Cardiac electrical activity is mediated by ion channels, and Kv4.2 plays a significant role, along with its binding partner, the Kvβ1.1 subunit. In the present study, we identify Kvβ1.1 as a sensor of pyridine nucleotides changes and modulator of Kv4.2 gating, action potential duration, and ECG in the mouse heart.
Keywords: Kvβ subunit, redox, potassium channel, heart, aldo-keto reductase, pyridine nucleotides
Abstract
The present study investigates the physiological role of Kvβ1 subunit for sensing pyridine nucleotide (NADH/NAD+) changes in the heart. We used Kvβ1.1 knockout (KO) or wild-type (WT) mice and established that Kvβ1.1 preferentially binds with Kv4.2 and senses the pyridine nucleotide changes in the heart. The cellular action potential duration (APD) obtained from WT cardiomyocytes showed longer APDs with lactate perfusion, which increases intracellular NADH levels, while the APDs remained unaltered in the Kvβ1.1 KO. Ex vivo monophasic action potentials showed a similar response, in which the APDs were prolonged in WT mouse hearts with lactate perfusion; however, the Kvβ1.1 KO mouse hearts did not show APD changes upon lactate perfusion. COS-7 cells coexpressing Kv4.2 and Kvβ1.1 were used for whole cell patch-clamp recordings to evaluate changes caused by NADH (lactate). These data reveal that Kvβ1.1 is required in the mediated inactivation of Kv4.2 currents, when NADH (lactate) levels are increased. In vivo, isoproterenol infusion led to increased NADH in the heart along with QTc prolongation in wild-type mice; regardless of the approach, our data show that Kvβ1.1 recognizes NADH changes and modulates Kv4.2 currents affecting AP and QTc durations. Overall, this study uses multiple levels of investigation, including the heterologous overexpression system, cardiomyocyte, ex vivo, and ECG, and clearly depicts that Kvβ1.1 is an obligatory sensor of NADH/NAD changes in vivo, with a physiological role in the heart.
NEW & NOTEWORTHY Cardiac electrical activity is mediated by ion channels, and Kv4.2 plays a significant role, along with its binding partner, the Kvβ1.1 subunit. In the present study, we identify Kvβ1.1 as a sensor of pyridine nucleotide changes and as a modulator of Kv4.2 gating, action potential duration, and ECG in the mouse heart.
cardiac injury including cardiac hypertrophy and myocardial ischemia demonstrate a decrease in NAD+ and sharp increase in NADH (5, 17, 32). Therefore, modulation of NAD+ by supplementing the substrate or activation of the NAD+ synthetic pathway increases intracellular NAD+, which has been demonstrated as a plausible avenue of cardiac intervention in recent years. Exogenous NAD+ injections resulted in significant rescue in agonist-induced cardiac hypertrophy in mice (33). Administration of nicotinamide mononucleotide and nicotinamide phosphoribosyltransferase resulted in a significant increase in NAD+ levels in the heart, as well as reduced infarct size and improved cardiac myocyte survival after ischemia reperfusion injury (14, 52). The increase in NAD+ levels leading to cardiac protection may be due to its ability to alter ion channel activity (15). Intracellular NADH was demonstrated to significantly alter the cardiac sodium channel (Nav1.5) and reduce peak currents, as well as inhibit the Na+/Ca2+ channel in ventricular myocytes (21, 23).
The shaker potassium channel subunits (Kvβ), which are members of the aldo-keto reductase superfamily, are highly expressed in the heart and bind to voltage-gated potassium channels Kv1 and Kv4 (8, 15, 36). Kv channels play a key role in cardiac repolarization, specifically, in the determination of the duration of the action potential plateau observed in phase 1 (2, 3, 27). In the mouse ventricle, it is well known that much of the Ito,f current is encoded by the molecular correlate Kv4.2/Kv4.3, which is responsible for the rapidly activating and inactivating potassium current (18, 30, 39). Further, the Kv4.2 channel plays a critical role in the early cardiac repolarization, as well as excitation contraction-coupling (38) and arrhythmias (4). Previous research also highlights the importance of potassium channel subunits, including KCHIP2 (12) and Kvβ1 (8), and their role in alterating Kv4.2/Kv4.3, affecting overall Ito current in cardiomyocytes and heterologous systems.
Previously, we have demonstrated that Kvβ1– Kvβ3 bind pyridine nucleotides with high affinity and alter Kv channel gating and regulation (15, 45, 47). The addition of NAD+ abolished Kvβ1-induced inactivation of Kv1.5 currents, whereas inclusion of NADH in the patch-pipette solutions supported inactivation (47). These reports, overall, support the idea that reduced pyridine nucleotides (NADPH or NADH) inactivate and oxidize pyridine nucleotides (NADP+ or NAD+), as well as abolish Kvβ1-mediated inactivation and gating of Kv currents. Previous reports have identified increased learning and memory, neuronal excitability, and synaptic plasticity in aged Kvβ1.1 knockout (KO) mice (25, 26). Kvβ1 KO mice also demonstrated a significant difference in Kv currents within left ventricular apex myocytes in 6–10-wk-old male mice (1). Moreover, Kvβ1.1 coimmunoprecipitates with Kv4.2, suggesting that Kvβ1.1 regulation of Kv4.2 activity may be of primary interest under pathophysiological stress. Recently, we have published data showing that Kvβ1.1 KO male and female mice have prolonged monophasic action potentials, as well as prolonged QTc durations (48). Electrically, male and female KO mice demonstrate similar phenotypes; however, hemodynamically, the female mice demonstrate elevated blood pressure, resulting in cardiac hypertrophy, while no significant alterations were noted in male mice.
Earlier studies using heterologous expression systems have identified that Kvβ subunits bind pyridine nucleotides [NAD(P)H/NAD(P)] with high affinity and modulate the gating and kinetics of the KV channel (15). Moreover, cardiac injury frequently involves elevated NADH/NAD+ redox potential. Hence, it is plausible that Kvβ1.1 is an essential player in relaying the inhibitory effects of increased NADH stress on cardiac repolarization. Therefore, we hypothesized that Kvβ1.1 is an essential mediator for pyridine nucleotide changes in the heart. We tested this hypothesis by using Kvβ1.1KO mice and assessed the physiological and biochemical consequences of pyridine nucleotide modulation.
MATERIALS AND METHODS
Animals.
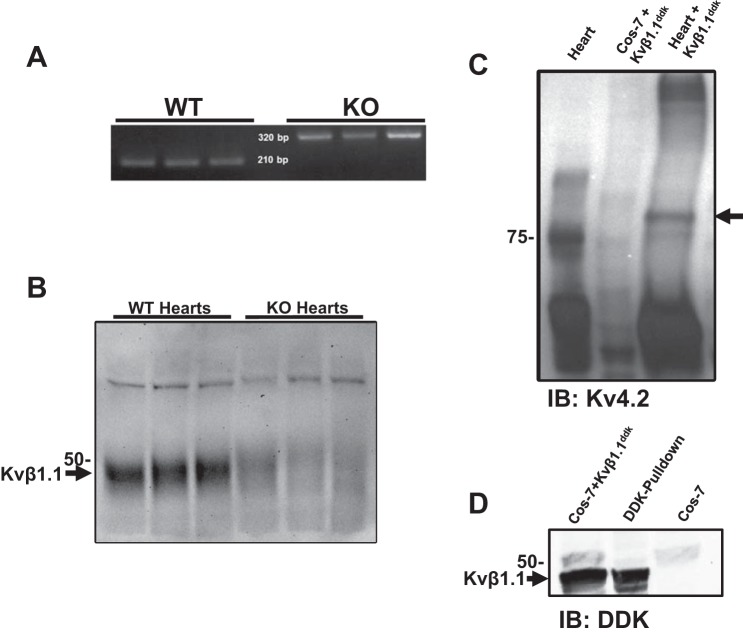
Kvβ1.1 (NP_034727.2) KO (global knockout) and wild-type (WT) mice (C57BL/6NJ) were obtained from Jackson Laboratories (Bar Harbor, ME) (48). Male mice of 16–20 wk of age were used and fed with food and water ad libitum. All animal work was approved in advance by the Institutional Animal Care and Use Committee at the University of South Florida (Tampa, FL). Mice were genotyped to confirm the genetic deletion of Kvβ1.1 (Fig. 1A).
Fig. 1.
Kvβ1.1 and Kv4.2 interaction. A: genotype of wild-type (WT) and knockout (KO) mice. PCR product separated by gel electrophoresis for identifying WT mice at 210 bp and KO mice at 320 bp. B: WT and KO heart homogenates. Primary antibody was Kvβ1.1 (1:200). C: WT heart homogenate (lane 1) and COS-7 cells transiently transfected with Kvβ1.1-DDK (lane 2) were combined and incubated with DDK-coated agarose beads overnight (lane 3). Primary antibody was Kv4.2 (1:500). D: COS-7 cells transfected with Kvβ1.1-DDK (lane 1) and incubated overnight with DDK coated agarose beads (lane 2), while untransfected COS-7 cells showed no DDK band (lane 3). Primary antibody was DDK (1:200).
Cardiomyocyte isolation.
Ventricular cardiomyocytes were isolated using an enzymatic dispersion technique. Briefly, hearts were cannulated and retrograde perfused with Ca2+-free isolation buffer containing (in mM: 117.3 NaCl, 5.3 KCl, 26.2 NaHCO3, 1 Na2HPO4, 20 HEPES, 10 taurine, 20 2,3-butanedione monoxime (BDM), and 6 d-glucose, at pH 7.4, for 5 min at 37°C. The perfusate was then switched to isolation buffer containing 0.4 mg/ml Liberase Blendzyme 4 (Roche, Indianapolis, IN) and 20 μM CaCl2, and perfused for 10 min. Following digestion, the apex was excised and triturated in the Ca2+-free isolation buffer containing 1% BSA. The resulting cell preparation was passed through ~29-μm polypropylene mesh to remove tissue debris. Isolated myocytes were then washed in isolation buffer without BDM, while adding CaCl2 in increments of 0.2-mM at 5-min intervals to reach a final concentration of 1.2 mM. Cells were then used for electrophysiological recordings within 4–5 h. Cells were placed on glass coverslips and perfused with an external solution containing (in mM) 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1.1 MgCl2, 10 HEPES, and 5.5 d-glucose, at pH 7.4 at room temperature.
Patch-clamp recording for isolated cardiomyocytes.
Current-clamp recordings were acquired on isolated cardiomyocytes using a perforated patch configuration. Isolated adult cardiomyocytes were plated on glass coverslips and allowed to rest in external solution for 10–30 min. The external solution for recordings consisted of (in mM): 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1.1 MgCl2, 10 HEPES, and 5.5 d-glucose, at pH 7.4. Patch pipettes were fabricated from borosilicate glass, pulled to a resistance of 1–1.5 MΩ. The internal (patch pipette) solution consisted of (in mM): 100 aspartic acid, 3.5 KCl, 1.0 MgCl2, 1.8 CaCl2, 4.5 NaCl, 10 EGTA, and 5 ATP, at pH 7.2. Membrane potential traces were acquired at room temperature using an Axopatch-200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) and pClamp 10 software (Molecular Devices). Electrical access to the cell was achieved through perforated-patch configuration using 240 μg/ml amphotericin B (0.1% DMSO), dissolved in the internal solution. Action potentials were evoked from the cells under current-clamp mode by applying 2-ms current pulses delivered at 1 Hz. Pulse amplitudes were 2× threshold levels (1–2 nA). Membrane potentials were sampled at 10 kHz. Control action potentials were recorded for 1 min. Cells were then perfused with external solution containing 10 mM lactate for 5 min, and action potentials were evoked again and recorded for 1 min using the same pulse parameters, as described before. Data were exported and analyzed using the peak analysis module of LabChart 7.2 (ADInstruments, Colorado Springs, CO).
Cell culture (COS-7) and transfection procedures.
COS-7 cells were purchased from American Type Culture Collection (Manassas, VA). COS-7 cells are green-monkey kidney fibroblast cells and were cultured in 5% CO2 incubator (Thermo Fisher Scientific, Waltham, MA) using standard DMEM medium (Invitrogen, Grand Island, NY) supplemented with 10% FBS (Invitrogen), and 1% penicillin and streptomycin antibiotics. For cDNA transfection experiments, the cells were transfected with 2–6 µg of either mouse Kv4.2 alone or in combination with Kvβ1.1-GFP (cat. nos. MC206092 and MG206299; Origene, Rockville, MD), at 70–80% confluence using Lipofectamine LTX transfection system (Invitrogen) (45). Cells were monitored for signs of toxicity every 24 h under an EVOS XL Core Light Microscope (Advanced Microscopy Group, Bothell, WA). No detectable cell loss or change in cell morphology was observed in transfected group. After 48 h of transfection, cells were used for electrophysiological recordings.
Patch-clamp recording for transfected COS-7 cells.
Whole cell patch-clamp recordings were performed on COS-7 cells. Briefly, COS-7 cells transfected with Kv4.2 with or without Kvβ1.1-GFP plasmids were trypsinized (0.25%) and washed with serum-free media, just before plating on glass coverslips and allowed to rest in external solution consisting of (in mM): 135 NaCl, 5.4 KCl, 1.1 MgCl2, 1.8 CaCl2, 10 HEPES, and 5.5 glucose at pH 7.4 for 10–30 min. Patch pipettes were fabricated from borosilicate glass, pulled to a resistance of 1–3 MΩ. The internal (patch pipette) solution consisted of (in mM): 100 aspartic acid, 35 KCl, 10 MgCl2, 1.8 CaCl2, 4.5 NaCl, 10 EGTA, 5 ATP, at pH 7.2 with KOH. Axopatch-200B patch-clamp amplifier (Molecular Devices) operated by pClamp 10 software (Molecular Devices) were used to record membrane currents, which were analyzed and digitized with 12-bit resolution. Patch pipettes with 1–3 MΩ resistance were used to obtain GΩ tight seals and membrane under the patch pipette was ruptured using negative pressure to achieve the whole cell configuration. Whole-cell currents were elicited by applying depolarizing voltage steps from −60 to +60 mV in 10-mV steps to the cells from a holding of −80 mV for 300 ms. The decay rates were determined by a single exponential fit to the inactivating phase of the current over (300 ms) a range of voltages from 0 to +60 mV. To analyze the current voltage relations, the Ipeak was measured at different voltages (−60 to +60 mV) and plotted versus membrane potential. Voltage dependence of inactivation was measured by using the two-pulse protocol, from a holding potential of −80 mV; different test potentials from −120 to +60 mV in 10-mV steps were applied for 300 ms. The steady-state inactivation curves were fit with a Boltzmann function.
Pull-down and immunoblotting.
To identify the interaction between Kvβ1.1 and Kv4.2 in the heart, we conducted a pull-down assay using ventricular tissue lysate. Briefly, 5 μg of DDK-tagged Kvβ1.1 plasmid (Origene, Rockville, MD) was transiently expressed (48 to 72 h) in COS-7 cells that were grown to 90% confluence in a 10-cm plate. Total cellular protein was extracted from Kvβ1.1-DDK expressing COS-7 and mice ventricles by homogenization using extraction buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 5 mM EDTA, and 1% Nonidet P40 (NP40) (Thermo Fisher Scientific, Waltham, MA), supplemented with 10 mM DDT, 1:100 protease inhibitor (Sigma-Aldrich, St. Louis, MO) and 1:100 protease inhibitor (Sigma-Aldrich). Tissue lysate was then centrifuged at 10,000 g for 10 min at 4°C, and the supernatant was collected. Protein quantification was performed using Pierce 660 assay (Thermo Fisher Scientific, Waltham, MA). Approximately 200 μg of DDK-tagged Kvβ1.1 Cos-7 lysate was incubated with Anti-DDK agarose beads (Origene, Rockville, MD) for 3 h at 4°C, and 500 μg of precleared ventricular tissue lysate was then added, and incubated overnight at 4°C. Bound proteins were then eluted, and immunoblot analysis was conducted using Kv4.2 antibody obtained from Millipore (Darmstadt, Germany).
Electrocardiography.
Mice were anesthetized with 2–3% isoflurane/oxygen anesthesia, lead II ECG was recorded with Power laboratory (ADInstruments) amplifier and data acquisition system, and analysis was performed by using LabChart 7.2. The end of the T wave is fixed at the point where the waveform returns to isoelectric line, and ECG parameters, including QTc, were assessed as reported before (6, 42).
Monophasic action potentials.
Monophasic action potentials (MAPs) were recorded from ex vivo heart preparations, as reported before (6, 48). Mice were injected with 1 mg heparin (180 USP; Sigma-Aldrich) and were euthanized with Somnasol (pentobarbital sodium, 50 mg/kg body wt; Henry Schein Animal Health, Dublin, OH) by intraperitoneal injection. Hearts were isolated through a bilateral thoracotomy and retrograde perfusion with Krebs-Henseleit buffer (in mM): 119 NaCl, 25 NaHCO3, 4 KCl, 1.2 KH2PO4, 1 MgCl2, 1.8 CaCl2, 10 d-glucose, and 2 sodium pyruvate, at pH 7.4) was carried out at a constant flow rate of 2.0 ml/min, 37°C. MAPs were recorded from left ventricular epicardial surface using contact electrode (Harvard Apparatus, Holliston, MA). Hearts were stabilized for 10 min, and MAP data were acquired using 8-channel PowerLab system (ADInstruments).
Modulation of NADH alters monophasic action potential durations.
MAPs were recorded from WT or KO mouse hearts to assess the activity in response to biochemical modulation of NADH (21). An increased NADH level in ex vivo heart tissue was accomplished by including 20 mM sodium lactate in the Krebs-Henseleit buffer. Baseline MAPs from LV were acquired with normal buffer without lactate. Subsequently, hearts were perfused for 20 min with a 20 mM lactate containing buffer, and MAPs were acquired using 8-channel PowerLab system (AD Instruments). Increase in NADH levels by perfusion for 20 min with high lactate vs. no lactate added buffer was confirmed in WT hearts. Further, NADH levels were also assessed and compared between the WT and KO mice after lactate buffer perfusion for 20 min.
Pyridine nucleotide assay.
Whole hearts from saline or isoproterenol hydrochloride (ISO) exposed WT and KO mice in addition to WT and KO hearts exposed with lactate were freeze clamped and stored at −80°C until analysis. Heart tissue was pulverized under liquid N2 in a mortar and pestle, and pyridine nucleotide; NADH/NAD+, ratio was assessed from 20 mg tissue of each sample by using EnzyChrome NAD+/NADH kit (Bioassays, Hayward, CA), according to the manufacturer’s recommendations. Sample absorbance was measured at 560 nm using a 96-well plate reader (Biotek, Winooski, VT) and was normalized to total protein level. The ratio of NADH/NAD+ was computed for all groups.
Quantitative real-time PCR.
Total RNA was isolated from left ventricles of hearts using the Exiqon miRCURY RNA isolation kit (Exiqon, Woburn, MA), according to the manufacturer's protocol. Complementary DNA from total RNA was synthesized, and quantitative real-time-PCR (qRT-PCR) analysis was performed for potassium channel subunit genes Kvβ1.2, Kvβ1.3, Kvβ2, and KCHIP2; potassium channel genes Kv4.2, 1.4, 1.5, sodium channel Nav1.5; and calcium regulators SERCA2, calcineurin, PI3K, and PLB (phospholamban). The cDNA synthesis and qRT-PCR procedures were performed as described previously (29, 48). The expression of mouse 18S (18S ribosomal RNA) was used as an internal control.
Western blot analysis.
Protein extracts from left ventricle (LV) of KO and WT mice hearts were isolated and quantified as described previously (6, 29) for Western blot analysis. Proteins were detected with a dilution of primary antibody as follows: 1:200 (Kv1.5), 1:500 (Kvβ1.1), 1:1000 (Kv4.2), and 1:10,000 (GAPDH). Primary antibodies Kv4.2 and GAPDH were obtained from Millipore (Darmstadt, Germany), Kv1.5 was obtained from Alomone (Jerusalem, Israel), Kvβ1.1 was obtained from Genetex (Irvine, CA), and Kvβ1.1 was obtained from Neuromab (Davis, CA). Immunoblots were quantified using Image J software, and means ± SE values were plotted as bar diagrams.
Mouse model of cardiac hypertrophy.
Age-matched Kvβ1.1 KO and WT mice were infused with either saline or ISO (Sigma-Aldrich) for 14 days at a dose of (30 mg·kg−1·day−1) using osmotic minipumps (model 2002, Durect; Alzet, Cupertino, CA), according to the previously published report (50). Mice were anesthetized with 2.5% isoflurane (Butler Schein, Dublin, OH); pumps were placed subcutaneously and monitored for 14 days.
Statistical analysis.
Statistical analyses were performed with Sigma Plot (v. 11.0). When comparing two groups, an independent Student’s t-test was used. Data are expressed as means ± SE, and P ≤ 0.05 were considered significant.
RESULTS
Kv4.2 interaction with Kvβ1.1.
We used KO mice that lack the Kvβ1.1 subunit by the insertion of a PGKneobpA/neo cassette in the first exon, which is responsible for coding Kvβ1.1 splice isoform (9) (Fig. 1A). As shown in Fig. 1B, Western blot confirmed the expression of Kvβ1.1 protein in the wild-type mouse heart; however, the Kvβ1.1 KO mouse showed the absence of Kvβ1.1 expression. COS-7 cells transfected with Kvβ1.1 tagged with DDK were utilized for pull-down assays. The Kvβ1.1-DDK was incubated with cardiac homogenate and DDK-coated agarose beads 12–16 h, precipitates were resolved using gel electrophoresis and immunoblotted with Kv4.2 antibody. As shown in Fig. 1C, Kvβ1.1 pulls down Kv4.2 from mouse heart lysates, demonstrating protein-protein interaction and binding. To validate the specificity of Kvβ1.1 binding to Kv4.2, COS-7 lysates overexpressing Kvβ1.1-DDK plasmid were pulled down with DDK-coated agarose beads alone and immunoblotted with anti-DDK antibody, which demonstrates Kvβ1.1-DDK expression (Fig. 1D).
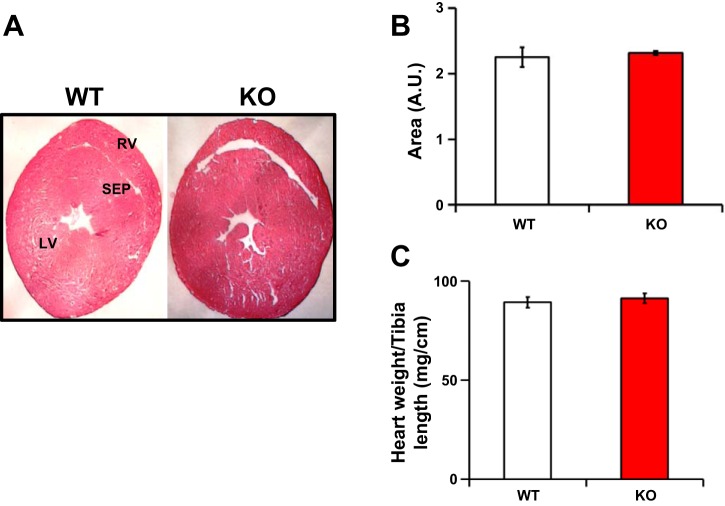
Kvβ1.1 KO hearts demonstrate similar physical dimensions.
Whole heart sections of both WT and KO mice demonstrate comparable morphometric measurements, including the right ventricle (RV), LV, and septum (Fig. 2A). Overall, area measurements from the cross sections demonstrate no significant difference (Fig. 2B). Heart weights normalized to tibia length also demonstrated similar weights in both WT and KO mice (Fig. 2C).
Fig. 2.
Physical parameters of the Kvβ1.1 knockout heart. A: Male hearts at 2-μm cross sections stained with hematoxylin and eosin (H&E) for identifying the differences in shape and size. LV, left ventricle; RV, right ventricle; SEP, septum. B: total H&E cross-sectional area (arbitrary units, AU) of the heart quantified and compared between WT and KO mice. The data represented are means ± SE (n = 3 hearts). C: normalized male heart weights by tibia length of both WT and KO mice. The data represent means ± SE (n = 10 mice).
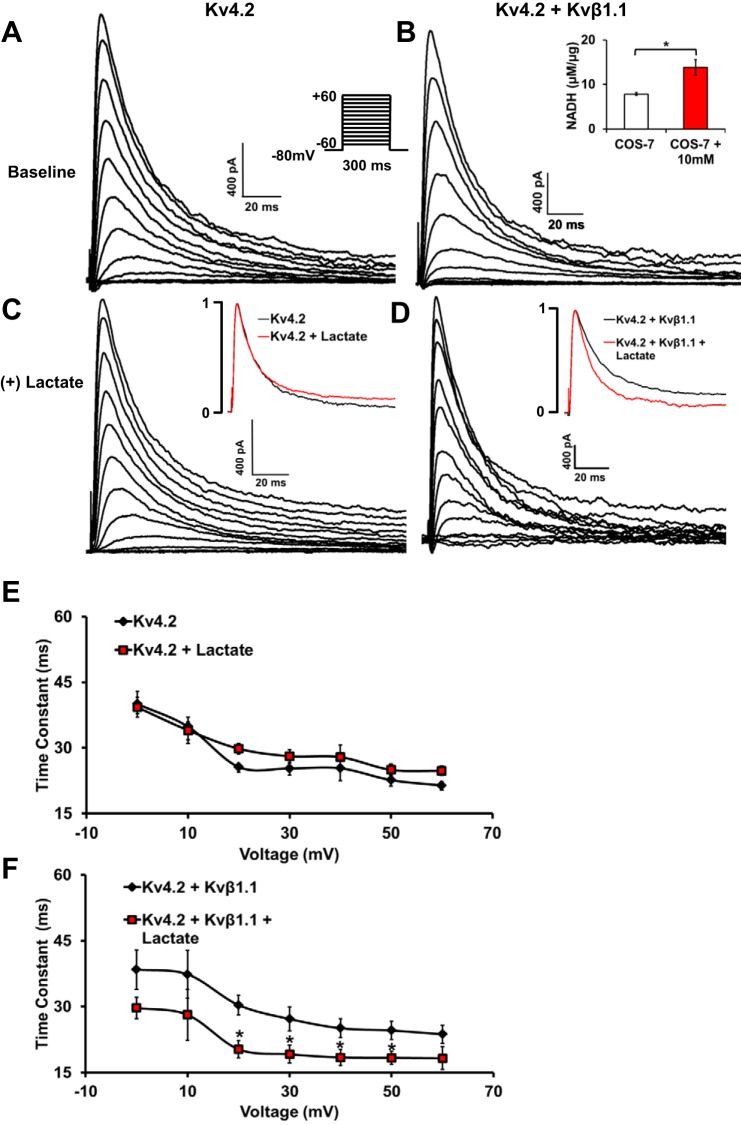
Kv4.2 inactivation decay (tau) in the presence of Kvβ1.1 and lactate.
We used COS-7 cells transfected with mKv4.2 with and without mKvβ1.1-GFP and perfused with 10-mM lactate solution to significantly increase the intracellular NADH levels (Fig. 3B, inset). Kv4.2 alone with and without lactate demonstrated no significant differences in overall current kinetics (Fig. 3, A and C), as well as in time constants (Fig. 3E) at voltages −20 to +60 mV. At baseline with no lactate exposure, Kv4.2+Kvβ1.1 demonstrated no significant current kinetics compared with Kv4.2 currents (Fig. 3, A and B). However, only in the presence of Kvβ1.1 did the addition of lactate result in a significant decrease in inactivation time constants at voltages of −20 to +60 mV (Fig. 3, D and F). However, inactivation time constants were not significantly different between Kv4.2 alone and Kv4.2+Kvβ1.1 groups, without lactate addition.
Fig. 3.
COS-7 cells transfected with Kv4.2 demonstrate redox (NADH/NAD)-dependent alterations in the presence of Kvβ1.1 A: current traces from representative COS-7 cells transfected with Kv4.2 alone. B: Kv4.2 with Kvβ1.1. Inset: NADH (µM/µg) levels with 10 mM lactate exposure. The data represent means ± SE (n = 3 hearts). *Significant difference represents P < 0.05. C: Kv4.2 alone with lactate (10 mM) exposure. D: Kv4.2 and Kvβ1.1 with lactate (10 mM) exposure; the voltage template is shown in Kv4.2 baseline. Inset: currents of baseline (black) and lactate (red) are shown at +40mV. E: time constant of decay demonstrates no significant difference between Kv4.2 with and without lactate exposure. The data represented are means ± SE (n = 10 cells in each group). F: time constant (tau) of decay demonstrates significant decrease in Kv4.2 with Kvβ1.1 in the presence of lactate exposure. The data represented are means ± SE (n = 10 cells in each group). *Significant difference represents P < 0.05.
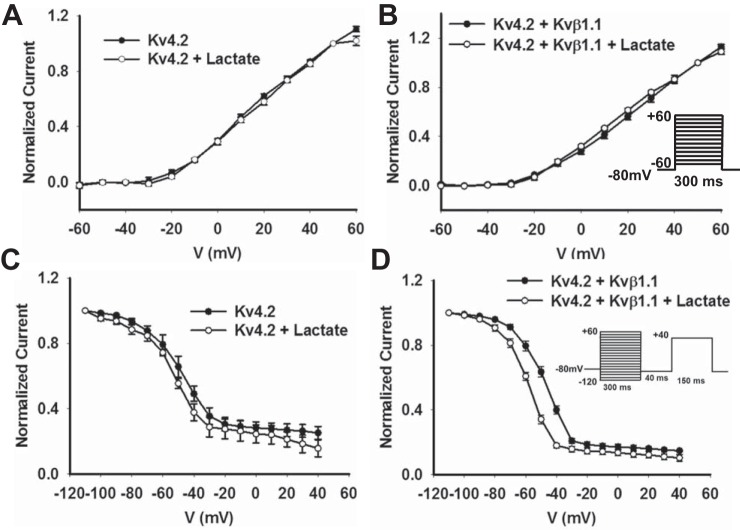
Current voltage relationship of Kv4.2 in the presence of Kvβ1.1 and modulation by lactate.
Analysis of current voltage (I-V) relationship of Kv4.2 exhibits an increase in current with channel activation at −20 mV, both before and after lactate exposure. The I-V curves were similar in Kv4.2+Kvβ1.1 group, and the addition of lactate caused no additional differences (Fig. 4, A and B). For the voltage dependence of inactivation measurements recorded by using the two-pulse protocol (Fig. 4), the I-V curves showed a steep decrease in the inactivation profile in both Kv4.2 alone and +lactate with a small nonsignificant hyperpolarization shift (P = NS at −40mV). The V1/2 of inactivation demonstrated that there was no significant difference between Kv4.2 alone and with the addition of lactate (−50.2 ± 3.1 mV and −52.3 ± 1.8 mV, P = 0.442). While Kv4.2 + Kvβ1.1 demonstrated a significant hyperpolarizing shift with the addition of lactate with a V1/2 of inactivation (−48.3 ± 1.1 mV and −58.6 ± 0.9 mV, P = 0.01) (Fig. 4D). Kv4.2 alone and Kv4.2 + Kvβ1.1 demonstrated no significant hyperpolarizing shift. These data suggest that the addition of lactate causes an increase in the Kvβ1.1-mediated hyperpolarization shift in Kv4.2 currents.
Fig. 4.
COS-7 cells transfected with Kv4.2 demonstrate alterations in inactivation in the presence of Kvβ1.1 and lactate. A: normalized current curve for Kv4.2 with and without lactate demonstrate no significant difference in activation. The data represented are means ± SE (n = 10 cells in each group). B: normalized current curve for Kv4.2+Kvβ1.1 with and without lactate demonstrate no significant difference in activation. The voltage dependence of activation was determined by normalizing outward currents at indicated voltages to +50 mV. The data represented are means ± SE (n = 10 cells in each group). The voltage template is shown in Kv4.2 and Kvβ1.1 baseline. C: normalized current curve for Kv4.2 with and without lactate demonstrates no significant difference in the voltage-dependent inactivation curve. The data represented are means ± SE (n = 10 cells in each group). D: normalized current curve for Kv4.2+Kvβ1.1 with and without lactate demonstrate a significant hyperpolarizing shift in the presence of lactate. The voltage dependence of inactivation was determined by normalizing outward currents at indicated voltages to −110 mV. The data represented are means ± SE (n = 10 cells in each group). The voltage template is shown in Kv4.2 and Kvβ1.1 lactate.
Isolated cardiomyocyte action potentials.
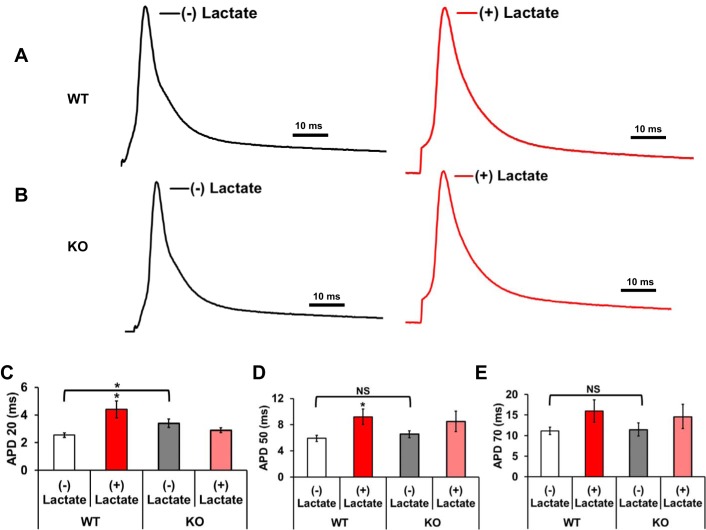
Left ventricular apex adult cardiomyocytes were isolated from the hearts of 16–20-wk WT and KO mice and were subjected to current-clamp recordings. Baseline action potentials were recorded after which external buffer was switched to a buffer containing lactate (10 mM) and were allowed to perfuse for 10 min. WT cardiomyocytes demonstrated significant increases in the action potential durations at APD 20 and 50% (P < 0.05), repolarization along with an increase at APD 70 (P < 0.1) after exposure with 10 mM lactate (Fig. 5, C–E). However, KO myocytes demonstrated no significant difference in APD durations (APD 20–70) after lactate exposure compared with no lactate (Fig. 5, C–E). Action potential durations between WT and KO myocytes at baseline or (−) lactate demonstrated a significant increase in KO at APD 20; however, while increased at APD 50 and 70, they were not significantly different from WT. These data suggest that by increasing the intracellular NADH levels by lactate perfusion, the action potential duration is significantly prolonged in WT myocytes. However, in the Kvβ1.1 KO cardiomyocyte, the increase in NADH by lactate fails to prolong the APD, suggesting a significant role for Kvβ1.1 in cardiac action potential regulation under high intracellular NADH levels.
Fig. 5.
Isolated cardiomyocyte action potentials (AP). A: representative AP traces from a single cell isolated from a WT mouse heart recorded in the absence (baseline, black line) and presence of 10 mM lactate (lactate, red line) in the external solution. B: representative APs from a single cell isolated from a KO mouse heart recorded in the absence (baseline, black line) and presence of 10 mM lactate (lactate, red line) in the external solution. APs were evoked from the cells under current-clamp mode by applying 2-ms current pulses delivered at 1 Hz. Representative AP traces have been edited to remove the stimulation artifact. C: action potential durations (APDs) at 20% (APD 20) repolarization lactate [white bar (−)] and lactate exposure [red bar (+)] in WT mice and lactate [gray bar (−)] and lactate [pink bar (+)] in KO mice. D: APDs at 50% (APD 50) repolarization lactate [white bar (−)] and lactate exposure [red bar (+)] in WT mice and lactate [gray bar (−)] and lactate [pink bar (+)] in KO mice. E: APDs at 70% (APD 70) repolarization lactate [white bar (−)] and lactate exposure [red bar) (+)] in WT mice and lactate [gray bar (−)] and lactate [pink bar (+)] in KO mice. The data represented are means ± SE (n = 7–9 cells in each group). Scale bars = 10 ms, *Significant difference represents P ≤ 0.05, and NS represents not statistically significant.
Changes in monophasic action potential in lactate perfused hearts.
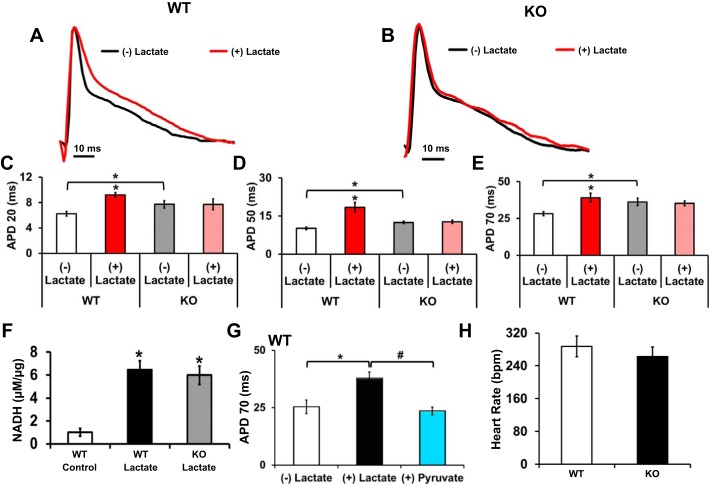
Ex vivo MAP traces were recorded from WT and KO hearts before and after lactate perfusion with a modified Krebs-Henseleit buffer consisting of 1 mM pyruvate and 20 mM lactate (Fig. 6, A and B). MAP waveforms show typical triangular peaks with a rapid depolarization upstroke followed by a downward spike representing repolarization activity. Analysis of MAP durations (ms) at APD 20, 50, and 70% repolarization demonstrated significant prolongation in the WT hearts after 10 min of lactate perfusion (Fig. 6, C–E). No significant prolongation was noted in KO hearts after 10 min of lactate exposure (Fig. 6, C–E). KO mouse hearts showed APD prolongation at baseline as compared with WT hearts at APD 20–70% (Fig. 6, C–E). The addition of lactate to the ex vivo WT and KO hearts demonstrated a significant increase in NADH (µM/µg) after lactate exposure (Fig. 6F). To determine that lactate-based alterations lead to electrical changes by modulating NADH levels and are not as a result of nonspecific effects, we used pyruvate in the buffer and perfused WT hearts as a rescue strategy. After 10 min of pyruvate perfusion, the APDs returned to levels similar to baseline (Fig. 6G). Heart rate of WT and KO hearts at baseline or (−) lactate recordings demonstrated no significant difference indicating that changes observed to APs were not due to heart rate variation (Fig. 6H). These results indicate that lactate perfusion leads to cardiac NADH increase and prolongation in action potential duration in wild-type mouse hearts, but not in KO hearts. Hence, it is plausible that the repolarization phase can be altered by modulation of NADH levels and that the Kvβ1.1 subunit is a key sensory component to relay the NADH alterations.
Fig. 6.
Pyridine nucleotide modulation alters monophasic action potentials (MAPs) A: representative MAP trace from a WT mouse heart recorded in the absence [(−) lactate, black line] and the presence of 20 mM lactate [(+) lactate, red line] in the external solution. B: representative MAP trace from a KO mouse heart recorded in the absence [(−) lactate, black line] and presence of 20 mM lactate [(+) lactate, red line] in the external solution. C: LV surface MAP durations at 20% (APD 20) in WT (+) lactate demonstrated a significant increase in duration, while KO group MAPs were indistinguishable. D: LV surface MAPs at 50% (APD 50) in WT (+) lactate demonstrated a significant increase in duration, while KO group APs were indistinguishable. E: LV surface MAPS at 70% (APD 70) in WT (+) lactate demonstrated a significant increase in duration, while KO group MAPs were indistinguishable. The data are represented as means ± SE (n = 7–10). *Significant difference, P < 0.05 (−) lactate vs. (+) lactate and WT (−) lactate vs. KO (−) lactate. F: relative NADH levels were examined in hearts after lactate perfusion from WT or KO when compared with WT hearts perfused with no lactate (Control). *Significant difference, P < 0.05, compared with control; n = 4. Bar represents 10-ms scale. G: LV surface action potentials at APD 70 in WT (−) lactate, (+) lactate, and (+) pyruvate demonstrated a significant increase in duration (lactate) and then a significant reduction in duration (pyruvate). The data are represented as means ± SE (n = 6). *Significant difference, P < 0.05 lactate vs. baseline. #Significant difference, P < 0.05 lactate vs. pyruvate. H: heart rate from LV surface action potentials. Data are represented as means ± SE (n = 7–10).
Changes in monophasic action potentials in ISO-infused hearts.
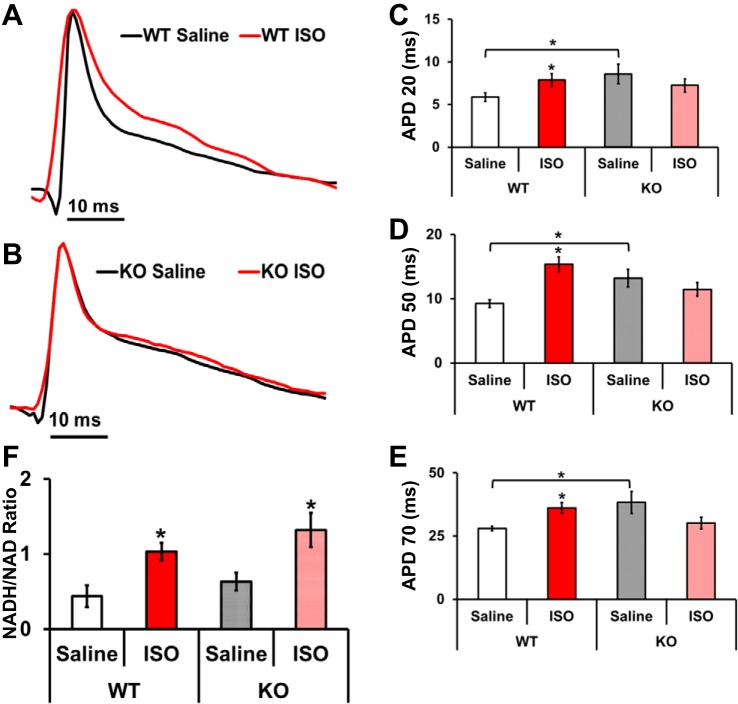
Isoproterenol infusion leads to increased intracellular NADH levels, and we used this as a strategy to identify electrical differences between WT and KO mouse hearts. We recorded MAPs from saline or ISO-infused WT and KO mice hearts (Fig. 7, A and B). Analysis of MAP traces demonstrates that chronic ISO exposure of WT mice prolongs cardiac APDs. As shown in Fig. 7, C–E, ISO treatment of WT mice led to a significant prolongation of APD at 20, 50, and 70% return repolarization, when compared with that of saline (Fig. 7, C–E). Contrarily, no significant differences were observed in the APDs of saline vs. ISO-exposed KO mice at APD 20, 50, and 70 (Fig. 7, C–E). Previous reports suggest that remodeling associated with cardiac hypertrophy also results in a significant shift in NADH/NAD+ levels. Hence, we reasoned that elevated NADH may reduce NADH/NAD+ ratio and contribute to the APD prolongation seen in WT mouse hearts. Indeed, infusion of ISO resulted to a significant increase in cardiac NADH/NAD+ ratios in both WT and KO mice (Fig. 7F). These results suggest that NADH elevation can lead to APD prolongation and that Kvβ1.1 subunit is essential to electrical signaling.
Fig. 7.
MAP changes in isoproterenol (ISO)-treated mouse hearts. A: representative MAP traces from a WT mouse heart infused (osmotic mini pumps) with saline (black line) and isoproterenol (red line). B: representative MAP traces from a KO mouse heart infused (osmotic mini pumps) with saline (black line) and isoproterenol (red line). C: LV surface MAPs at 20% repolarization (APD 20) in WT ISO demonstrated a significant increase in duration, while KO-ISO MAPs were indistinguishable from KO-Saline. D: LV surface MAP at 50% repolarization (APD 50) in WT ISO demonstrated a significant increase in duration, while KO-ISO MAPS were indistinguishable from KO-Saline. E: LV surface MAPs at 70% repolarization (APD 70) in WT ISO demonstrated a significant increase in duration, while KO-ISO action potentials were indistinguishable from KO-Saline. The data are represented as means ± SE (n = 7). *Significant difference, P < 0.05 ISO vs. saline and WT saline vs. KO saline. F: NADH/NAD ratio of WT and KO hearts from saline or ISO. Both WT and KO-ISO hearts showed a significant increase in NADH/NAD ratios. Bars show means ± SE; n = 4. *Significant difference, P < 0.05 ISO vs. saline.
ECG activity in ISO-infused Kvβ1.1 KO hearts.
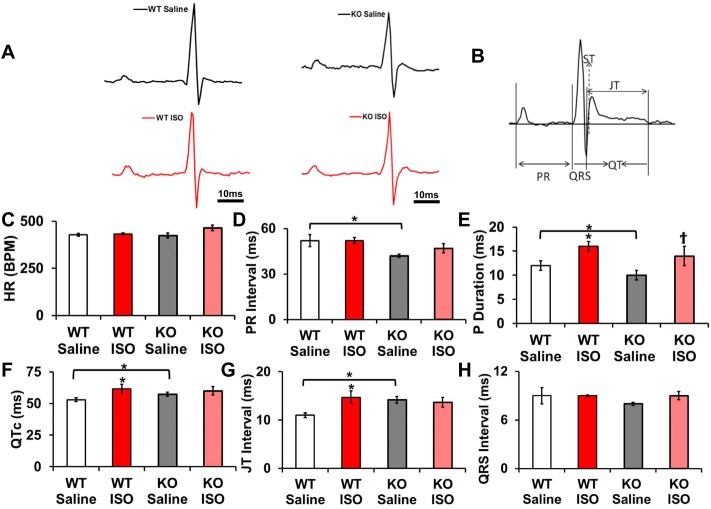
To evaluate the electrical activity in the mice, we used lead II ECG recordings. The WT and KO mice were infused with either saline or ISO, and ECG recordings were obtained on the 14th day (Fig. 8A). Heart rate variation between all groups demonstrated no significant difference (Fig. 8C). The PR interval along with the P duration demonstrates a significant decrease in KO saline (baseline) compared with WT controls. P duration in both WT-ISO and KO-ISO were significantly increased from their respective controls (Fig. 8E). WT-ISO mice demonstrated significantly increased QTc, and JT intervals compared with the saline group, suggesting a decreased repolarization reserve after ISO infusion (Fig. 8, F and G). The QTc and JT interval in the KO-Saline and KO-ISO mice remain unchanged, indicating that electrical activity was unaltered with isoproterenol infusion (Fig. 8, F and G). The mice showed a significant QTc and JT interval prolongation at baseline between WT and KO mice (Fig. 8, F and G). QRS intervals remained unaltered in all groups (Fig. 8H). ECG data clearly validate the notion that isoproterenol-induced QT prolongation is attenuated in the KO compared with WT.
Fig. 8.
ECG parameters. A: representative lead II ECG traces from anesthetized mice after saline or ISO infusion of WT [Saline (black line) and ISO (red line)] and KO [Saline (black line) and ISO (red line)] scale bar represents 10 ms. B: representative mouse ECG diagram on different ECG parameters measured. C: heart rates in WT and KO groups. Data are represented are means ± SE; n = 10–12. D: PR interval durations in WT and KO groups. The data are represented as means ± SE; n = 10–12. *Significant difference, P < 0.05 WT saline vs. KO saline. E: P durations in WT and KO groups. Data are represented as means ± SE; n = 10–12. *P < 0.05 WT ISO vs. WT saline, as well as KO vs. WT-Saline. †P < 0.05 KO Saline vs. KO ISO. F: QTc interval durations in WT and KO groups. The data are represented as means ± SE; n = 10–12. *P < 0.05 ISO vs. saline, as well as KO vs. WT-Saline. G: JT interval durations in WT and KO groups. The data are represented as means ± SE; n = 10–12. *P < 0.05 ISO vs. Saline, as well as KO vs. WT-Saline. H: QRS interval durations in WT and KO groups. The data are represented as means ± SE; n = 10–12.
Cardiac ion channel expression.
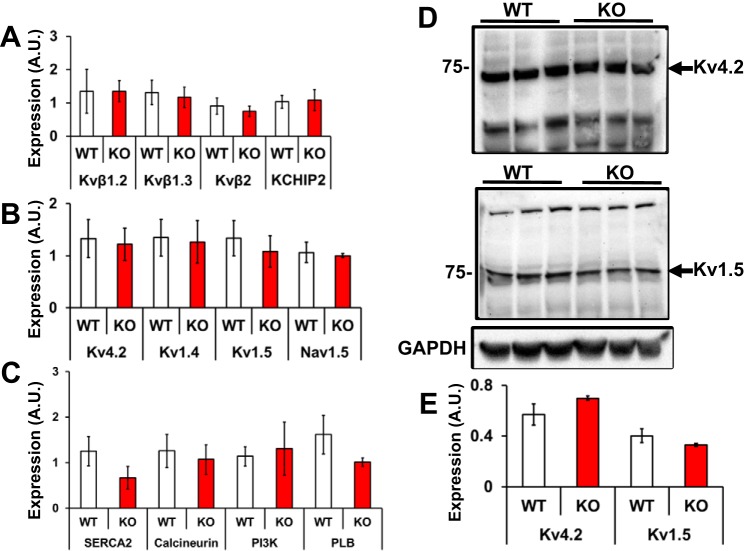
To gain insights into the transcriptional status of key Kv channels in Kvβ1.1 KO hearts, qRT-PCR assay was used to assess the expression of Kv channel subunit genes, including Kvβ1.2, Kvβ1.3, Kvβ2, and KChIP2, which showed no significant differences between WT and KO hearts (Fig. 9A). Also, key ion channels known to interact with Kvβ1.1 including Kv4.2, Kv1.4, and Kv1.5, as well as ion channels affected by pyridine nucleotides, Nav1.5, were also assayed, which revealed no significant differences between WT and KO hearts (Fig. 9B) (21). Furthermore, expression of key mediators of calcium signaling, including SERCA2, calcineurin, PI3K, and phospholamban (PLB), was found to be comparable in the WT and KO hearts (Fig. 9C). These results suggest that the electrical changes observed are, therefore, likely caused by kinetic alterations as opposed to gene expression changes. Whole hearts were homogenized and membrane extracts were separated for Western blot analysis of Kv4.2 and Kv1.5 expression, revealing no significant difference between WT and KO membrane fractions (Fig. 9, D and E).
Fig. 9.
Gene expression. A: mRNA expression of β subunits, including Kvβ1.2 Kvβ1.3, Kvβ2, and KCHIP2, the data are represented as means ± SE; n = 3. B: gene expression of key cardiac channels, including Kv4.2 Kv1.4, Kv1.5, and Nav1.5; the data represented are means ± SE (n = 3). C: gene expression of key calcium regulators SERCA2, calcineurin, PI3K, and PLB (phospholamban). Data are represented as means ± SE; n = 3. D: protein expression of key KV channels in the heart. Kv4.2 and Kv1.5 demonstrate no significant difference in membrane expression between WT and KO. E: data are represented as means ± SE; n = 3.
DISCUSSION
In the present study, we identified Kvβ1.1 as a major physiological regulator in the heart. Modulation of pyridine nucleotides is sensed by the Kvβ1.1 subunit and helps relay the biochemical information for regulating the electrical activity.
We identified that the cellular action potential is modified with the addition of lactate and regulated by the presence of Kvβ1.1. The basal tonic regulation of action potential is tightly coupled to the ion channel function via Kv4.2, through which the action potential is significantly regulated. Identification of increased intracellular NADH levels via perfusion of lactate was utilized as a model to probe its effects on cardiac action potential and KV current changes (21). In presence of Kvβ1.1 subunit, using the WT cardiomyocytes, we identified that the action potential is prolonged upon NADH increase, and lack of Kvβ1.1 subunit significantly diminished the modulatory role of NADH on the action potential. These experiments for the first time clearly demonstrate that Kvβ1.1 is necessary for regulation of basal action potential duration, as well as imparts the ability to sense the changes in the pyridine nucleotides in the cardiomyocytes in a precise fashion.
Previous studies identified that Kvβ subunit belongs to the aldo-keto reductase superfamily. The Kvβ1 belongs to the AKR6 family and depicts very tight binding to NADH/NAD+. Crystal structure analysis revealed that NADP+ is very tightly bound to the Kvβ subunit and cocrystallizes with the Kvβ protein (13). Affinity studies performed to identify the dissociation constant (Kd) showed that that the affinity is in the micromolar range (22, 46). Therefore, the fundamental information for the binding characteristics and ion channel modulation was previously published; however, the physiological roles of Kvβ subunits remain unknown. In the present study, we connect the biochemical basis and the ion channel physiology to cardiac electrical activity. On the basis of the biochemical changes caused by modulation of NADH/NAD+, the KV current is regulated by Kvβ1.1 subunit in a subtle but significant manner and that these connections ultimately lead to physiological changes, including action potential and ECG changes in the mice heart.
While cardiac action potential is an ensemble of many ionic currents (K, Na, Ca) and its activity during depolarization and repolarization depend on many ion channels (11), the role of Kvβ1.1 seems to be tightly coupled to KV current modulation affecting the repolarization in the presence of NADH/NAD+. Previously, we identified that KV current is modulated by Kvβ subunits in the presence of oxidized (NAD+/NADP+) and reduced pyridine nucleotides (NADH/NADPH) (45–47). Under reducing (NADH) conditions, the Kv current inactivation was supported while the addition of oxidized pyridine nucleotides (NAD+) provided Kv current activation. These studies form the experimental basis for testing the physiological roles of Kvβ1.1 in the heart. Using heterologous COS-7 expression system, we coexpressed Kv4.2 along with the Kvβ1.1 subunit to identify the influence of NADH via lactate perfusion. As noted previously with Kv1.5+Kvβ1 pairing, we found that Kvβ1.1 produces a faster inactivation tau with Kv4.2 in the presence of NADH. Further, we demonstrated that steady-state inactivation of Kv4.2 was significantly shifted to a more hyperpolarized state by Kvβ1.1 in the presence of elevated NADH.
The ex vivo MAPs show that action potentials were significantly prolonged in wild-type hearts upon addition of lactate, whereas the action potential prolongation was completely reversed with the addition of pyruvate, which is a known energy substrate that can restore the intracellular levels of NAD+, clearly identifying the role of NADH/NAD+ in cardiac electrical activity. Moreover, the lack of Kvβ1.1 subunit failed to impart the ability of heart to respond to NADH/NAD+ changes, pointing to the importance of the Kvβ1.1 subunit in cardiac physiology both at the cellular and tissue level. We next asked the physiological significance of Kvβ1.1 in cardiac physiology in regard to ECG changes and whether Kvβ1.1 has a physiological role in terms of modulating ECG. The cardiac lead II ECG signal allows monitoring the left ventricular activity in a precise manner (7). To test this, we used the Kvβ1.1 knockout mice and recorded ECG from wild-type and KO mice and identified that lack of Kvβ1.1 subunit leads to prolonged QTc and JT interval in 16–20-wk-old mice. The QTc is a standard measure of cardiac ventricular depolarization and repolarization activity, providing the ability to delineate the contribution of Kvβ1.1 to ECG changes (35). Furthermore, the KO mice demonstrated a significant decrease in P duration and PR interval compared with WT mice. P duration and PR interval changes have demonstrated proarrhythmic phenotypes (20). Although the significant decrease in PR duration in KO has yet to be defined, quantitative trait loci mapping demonstrated that chromosome 3 influences the variance of the PR interval; interestingly, Kvβ1.1 (KCNAB1) is found on chromosome 3 (40). A significant increase in P duration in WT and KO after ISO exposure has been previously demonstrated in hypertrophic models (37, 43). These data demonstrate a significant physiological role for Kvβ1.1 subunit in the heart, in which coupling of Kvβ1.1 subunit to the Kv channel can significantly alter the cardiac action potential and ECG parameters. We further investigated the role of Kvβ1.1 subunit in the physiological changes in cardiac NADH/NAD+ levels. For this, we used the well-established cardiac hypertrophy model by infusing isoproterenol for 14 days in wild-type or KO mice. Hypertrophy and injury caused by ISO infusion have been shown to cause prolongation of QTc (44) and MAPs (10), as well as a significant decrease in Kv4.2 and Kv4.3 current (28). Although hypertrophy can result in significant cardiac alterations, including the increase in PKA, which regulates many regulatory proteins in cardiac contraction-relaxation cycle, such as ryanodine receptor 2 and L-type Ca channels, it is important to note that QRS interval was not altered after ISO exposure in either WT or KO mice (49). In addition, numerous hypertrophic research has demonstrated that L-type calcium currents remained unchanged (16). Furthermore, preliminary evidence demonstrates that reduced Ito density represents a very early event in response to decreased pump performance (24, 51). Therefore, decreased Ito appears to be a significant contributor to action potential prolongation in cardiac hypertrophy (51).
Isoproterenol perfusion caused a significant increase in cardiac NADH/NAD+ ratio levels, leading to prolonged QTc intervals in the wild-type mice; however, the KO mice lacking the Kvβ1.1 subunit failed to respond to the NADH/NAD+ ratio increase caused by cardiac hypertrophy. Since the KO mice failed to demonstrate further prolongation in the QTc with ISO treatment, it is likely that the NADH generated due to hypertrophic response is not sensed and, hence, NADH-induced QTc prolongation was abolished in the KO mice. Therefore, on the basis of the cellular model using Kv4.2+Kvβ1.1 expression system, cardiomyocyte action potential, and tissue ex vivo action potentials, we identified and established the physiological responses of the heart in the presence and absence of the Kvβ1.1 subunit for its ability to sense change in NADH/NAD+ levels. In a recent study we published the sex differences between Kvβ1.1 KO mice and demonstrated that loss of Kvβ1.1 leads to similar electrical differences in male and female KO mice, in which the ECG and the monophasic action potentials were prolonged similarly due to lack of Kvβ1.1 in the KO mice (48). In the same study, we tested additional cardiac phenotype and identified that the female Kvβ1.1 KO mice developed enlarged hearts compared with female wild type, whereas the male Kvβ1.1 did not develop cardiac hypertrophy compared with male wild-type mouse hearts. Therefore, these data clearly identify that the electrical activity is similar in both male and female Kvβ1.1 KO mice. Since Kvβ1.1 bind with NADH and NAD with high affinity and the genetic deletion of Kvβ1.1 leads to similar electrical activities in male and female mice, it clearly points to the notion that the sensing of pyridine nucleotide by Kvβ1.1 will be similar in both male and female mouse hearts. Overall, these changes point to the idea that because Kvβ1.1 is an obligatory mediator for sensing NADH/NAD+ changes in the heart, it is likely that ECG changes caused by cardiac hypertrophy are due to high NADH levels, and the presence of Kvβ1.1 allows the heart to sense the pyridine nucleotide changes.
Heterologous expression studies using Xenopus oocytes or mammalian expression system established that Kvβ1 could bind to multiple KV channel partners (19, 34, 41). Rat heart studies identified that Kvβ1.1 binds Kv1.5, while other studies showed that Kvβ1.1 binds to the Kv4 channel (31, 53). Coimmunoprecipitation in mouse heart revealed that Kvβ1.1 binds with Kv4.2 and is likely a preferential binding partner in the mouse heart (1). By using the pull-down approach in the present study, we identify the binding of Kvβ1.1 with Kv4.2, which is in agreement with a previous report (1). Overall, these studies provide a strong basis that Kvβ1.1 can bind to Kv4.2 and other Kv channels, and, therefore, likely contributes to the cardiac electrical activity in a physiologically significantly manner.
Conclusions.
Overall in the present study, we demonstrate that Kvβ1.1 subunit offers sensing of changes in NADH/NAD+ in the heart. The modulation of Kv4.2 currents in the presence and absence of Kvβ1.1 under increased NADH levels points toward the ability of Kvβ1.1 subunit to mediate inactivation of Kv4.2 currents. The changes in action potential duration and contribution of Kvβ1.1 in cardiomyocyte and ex vivo hearts identify the specific roles of Kvβ1.1 mediation in sensing NADH changes under both cellular and ex vivo settings. In addition, the in vivo changes at the ECG level using the cardiac hypertrophy model, which cause increased intracellular NADH levels, clearly shows that the electrical activity and changes to NADH increase are mediated by Kvβ1.1 subunit since the KO mice failed to respond to hypertrophic stimulation. Taken together, the physiological changes and the biochemical basis provide novel mechanistic insights with distinct Kvβ subunit-mediated responses in cardiovascular physiology.
GRANTS
Authors acknowledge the grant support received from National Institutes of Health Grant HL-102171 (to S. M. Tipparaju).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.T., K.C.C., and C.K. performed experiments; J.T. analyzed data; J.T. drafted manuscript; J.T., A.B., and S.M.T. edited and revised manuscript; J.T., K.C.C., C.K., J.C., and S.M.T. approved final version of manuscript; K.C.C., J.C., A.B., and S.M.T. interpreted results of experiments; S.M.T. conceived and designed research.
ACKNOWLEDGMENTS
The authors also acknowledge that part of the study was performed toward a doctoral dissertation (J. Tur).
REFERENCES
- 1.Aimond F, Kwak SP, Rhodes KJ, Nerbonne JM. Accessory Kvβ1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes. Circ Res 96: 451–458, 2005. doi: 10.1161/01.RES.0000156890.25876.63. [DOI] [PubMed] [Google Scholar]
- 2.Akar FG, Wu RC, Deschenes I, Armoundas AA, Piacentino V III, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol 286: H602–H609, 2004. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- 3.Akar FG, Wu RC, Juang GJ, Tian Y, Burysek M, Disilvestre D, Xiong W, Armoundas AA, Tomaselli GF. Molecular mechanisms underlying K+ current downregulation in canine tachycardia-induced heart failure. Am J Physiol Heart Circ Physiol 288: H2887–H2896, 2005. doi: 10.1152/ajpheart.00320.2004. [DOI] [PubMed] [Google Scholar]
- 4.Calloe K, Cordeiro JM, Di Diego JM, Hansen RS, Grunnet M, Olesen SP, Antzelevitch C. A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res 81: 686–694, 2009. doi: 10.1093/cvr/cvn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceconi C, Bernocchi P, Boraso A, Cargnoni A, Pepi P, Curello S, Ferrari R. New insights on myocardial pyridine nucleotides and thiol redox state in ischemia and reperfusion damage. Cardiovasc Res 47: 586–594, 2000. doi: 10.1016/S0008-6363(00)00104-8. [DOI] [PubMed] [Google Scholar]
- 6.Chapalamadugu KC, Panguluri SK, Bennett ES, Kolliputi N, Tipparaju SM. High level of oxygen treatment causes cardiotoxicity with arrhythmias and redox modulation. Toxicol Appl Pharmacol 282: 100–107, 2015. doi: 10.1016/j.taap.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danik S, Cabo C, Chiello C, Kang S, Wit AL, Coromilas J. Correlation of repolarization of ventricular monophasic action potential with ECG in the murine heart. Am J Physiol Heart Circ Physiol 283: H372–H381, 2002. doi: 10.1152/ajpheart.01091.2001. [DOI] [PubMed] [Google Scholar]
- 8.Deschênes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett 528: 183–188, 2002. doi: 10.1016/S0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- 9.Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvβ1.1-deficient mice with impaired learning. Learn Mem 5: 257–273, 1998. doi: 10.1101/lm.5.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillis AM, Mathison HJ, Patel C, Lester WM. Quinidine pharmacodynamics in normal and isoproterenol-induced hypertrophied blood-perfused working rabbit hearts. J Cardiovasc Pharmacol 27: 916–926, 1996. doi: 10.1097/00005344-199606000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol 2: 185–194, 2009. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 12.Grubb S, Aistrup GL, Koivumäki JT, Speerschneider T, Gottlieb LA, Mutsaers NA, Olesen SP, Calloe K, Thomsen MB. Preservation of cardiac function by prolonged action potentials in mice deficient of KChIP2. Am J Physiol Heart Circ Physiol 309: H481–H489, 2015. doi: 10.1152/ajpheart.00166.2015. [DOI] [PubMed] [Google Scholar]
- 13.Gulbis JM, Mann S, MacKinnon R. Structure of a voltage-dependent K+ channel β subunit. Cell 97: 943–952, 1999. doi: 10.1016/S0092-8674(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 14.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res 105: 481–491, 2009. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilfoil PJ, Tipparaju SM, Barski OA, Bhatnagar A. Regulation of ion channels by pyridine nucleotides. Circ Res 112: 721–741, 2013. doi: 10.1161/CIRCRESAHA.111.247940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiman RB, Houser SR. Calcium currents in normal and hypertrophied isolated feline ventricular myocytes. Am J Physiol Heart Circ Physiol 255: H1434–H1442, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Neely JR. Effects of ischemia and reperfusion on pyruvate dehydrogenase activity in isolated rat hearts. J Mol Cell Cardiol 15: 359–367, 1983. doi: 10.1016/0022-2828(83)90320-6. [DOI] [PubMed] [Google Scholar]
- 18.Kong W, Po S, Yamagishi T, Ashen MD, Stetten G, Tomaselli GF. Isolation and characterization of the human gene encoding Ito: further diversity by alternative mRNA splicing. Am J Physiol Heart Circ Physiol 275: H1963–H1970, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Kuryshev YA, Wible BA, Gudz TI, Ramirez AN, Brown AM. KChAP/Kvβ1.2 interactions and their effects on cardiac Kv channel expression. Am J Physiol Cell Physiol 281: C290–C299, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Lim WW, Baumert M, Neo M, Kuklik P, Ganesan AN, Lau DH, Tsoutsman T, Semsarian C, Sanders P, Saint DA. Slowed atrial and atrioventricular conduction and depressed HRV in a murine model of hypertrophic cardiomyopathy. Clin Exp Pharmacol Physiol 43: 95–101, 2016. doi: 10.1111/1440-1681.12498. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Sanyal S, Gao G, Gurung IS, Zhu X, Gaconnet G, Kerchner LJ, Shang LL, Huang CL, Grace A, London B, Dudley SC Jr. Cardiac Na+ current regulation by pyridine nucleotides. Circ Res 105: 737–745, 2009. doi: 10.1161/CIRCRESAHA.109.197277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu SQ, Jin H, Zacarias A, Srivastava S, Bhatnagar A. Binding of pyridine nucleotide coenzymes to the β-subunit of the voltage-sensitive K+ channel. J Biol Chem 276: 11812–11820, 2001. doi: 10.1074/jbc.M008259200. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, O’Rourke B. Regulation of the Na+/Ca2+ exchanger by pyridine nucleotide redox potential in ventricular myocytes. J Biol Chem 288: 31984–31992, 2013. doi: 10.1074/jbc.M113.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation 85: 1175–1188, 1992. doi: 10.1161/01.CIR.85.3.1175. [DOI] [PubMed] [Google Scholar]
- 25.Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, Silva AJ. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvβ1.1 knockout mice. Curr Biol 14: 1907–1915, 2004. doi: 10.1016/j.cub.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Need AC, Irvine EE, Giese KP. Learning and memory impairments in Kv β 1.1-null mutants are rescued by environmental enrichment or ageing. Eur J Neurosci 18: 1640–1644, 2003. doi: 10.1046/j.1460-9568.2003.02889.x. [DOI] [PubMed] [Google Scholar]
- 27.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 28.Panama BK, Korogyi AS, Aschar-Sobbi R, Oh Y, Gray CB, Gang H, Brown JH, Kirshenbaum LA, Backx PH. Reductions in the cardiac transient outward K+ current Ito caused by chronic β-adrenergic receptor stimulation are partly rescued by inhibition of nuclear factor-κB. J Biol Chem 291: 4156–4165, 2016. doi: 10.1074/jbc.M115.694984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panguluri SK, Tur J, Fukumoto J, Deng W, Sneed KB, Kolliputi N, Bennett ES, Tipparaju SM. Hyperoxia-induced hypertrophy and ion channel remodeling in left ventricle. Am J Physiol Heart Circ Physiol 304: H1651–H1661, 2013. doi: 10.1152/ajpheart.00474.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SP, Campbell DL. Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol 569: 7–39, 2005. doi: 10.1113/jphysiol.2005.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez-García MT, López-López JR, González C. Kvβ1.2 subunit coexpression in HEK293 cells confers O2 sensitivity to kv4.2 but not to Shaker channels. J Gen Physiol 113: 897–907, 1999. doi: 10.1085/jgp.113.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J Biol Chem 280: 43121–43130, 2005. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 33.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 285: 3133–3144, 2010. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 90: 755–796, 2010. doi: 10.1152/physrev.00020.2009. [DOI] [PubMed] [Google Scholar]
- 35.Postema PG, Wilde AA. The measurement of the QT interval. Curr Cardiol Rev 10: 287–294, 2014. doi: 10.2174/1573403X10666140514103612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature 369: 289–294, 1994. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg MA, Das S, Pinzon PQ, Knight AC, Sosnovik DE, Ellinor PT, Rosenzweig A. A novel transgenic mouse model of cardiac hypertrophy and atrial fibrillation. J Atr Fibrillation 2: 1–15, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito). J Physiol 546: 5–18, 2003. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev 94: 609–653, 2014. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 40.Scicluna BP, Tanck MW, Remme CA, Beekman L, Coronel R, Wilde AA, Bezzina CR. Quantitative trait loci for electrocardiographic parameters and arrhythmia in the mouse. J Mol Cell Cardiol 50: 380–389, 2011. doi: 10.1016/j.yjmcc.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Sewing S, Roeper J, Pongs O. Kvβ1 subunit binding specific for shaker-related potassium channel alpha subunits. Neuron 16: 455–463, 1996. doi: 10.1016/S0896-6273(00)80063-X. [DOI] [PubMed] [Google Scholar]
- 42.Speerschneider T, Thomsen MB. Physiology and analysis of the electrocardiographic T wave in mice. Acta Physiol (Oxf) 209: 262–271, 2013. doi: 10.1111/apha.12172. [DOI] [PubMed] [Google Scholar]
- 43.Sysa-Shah P, Sørensen LL, Abraham MR, Gabrielson KL. Electrocardiographic Characterization of cardiac hypertrophy in mice that overexpress the ErbB2 receptor tyrosine kinase. Comp Med 65: 295–307, 2015. [PMC free article] [PubMed] [Google Scholar]
- 44.Tang T, Lai NC, Wright AT, Gao MH, Lee P, Guo T, Tang R, McCulloch AD, Hammond HK. Adenylyl cyclase 6 deletion increases mortality during sustained β-adrenergic receptor stimulation. J Mol Cell Cardiol 60: 60–67, 2013. doi: 10.1016/j.yjmcc.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tipparaju SM, Li XP, Kilfoil PJ, Xue B, Uversky VN, Bhatnagar A, Barski OA. Interactions between the C-terminus of Kv1.5 and Kvβ regulate pyridine nucleotide-dependent changes in channel gating. Pflügers Arch 463: 799–818, 2012. doi: 10.1007/s00424-012-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tipparaju SM, Liu S-Q, Barski OA, Bhatnagar A. NADPH binding to β-subunit regulates inactivation of voltage-gated K+ channels. Biochem Biophys Res Commun 359: 269–276, 2007. doi: 10.1016/j.bbrc.2007.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tipparaju SM, Saxena N, Liu SQ, Kumar R, Bhatnagar A. Differential regulation of voltage-gated K+ channels by oxidized and reduced pyridine nucleotide coenzymes. Am J Physiol Cell Physiol 288: C366–C376, 2005. doi: 10.1152/ajpcell.00354.2004. [DOI] [PubMed] [Google Scholar]
- 48.Tur J, Chapalamadugu KC, Padawer T, Badole SL, Kilfoil PJ, Bhatnagar A, and Tipparaju SM. Deletion of Kvβ1.1 subunit leads to electrical and haemodynamic changes causing cardiac hypertrophy in female murine hearts. Exp Physiol 101: 494–508, 2016. doi: 10.1113/EP085405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidal M, Wieland T, Lohse MJ, Lorenz K. β-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gβγ/Erk-dependent pathway. Cardiovasc Res 96: 255–264, 2012. doi: 10.1093/cvr/cvs249. [DOI] [PubMed] [Google Scholar]
- 50.Webb IG, Nishino Y, Clark JE, Murdoch C, Walker SJ, Makowski MR, Botnar RM, Redwood SR, Shah AM, Marber MS. Constitutive glycogen synthase kinase-3α/β activity protects against chronic β-adrenergic remodelling of the heart. Cardiovasc Res 87: 494–503, 2010. doi: 10.1093/cvr/cvq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wickenden AD, Kaprielian R, Kassiri Z, Tsoporis JN, Tsushima R, Fishman GI, Backx PH. The role of action potential prolongation and altered intracellular calcium handling in the pathogenesis of heart failure. Cardiovasc Res 37: 312–323, 1998. doi: 10.1016/S0008-6363(97)00256-3. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One 9: e98972, 2014. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang EK, Alvira MR, Levitan ES, Takimoto K. Kvβ subunits increase expression of Kv4.3 channels by interacting with their C termini. J Biol Chem 276: 4839–4844, 2001. doi: 10.1074/jbc.M004768200. [DOI] [PubMed] [Google Scholar]