Abstract
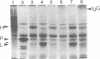
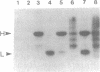
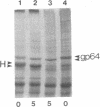
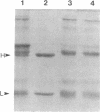
A murine immunoglobulin heterodimer has been expressed in a baculovirus expression system. This was achieved by using both double infection of insect cells with separate heavy- and light-chain-expressing viruses and infection with a double-recombinant virus containing both the immunoglobulin heavy- and light-chain cDNAs. In both cases, the polypeptide chains were correctly processed, glycosylated, and assembled into normal H2L2 (H = heavy, L = light) immunoglobulin monomers. These molecules bound antigen and expressed both polyclonal idiotype and monoclonal idiotopes. Furthermore, the transfer vectors described have been modified to contain the F1 origin of replication for the production of single-stranded DNA, which facilitates site-specific mutations of either the polyhedrin promoter or the inserted foreign gene. Use of this system should significantly advance the analysis of the structural bases for both idiotype expression and antigen binding by immunoglobulin. More importantly, it provides a generic method for the high-level expression of antibodies of diverse interest.
Full text
PDF
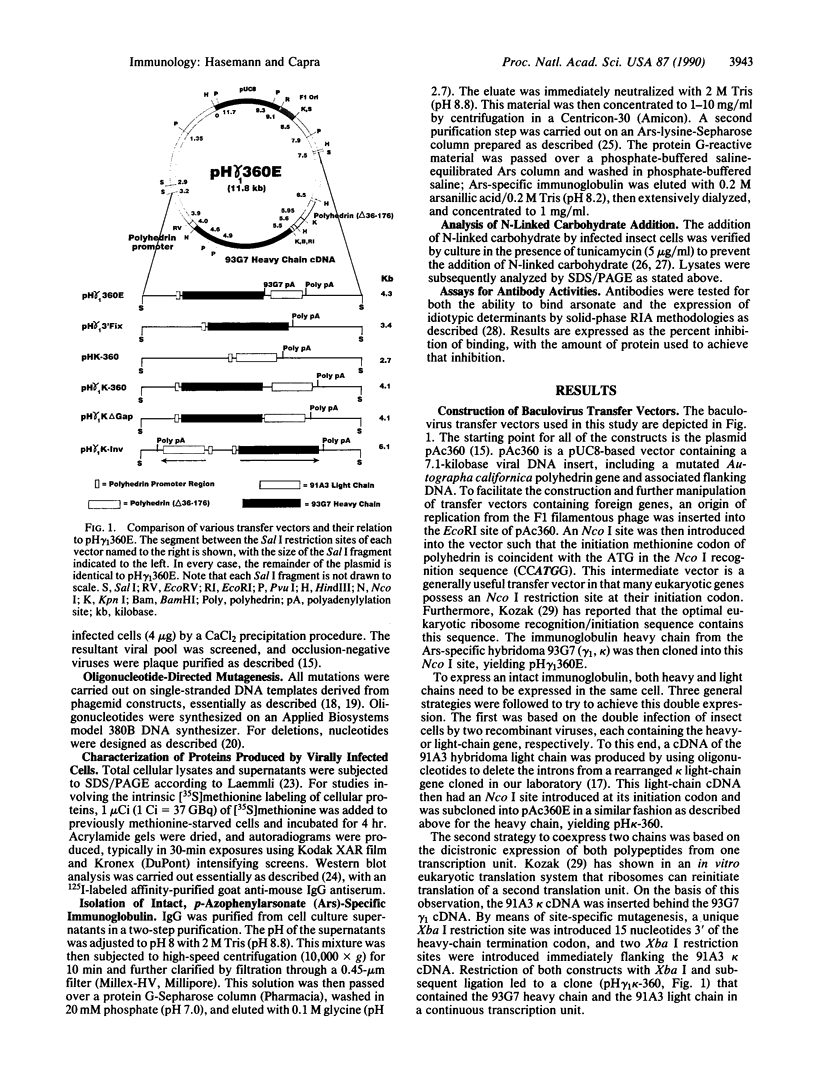

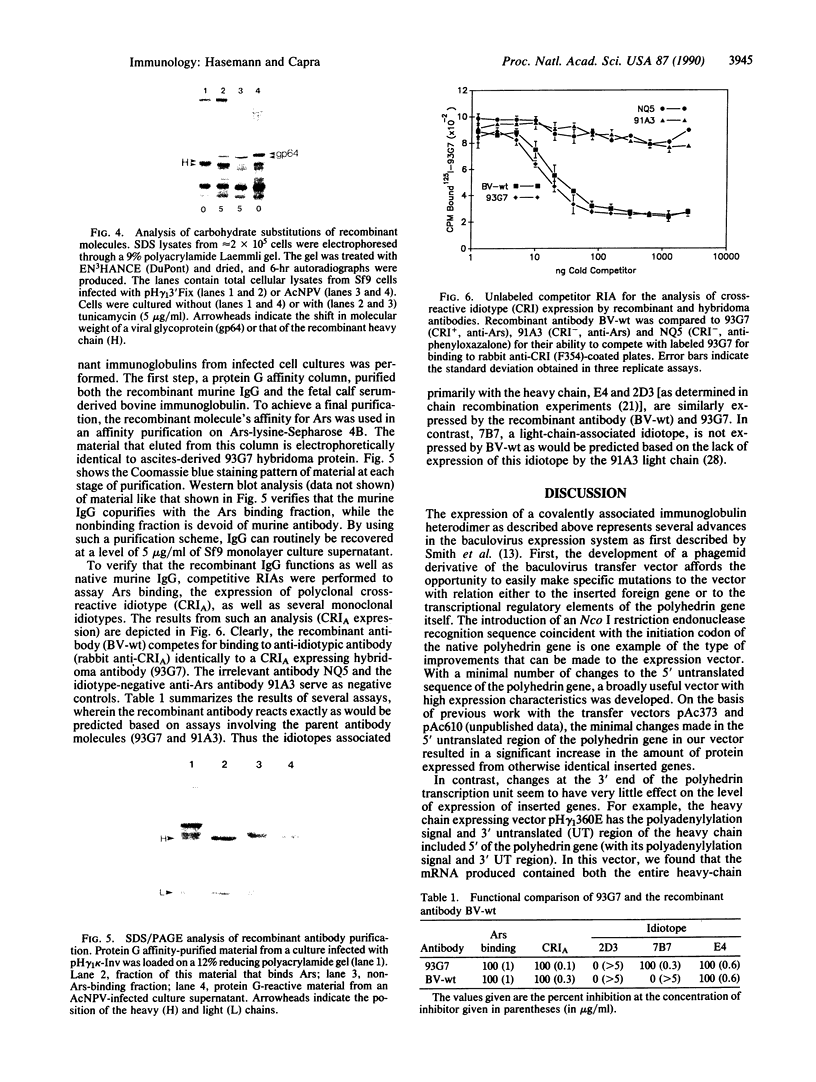

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Clark A. F., Capra J. D. Ubiquitous nonimmunoglobulin p-azobenzenearsonate-binding molecules from lymphoid cells. J Exp Med. 1982 Feb 1;155(2):611–616. doi: 10.1084/jem.155.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghtedarzadeh M. K., Henikoff S. Use of oligonucleotides to generate large deletions. Nucleic Acids Res. 1986 Jun 25;14(12):5115–5115. doi: 10.1093/nar/14.12.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery V. C., Bishop D. H. The development of multiple expression vectors for high level synthesis of eukaryotic proteins: expression of LCMV-N and AcNPV polyhedrin protein by a recombinant baculovirus. Protein Eng. 1987 Aug-Sep;1(4):359–366. doi: 10.1093/protein/1.4.359. [DOI] [PubMed] [Google Scholar]
- Herrera R., Lebwohl D., Garcia de Herreros A., Kallen R. G., Rosen O. M. Synthesis, purification, and characterization of the cytoplasmic domain of the human insulin receptor using a baculovirus expression system. J Biol Chem. 1988 Apr 25;263(12):5560–5568. [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Fellows R. E., Lebovitz H. E. The evolutionary origins of the immunoglobulins. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1762–1769. doi: 10.1073/pnas.56.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske D., Milner E. C., Leo O., Moser M., Marvel J., Urbain J., Capra J. D. Molecular mapping of idiotopes of anti-arsonate antibodies. J Immunol. 1986 Apr 1;136(7):2568–2574. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leo O., Slaoui M., Marvel J., Milner E. C., Hiernaux J., Moser M., Capra J. D., Urbain J. Idiotypic analysis of polyclonal and monoclonal anti-p-azophenylarsonate antibodies of BALB/c mice expressing the major cross-reactive idiotype of the A/J strain. J Immunol. 1985 Mar;134(3):1734–1739. [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Maniatis T. Drosophila Krüppel gene product produced in a baculovirus expression system is a nuclear phosphoprotein that binds to DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5700–5704. doi: 10.1073/pnas.84.16.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Schaffner W. Correct transcription of a cloned mouse immunoglobulin gene in vivo. Proc Natl Acad Sci U S A. 1983 Jan;80(2):417–421. doi: 10.1073/pnas.80.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. M., Reichelderfer C. F., Johansson B. E., Kilbourne E. D., Acs G. Complementation of recombinant baculoviruses by coinfection with wild-type virus facilitates production in insect larvae of antigenic proteins of hepatitis B virus and influenza virus. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1453–1456. doi: 10.1073/pnas.86.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Baltimore D. Regulated expression of an immunoglobulin kappa gene introduced into a mouse lymphoid cell line. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7862–7865. doi: 10.1073/pnas.79.24.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I., Capra J. D. V kappa and J kappa gene segments of A/J Ars-A antibodies: somatic recombination generates the essential arginine at the junction of the variable and joining regions. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1085–1089. doi: 10.1073/pnas.84.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J., Rabbitts T. H., Estess P., Slaughter C., Tucker P. W., Capra J. D. Somatic mutation in genes for the variable portion of the immunoglobulin heavy chain. Science. 1982 Apr 16;216(4543):309–311. doi: 10.1126/science.6801765. [DOI] [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Ju G., Ericson B. L., Moschera J., Lahm H. W., Chizzonite R., Summers M. D. Modification and secretion of human interleukin 2 produced in insect cells by a baculovirus expression vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8404–8408. doi: 10.1073/pnas.82.24.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Angelo C., Smith G. E., Summers M. D., Krug R. M. Two of the three influenza viral polymerase proteins expressed by using baculovirus vectors form a complex in insect cells. J Virol. 1987 Feb;61(2):361–365. doi: 10.1128/jvi.61.2.361-365.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]