Abstract
Free radical toxicity due to poorly maintained cellular redox levels is crucial events that have been associated with the pathogenesis of Guillain–Barre syndrome (GBS) patients. Uric acid (UA) and albumin correlate with oxidative stress in some degree. We aimed to evaluate the relationship between GBS and serum levels of UA and albumin in the present study.
The serum levels of UA and albumin were determined in 203 individuals including 88 patients with GBS and 153 healthy controls (HC).
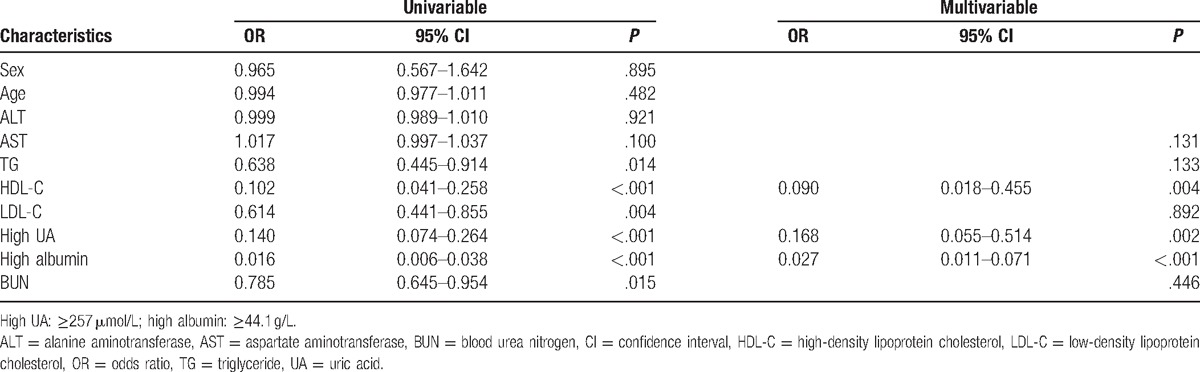
We found that serum levels of UA and albumin in patients with GBS were significantly lower than those in HC group. Besides, similar phenomenon was observed when the male and female subgroups were estimated, respectively. Additionally, we found that there is no statistic difference among subgroups of GBS regarding UA and albumin. The univariate analysis revealed that both the high UA and high albumin were protective factors for patients with GBS (odds ratio [OR] 0.140; 95% confidence interval [CI]: 0.074–0.264; P < .001 and OR 0.016; 95% CI: 0.006–0.038; P < .001, respectively). It was further confirmed by the multivariable logistic regression analysis after adjusting for other potential confounding factors (OR 0.168; 95% CI: 0.055–0.514; P = .002 and OR 0.027; 95% CI: 0.011–0.071; P < .001, respectively).
In conclusion, we found that patients with GBS had significantly low serum UA and albumin levels. Moreover, we demonstrated that both the high UA and high albumin were protective factors for patients with GBS.
Keywords: antioxidants, Guillain–Barre syndrome, serum albumin, uric acid
1. Introduction
As chemically reactive molecules, reactive oxygen species (ROS) including free radicals (superoxide and hydroxyl radicals) and nonradical species (hydrogen peroxide) can be produced at various conditions in a multitude of ways. It has been proven that excessive ROS production is responsible for inflammatory and autoimmune-mediated tissue destruction, which will inevitably impair cellular metabolism and result in cell death. To maintain the appropriate cellular redox balance, antioxidant defense systems should control excessive ROS production by scavenging or reducing ROS levels.[1] A state of oxidative stress results from imbalance between the production of antioxidant defense mechanisms and ROS. Uric acid (UA) is the main final product of the common pathway of purine nucleotides metabolism. As a naturally occurring antioxidant in human blood,[2] UA can be capable of scavenging peroxynitrite (PN).[3] An overwhelming amount of studies evidenced that serum albumin possesses potent antioxidant properties, which inhibit production of free hydroxyl radicals[4] and have the ability to scavenge peroxy radicals.[5,6] Moreover, it has been proposed that albumin is a major known antioxidant role in extracellular fluids.[7] As a result, there is no doubt that UA and albumin correlate with oxidative stress in some degree.
As an autoimmune disease of the nervous system, Guillain–Barre syndrome (GBS) is an acute peripheral inflammatory neuropathy and the most common cause of nonpoliovirus acute flaccid paralysis around the world with an incidence of 1.2 to 2.3/100,000 people a year.[8–10] It has been proven that free radical toxicity due to poorly maintained cellular redox levels is crucial events that have been associated with the pathogenesis of patients with GBS.[11] Besides, a decrease was shown for serum antioxidant activity in both GBS and multiple sclerosis (MS) patients when compared with the control.[11] GBS, neuromyelitis optica (NMO), and MS are all the inflammatory demyelinating autoimmune diseases of the nervous system and they have much in common in the pathogenesis. A multitude of literature suggests that excessive production of the ROS or reactive nitrogen species (RNS) that lead to oxidative and nitrosative stress is the major determinant in the pathogenesis of inflammatory and autoimmune diseases of the nervous system such as NMO,[1] MS,[12] myasthenia gravis (MG)[13] and the like. Previous reports have proven that lower serum UA levels were observed in patients with MS than the healthy control (HC) groups.[3,14,15] Additionally, lower serum UA and albumin levels were observed in patients with NMO[1] and MG[13,16] than the HC groups. Furthermore, the data and findings of Fuhua Peng's study[17] suggest that patients with GBS may have lower serum UA levels than those in the HC group.
Based on the above observations, we drive the hypothesis that UA and albumin may be critical in GBS and that patients with GBS had significantly lower serum UA and albumin levels than those in the HC group. Considering that the sampling size of Fuhua Peng's study[17] was too small and its statistical analyses lack rigor and serum levels of albumin, we aimed to evaluate the relationship between GBS and serum levels of UA and albumin in the present study.
2. Patients and methods
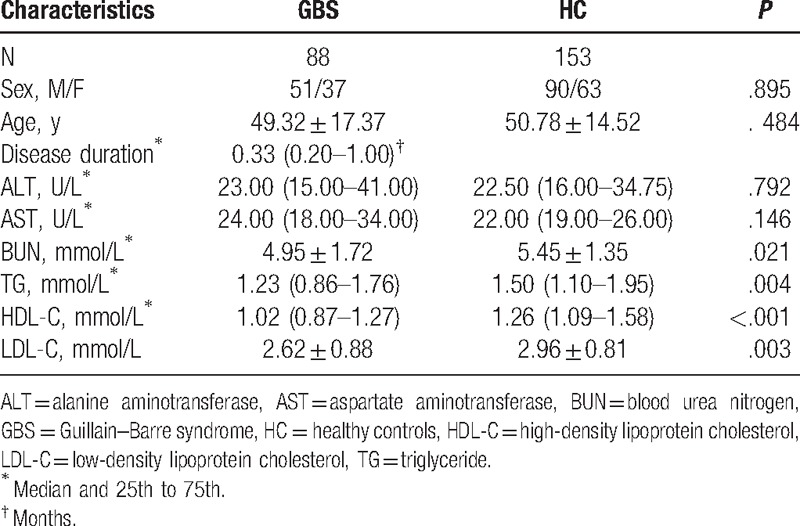
We enrolled 203 individuals comprising 88 patients with GBS and 153 HC who underwent a health examination in the First Affiliated Hospital of Wenzhou Medical University, from June 2009 to August 2016. Moreover, serum samples were collected from 88 patients with GBS before beginning any treatment on admission and 153 HC who underwent a health examination. The basic demographic and clinical characteristics of GBS patients and HC were presented in Table 1. Venous blood was drawn and was used for the analysis of biochemical measurements by venipuncture using a Clinical Analyzer Beckman Coulter AU5831 (Beckman Coulter, CA). The measurements included serum UA, albumin, alanine aminotransferase (ALT) (normal range: 5–55 U/L), aspartate aminotransferase (AST) (normal range: 5–60 U/L), blood urea nitrogen, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol.
Table 1.
Demographic and clinical characteristics of patients with GBS and HC groups.
All the patients with GBS were hospitalized and the diagnosis of GBS was based on the universally accepted Asbury diagnostic criteria (1990). The exclusion criteria were as follows: treatment with steroids, liver disease, abnormal ranges of ALT and AST concentrations, as well as with diabetes, gout, and renal failure.[1] Finally, 88 patients were enrolled. This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
3. Statistical analysis
The statistical software Statistical Program for Social Sciences (version 21.0, SPSS Inc, Chicago, IL) and Medcalc 11.4.2 (MedCalc Software, Mariakerke, Belgium) were used for all statistical analyses. The comparisons of the data with normal distribution between patients with GBS and HC were performed using independent Student t test. Otherwise, the Mann–Whitney U test was used. Enumeration data were expressed as numbers and percentiles and compared by Chi-squared test. The comparisons of the data among subgroups of patients with GBS were performed using 1-way analysis of variance. Factors with P ≤ .10 in the univariate analysis were included into the multivariate analyses as independent variables. The influence of serum UA and albumin levels on GBS was estimated by binary logistic regression analysis, after adjusting for some potential confounding variables. Results were expressed as adjusted odds ratios (ORs) (95% confidence intervals [CIs]). All P values are 2-sided and P values <.05 were considered statistically significant.
4. Results
The basic demographic and clinical characteristics of GBS patients and HC were presented in Table 1. There were no significant differences between the 2 groups regarding age, gender, ALT, and AST.
In our study, the average serum UA level of all subjects was 321.00 ± 101.95 μmol/L. As shown in Table 2, serum UA levels of patients with GBS (267.74 ± 104.96 μmol/L) were significantly lower than those in the HC group (350.58 ± 87.47 μmol/L, P < .001). Moreover, previous reports have proven that gender has an important effect on serum UA levels. Therefore, in our study, each group was further divided into 2 subgroups of women and men according to gender. In male subgroups, we found that serum UA levels of patients with GBS (296.42 ± 112.91 μmol/L) were significantly lower than those in the male HC subgroup (388.26 ± 80.09 μmol/L, P < .001) (Table 2; Fig. 1). Likewise, in female subgroups, we also found that serum UA levels of female patients with GBS (230.54 ± 80.88 μmol/L) were also significantly lower than those in the female HC group (296.76 ± 67.42 μmol/L, P < .001) (Table 2; Fig. 1). Women in the HC group had significantly lower serum UA levels than those in men in the HC group (P < .001). Moreover, a similar phenomenon was found between female and male patients with GBS (P = .002).
Table 2.
Serum UA and albumin levels in patients with GBS and HC group (mean ± SD).
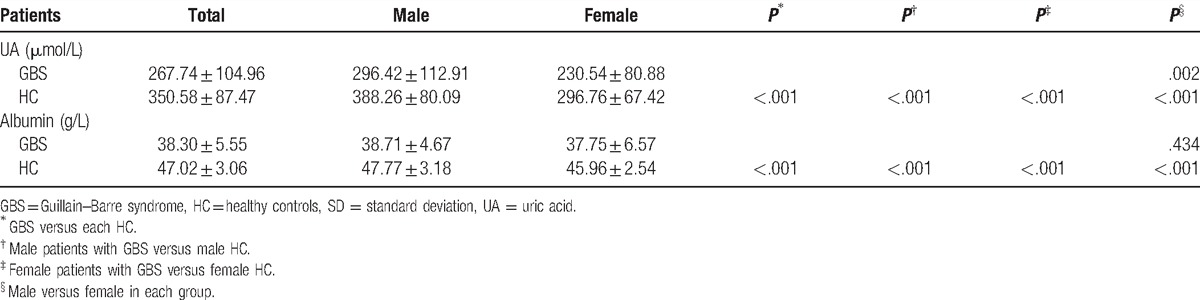
Figure 1.
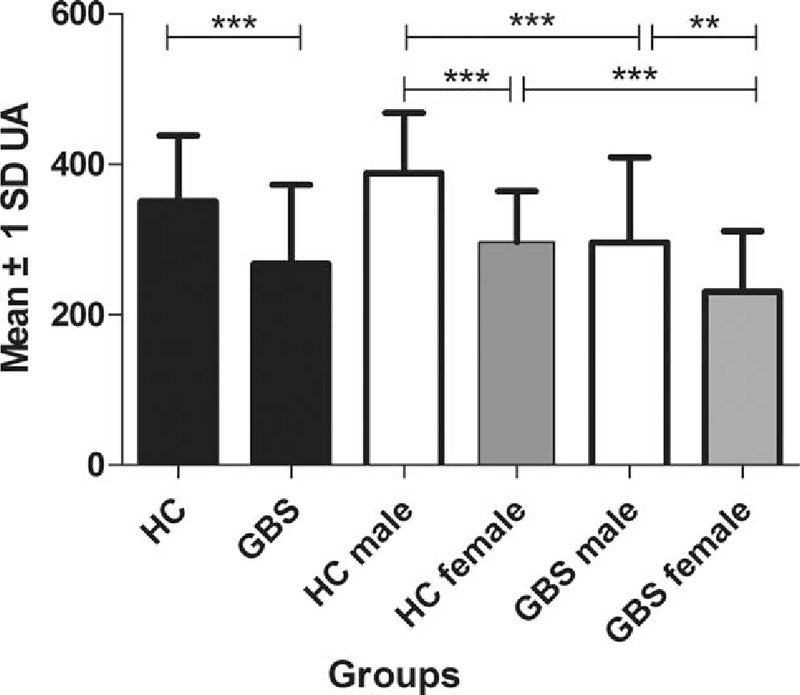
Serum uric acid levels (μmol/L) in HC and patients with GBS. GBS = Guillain–Barre syndrome, HC = healthy controls, UA = uric acid. ∗∗P < .01, ∗∗∗P < .001.
In our study, the average serum levels of albumin in all subjects were 43.88 ± 5.88 g/L. As shown in Table 2, serum levels of albumin in patients with GBS were significantly lower than those in the HC group (P < .001) (Table 2; Fig. 2). In addition, as serum UA levels have been shown to be dependent upon gender, we further divided each group into 2 subgroups of women and men in order to remove the effect of gender. We found that serum levels of albumin in male patients with GBS were significantly lower when compared with in the male HC group (P < .001), a similar phenomenon found in female patients with GBS and the female HC group (P < .001) (Table 2; Fig. 2). Women in the HC group had significantly lower serum albumin levels than those in men in the HC group (P < .001), while serum levels of albumin in female patients with GBS were mildly lower than those in male patients with GBS. However, there is no significant difference between female and male patients with GBS (P = .434).
Figure 2.
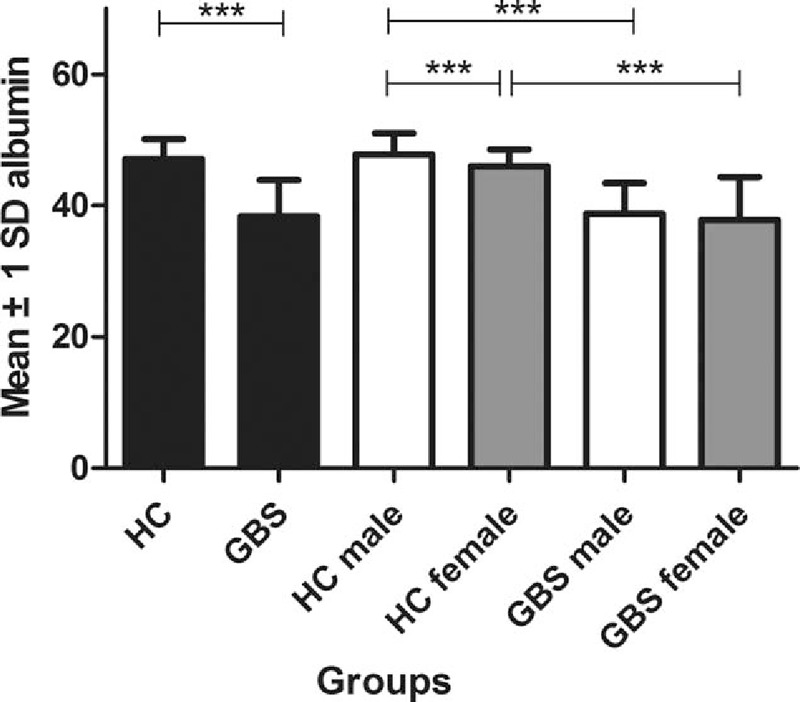
Serum albumin levels (g/L) in HC and patients with GBS. GBS = Guillain–Barre syndrome, HC = healthy controls. ∗∗∗P < .001.
In addition, in order to better evaluate the relation between serum UA and albumin concentration and the clinical subtype of GBS, patients with GBS were divided into several subgroups based on the clinical classification (Table 3). However, there is nonsignificant difference among subgroups of GBS regarding UA and albumin (P = .780 and P = .451, respectively) (Table 3).
Table 3.
Serum UA and albumin levels in patients with Guillain–Barre syndrome according to clinical classification.

The cut-off value of the UA for predicting the presence of GBS was determined to be 257. Receiver operator characteristic curve (ROC) analysis revealed a 0.727 area under the curve (0.666–0.783 CI), a 52.94% sensitivity, and a 86.93% specificity with the UA cut-off value (Fig. 3). The cut-off value of the albumin for its ability to differentiate GBS patients from HC was determined as 44.1. The ROC analysis revealed a 0.944 area under the curve (0.907–0.970 CI), a 93.02% sensitivity, and a 84.97% specificity (Fig. 3). The ROC curves revealed that UA and albumin had high discriminative ability for differentiating GBS patients from HC.
Figure 3.
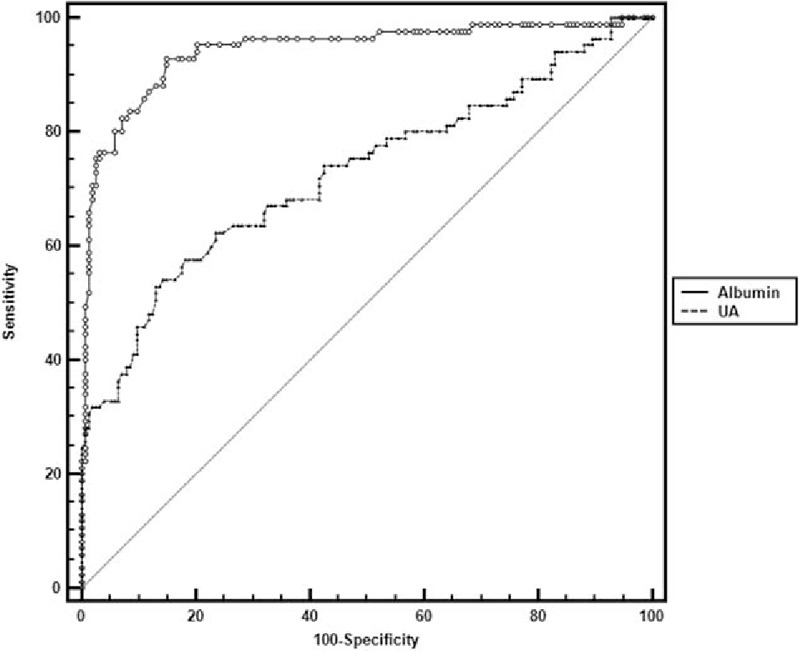
Receiver operator characteristic curve shows specificity and sensitivity percentages of UA and albumin in patients with GBS. UA: area under the curve 0.727, 95% CI: 0.666 to 0.783, cut-off value 257 with sensitivity 52.94%, specificity 86.93%; albumin: area under the curve 0.944, 95% CI: 0.907 to 0.970, cut-off value 44.1 with sensitivity 93.02%, specificity 84.97%. CI = confidence interval, GBS = Guillain–Barre syndrome, UA = uric acid.
According to the cut-off values of UA and albumin, we defined as follows: high UA (≥257) and low UA (<257) as well as high albumin (≥44.1) and low albumin (<44.1). A multivariable logistic regression analysis was performed to evaluate whether the UA and albumin were protective factors for GBS. As shown in Table 4, the univariate analysis revealed that both the high UA and high albumin were protective factors for patients with GBS (OR 0.140; 95% CI: 0.074–0.264; P < .001 and OR 0.016; 95% CI: 0.006–0.038; P < .001, respectively). It was further confirmed by the multivariable logistic regression analysis after adjusting for other potential confounding factors (OR 0.168; 95% CI: 0.055–0.514; P = .002 and OR 0.027; 95% CI: 0.011–0.071; P < .001, respectively).
Table 4.
Adjusted ORs for serum UA and albumin levels.
5. Discussion
In this study, we confirmed that patients with GBS had significantly lower serum UA and albumin levels than those in the HC group. Moreover, similar phenomenon was observed when the male and female subgroups were investigated, respectively, which were consistent with the data and findings of previous study.[17] In other words, we confirmed our previous hypothesis. Hence, we can draw the conclusion that there are decreasing serum levels of UA and albumin in patients with GBS. Furthermore, ROC curves revealed that UA and albumin have high discriminative ability for differentiating patients with GBS from HC.
Numerous studies suggest that nitrated proteins have been identified in the initial stages of various neurodegenerative diseases,[18–21] evidencing that nitric oxide (NO)-mediated oxidative damage has been involved. As a mildly reactive and toxic substance under normal and abnormal conditions, NO can create a more potent oxidant called peroxynitrite (ONOO−) via a diffusion-limited reaction with superoxide.[22] Moreover, cell structures including lipids, DNA, and proteins can be oxidized by PN through either direct reactions or nitrogen dioxide (NO2) and carbonate radical (CO3−), 2 highly reactive free radicals derived from spontaneous hemolysis.[23] In addition, a multitude of literature shows that excessive ROS or RNS production that lead to oxidative and nitrosative stress is the major determinant in the pathogenesis of inflammatory and autoimmune diseases of the nervous system such as NMO,[1] MS,[12] MG,[13] and the like.
Furthermore, the nervous system in human consists of central nervous system and peripheral nervous system. GBS, NMO, and MS are all the inflammatory demyelinating autoimmune diseases of the nervous system, whose symbolic characteristics are demyelination and axonal damage, and they have much in common in the pathogenesis. One of the biggest differences of pathogenesis is that the GBS is in the peripheral nervous system while the NMO and MS are in the central nervous system. It is well established that active oxygen plays a role in a variety of neuropathies and myopathies.[24] Besides, previous literature suggests that free radical toxicity due to poorly maintained cellular redox levels is crucial events that have been associated with the pathogenesis of GBS patients.[11] ROS are not only generally believed to be produced by activated inflammatory cells, but also involved in demyelination and axonal damage.[25] As a naturally occurring antioxidant in human blood,[2] UA has the ability to scavenge PN.[3] Serum albumin is in possession of potent antioxidant properties, which inhibit production of free hydroxyl radicals[4] and can be capable of scavenging peroxy radicals.[5,6] Besides, albumin is a major known antioxidant role in extracellular fluids[7] and plays a decisive role in redox species distribution of thiols in plasma in MS, acting by oxidations and albumin-dependent thiol/disulfide (SH/SS) exchange reactions.[26]
Importantly, in our study, we demonstrated that both the high UA and high albumin were protective factors for patients with GBS in the multivariable logistic regression analysis after adjusting for other potential confounding factors. There is evidence to demonstrate that treatment with UA promotes functional recovery in experimental autoimmune encephalomyelitis, which is an animal model of MS.[3,14] Moreover, it has been proven that lower serum UA levels were observed in patients with MS than the HC groups or patients suffering from other neurological diseases.[3,14,15] In addition, it has been confirmed that serum UA and albumin levels were lower in patients with NMO[1] and MG[13,16] than the HC groups.
In the present study, we found that the serum levels of UA in patients with GBS and HC groups were significantly lower in women than in men. Besides, the serum levels of albumin in GBS patients and HC groups were lower in women than in men. Our findings agreed with previous study.[16,17] The lower serum antioxidant level in females maybe relate that incidence of many autoimmune diseases in the nervous system was higher in women than in men.
Though it still remained uncertain whether low serum UA and albumin level can contribute to GBS inflammation activity or served as a consequence of GBS activity, that is the first English clinical study showing that patients with GBS had significantly low serum UA and albumin levels. Moreover, we demonstrated that both the high UA and high albumin were protective factors for patients with GBS. As patients with GBS had decreased UA and albumin serum levels, administration of these substances or their precursors appears to be beneficial to the patients with GBS.
There were several limitations to our study. Our research is limited to a single center due to various reasons. Although the sample size of our study was relatively small, our results were consistent with the expected protective effects of UA and albumin.
In conclusion, we found that patients with GBS had significantly low serum UA and albumin levels. Moreover, we demonstrated that both the high UA and high albumin were protective factors for patients with GBS.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, CI = confidence interval, GBS = Guillain–Barre syndrome, HC = healthy controls, MG = myasthenia gravis, MS = multiple sclerosis, NMO = neuromyelitis optica, OR = odds ratio, PN = peroxynitrite, RNS = reactive nitrogen species, ROS = reactive oxygen species, UA = uric acid.
ZS, ZC, YX, and BW have contributed equally to this work and should be considered as co-first authors.
The study was financially supported by the Natural Science Foundation of Zhejiang Province (No. LY13H090010) and the Natural Science Foundation of Zhejiang Province (No. LQ15H090008).
The authors have no conflicts of interest to disclose.
References
- [1].Peng F, Yang Y, Liu J, et al. Low antioxidant status of serum uric acid, bilirubin and albumin in patients with neuromyelitis optica. Eur J Neurol 2012;19:277–83. [DOI] [PubMed] [Google Scholar]
- [2].So A, Thorens B. Uric acid transport and disease. J Clin Invest 2010;120:1791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hooper DC, Bagasra O, Marini JC, et al. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc Natl Acad Sci USA 1997;94:2528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marx G, Chevion M. Site-specific modification of albumin by free radicals. Reaction with copper(II) and ascorbate. Biochem J 1986;236:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wayner DD, Burton GW, Ingold KU, et al. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett 1985;187:33–7. [DOI] [PubMed] [Google Scholar]
- [6].Halliwell B. Albumin—an important extracellular antioxidant? Biochem Pharmacol 1988;37:569–71. [DOI] [PubMed] [Google Scholar]
- [7].Roche M, Rondeau P, Singh NR, et al. The antioxidant properties of serum albumin. FEBS Lett 2008;582:1783–7. [DOI] [PubMed] [Google Scholar]
- [8].van den Berg B, Walgaard C, Drenthen J, et al. Guillain–Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 2014;10:469–82. [DOI] [PubMed] [Google Scholar]
- [9].Yuki N, Hartung HP. Guillain–Barre syndrome. N Engl J Med 2012;366:2294–304. [DOI] [PubMed] [Google Scholar]
- [10].Walgaard C, Jacobs BC, van Doorn PA. Emerging drugs for Guillain–Barre syndrome. Exp Opin Emerg Drugs 2011;16:105–20. [DOI] [PubMed] [Google Scholar]
- [11].Ghabaee M, Jabedari B, Al-E-Eshagh N, et al. Serum and cerebrospinal fluid antioxidant activity and lipid peroxidation in Guillain–Barre syndrome and multiple sclerosis patients. Int J Neurosci 2010;120:301–4. [DOI] [PubMed] [Google Scholar]
- [12].Ghafourifar P, Mousavizadeh K, Parihar MS, et al. Mitochondria in multiple sclerosis. Front Biosci 2008;13:3116–26. [DOI] [PubMed] [Google Scholar]
- [13].Fuhua P, Xuhui D, Zhiyang Z, et al. Antioxidant status of bilirubin and uric acid in patients with myasthenia gravis. Neuroimmunomodulation 2012;19:43–9. [DOI] [PubMed] [Google Scholar]
- [14].Hooper DC, Spitsin S, Kean RB, et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA 1998;95:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sotgiu S, Pugliatti M, Sanna A, et al. Serum uric acid and multiple sclerosis. Neurol Sci 2002;23:183–8. [DOI] [PubMed] [Google Scholar]
- [16].Yang D, Su Z, Wu S, et al. Low antioxidant status of serum bilirubin, uric acid, albumin and creatinine in patients with myasthenia gravis. Int J Neurosci 2016;126:1120–6. [DOI] [PubMed] [Google Scholar]
- [17].Peng F, Zhang B, Zhong X, et al. Serum uric acid levels of patients with multiple sclerosis and other neurological diseases. Mult Scler 2008;14:188–96. [DOI] [PubMed] [Google Scholar]
- [18].Casoni F, Basso M, Massignan T, et al. Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: possible multifunctional role in the pathogenesis. J Biol Chem 2005;280:16295–304. [DOI] [PubMed] [Google Scholar]
- [19].Greenacre SA, Ischiropoulos H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic Res 2001;34:541–81. [DOI] [PubMed] [Google Scholar]
- [20].Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Smith MA, Richey Harris PL, Sayre LM, et al. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci 1997;17:2653–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beckman JS, Beckman TW, Chen J, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990;87:1620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Drechsel DA, Estevez AG, Barbeito L, et al. Nitric oxide-mediated oxidative damage and the progressive demise of motor neurons in ALS. Neurotox Res 2012;22:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Davison A, Tibbits G, Shi ZG, et al. Active oxygen in neuromuscular disorders. Mol Cell Biochem 1988;84:199–216. [DOI] [PubMed] [Google Scholar]
- [25].Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 2004;251:261–8. [DOI] [PubMed] [Google Scholar]
- [26].Di Giuseppe D, Ulivelli M, Bartalini S, et al. Regulation of redox forms of plasma thiols by albumin in multiple sclerosis after fasting and methionine loading test. Amino acids 2010;38:1461–71. [DOI] [PubMed] [Google Scholar]


