Abstract
Cholesterol homeostasis relies on an intricate network of cellular processes whose deregulation in response to Western type high-fat/cholesterol diets can lead to several life-threatening pathologies. Significant advances have been made in resolving the molecular identity and regulatory function of transcription factors sensitive to fat, cholesterol, or bile acids, but whether body senses the presence of both fat and cholesterol simultaneously is not known. Assessing the impact of a high-fat/cholesterol load, rather than an individual component alone, on cholesterol homeostasis is more physiologically relevant because Western diets deliver both fat and cholesterol at the same time. Moreover, dietary fat and dietary cholesterol are reported to act synergistically to impair liver cholesterol homeostasis. A key insight into the role of protein kinase C-β (PKCβ) in hepatic adaptation to high-fat/cholesterol diets was gained recently through the use of knockout mice. The emerging evidence indicates that PKCβ is an important regulator of cholesterol homeostasis that ensures normal adaptation to high-fat/cholesterol intake. Consistent with this function, high-fat/cholesterol diets induce PKCβ expression and signaling in the intestine and liver, while systemic PKCβ deficiency promotes accumulation of cholesterol in the liver and bile. PKCβ disruption results in profound dysregulation of hepatic cholesterol and bile homeostasis and imparts sensitivity to cholesterol gallstone formation. The available results support involvement of a two-pronged mechanism by which intestine and liver PKCβ signaling converge on liver ERK1/2 to dictate diet-induced cholesterol and bile acid homeostasis. Collectively, PKCβ is an integrator of dietary fat/cholesterol signal and mediates changes to cholesterol homeostasis.
Keywords: high-fat/cholesterol diets, protein kinase Cβ induction, FGF15, extracellular/mitogen-activated protein kinase, hepatic cholesterol homeostasis, cholesterol associated diseases
the western diet is an important environmental factor that predisposes to various metabolic diseases, including atherosclerosis and cholesterol gallstone formation (24, 44, 47, 73, 83, 99). Such diet usually consists of complex combinations of lipids (cholesterol, fat, etc.) that might act synergistically with intestinal bile acids to aggravate dysregulation of cholesterol homeostasis (43, 85, 90, 94, 100). Abnormal levels of cholesterol can have serious cellular consequences and can affect onset of the above-mentioned cholesterol-related metabolic diseases (24, 44, 83).
The liver plays an important role in maintaining body’s cholesterol homeostasis by regulating absorption and synthesis to prevent net accumulation of cholesterol in the plasma and tissues (28, 29). The small intestine also has a major impact on cholesterol homeostasis at the level of cholesterol and bile absorption, fecal excretion, and de novo synthesis. Dietary fats have been shown to alter several metabolic pathways in the liver and intestine, the combined effects of which are reflected by elevations in liver cholesterol levels as well as plasma lipid and lipoprotein profiles. Stringent adaptation of cholesterol homeostasis to dietary fat/cholesterol intake is essential, because the Western diet delivers fat and cholesterol simultaneously from animal sources. In addition, recent studies have shown that dietary fat and dietary cholesterol act synergistically to impair liver metabolism, including cholesterol homeostasis (43, 85, 90, 94, 100). Although significant advances have been made in resolving the molecular identity of individual transcription factors sensitive to fat, cholesterol, or bile acids (11, 26, 48, 49), the dietary signals that sense a combined load of dietary fat and cholesterol and fine-tune cholesterol regulatory network to handle such dietary load are only partially understood. In particular, the critical signaling links and the underlying mechanisms in the body during gut and liver adaptations to such diets remain insufficiently explored. Above all, it is also unclear whether body mounts a specific “defense” mechanism to counteract detrimental effects of the combined fat/cholesterol dietary load on hepatic and whole-body cholesterol homeostasis. Assessing the impact of a high-fat/cholesterol load, rather than an individual component alone, on cholesterol homeostasis should be more physiologically relevant.
Liver, intestine, and cross talk between them play a critical role in cholesterol homeostasis via regulatory networks of transcription factors that translate signals evoked by dietary cholesterol into selective gene expression. Cholesterol homeostatic control is achieved by coordinated actions of several transcription factors, and the recent work from several laboratories suggests that the sterol regulatory element-binding protein (SREBP) and liver X receptor (LXR) transcriptional pathways work in a coordinated and reciprocal fashion to maintain cellular and systemic cholesterol homeostasis (Fig. 1) (11, 29, 49). SREBP regulates the biosynthesis and uptake of cholesterol, whereas LXR family is critical for the elimination of excess cholesterol. The SREBP and LXR transcription factors also work together with the farnesoid X receptor (FXR) to integrate cholesterol homeostasis through regulating bile acid metabolism. In the enterohepatic system, FXR plays a major role in determining the expression levels of genes involved in the maintenance of cholesterol, bile acid, and triglyceride homeostasis. FXR-regulated fibroblast growth factor 15/19 (FGF15/19) is reported to regulate cholesterol 7α-hydroxylase (Cyp7a1) and sterol 12α-hydroxylase gene (Cyp8b1) expression and thereby bile acid biosynthesis and hydrophobicity (75). Cyp7a1 is the rate-limiting enzyme in bile acid biosynthesis, whereas Cyp8b1 is critical in regulating bile hydrophobicity of the bile acid pool by regulating the cholic acid-to-chenodeoxycholic acid ratio (14).
Fig. 1.
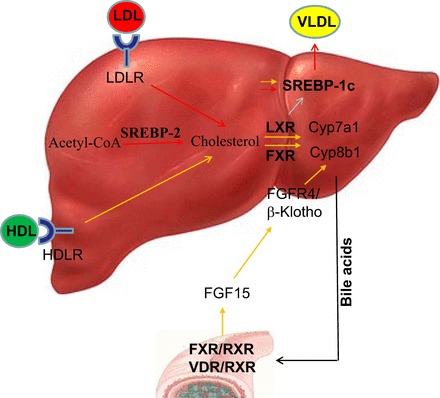
An overview of key signaling kinases (PKCs and ERK1/2) targeting transcription factors involved critical for cholesterol homeostasis. Cholesterol homeostasis is governed by 3 key transcription factors: SREBP-2, LXR, and FXR. SREBP-2 upregulates target genes involved in cholesterol biosynthesis and uptake in response to low-cellular cholesterol, whereas cholesterol catabolism and efflux are promoted through LXR in response to excess cholesterol. Bile acids activate FXR, which reduces conversion of cholesterol to bile acids by downregulating the expression of enzymes involved in bile acid synthesis, such as Cyp7a1 and Cyp8b1. It also promotes fatty acid synthesis via induction of SREBP-1c and its targets. Secretion of triglyceride-rich very low-density lipoproteins by the liver transports lipids to peripheral tissues. Bile acid-sensitive FXR reduces conversion of cholesterol to bile acids by downregulating expression of Cyp7a1 and Cyp8b1 genes. FXR also promotes the transport of bile acids to the gallbladder via bile salt export pump, multidrug resistance proteins 2 and 3. Within the intestine, FXR reduces the bile acid absorption via downregulation of the apical sodium-dependent bile acid transporter, promotes bile acid movement across the enterocytes via ileal bile acid binding protein, and promotes recycling of bile acids to the liver. FXR also promotes the release of FGF15 in mice or FGF19 in humans from the intestine. FGF15/19 travels to the liver, acting on FGF4 receptor to reduce Cyp7a1 expression and thus repress bile acid synthesis. FXR reduces lipogenesis via inhibition of SREBP-1c. Red and orange arrows indicate ERK1/2- and PKC-dependent metabolic steps, respectively. HDL, High-density lipoprotein; HDLR, HDL receptor; LDL, Low-density lipoprotein; LDLR, LDL receptor; VLDL, Very low density lipoprotein.
In addition to the above classical regulators of cholesterol and bile metabolism, endogenous bile acids can also activate the pregnane X receptor and constitutive androstane receptor, which are known to regulate genes responsible for the detoxification and elimination of a broad spectrum of potentially toxic endogenous and exogenous compounds (21, 84, 92). Likewise, vitamin D receptor (VDR) is also an intestinal sensor for secondary bile acids such as lithocholic acid (62). Altered functions of these nuclear receptors are involved in both pathogenesis and adaptation to cholestatic liver diseases (93). The above nuclear receptors utilize distinct combinations of transcriptional cofactors to effectively regulate their target genes at the transcriptional level (77, 86).
Accumulating evidence supports that above transcription factors are subject to extensive transcriptional, posttranscriptional, and posttranslational regulation (Table 1). While posttranslational regulatory mechanisms (such as phosphorylation and acetylation) control activity and subcellular localization for rapid changes in activity, transcriptional mechanisms account for intermediate and long-term changes in expression. The coordinated transcriptional and posttranscriptional regulation of transcription factors and cofactors enables the liver to rapidly respond to changes in cholesterol and bile acid homeostasis (55).
Table 1.
Summary of the reported effects of PKCs and ERK1/2 on proteins involved in cholesterol homeostasis
| Kinase | Protein and Effect of Phosphorylation on Function | Known Role of Phosphorylated Protein | References |
|---|---|---|---|
| PKCα | LXR↓ | Cholesterol transport and modulation; reverse cholesterol transport; cholesterol uptake; bile acid metabolism intestinal absorption and excretion | 16 |
| PKCα/βΙ | FXR↑ | Bile acid synthesis, secretion, transport and detoxification | 87 |
| PKC | ABCA1↑ | Cholesterol efflux | 90, 92 |
| PKCβ | VDR↑ | Bile acid metabolism | 32 |
| PKC | PXR↓ | Bile acid metabolism | 18 |
| PKC | RXR↑ | Bile acid metabolism | 94 |
| ERK-1/2 | SREBP-2↑ | Cholesterol biosynthesis | 3, 47 |
| ERK-1/2 | SHP↓ | Bile acid metabolism | 60 |
| GSK-3β | SREBPs | Cholesterol and fatty acid biosynthesis | 87 |
Up arrow indicates activation, and down arrow indicates repression.
Besides classical transcriptional regulators of cholesterol metabolism, recent studies have highlighted the importance of noncoding RNAs, termed microRNAs (miRNAs), as important posttranscriptional regulators of cholesterol homeostasis (22, 67). In particular, microRNA-33 (miR-33) has been shown to downregulate expression of ABCA1 and ABCG1 to reduce cholesterol efflux and high-density lipoprotein biogenesis (80, 81). Other miRNAs, such as miR-122, miR-370, miR-378, miR-125a, miR-27, and miR-355, have also been shown to regulate cholesterol homeostasis (22).
Studies Implicating PKC in the Regulation of Diet-Induced Cholesterol and Bile Acid Homeostasis
Protein kinase C (PKC) family plays a central role in transducing extracellular signals into a variety of intracellular responses ranging from cell proliferation to apoptosis (70). The PKC family comprises a family of lipid-activated enzymes that are structurally and functionally similar and are categorized into conventional (α, βI, βII, and γ; require diacylglycerol and calcium for activation), novel (δ, ε, η, and θ; require only diacylglycerol for activation), and atypical (ζ and λ; require neither diacylglycerol nor calcium for activation) isoforms (71). The general structure of a PKC molecule consists of a catalytic and a regulatory domain found at the COOH- and NH2-terminus, respectively. In the inactive state, the regulatory region (composed of a conserved C1 and C2 domain) is bound to the catalytic region and inhibits the activity of the enzyme. Dissociation of this intramolecular inhibitory interaction results in activation of the enzyme. Although functional role of individual PKC isoenzymes, many of which are coexpressed in the same cell, are poorly studied, emerging studies support that different PKC isoforms display highly distinct functions in vivo (87). The functional differences are due in part to the differential expression profiles of isoforms and partially caused by the various biochemical properties responsible for differential integration of the PKC isoforms into signaling networks and transcriptional complexes.
Several previous studies have indirectly suggested roles of the PKC family in modulating the cholesterol and bile acid homeostasis and are briefly summarized here: First, dietary constituents (bile acids, cholesterol, and to some extent fatty acids) as well as diacylglycerol are reported to activate PKCs including PKCβ, and according to many reports do so in a synergistic manner (4, 17, 27, 33, 46, 56, 60, 69, 74, 78). Accordingly, uptake of various lipoproteins is associated with the induction of PKCβ expression in various cell culture models (6, 10, 72, 82). PKC isoforms have also been implicated in the regulation of lipoprotein uptake (2, 10, 37, 54), and reverse cholesterol transport, such as modulating Abca1 stability and ApoAI-dependent efflux (98, 101, 104). Furthermore, tauroursodeoxycholic acid used for the treatment of cholestatic liver disease stimulates excretion of bile acids through Ca2+-dependent PKC activation (7). Lastly, PKCβ (Chr 7, 117.5 Mb, 65.7 cM) can be a positional candidate for Lith 22 gallstone susceptibility loci detected on chromosome 7, with peak linkage at 65 cM (51, 61). This linkage has a mechanistic basis since we found that PKCβ−/− mice, and not PKCδ−/− mice, show sensitivity to diet-induced gallstone formation (39). Second, PKCs have been shown to phosphorylate and regulate the activity of several nuclear receptors (Table 1). For example, VDR DNA binding and transactivation is inhibited by PKCβ (36), which is involved in regulating ileum fibroblast growth factor 15 (FGF15) expression and thereby bile acid synthesis (88); PKC phosphorylation of nuclear receptors, such as retinoid X receptor-α (RXRα), is shown to promote its cytoplasmic localization in cell culture models (95); Both PKCα and β are shown to phosphorylate and activate transactivation activity of farnesoid X receptor (FXR) (25), whereas PKCα is reported to inactivate LXRα transactivation (18); and human retinoid A receptor-α (RARα) can be phosphorylated by PKC resulting in a decrease in DNA binding activity, dimerization with RXRα, and transactivation (19). Recently, treatment by PKC activator phorbol 12-myristate 13-acetate (TPA) was shown to induce mitochondrial localization of orphan nuclear receptor Nurr77 and RXRα (9, 12, 31), as well as promote degradation of RXRα (103). Third, PKCβ can also regulate cholesterol homeostasis by modulating insulin signaling in the liver, as PKCβ is reported to disrupt insulin signaling cascade (45, 58, 59, 79). PKCβ is also shown to mediate insulin-induced liver SREBP-1c expression (102), and downstream mitogen/extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) is reported to modulate SREBP-2 transactivation (3, 52) (Table 1) and cholesterol-7α-hydroxylase (Cyp7a1) expression via small heterodimer partner modification (65). Moreover, PKCs are shown to inhibit glycogen synthase kinase-3β (30), which is shown to modulate SREBP degradation (96); Finally, ERK1/2 inhibition promotes very low-density lipoprotein (VLDL) assembly/secretion (97), whereas we have reported earlier that ERK1/2 also controls hepatic low-density lipoprotein (LDL) receptor expression levels (20, 50, 53, 54, 91). Despite the implications of PKCs in cellular mechanisms critically important to regulation of cholesterol and bile acid homeostasis by in vitro studies and cell culture models, no in vivo evidence existed to suggest a physiologically relevant role for specific PKC isoforms in this process. Understanding the individual role of specific PKC isoforms has become more important with the development of several successful drugs that target this cell signaling pathway (16, 66).
Emerging Role of PKCβ in Adaptiveness to High-Fat/Cholesterol Diet
PKCβ belongs to a conventional PKC subfamily whose members require DAG and calcium for activation (70). Despite similarity of stimulatory agonists, it appears that individual PKC isoforms in this subfamily perform unique functions. For example, unlike systemic deletion of either PKCα (57) or PKCγ (1), PKCβ deletion in mice was found to have a significant impact on the body’s lipid and glucose metabolism (5, 37–42, 63). Emerging evidence suggests that PKCβ action is not only limited to triglyceride metabolism; its induction in the liver and intestinal tissues by high-fat/cholesterol diets, with or without cholic acid, enables this kinase to function as a metabolic adaptor of cholesterol homeostasis (39, 42).
Emergence of PKCβ as a specific regulator of cholesterol homeostasis is mainly supported by our observation that high-fat/cholesterol diets induce intestine and liver PKCβ expression, whereas deficiency of PKCβ expression in mice has profound effects on hepatic cholesterol metabolism, including increases in liver and plasma cholesterol, hepatic bile cholesterol saturation index, and hydrophobicity, as well as hypersensitivity to gallstone formation (39, 42). It is also accompanied by ERK1/2 activation and reduced expression of both Cyp7a1 and Cyp8b1 genes in the liver (39).
These results suggest that PKCβ is a critical link in an adaptive response for proper handling of high dietary fat and cholesterol intake by modulating hepatic ERK1/2 activity to coordinately regulate the cellular cholesterol biosynthesis, uptake, and degradation. Our data are consistent with a two-pronged mechanism by which intestinal and liver PKCβ deficiency converges on liver ERK1/2 to modify the expression of genes involved in cholesterol and bile acid homeostasis (Fig. 2). One pathway may cause the stimulation of intestinal FGF15 expression, leading to an increase in ERK1/2 activity in the liver via fibroblast growth factor receptor 4 and coreceptor β-Klotho, resulting in suppression of Cyp7a1 and Cyp8b1 gene transcription and the reduction of cholesterol catabolism (39). The other pathway may rely on the negative regulation of the Raf-1/MEK/ERK signaling axis by PKCβ itself in the liver, resulting in further activation of ERK1/2 to reduce cholesterol catabolism in PKCβ−/− mice. Interestingly, negative in vivo regulation of the hepatic Raf/MEK/ERK cascade by PKCβ is unexpected, since two earlier in vitro studies using cell culture models suggest that PKCβ is required for ERK1/2 activation (23, 32). Both mechanisms may cooperate to maximize the effectiveness of PKCβ deficiency on hepatic ERK1/2 activation. It is interesting to note that ERK1/2 plays an important role in controlling hepatic LDL receptor expression (20, 50, 53, 54, 91), SREBP-2 expression (52), VLDL assembly (96), and cholesterol efflux (68, 104). The net effect of systemic PKCβ deficiency would be to promote hepatic cholesterol accumulation, as was observed for PKCβ−/− mice. This model has the potential to explain how PKCβ, either directly or indirectly through ERK1/2, caused differential expression of the genes involved in the cholesterol uptake, biosynthesis, and catabolic reduction of the hepatic cholesterol burden. Establishing PKCβ as the prime kinase involved in fine tuning hepatic ERK1/2 activation in vivo in response to high-fat/cholesterol diet introduces a new model that can be used to investigate the FGF15 regulatory mechanisms within a functional context.
Fig. 2.
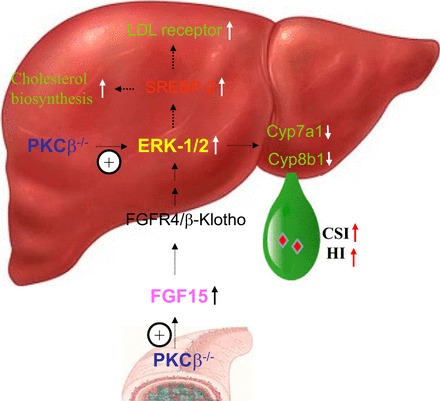
Proposed model for the mechanism by which PKCβ acts as a major regulator of cholesterol homeostasis, allowing adaptation to high-fat/cholesterol intake. It appears that PKCβ defends hepatic cholesterol homeostasis by inducing cholesterol excretion pathways, while reducing cholesterol synthesis and uptake pathways. In such a way, PKCβ may modulate bile lipid composition and thus the susceptibility to cholesterol gallstone formation. The underlying mechanism is consistent with a 2-pronged mechanism by which PKCβ deficiency contributes to dysfunctional cholesterol homeostasis through disturbing PKCβ/FGF15/ERK and Raf-1/MEK/ERK regulatory axes critical for hepatic and systemic cholesterol homeostasis. Emerging evidence indicates that these nuclear receptors have essential roles not only in the regulation of cholesterol and bile acid metabolism, but also in the integration of sterol, fatty acid, and glucose metabolism. CSI, bile cholesterol saturation index; HI, bile hydrophobicity index.
It is possible that greater insulin sensitivity might also play a role in increased ERK1/2 activation in PKCβ−/− liver of animals fed a high-fat/cholesterol diet. PKCβ has been reported to inhibit several components of the insulin signaling cascade (45, 79). Overexpression of PKCβ in the liver, similar to its overexpression in muscle (35), may result in hepatic insulin resistance, a condition known to affect ERK1/2 activation and the expression of both Cyp7a1 and Cyp8b1 genes. Insulin has been shown to initiate signaling cascades through protein kinase B (Akt) and ERK1/2 signaling pathways and has also been shown to act synergistically with FGF15 to activate these critical signaling pathways (76, 89). Further studies are needed to determine how insulin- and FGF15-specific phosphorylation of ERK1/2 is coordinated with PKCβ-dependent signaling events to respond appropriately to environmental conditions. It is possible the detrimental effect on cholesterol homeostasis exerted by PKCβ deficiency may supersede the potential protective effect offered by insulin sensitivity on gallstone formation (8).
A final consideration is that nutrition plays a major role in determining health of not only the individual, but also of the next generation. There is emerging evidence that, in addition to more traditional regulatory schemes outlined above (Fig. 2), cholesterol homeostasis is also governed by epigenetic mechanisms such as histone phosphorylation and acetylation (34). Interestingly, both PKC and ERK signaling can affect chromatin modifications in multiple ways through phosphorylation of transcription factors, which recruit chromatin-modifying complexes, and/or through direct phosphorylation of histones (13, 15, 37, 38, 64). PKCβ and downstream ERK1/2, being histone kinases, can change gene expression by modifying epigenetic marks and can thus directly link dietary lipids with epigenetic changes.
Concluding Remarks
Frequent metabolic abnormalities such as atherosclerosis and gallstone formation are related to impaired cholesterol homeostasis (24, 44, 73, 99). Understanding of cholesterol homeostasis in response to dietary fat or cholesterol has advanced significantly (11, 26, 48, 49), however much insight is needed into the mechanisms that tend to minimize fluctuations in the amount of cholesterol in the body by combined high-fat/cholesterol load. We provide a brief overview of how studies based on PKCβ−/− mice may be instrumental in leading to an understanding of the defense mechanism counteracting the deleterious effects of lithogenic stress and some of its implications. Emerging evidence supports the possibility that PKCβ deficiency triggers a cascade of reactions aimed at decreasing hepatic cholesterol to maintain homeostasis. The underlying mechanism appears to involve PKCβ-mediated regulatory loops, leading to the upregulation of genes involved in hepatic bile synthesis, while hepatocellular uptake of cholesterol and cholesterol biosynthesis are inhibited (42). The mechanism through which PKCβ regulates cholesterol homeostasis is incompletely defined, in particular the regulation of ileum FGF15 expression and its physiological significance. Another unanswered question that is of considerable interest is the nature of the signal that initiates activation of PKCβ expression. Is the PKCβ promoter targeted by nuclear receptors and SREBPs? More detailed information is also needed to define the downstream components of the PKCβ signaling pathway regulated by dietary fat/cholesterol intake. For example, is cholesterol efflux regulated by PKCβ and/or ERK (68, 101, 104)? Are there unidentified PKCβ/ERK-dependent targets involved in the effect? Does PKCβ serve as a point of cross talk between signaling pathways by integrating transcriptional inputs on different gene networks? Does PKCβ overexpression in the liver correct the diet-induced dysregulated cholesterol homeostasis? These and many other questions remain to be answered before an understanding of how PKCβ signaling precisely regulate cholesterol and bile acid homeostasis. An important and emergent area, in terms of both physiology and therapeutic exploitation, is the role liver and intestinal PKCβ play in maintaining cholesterol and bile acid homeostasis. It is also anticipated that a greater understanding of the role of PKCβ in diet-induced cholesterol homeostasis may lead to more effective therapeutic strategies for highly prevalent cholesterol-related diseases. Furthermore, if epigenetic events are controllable through dietary interventions via PKCβ, then a new therapeutic approach to atherosclerosis and gallstones is possible. The connections between signaling pathways such as PKCβ to lipid metabolism and epigenetic gene regulation make the explanation of this concept possible in the near future. Finally, even though new insights have been obtained using animal models, more studies are needed to establish a definite relevance of PKCβ to human metabolic diseases.
GRANTS
This review article is based on works supported in part by grants from the Ohio State University Wexner Medical Center and the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.M. prepared figures; D.M. drafted manuscript; D.M. and K.D.M. edited and revised manuscript; K.D.M. approved final version of manuscript.
REFERENCES
- 1.Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC γ-mutant mice. Cell 75: 1253–1262, 1993. doi: 10.1016/0092-8674(93)90613-U. [DOI] [PubMed] [Google Scholar]
- 2.Amos S, Mut M, diPierro CG, Carpenter JE, Xiao A, Kohutek ZA, Redpath GT, Zhao Y, Wang J, Shaffrey ME, Hussaini IM. Protein kinase C-α-mediated regulation of low-density lipoprotein receptor related protein and urokinase increases astrocytoma invasion. Cancer Res 67: 10241–10251, 2007. doi: 10.1158/0008-5472.CAN-07-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arito M, Horiba T, Hachimura S, Inoue J, Sato R. Growth factor-induced phosphorylation of sterol regulatory element-binding proteins inhibits sumoylation, thereby stimulating the expression of their target genes, low density lipoprotein uptake, and lipid synthesis. J Biol Chem 283: 15224–15231, 2008. doi: 10.1074/jbc.M800910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong D, Zidovetzki R. Amplification of diacylglycerol activation of protein kinase C by cholesterol. Biophys J 94: 4700–4710, 2008. doi: 10.1529/biophysj.107.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansode RR, Huang W, Roy SK, Mehta M, Mehta KD. Protein kinase Cβ deficiency increases fatty acid oxidation and reduces fat storage. J Biol Chem 283: 231–236, 2008. doi: 10.1074/jbc.M707268200. [DOI] [PubMed] [Google Scholar]
- 6.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Lüscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 121: 2693–2708, 2011. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beuers U, Throckmorton DC, Anderson MS, Isales CM, Thasler W, Kullak-Ublick GA, Sauter G, Koebe HG, Paumgartner G, Boyer JL. Tauroursodeoxycholic acid activates protein kinase C in isolated rat hepatocytes. Gastroenterology 110: 1553–1563, 1996. doi: 10.1053/gast.1996.v110.pm8613063. [DOI] [PubMed] [Google Scholar]
- 8.Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med 14: 778–782, 2008. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanquart C, Mansouri R, Paumelle R, Fruchart JC, Staels B, Glineur C. The protein kinase C signaling pathway regulates a molecular switch between transactivation and transrepression activity of the peroxisome proliferator-activated receptor α. Mol Endocrinol 18: 1906–1918, 2004. doi: 10.1210/me.2003-0327. [DOI] [PubMed] [Google Scholar]
- 10.Brunet R, How M, Trigatti BL. Modulators of protein kinase C affect SR-BI-dependent HDL lipid uptake in transfected HepG2 cells. Cholesterol 2011: 687939, 2011. doi: 10.1155/2011/687939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol 13: 213–224, 2012. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, Han YH, Dawson MI, Zhang XK. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol 24: 9705–9725, 2004. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell 103: 263–271, 2000. doi: 10.1016/S0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 14.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 50: 1955–1966, 2009. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke DL, Sutcliffe A, Deacon K, Bradbury D, Corbett L, Knox AJ. PKCbetaII augments NF-kappaB-dependent transcription at the CCL11 promoter via p300/CBP-associated factor recruitment and histone H4 acetylation. J Immunol 181: 3503–3514, 2008. doi: 10.4049/jimmunol.181.5.3503. [DOI] [PubMed] [Google Scholar]
- 16.Cohen P, Alessi DR. Kinase drug discovery—what’s next in the field? ACS Chem Biol 8: 96–104, 2013. doi: 10.1021/cb300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson LA, Brown RE, Chang WC, Morris JS, Wang N, Carroll RJ, Turner ND, Lupton JR, Chapkin RS. Morphodensitometric analysis of protein kinase C beta(II) expression in rat colon: modulation by diet and relation to in situ cell proliferation and apoptosis. Carcinogenesis 21: 1513–1519, 2000. doi: 10.1093/carcin/21.8.1513. [DOI] [PubMed] [Google Scholar]
- 18.Delvecchio CJ, Capone JP. Protein kinase C alpha modulates liver X receptor alpha transactivation. J Endocrinol 197: 121–130, 2008. doi: 10.1677/JOE-07-0525. [DOI] [PubMed] [Google Scholar]
- 19.Delmotte MH, Tahayato A, Formstecher P, Lefebvre P. Serine 157, a retinoic acid receptor α residue phosphorylated by protein kinase C in vitro, is involved in RXR.RARalpha heterodimerization and transcriptional activity. J Biol Chem 274: 38225–38231, 1999. doi: 10.1074/jbc.274.53.38225. [DOI] [PubMed] [Google Scholar]
- 20.Dhawan P, Bell A, Kumar A, Golden C, Mehta KD. Critical role of p42/44MAPK activation in anisomycin and hepatocyte growth factor-induced LDL receptor expression: activation of Raf-1/MEK-1/2/p42/44MAPK cascade alone is sufficient to induce LDL receptor expression. J Lipid Res 40: 1911–1919, 1999. [PubMed] [Google Scholar]
- 21.Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol 69: 867–873, 2005. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Hernando C, Moore KJ, Sessa WC. MicroRNA modulation of cholesterol homeostasis. Arterioscler Thromb Vasc Biol 31: 2378–2382, 2011. doi: 10.1161/ATVBAHA.111.226688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Formisano P, Oriente F, Fiory F, Caruso M, Miele C, Maitan MA, Andreozzi F, Vigliotta G, Condorelli G, Beguinot F. Insulin-activated protein kinase Cbeta bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol Cell Biol 20: 6323–6333, 2000. doi: 10.1128/MCB.20.17.6323-6333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaby AR. Nutritional approaches to prevention and treatment of gallstones. Altern Med Rev 14: 258–267, 2009. [PubMed] [Google Scholar]
- 25.Gineste R, Sirvent A, Paumelle R, Helleboid S, Aquilina A, Darteil R, Hum DW, Fruchart JC, Staels B. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol Endocrinol 22: 2433–2447, 2008. doi: 10.1210/me.2008-0092. [DOI] [PubMed] [Google Scholar]
- 26.Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Adv Nutr 3: 127–134, 2012. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg EM, Zidovetzki R. Effects of dipalmitoylglycerol and fatty acids on membrane structure and protein kinase C activity. Biophys J 73: 2603–2614, 1997. doi: 10.1016/S0006-3495(97)78290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 343: 425–430, 1990. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell 124: 35–46, 2006. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem 267: 16878–16882, 1992. [PubMed] [Google Scholar]
- 31.Gray JP, Burns KA, Leas TL, Perdew GH, Vanden Heuvel JP. Regulation of peroxisome proliferator-activated receptor α by protein kinase C. Biochemistry 44: 10313–10321, 2005. doi: 10.1021/bi050721g. [DOI] [PubMed] [Google Scholar]
- 32.Guo K, Liu Y, Zhou H, Dai Z, Zhang J, Sun R, Chen J, Sun Q, Lu W, Kang X, Chen P. Involvement of protein kinase C beta-extracellular signal-regulating kinase 1/2/p38 mitogen-activated protein kinase-heat shock protein 27 activation in hepatocellular carcinoma cell motility and invasion. Cancer Sci 99: 486–496, 2008. doi: 10.1111/j.1349-7006.2007.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haeffner EW, Wittmann U. Free cholesterol induces activation but not translocation of protein kinase C in cultured ascites tumour cells. Cell Signal 6: 201–207, 1994. doi: 10.1016/0898-6568(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 34.Handy DE, Castro R, Loscalzo J. Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation 123: 2145–2156, 2011. doi: 10.1161/CIRCULATIONAHA.110.956839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennige AM, Heni M, Machann J, Staiger H, Sartorius T, Hoene M, Lehmann R, Weigert C, Peter A, Bornemann A, Kroeber S, Pujol A, Franckhauser S, Bosch F, Schick F, Lammers R, Häring HU. Enforced expression of protein kinase C in skeletal muscle causes physical inactivity, fatty liver and insulin resistance in the brain. J Cell Mol Med 14: 903–913, 2010. doi: 10.1111/j.1582-4934.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh JC, Jurutka PW, Galligan MA, Terpening CM, Haussler CA, Samuels DS, Shimizu Y, Shimizu N, Haussler MR. Human vitamin D receptor is selectively phosphorylated by protein kinase C on serine 51, a residue crucial to its trans-activation function. Proc Natl Acad Sci USA 88: 9315–9319, 1991. doi: 10.1073/pnas.88.20.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W, Mishra V, Batra S, Dillon I, Mehta KD. Phorbol ester promotes histone H3-Ser10 phosphorylation at the LDL receptor promoter in a protein kinase C-dependent manner in human hepatoma cells. J Lipid Res 45: 1519–1527, 2004. doi: 10.1194/jlr.M400088-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Batra S, Atkins BA, Mishra V, Mehta KD. Increases in intracellular calcium dephosphorylate histone H3 at serine 10 in human hepatoma cells: potential role of protein phosphatase 2A-protein kinase CbetaII complex. J Cell Physiol 205: 37–46, 2005. doi: 10.1002/jcp.20372. [DOI] [PubMed] [Google Scholar]
- 39.Huang W, Bansode RR, Xie Y, Rowland L, Mehta M, Davidson NO, Mehta KD. Disruption of the murine protein kinase Cbeta gene promotes gallstone formation and alters biliary lipid and hepatic cholesterol metabolism. J Biol Chem 286: 22795–22805, 2011. doi: 10.1074/jbc.M111.250282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W, Bansode R, Mehta M, Mehta KD. Loss of protein kinase Cbeta function protects mice against diet-induced obesity and development of hepatic steatosis and insulin resistance. Hepatology 49: 1525–1536, 2009. doi: 10.1002/hep.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang W, Bansode RR, Bal NC, Mehta M, Mehta KD. Protein kinase Cβ deficiency attenuates obesity syndrome of ob/ob mice by promoting white adipose tissue remodeling. J Lipid Res 53: 368–378, 2012. doi: 10.1194/jlr.M019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang W, Mehta KD. Modulation of hepatic protein kinase Cβ expression in metabolic adaptation to a lithogenic diet. Cell Mol Gastroenterol Hepatol 1: 395–405, 2015. doi: 10.1016/j.jcmgh.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichimura M, Kawase M, Masuzumi M, Sakaki M, Nagata Y, Tanaka K, Suruga K, Tamaru S, Kato S, Tsuneyama K, Omagari K. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague-Dawley rats. Hepatol Res 45: 458–469, 2015. doi: 10.1111/hepr.12358. [DOI] [PubMed] [Google Scholar]
- 44.Ioannou GN. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab 27: 84–95, 2016. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Ishizuka T, Kajita K, Natsume Y, Kawai Y, Kanoh Y, Miura A, Ishizawa M, Uno Y, Morita H, Yasuda K. Protein kinase C (PKC) beta modulates serine phosphorylation of insulin receptor substrate-1 (IRS-1)—effect of overexpression of PKCbeta on insulin signal transduction. Endocr Res 30: 287–299, 2004. doi: 10.1081/ERC-120039580. [DOI] [PubMed] [Google Scholar]
- 46.Jalili T, Manning J, Kim S. Increased translocation of cardiac protein kinase C β2 accompanies mild cardiac hypertrophy in rats fed saturated fat. J Nutr 133: 358–361, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Johnston DE, Kaplan MM. Pathogenesis and treatment of gallstones. N Engl J Med 328: 412–421, 1993. doi: 10.1056/NEJM199302113280608. [DOI] [PubMed] [Google Scholar]
- 48.Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr 33: 249–269, 2013. doi: 10.1146/annurev-nutr-071812-161139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol 68: 159–191, 2006. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 50.Kapoor GS, Atkins BA, Mehta KD. Activation of Raf-1/MEK-1/2/p42/44(MAPK) cascade alone is sufficient to uncouple LDL receptor expression from cell growth. Mol Cell Biochem 236: 13–22, 2002. doi: 10.1023/A:1016185928871. [DOI] [PubMed] [Google Scholar]
- 51.Kofler K, Erdel M, Utermann G, Baier G. Molecular genetics and structural genomics of the human protein kinase C gene module. Genome Biol 3: research0014.1–research0014.10, 2002. doi: 10.1186/gb-2002-3-3-research0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotzka J, Lehr S, Roth G, Avci H, Knebel B, Muller-Wieland D. Insulin-activated Erk-mitogen-activated protein kinases phosphorylate sterol regulatory element-binding Protein-2 at serine residues 432 and 455 in vivo. J Biol Chem 279: 22404–22411, 2004. doi: 10.1074/jbc.M401198200. [DOI] [PubMed] [Google Scholar]
- 53.Kumar A, Middleton A, Chambers TC, Mehta KD. Differential roles of extracellular signal-regulated kinase-1/2 and p38(MAPK) in interleukin-1β- and tumor necrosis factor-α-induced low density lipoprotein receptor expression in HepG2 cells. J Biol Chem 273: 15742–15748, 1998. doi: 10.1074/jbc.273.25.15742. [DOI] [PubMed] [Google Scholar]
- 54.Kumar A, Chambers TC, Cloud-Heflin BA, Mehta KD. Phorbol ester-induced low density lipoprotein receptor gene expression in HepG2 cells involves protein kinase C-mediated p42/44 MAP kinase activation. J Lipid Res 38: 2240–2248, 1997. [PubMed] [Google Scholar]
- 55.Lagace TA. PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells. Curr Opin Lipidol 25: 387–393, 2014. doi: 10.1097/MOL.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau BW, Colella M, Ruder WC, Ranieri M, Curci S, Hofer AM. Deoxycholic acid activates protein kinase C and phospholipase C via increased Ca2+ entry at plasma membrane. Gastroenterology 128: 695–707, 2005. doi: 10.1053/j.gastro.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 57.Leitges M, Plomann M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y, Farese RV. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol 16: 847–858, 2002. doi: 10.1210/mend.16.4.0809. [DOI] [PubMed] [Google Scholar]
- 58.Li T, Kong X, Owsley E, Ellis E, Strom S, Chiang JYL. Insulin regulation of cholesterol 7α-hydroxylase expression in human hepatocytes: roles of forkhead box O1 and sterol regulatory element-binding protein 1c. J Biol Chem 281: 28745–28754, 2006. doi: 10.1074/jbc.M605815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li T, Francl JM, Boehme S, Ochoa A, Zhang Y, Klaassen CD, Erickson SK, Chiang JY. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J Biol Chem 287: 1861–1873, 2012. doi: 10.1074/jbc.M111.305789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Looby E, Long A, Kelleher D, Volkov Y. Bile acid deoxycholate induces differential subcellular localisation of the PKC isoenzymes beta 1, epsilon and delta in colonic epithelial cells in a sodium butyrate insensitive manner. Int J Cancer 114: 887–895, 2005. doi: 10.1002/ijc.20803. [DOI] [PubMed] [Google Scholar]
- 61.Lyons MA, Wittenburg H. Cholesterol gallstone susceptibility loci: a mouse map, candidate gene evaluation, and guide to human LITH genes. Gastroenterology 131: 1943–1970, 2006. doi: 10.1053/j.gastro.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 62.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science 296: 1313–1316, 2002. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 63.Mehta NK, Mehta KD. Protein kinase C-β: An emerging connection between nutrient excess and obesity. Biochim Biophys Acta 1841: 1491–1497, 2014. doi: 10.1016/j.bbalip.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 64.Metzger E, Imhof A, Patel D, Kahl P, Hoffmeyer K, Friedrichs N, Müller JM, Greschik H, Kirfel J, Ji S, Kunowska N, Beisenherz-Huss C, Günther T, Buettner R, Schüle R. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature 464: 792–796, 2010. doi: 10.1038/nature08839. [DOI] [PubMed] [Google Scholar]
- 65.Miao J, Xiao Z, Kanamaluru D, Min G, Yau PM, Veenstra TD, Ellis E, Strom S, Suino-Powell K, Xu HE, Kemper JK. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev 23: 986–996, 2009. doi: 10.1101/gad.1773909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov 11: 937–957, 2012. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore KJ, Rayner KJ, Suárez Y, Fernández-Hernando C. microRNAs and cholesterol metabolism. Trends Endocrinol Metab 21: 699–706, 2010. doi: 10.1016/j.tem.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mulay V, Wood P, Manetsch M, Darabi M, Cairns R, Hoque M, Chan KC, Reverter M, Alvarez-Guaita A, Rye KA, Rentero C, Heeren J, Enrich C, Grewal T. Inhibition of mitogen-activated protein kinase Erk1/2 promotes protein degradation of ATP binding cassette transporters A1 and G1 in CHO and HuH7 cells. PLoS One 8: e62667, 2013. doi: 10.1371/journal.pone.0062667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murakami K, Chan SY, Routtenberg A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem 261: 15424–15429, 1986. [PubMed] [Google Scholar]
- 70.Newton AC. Lipid activation of protein kinases. J Lipid Res 50, Suppl: S266–S271, 2009. doi: 10.1194/jlr.R800064-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem 270: 28495–28498, 1995. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 72.Osto E, Kouroedov A, Mocharla P, Akhmedov A, Besler C, Rohrer L, von Eckardstein A, Iliceto S, Volpe M, Lüscher TF, Cosentino F. Inhibition of protein kinase Cbeta prevents foam cell formation by reducing scavenger receptor A expression in human macrophages. Circulation 118: 2174–2182, 2008. doi: 10.1161/CIRCULATIONAHA.108.789537. [DOI] [PubMed] [Google Scholar]
- 73.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF Jr, Smith SC Jr, Stone NJ, Taubert KA; American Heart Association Science Advisory and Coordinating Committee . AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation 106: 388–391, 2002. doi: 10.1161/01.CIR.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 74.Pongracz J, Clark P, Neoptolemos JP, Lord JM. Expression of protein kinase C isoenzymes in colorectal cancer tissue and their differential activation by different bile acids. Int J Cancer 61: 35–39, 1995. doi: 10.1002/ijc.2910610107. [DOI] [PubMed] [Google Scholar]
- 75.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 26: 312–324, 2012. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab 13: 729–738, 2011. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol 66: 315–360, 2004. doi: 10.1146/annurev.physiol.66.032802.155556. [DOI] [PubMed] [Google Scholar]
- 78.Rao YP, Stravitz RT, Vlahcevic ZR, Gurley EC, Sando JJ, Hylemon PB. Activation of protein kinase C alpha and delta by bile acids: correlation with bile acid structure and diacylglycerol formation. J Lipid Res 38: 2446–2454, 1997. [PubMed] [Google Scholar]
- 79.Rao X, Zhong J, Xu X, Jordan B, Maurya S, Braunstein Z, Wang TY, Huang W, Aggarwal S, Periasamy M, Rajagopalan S, Mehta K, Sun Q. Exercise protects against diet-induced insulin resistance through downregulation of protein kinase Cβ in mice. PLoS One 8: e81364, 2013. doi: 10.1371/journal.pone.0081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernández-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328: 1570–1573, 2010. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rayner KJ, Moore KJ. MicroRNA control of high-density lipoprotein metabolism and function. Circ Res 114: 183–192, 2014. doi: 10.1161/CIRCRESAHA.114.300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren S, Shatadal S, Shen GX. Protein kinase C-β mediates lipoprotein-induced generation of PAI-1 from vascular endothelial cells. Am J Physiol Endocrinol Metab 278: E656–E662, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, Roger VL, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saini SP, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, Ren S, Moore DD, Evans RM, Xie W. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol 65: 292–300, 2004. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- 85.Savard C, Tartaglione EV, Kuver R, Haigh WG, Farrell GC, Subramanian S, Chait A, Yeh MM, Quinn LS, Ioannou GN. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 57: 81–92, 2013. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sever R, Glass CK. Signaling by nuclear receptors. Cold Spring Harb Perspect Biol 5: a016709, 2013. doi: 10.1101/cshperspect.a016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmitz-Peiffer C. The tail wagging the dog—regulation of lipid metabolism by protein kinase C. FEBS J 280: 5371–5383, 2013. doi: 10.1111/febs.12285. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem 285: 14486–14494, 2010. doi: 10.1074/jbc.M110.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin DJ, Osborne TF. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem 284: 11110–11120, 2009. doi: 10.1074/jbc.M808747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shinomura T, Asaoka Y, Oka M, Yoshida K, Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc Natl Acad Sci USA 88: 5149–5153, 1991. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh RP, Dhawan P, Golden C, Kapoor GS, Mehta KD. One-way cross-talk between p38MAPK and p42/44MAPK: inhibition of p38MAPK induces low density lipoprotein receptor expression through activation of p42/44MAPK cascade. J Biol Chem 274: 19593–19600, 1999. doi: 10.1074/jbc.274.28.19593. [DOI] [PubMed] [Google Scholar]
- 92.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98: 3369–3374, 2001. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sonoda J, Pei L, Evans RM. Nuclear receptors: decoding metabolic disease. FEBS Lett 582: 2–9, 2008. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, Haigh WG, Yeh MM, Kowdley KV, O’Brien KD, Pennathur S, Chait A. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res 52: 1626–1635, 2011. doi: 10.1194/jlr.M016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun K, Montana V, Chellappa K, Brelivet Y, Moras D, Maeda Y, Parpura V, Paschal BM, Sladek FM. Phosphorylation of a conserved serine in the deoxyribonucleic acid binding domain of nuclear receptors alters intracellular localization. Mol Endocrinol 21: 1297–1311, 2007. doi: 10.1210/me.2006-0300. [DOI] [PubMed] [Google Scholar]
- 96.Sundqvist A, Bengoechea-Alonso MT, Ye X, Lukiyanchuk V, Jin J, Harper JW, Ericsson J. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab 1: 379–391, 2005. doi: 10.1016/j.cmet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Tsai J, Qiu W, Kohen-Avramoglu R, Adeli K. MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Arterioscler Thromb Vasc Biol 27: 211–218, 2007. doi: 10.1161/01.ATV.0000249861.80471.96. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C δ pathway. J Lipid Res 48: 1062–1068, 2007. doi: 10.1194/jlr.M600437-JLR200. [DOI] [PubMed] [Google Scholar]
- 99.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res 50, Suppl: S406–S411, 2009. doi: 10.1194/jlr.R800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lütjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B, van Bilsen M, Shiri-Sverdlov R, Hofker MH. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 48: 474–486, 2008. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 101.Yamauchi Y, Hayashi M, Abe-Dohmae S, Yokoyama S. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J Biol Chem 278: 47890–47897, 2003. doi: 10.1074/jbc.M306258200. [DOI] [PubMed] [Google Scholar]
- 102.Yamamoto T, Watanabe K, Inoue N, Nakagawa Y, Ishigaki N, Matsuzaka T, Takeuchi Y, Kobayashi K, Yatoh S, Takahashi A, Suzuki H, Yahagi N, Gotoda T, Yamada N, Shimano H. Protein kinase Cbeta mediates hepatic induction of sterol-regulatory element binding protein-1c by insulin. J Lipid Res 51: 1859–1870, 2010. doi: 10.1194/jlr.M004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye XF, Liu S, Wu Q, Lin XF, Zhang B, Wu JF, Zhang MQ, Su WJ. Degradation of retinoid X receptor alpha by TPA through proteasome pathway in gastric cancer cells. World J Gastroenterol 9: 1915–1919, 2003. doi: 10.3748/wjg.v9.i9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou X, Yin Z, Guo X, Hajjar DP, Han J. Inhibition of ERK1/2 and activation of liver X receptor synergistically induce macrophage ABCA1 expression and cholesterol efflux. J Biol Chem 285: 6316–6326, 2010. doi: 10.1074/jbc.M109.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]


