55, 70), as well as upregulating the transcription of the translational machinery itself (mRNA, ribosomes) (19). Thus resistance exercise stimulates mTORC1 activity, promoting increases in the rates of myofibrillar MPS through both increased translational efficiency (protein synthesized per unit of mRNA) and translational capacity (the abundance of the translational machinery; ribosomes) (5, 29).
The relative contribution of resistance exercise-induced changes in translational capacity and translational efficiency to the ensuing hypertrophic phenotype has yet to be fully defined. To date, many studies informing the time-course change in translational responses to resistance exercise are in fact conducted in rodents (18, 43, 91). One of the most recent, but also comprehensive, studies using this model has demonstrated that resistance exercise results in a transient increase in mTORC1 activity (up to 18 h postexercise) but a more prolonged stimulation of protein synthesis (0–36 h) (91). Interestingly, readouts of ribosomal biogenesis were also increased at 12–36 h of recovery, which preceded a trend for an increase in total RNA at 18–36 h. Given that ~85% of RNA is ribosomal RNA (rRNA), it is possible that while the initial increase in MPS was predominantly attributed to mTORC1-mediated translational efficiency, the sustained elevations in MPS during the latter stages of recovery might have been supported by increased translational capacity. It is, however, important to note that treatment with the mTORC1 inhibitor rapamycin significantly reduced RNA content as well as the expression of the internal transcribed spacer 1 (estimate of ribosomal DNA transcription rate) at 18 h but only marginally reduced rates of MPS. This finding suggests that other factors, in addition to mTORC1 activity and improved translational capacity, contributed to sustaining rates of MPS during recovery in this study (91).
As a result of important proof-of-concept data generated in rodent models of resistance exercise, the response of the ribosomal pool to resistance exercise in humans is beginning to be understood (32, 33, 83, 84). Studies have shown that acute resistance exercise increases readouts of ribosomal biogenesis as well total RNA in the hours following a single resistance exercise bout (33, 63, 83). Episodic increases in the expression and content of ribosomal proteins with each successive bout of resistance exercise have been proposed to support long-term gains in skeletal muscle mass with resistance exercise training (14, 91). Indeed, it is known that even two bouts of resistance exercise are sufficient to increase total RNA content (10). Increases in total RNA and 45S pre-rRNA have also been reported following 3 wk (14) and 6 wk (63) of resistance exercise training respectively, but these increases may be short-lived since ribosomal gene expression (45S pre-rRNA) and ribosomal transcription (c-Myc) expression have been shown to return to baseline levels after 12 wk of training (63). Similarly, others have demonstrated that in response to 20 wk of resistance exercise training there is a downregulation in the expression of rRNA in individuals who displayed the greatest magnitude of muscle hypertrophy (67). Reconciling the incongruent findings between studies is difficult due to obvious differences in experimental designs and participant cohorts. However, we speculate that at the onset of resistance training there is an initial increase in ribosomal capacity to support an overall "nonspecific" protein remodeling response followed by attenuation of ribosomal content over time that accompanies a more "specific" response (Fig. 1). This concept differs slightly from those primarily based on rodent studies of hypertrophy (90) that have employed a synergist ablation model of overload where a constant load is applied and magnitudes of up to 40% hypertrophy are achieved within a matter of days (40). Such magnitudes of muscle growth are hardly indicative of those observed in human models of resistance exercise training and it is therefore unlikely that they would share the same biological signature of hypertrophy. More work is now needed that brings together a detailed time course of gene transcription, ribosomal protein content, and muscle hypertrophy during a period of resistance exercise training in humans.
Fig. 1.
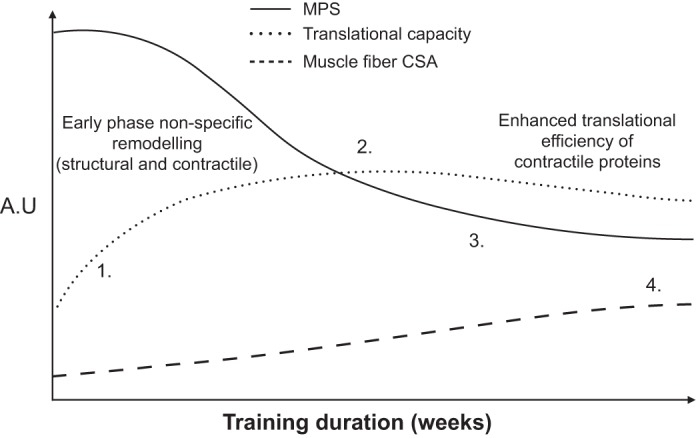
A proposed framework of changes in muscle protein synthesis (MPS), translational capacity, whole muscle, and muscle fiber cross-sectional area (CSA) in response to resistance exercise training. The overarching concept is that initial increases in MPS are a biological response to support remodeling of damaged muscle protein and eventually muscle hypertrophy. 1: The early stage increases in MPS are sustained partly by concomitant elevated translational capacity to support the remodeling of damaged structural and contractile elements of the muscle proteome. 2: Then, after the attenuation of exercise-induced muscle damage, there is a reduction in contribution of MPS to the remodeling of proteins related to the structural and architectural apparatus toward contractile muscle proteins. 3: After a period of time, the rates of MPS are subsequently regulated by the adaptive increase in translational efficiency. 4: This results in a detectable increase in skeletal muscle size and mass. All of these responses are deigned to support an expansion of the muscle protein pool, i.e., single fiber CSA. A.U., arbitrary units.
Changes in Muscle Protein Synthesis with Resistance Exercise Training
An acute bout of resistance exercise is known to stimulate rates of MPS (68). It is only following the ingestion of protein that rates of MPS exceed those of muscle protein breakdown, resulting in a positive muscle protein balance and net protein accretion (11, 12). Initial work from our laboratory (61) and later by others (93), demonstrated that the ingestion of 20 g of high-quality protein is sufficient to maximize postexercise rates of MPS during 4 h of recovery, with the consumption of 40 g eliciting no further increase in MPS but instead, stimulating whole body leucine oxidation (61). Extending these findings, Areta et al. (2) showed that the consumption of 20 g of high-quality protein every 3 h was superior for the stimulation of MPS over a 12-h recovery period as compared with ingesting 10 g every 1.5 h or 40 g every 6 h. However, an important caveat of these reports is that the exercise protocols were limited to lower body exercise regimens. The argument could therefore be made that in exercise protocols that utilize whole body protocols, the increase in contractile skeletal muscle mass may create a greater demand for ingested protein to remodel skeletal muscle proteins. A recent study, in which healthy young resistance-trained men performed whole body resistance exercise, showed that the ingestion of 40 g of protein resulted in a 16% greater stimulation of MPS during 5 h of recovery as compared with 20 g (54). Taken together with the results of previous work showing that the ingestion of 20 g of protein was sufficient saturate postexercise rates of MPS with lower body resistance exercise (2, 61, 93), these data (54) would suggest that the ingested protein dose to maximize rates of MPS following whole body resistance exercise could be greater than 20 g. However, it is important to note that this thesis was not specifically tested in the aforementioned study (54).
Although rates of MPS are elevated in the hours after a bout of resistance exercise, it is important to note that skeletal muscle can retain sensitivity to the anabolic influence of protein ingestion for up to 24 h (15) and likely up to at least 48 h (21). Moreover, it is unlikely that this increase is solely directed toward accretion of proteins to result in hypertrophy. Rather, the elevated rates of MPS during the early stages of recovery from resistance exercise are likely to be indicative of a greater remodeling of contractile and structural proteins. In humans, both MPS and MPB are known to be higher in the hours and days following resistance exercise with rates of MPS exceeding those of breakdown (68). Due to technical limitations associated with the measurement of MPB outside of a laboratory setting, our understanding of how rates of MPB change in the days during recovery from resistance exercise is limited. However, it has been shown that absolute and fractional integrated rates of MPS are increased early (i.e., <3 wk) during resistance training but are adaptively reduced over time (14, 24). We reported that in participants who performed resistance exercise training for 10 wk that integrated rates of MPS measured 48 h following a single bout of resistance exercise were significantly higher following the first bout of exercise but were then lower at the 3rd and 10th week of training (24). Moreover, there was a corresponding increase in Z-line streaming (indicative of muscle damage) after the first bout of resistance exercise training that was also diminished over the duration of the resistance exercise training protocol. When MPS was normalized to the area of Z-band streaming in an attempt to account for the relative contribution of MPS to repair of damaged proteins, the relative increase in MPS was no different across the resistance exercise training program. Based on these data (24), it would therefore appear that the initial increase in the rates of MPS during resistance exercise training is predominantly directed toward the remodeling/repair of damaged skeletal muscle protein, after which, MPS is subsequently refined, and targeted toward muscle hypertrophy (52). This thesis may also support our aforementioned assertion that there is an initial upregulation of translational capacity immediately following the onset of resistance exercise training to support elevated protein turnover that is then attenuated with more prolonged training (67).
Time Course of Muscle Hypertrophy with Resistance Exercise Training
Skeletal muscle is in a positive state of protein balance immediately following exercise when combined with protein feeding (11, 12, 68). Therefore, technically speaking, muscle hypertrophy, which we define as increases in the abundance of contractile units, commences during this time. However, despite the positive state of muscle protein balance after exercise and feeding, changes in skeletal muscle fiber cross-sectional area (CSA) assessed using histochemical staining are generally not detectable until at least 6–7 wk of training (42) (Fig. 2). One explanation for this disconnect between measurements of protein balance and CSA could be that resistance exercise leads to increased myofibrillar density (0–6 wk) followed by a delayed expansion of CSA. Indeed, the changes in the pennation angle of various muscles that occur rapidly with resistance exercise (14) training would allow a greater amount of contractile units to be placed in series into a myofiber without influencing muscle fiber CSA. However, as the myofibrils account for ~80% of fiber CSA, it is unlikely that sufficient changes in either the pennation angle or myofibrillar density would occur unless they were to negatively influence metabolic processes or myofilament interaction. It is more likely that the inability to measure changes in fiber CSA until the latter stages of resistance exercise training are attributed to a lack of measurement accuracy and statistical power due to low participant numbers. The latter point is particularly relevant when considering the heterogeneity in the hypertrophic response to training between subjects (22, 48), a factor highly dependent on training status (1), dietary intake (62), and potentially genetic predisposition (13). We acknowledge studies using advanced ultrasonography and magnetic resonance imaging of whole muscle CSA have reported significant increases in muscle CSA in response to resistance exercise training within 2–3 wk of training onset (14, 28, 80). However, recent data show that early increases in whole muscle CSA may largely be accounted for by damage-induced muscle swelling (24–26). It is also known that connective tissue mass may increase during resistance exercise training confounding interpretation of these data (14, 28, 80). Studies employing larger subject numbers may improve detection limits, but we would posit that true skeletal muscle fiber hypertrophy with resistance training is still unlikely to be detectable until at least 5–6 wk of resistance training.
Fig. 2.
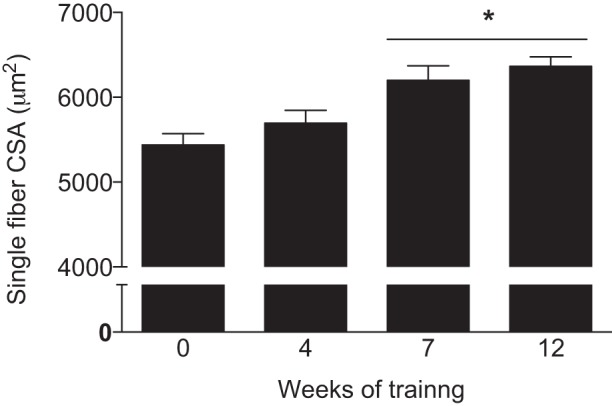
Redrawn with permission from Goreham et al. (42). Mean fiber area in response to resistance exercise training in untrained males (n = 7). Values are means ± SE. *Significantly different from week 0 (P < 0.05).
High-Intensity Interval Exercise and Muscle Protein Turnover
Exercise is not only employed as a means to enhance athletic performance but also to lower risk of chronic disease. While resistance- and endurance-type exercise training confer independent health benefits, other forms of exercise also have a significant impact of muscle protein turnover. High-intensity interval exercise (HIIE), used here to encompass both HIIE and sprint interval exercise (SIE), is known to increase cardiorespiratory fitness (37, 86) and stimulate muscle mitochondrial biogenesis (53, 74). The impact that HIIE has on muscle ultrastructural protein remodeling and/or hypertrophy is, however, yet to be fully characterized. It has been shown that myofibrillar MPS is elevated, although not to the same degree as that observed following resistance exercise, at 24 and 48 h following a bout of HIIE in untrained older men (8). Intriguingly, HIIE (and not resistance or aerobic exercise) was the only exercise modality to stimulate an increase in sarcoplasmic MPS, which the authors speculated was the result of increased mitochondrial protein synthesis (8). Furthermore, while the effect of SIE training on MPS was not specifically examined, integrative mixed-muscle and cytosolic muscle protein synthesis, with a trend for muscle mitochondrial protein synthesis, was greater in men than women during 3 wk of SIE (74). While these data do not necessarily indicate that MPS was increased in response to SIE, increases in citrate synthase and PGC-1α protein content in response to SIE training in both men and women were suggestive of increased mitochondrial protein synthesis during the period of SIE training (74). Taken together the results of these two trials (8, 74) are indicative that HIIE can stimulate MPS, particularly mitochondrial protein synthesis and allude, at least in older men, to a role for HIIE training to induce myofibrillar protein remodeling.
Despite an increase in myofibrillar MPS in response to acute HIIE in older men, short-term (≤6 wk) trials examining the effect of HIIE on muscle fiber CSA have not found that HIIE induces muscle hypertrophy (50, 51, 79). Joanisse et al. (51) reported no increase in type I nor II muscle fiber CSA and no expansion of the satellite cell pool in response to 6 wk of HIIE training, which can result in muscle hypertrophy (66, 87). Interestingly, despite no increase in satellite cell number, the authors did report an increase in the number of differentiating and terminally differentiating satellite cells following HIIE training (51), which they proposed was indicative of a role of satellite cells in muscle fiber remodeling. Furthermore, in response to HIIE training there is an increase in the number of satellite cells associated with hybrid muscle fibers (those expressing both myosin heavy chain I and II) as well as a greater number of differentiating satellite cells and centrally located nuclei associated with hybrid, compared with type I or II muscle fibers. Such findings provide further experimental evidence to support a role for HIIE to induce muscle fiber remodeling (50). Given the absence of HIIE-induced muscle hypertrophy following short-term training regimes, the increase in myofibrillar MPS seen in exercise-naïve older men is likely contributing to muscle fiber protein remodeling rather than protein accretion. While we presume, as detailed above, that increases in myofibrillar MPS early during HIIE training are contributing to muscle fiber remodeling, to date no study has examined the time course of the MPS response with HIIE training. Nonetheless, before examining time-course changes in MPS induced by HIIE, the effect of HIIE to increase myofibrillar MPS needs to be confirmed in young individuals. Furthermore, an examination of the longer-term (> 6 wk) effects of HIIE on muscle fiber CSA would be informative.
The Role of Easily Releasable Myofilaments in Skeletal Muscle Protein Turnover
As we have discussed, although acute gene and translational responses to exercise are a fundamental part of the remodeling process, there is often a poor correlation between the magnitude of these responses and actual rates of MPS (2, 58). Therefore, to gain a more accurate understanding of how exercise influences skeletal muscle remodeling, researchers often rely on direct measurements of MPS in conjunction with readouts of translational and transcriptional control (20, 56). However, the biological processes by which newly synthesized myofibrillar proteins are incorporated into the functional myofibrillar protein lattice still remain largely unknown. Studies in animal models have alluded to the existence of a group of myofilaments termed "easily releasable myofilaments" (23, 64). This group of myofilaments appear to act as intermediates and are readily released and degraded in response to disuse or starvation to release amino acids for gluconeogenesis (7, 23, 64). If these fibers are readily released in times of atrophy, it is conceivable that they also are rapidly constructed in times of growth in humans. To our knowledge, there are no data relating to exercise-induced changes in the turnover of these filaments in human models of exercise training. It has been suggested that following translation, easily releasable myofilaments are constructed in the sarcoplasm then move (via an undefined mechanism) to the lattice where they exchange with other myofibrillar proteins (64). It is important to consider that the spacing between the various elements of the myofibrillar lattice can vary from 20 to 50 nm (9). Therefore, while small molecular mass proteins such as troponin C (~7.5-nm long, ~2.5-nm wide) (73) would have little problem navigating through the lattice, the likelihood that larger proteins such as myosin (~160- to 165-nm long), and titin (2,500–3,000 kDa, >1-µm long) (49) would be capable of simply diffusing into the preexisting protein lattice requires experimental corroboration. More work in humans is now needed to identify the role(s) of easily releasable myofilaments and how they respond to changes in contractile activity. Such data may provide important information for not only the examination of resistance exercised-induced muscle growth but also the etiology of muscle loss during periods of disease and muscle disuse.
Conclusion
By combining proof-of-principle studies in animals with clinically relevant human work, in recent years our understanding of the molecular and cellular processes by which exercise alters skeletal muscle phenotype has been significantly advanced. Nevertheless, there are still a number of important questions that remain unanswered. For instance, how acute changes in gene expression and ribosomal proteins relate to skeletal muscle growth is still unknown. Novel technological developments such as the ability to assess the fractional synthetic rate of individual muscle proteins (81, 82) in conjunction with nonbiased microarray analysis of gene expression (56) will no doubt provide critical information in this area. Another important question that remains unanswered is exactly what are the processes that regulate the deposition of newly synthesized muscle proteins into the myofibrillar lattice following translation/termination and are these processes altered by age, sex, and/or disease? Certainly existing studies in animal models of research suggest a potential role for easily releasable myofilaments (23, 64), but more work is now needed in humans to corroborate these interesting and potentially clinically relevant data. Finally, there are a number of other factors such as changes in miRNA activity (27, 96) that impact posttranscriptional responses to exercise, leading to a fundamental disconnect between changes in gene expression and protein abundance (89). Future studies that elucidate these processes also promise to enhance our understanding regarding the role of exercise in skeletal muscle biology.
AUTHOR CONTRIBUTIONS
C.M. prepared figures; C.M., M.C.D., and S.M.P. drafted manuscript; C.M., M.C.D., and S.M.P. edited and revised manuscript; C.M., M.C.D., and S.M.P. approved final version of manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
GRANTS
S. M. Phillips acknowledges the Canadian Institutes for Health Research, the National Science and Engineering Research Council of Canada, and the Canada Research Chairs Program for support during the completion of this work.
REFERENCES
- 1.Alway SE, Grumbt WH, Stray-Gundersen J, Gonyea WJ. Effects of resistance training on elbow flexors of highly competitive bodybuilders. J Appl Physiol (1985) 72: 1512–1521, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 591: 2319–2331, 2013. doi: 10.1113/jphysiol.2012.244897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 5.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15: 405–411, 2012. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Belcastro AN, Scrubb J, Gilchrist JS. Regulation of ATP-stimulated releasable myofilaments from cardiac and skeletal muscle myofibrils. Mol Cell Biochem 103: 113–120, 1991. doi: 10.1007/BF00227477. [DOI] [PubMed] [Google Scholar]
- 8.Bell KE, Séguin C, Parise G, Baker SK, Phillips SM. Day-to-day changes in muscle protein synthesis in recovery from resistance, aerobic, and high-intensity interval exercise in older men. J Gerontol A Biol Sci Med Sci 70: 1024–1029, 2015. doi: 10.1093/gerona/glu313. [DOI] [PubMed] [Google Scholar]
- 9.Bennett P, Craig R, Starr R, Offer G. The ultrastructural location of C-protein, X-protein and H-protein in rabbit muscle. J Muscle Res Cell Motil 7: 550–567, 1986. doi: 10.1007/BF01753571. [DOI] [PubMed] [Google Scholar]
- 10.Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol (1985) 98: 482–488, 2005. doi: 10.1152/japplphysiol.00895.2004. [DOI] [PubMed] [Google Scholar]
- 11.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinl Metab 268: E514–E520, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab 273: E122–E129, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard C, Rankinen T, Timmons JA. Genomics and genetics in the biology of adaptation to exercise. Compr Physiol 1: 1603–1648, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 29: 4485–4496, 2015. doi: 10.1096/fj.15-273755. [DOI] [PubMed] [Google Scholar]
- 15.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141: 568–573, 2011. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- 16.Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 88: 50–60, 2002. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- 17.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 96: 1454–1464, 2012. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 18.Chaillou T, Kirby TJ, McCarthy JJ. Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. J Cell Physiol 229: 1584–1594, 2014. doi: 10.1002/jcp.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauvin C, Koka V, Nouschi A, Mieulet V, Hoareau-Aveilla C, Dreazen A, Cagnard N, Carpentier W, Kiss T, Meyuhas O, Pende M. Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene 33: 474–483, 2014. doi: 10.1038/onc.2012.606. [DOI] [PubMed] [Google Scholar]
- 20.Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 590: 2751–2765, 2012. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Churchward-Venne TA, Burd NA, Phillips SM. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab (Lond) 9: 40, 2012. doi: 10.1186/1743-7075-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churchward-Venne TA, Tieland M, Verdijk LB, Leenders M, Dirks ML, de Groot LC, van Loon LJ. There are no nonresponders to resistance-type exercise training in older men and women. J Am Med Dir Assoc 16: 400–411, 2015. doi: 10.1016/j.jamda.2015.01.071. [DOI] [PubMed] [Google Scholar]
- 23.Dahlmann B, Rutschmann M, Reinauer H. Effect of starvation or treatment with corticosterone on the amount of easily releasable myofilaments in rat skeletal muscles. Biochem J 234: 659–664, 1986. doi: 10.1042/bj2340659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrão ME, Jannig PR, Costa LA, Bacurau AV, Snijders T, Parise G, Tricoli V, Roschel H, Ugrinowitsch C. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 594: 5209–5222, 2016. doi: 10.1113/JP272472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damas F, Phillips SM, Lixandrão ME, Vechin FC, Libardi CA, Roschel H, Tricoli V, Ugrinowitsch C. Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol 116: 49–56, 2016. doi: 10.1007/s00421-015-3243-4. [DOI] [PubMed] [Google Scholar]
- 26.Damas F, Phillips SM, Lixandrão ME, Vechin FC, Libardi CA, Roschel H, Tricoli V, Ugrinowitsch C. An inability to distinguish edematous swelling from true hypertrophy still prevents a completely accurate interpretation of the time course of muscle hypertrophy. Eur J Appl Physiol 116: 445–446, 2016. doi: 10.1007/s00421-015-3287-5. [DOI] [PubMed] [Google Scholar]
- 27.Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol (1985) 110: 309–317, 2011. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- 28.DeFreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR II. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol 111: 2785–2790, 2011. doi: 10.1007/s00421-011-1905-4. [DOI] [PubMed] [Google Scholar]
- 29.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan B, O’Connor PL, Zierath JR, O’Gorman DJ. Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training in human skeletal muscle. PLoS One 8: e74098, 2013. doi: 10.1371/journal.pone.0074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309: E72–E83, 2015. doi: 10.1152/ajpendo.00050.2015. [DOI] [PubMed] [Google Scholar]
- 33.Figueiredo VC, Roberts LA, Markworth JF, Barnett MP, Coombes JS, Raastad T, Peake JM, Cameron-Smith D. Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiol Rep 4: e12670, 2016. doi: 10.14814/phy2.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flück M, Hoppeler H. Molecular basis of skeletal muscle plasticity--from gene to form and function. Rev Physiol Biochem Pharmacol 146: 159–216, 2003. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- 35.Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575: 901–911, 2006. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res 92: 518–524, 2003. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 37.Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity (Silver Spring) 21: 2249–2255, 2013. doi: 10.1002/oby.20379. [DOI] [PubMed] [Google Scholar]
- 38.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 13: 1422–1437, 1999. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 299: R533–R540, 2010. doi: 10.1152/ajpregu.00077.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg AL, Etlinger JD, Goldspink DF, Jablecki C. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports 7: 185–198, 1975. [PubMed] [Google Scholar]
- 41.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589: 5485–5501, 2011. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goreham C, Green HJ, Ball-Burnett M, Ranney D. High-resistance training and muscle metabolism during prolonged exercise. Am J Physiol Endocrinol Metab 276: E489–E496, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Hamosch M, Lesch M, Baron J, Kaufman S. Enhanced protein synthesis in a cell-free system from hypertrophied skeletal muscle. Science 157: 935–937, 1967. doi: 10.1126/science.157.3791.935. [DOI] [PubMed] [Google Scholar]
- 44.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967. [PubMed] [Google Scholar]
- 45.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569–580, 2005. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol (1985) 90: 1137–1157, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Hood DA, Tryon LD, Vainshtein A, Memme J, Chen C, Pauly M, Crilly MJ, Carter H. Exercise and the regulation of mitochondrial yurnover. Prog Mol Biol Transl Sci 135: 99–127, 2015. doi: 10.1016/bs.pmbts.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37: 964–972, 2005. [PubMed] [Google Scholar]
- 49.Hughes DC, Wallace MA, Baar K. Effects of aging, exercise and disease on force transfer in skeletal muscle. Am J Physiol Endocrinol Metab 309: E1–E10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joanisse S, Gillen JB, Bellamy LM, McKay BR, Tarnopolsky MA, Gibala MJ, Parise G. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J 27: 4596–4605, 2013. doi: 10.1096/fj.13-229799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joanisse S, McKay BR, Nederveen JP, Scribbans TD, Gurd BJ, Gillen JB, Gibala MJ, Tarnopolsky M, Parise G. Satellite cell activity, without expansion, after nonhypertrophic stimuli. Am J Physiol Regul Integr Comp Physiol 309: R1101–R1111, 2015. doi: 10.1152/ajpregu.00249.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurent GJ, Millward DJ. Protein turnover during skeletal muscle hypertrophy. Fed Proc 39: 42–47, 1980. [PubMed] [Google Scholar]
- 53.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588: 1011–1022, 2010. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, Lawrence CE, Wallis GA, Tipton KD. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep 4: e12893, 2016. doi: 10.14814/phy2.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol 15: 4990–4997, 1995. doi: 10.1128/MCB.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahoney DJ, Tarnopolsky MA. Understanding skeletal muscle adaptation to exercise training in humans: contributions from microarray studies. Phys Med Rehabil Clin N Am 16: 859–873, 2005. doi: 10.1016/j.pmr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 57.McGlory C, Phillips SM. Assessing the regulation of skeletal muscle plasticity in response to protein ingestion and resistance exercise: recent developments. Curr Opin Clin Nutr Metab Care 17: 412–417, 2014. doi: 10.1097/MCO.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 58.McGlory C, Wardle SL, Macnaughton LS, Witard OC, Scott F, Dick J, Bell JG, Phillips SM, Galloway SD, Hamilton DL, Tipton KD. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol Rep 4: e12715, 2016. doi: 10.14814/phy2.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGlory C, White A, Treins C, Drust B, Close GL, Maclaren DP, Campbell IT, Philp A, Schenk S, Morton JP, Hamilton DL. Application of the [γ-32P] ATP kinase assay to study anabolic signaling in human skeletal muscle. J Appl Physiol (1985) 116: 504–513, 2014. doi: 10.1152/japplphysiol.01072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell CJ, Churchward-Venne TA, West DWD, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol (1985) 113: 71–77, 2012. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 62.Morton RW, McGlory C, Phillips SM. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front Physiol 6: 245, 2015. doi: 10.3389/fphys.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nader GA, von Walden F, Liu C, Lindvall J, Gutmann L, Pistilli EE, Gordon PM. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (1985) 116: 693–702, 2014. doi: 10.1152/japplphysiol.01366.2013. [DOI] [PubMed] [Google Scholar]
- 64.Neti G, Novak SM, Thompson VF, Goll DE. Properties of easily releasable myofilaments: are they the first step in myofibrillar protein turnover? Am J Physiol Cell Physiol 296: C1383–C1390, 2009. doi: 10.1152/ajpcell.00022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 104: 1736–1742, 2008. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 67.Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA, Atherton PJ. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet 9: e1003389, 2013. doi: 10.1371/journal.pgen.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab 279: E806–E814, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol 31: 43–57, 1999. doi: 10.1016/S1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 71.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 63: 519–523, 1982. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz JR, Sui X, Lobelo F, Morrow JR, Jackson AW, Sjöström M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. BMJ 337: a439, 2008. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Satyshur KA, Rao ST, Pyzalska D, Drendel W, Greaser M, Sundaralingam M. Refined structure of chicken skeletal muscle troponin C in the two-calcium state at 2-A resolution. J Biol Chem 263: 1628–1647, 1988. [PubMed] [Google Scholar]
- 74.Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC, Paris HL, Szallar SE, Wood LM, Peelor FF III, Holmes WE, Hellerstein MK, Bell C, Hamilton KL, Miller BF. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28: 2705–2714, 2014. doi: 10.1096/fj.13-246595. [DOI] [PubMed] [Google Scholar]
- 76.Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576: 1–14, 2002. doi: 10.1016/S0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 77.Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci 1147: 321–334, 2008. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem 97: 673–683, 2006. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- 79.Schmitt J, Lindner N, Reuss-Borst M, Holmberg HC, Sperlich B. A 3-week multimodal intervention involving high-intensity interval training in female cancer survivors: a randomized controlled trial. Physiol Rep 4: e12693, 2016. doi: 10.14814/phy2.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol (1985) 102: 368–373, 2007. doi: 10.1152/japplphysiol.00789.2006. [DOI] [PubMed] [Google Scholar]
- 81.Shankaran M, King CL, Angel TE, Holmes WE, Li KW, Colangelo M, Price JC, Turner SM, Bell C, Hamilton KL, Miller BF, Hellerstein MK. Circulating protein synthesis rates reveal skeletal muscle proteome dynamics. J Clin Invest 126: 288–302, 2016. doi: 10.1172/JCI79639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shankaran M, Shearer TW, Stimpson SA, Turner SM, King C, Wong PY, Shen Y, Turnbull PS, Kramer F, Clifton L, Russell A, Hellerstein MK, Evans WJ. Proteome-wide muscle protein fractional synthesis rates predict muscle mass gain in response to a selective androgen receptor modulator in rats. Am J Physiol Endocrinol Metab 310: E405–E417, 2016. doi: 10.1152/ajpendo.00257.2015. [DOI] [PubMed] [Google Scholar]
- 83.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stec MJ, Mayhew DL, Bamman MM. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol (1985) 119: 851–857, 2015. doi: 10.1152/japplphysiol.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol (1985) 102: 1439–1447, 2007. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- 86.Tjønna AE, Leinan IM, Bartnes AT, Jenssen BM, Gibala MJ, Winett RA, Wisløff U. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PLoS One 8: e65382, 2013. doi: 10.1371/journal.pone.0065382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verdijk LB, Gleeson BG, Jonkers RAM, Meijer K, Savelberg HH, Dendale P, van Loon LJC. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 64: 332–339, 2009. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vissing K, Schjerling P. Simplified data access on human skeletal muscle transcriptome responses to differentiated exercise. Sci Data 1: 140041, 2014. doi: 10.1038/sdata.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13: 227–232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wen Y, Alimov AP, McCarthy JJ. Ribosome biogenesis is necessary for skeletal muscle hypertrophy. Exerc Sport Sci Rev 44: 110–115, 2016. doi: 10.1249/JES.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.West DW, Baehr LM, Marcotte GR, Chason CM, Tolento L, Gomes AV, Bodine SC, Baar K. Acute resistance exercise activates rapamycin-sensitive and -insensitive mechanisms that control translational activity and capacity in skeletal muscle. J Physiol 594: 453–468, 2016. doi: 10.1113/JP271365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99: 86–95, 2014. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- 94.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006. [DOI] [PubMed] [Google Scholar]
- 95.Yu M, Stepto NK, Chibalin AV, Fryer LG, Carling D, Krook A, Hawley JA, Zierath JR. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. J Physiol 546: 327–335, 2003. doi: 10.1113/jphysiol.2002.034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zacharewicz E, Lamon S, Russell AP. MicroRNAs in skeletal muscle and their regulation with exercise, ageing, and disease. Front Physiol 4: 266, 2013. doi: 10.3389/fphys.2013.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]


