Abstract
It has been postulated that the segregation of nucleus and cytoplasm supported the development of increased organismal complexity. For example, separating transcription and translation allows for mRNA splicing, while the sequestration of genomic DNA supports the innate immune system’s ability to equate cytoplasmic DNA with pathogens. Consistent with the importance of nucleocytoplasmic compartmentalization in a broad array of cellular processes, defects in maintaining discrete nuclear and cytoplasmic compartments, either due to loss of nuclear pore complex integrity, disrupted nuclear transport or ruptures of the nuclear envelope, lead to cellular dysfunction, cell death and disease. Here, we discuss recent insights into how loss of compartmentalization can arise as well as the consequences for cellular and organismal homeostasis.
Keywords: nuclear transport, nuclear rupture, mechanics, laminopathy, c9orf72, dipeptide repeat, hexanucleotide repeat expansion
Introduction
Fundamental mechanisms that maintain the segregation of the nuclear and cytosolic contents have classically been examined under the auspices of “basic” research. Recently, several studies have now identified compromised nuclear compartmentalization as a hallmark of disease, from normal aging to genetically-linked neurodegenerative disease, and potentially even to cellular transformation. Here, we consider how loss of nuclear compartmentalization could contribute to these diseases through the lens of our foundational knowledge of the nuclear transport system, which promises to provide deep insight into disease mechanisms and future potential for targeted molecular intervention.
Establishing stable nuclear and cytoplasmic compartments
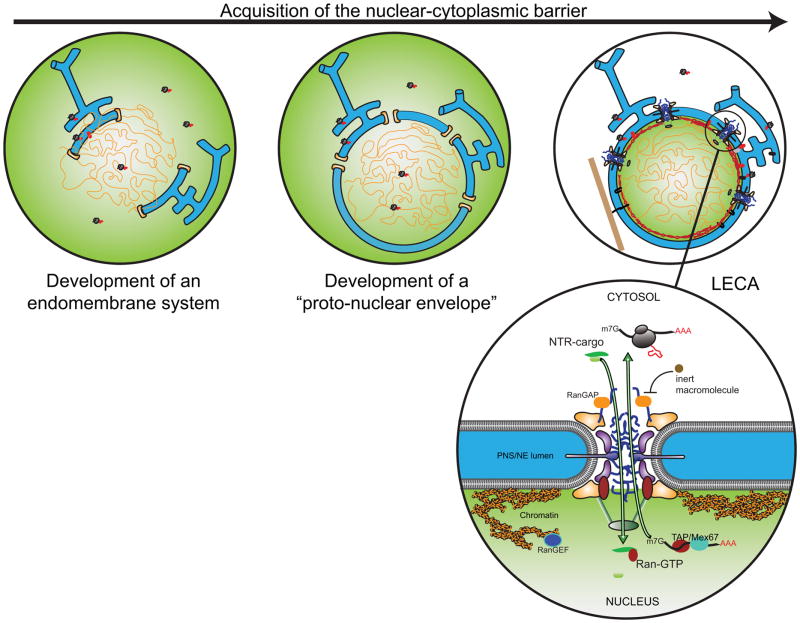
The two lipid bilayers that compose the nuclear envelope (NE) form a barrier that effectively compartmentalizes eukaryotic cells into nucleus and cytoplasm; as these membranes are continuous with the endoplasmic reticulum (ER), the NE is essentially an ER subcompartment. The physical integration of the NE and ER suggests that establishment of a distinct nuclear compartment likely arose in concert with (or subsequent to) the acquisition of an endomembrane system, likely through an intermediate state (or proto-nuclear envelope) in which free diffusion could occur between a membrane-encircled genome and the surrounding cell [1,2] (Fig. 1). The protocoatomer hypothesis suggests that a common membrane bending (or stabilizing) machinery arose early in evolution and later became specialized as a critical scaffold for the docking of nuclear pore complexes (NPCs) into the NE [2]. Indeed, the scaffold of the NPC is made up of about a dozen constituent proteins (called nucleoporins or nups) that bear a striking structural similarity to the clathrin and COP coat complexes [3]. The initial NPC membrane coat, which resides at the “pore membrane” where the inner and outer nuclear membranes converge, likely provided the structural basis for ensuring that all macromolecules were routed through pores in the NE (Fig. 1). The later formation of a diffusion barrier by nups rich in phenylalanine-glycine (FG) repeats anchored to this membrane coat likely laid the groundwork for the future regulation of nuclear-cytoplasmic exchange of macromolecules by NPCs and soluble nuclear transport receptors (NTRs; Fig. 1) [4,5].
Figure 1.
Schematic of likely steps leading to the formation of the nucleocytoplasmic barrier. At left, a primitive endomembrane system (blue) is developed with connections to the genome mediated by membrane proteins and an ancient proto-coatomer (bracket shapes) capable of stabilizing membrane curvature. Ribosomes are grey with red nascent polypeptide. In middle, the protocoatomer provides the building blocks for the NPC architecture, which drives exchange through pores in the “proto-nuclear envelope”. The contents of the nucleus and cytoplasm are not fully segregated until the last eukaryotic common ancestor (LECA; right), which would incorporate the FG-nups into the NPC scaffold, allowing the generation of a size-selective diffusion barrier and active nuclear transport mediated by NTRs (green in inset). Inset of NPC showing the major architectural units including the inner rings (purple), outer rings (orange), FG-nups (blue squiggles) and nuclear basket (green). Ran-GTP is found predominantly in the nucleus, where it releases import cargoes from NTRs and stabilizes export complexes.
The establishment of NPCs as a barrier to free diffusion for macromolecules with a Stokes radius of >~2.5 nm, corresponding to a protein of ~35–40 kDa in mass [6] would present a clear impediment to the exchange of macromolecules necessary for a number of essential cellular processes. One of the intuitive consequences of this compartmentalization is the physical segregation of the genome (and therefore transcription) away from ribosomes (and therefore translation). Indeed, it has been postulated that the origin of introns and the mRNA splicing machinery is intimately linked to the establishment of separate nuclear and cytoplasmic compartments [7]. Moreover, the ribosome is built upon its rRNA core, and acts on an mRNA template; both RNAs are synthesized from a nuclear DNA template, necessitating mechanisms to export these large cargos to the cytoplasm, where mature ribosomes reside and translation take place [8]. Accessory proteins (NTRs, some of which are also called karyopherins or importins/exportins) that bind such large molecules promote their passage through the FG-repeat rich NPC core, while the directionality of transport is contributed by asymmetric nuclear and/or cytosolic factors, most notably the Ran GTPase, which is in the GTP-bound form in the nucleus and the GDP-bound form in the cytoplasm [9](Fig. 1). Thus, the steady-state localization of a given factor is predominantly determined by 1) its size (either alone or in complex), which will define its ability to equilibrate in a passive fashion between nucleus and cytoplasm, 2) whether active transport pathways drive its import or export to/from the nucleus and 3) the retention of a factor by its interactions with architectural components restricted to either compartment [10]. As we will discuss below, both passive and active transport will both be affected by perturbations that disrupt the nucleocytoplasmic barrier either through the loss of NPC function, or, mechanical disruption of the NE membranes.
To limit mechanical disruption of the NE, the nucleus is stabilized by the nuclear lamina, an integrated scaffold composed of nuclear lamins, integral inner nuclear membrane (INM) proteins and (predominantly heterochromatic) chromatin [11]. A- and B-type lamins, members of the intermediate filament family, each form distinct but inter-dependent protein networks that line the INM as recently visualized with super-resolution approaches [12,13]. It is possible that imparting mechanical stability also limits NE dynamics, as exemplified by the lamin-based immobilization of NPCs [14]. Indeed, the mobility of NPCs might serve as a bellwether that reflects the plasticity of the NE system. For example, in embryonic Drosophila cells NPCs are highly mobile; this mobility is later restricted by upregulation of lamins and lamin binding proteins during differentiation [15]. Indeed, in terminally differentiated neurons, scaffold nucleoporins are some of the most stable proteins, turning over in timespans of months to years [16,17]. This lack of turnover is not without consequence, however, as it opens the door for NPCs to accumulate damage with age.
Leaky NPCs, NPC dysfunction and disease
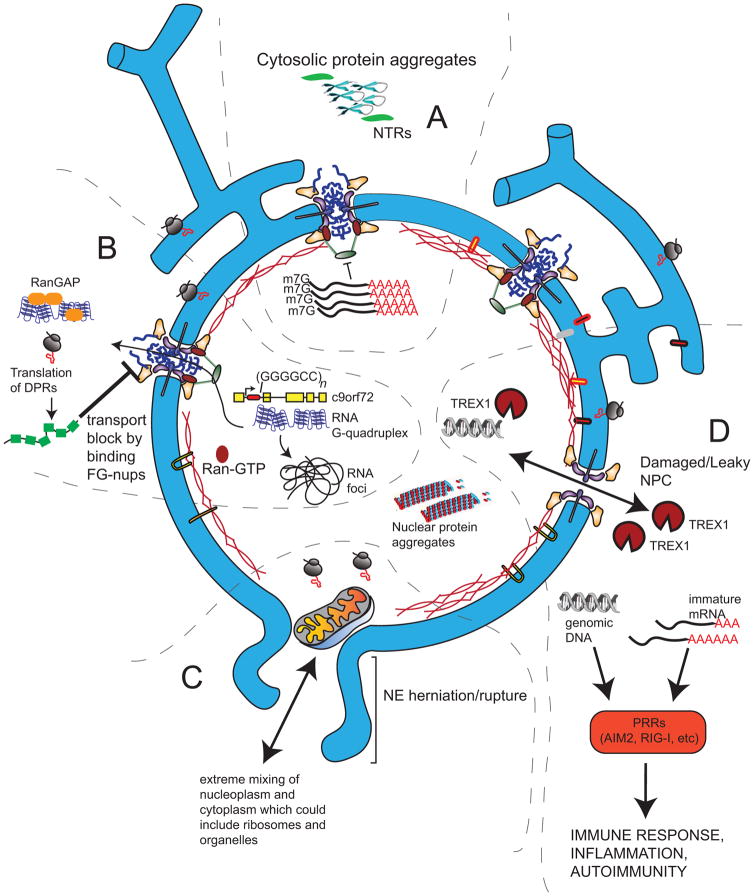
Indeed, oxidative damage to NPCs has been observed in C. elegans and in old rat brains leading to a disruption of the NPC diffusion barrier and the nuclear aggregation of cytosolic proteins, a typical phenotype of neurodegenerative disease pathology [18] (Fig. 2). Analogously, toxic cytosolic protein aggregates accumulate in neurodegenerative disease [19] and these might also impact nuclear compartmentalization by sequestering specific NTRs [20] (Fig. 2A). More recently, the loss of nuclear compartmentalization was shown to impact the progression of neurodegenerative diseases like amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), but through a distinct mechanism. Forms of these diseases are thought to be caused by the expression of a hexanucleotide (GGGGCC) repeat expansion (HRE) in an intron of the C9orf72 gene, but it remains debated whether the toxicity of the repeats stems from the resulting transcript, which may form parallel arrays of G-quadruplexes capable of binding other proteins and RNAs [21], and/or non-AUG translation, producing small dipeptide repeat (DPR) proteins (Fig 2B). Nonetheless, from yeast to mammals, the overexpression of these repeats leads to cellular toxicity, suggesting the evolutionary conservation of molecular components that, when disrupted, help drive the disease.
Figure 2.
Consequences of disruptions of nucleocytoplasmic compartmentalization. A. Cytosolic aggregates of β-sheet proteins can sequester NTRs and mRNA export factors, leading to the accumulation of mRNA in the nucleus. B. The transcription of the HRE of the C9orf72 intron (red) leads to the production of parallel RNA G-quadruplexes that can accumulate in RNA foci or be exported (through an unknown mechanism) into the cytosol, where they can interact with other proteins (like RanGAP) or be translated into DPRs. DPRs can direct bind to the FG-nups within the NPC to block nuclear transport. C. In more extreme circumstances a nuclear rupture event can allow even large organelles into the nucleus and will expose genomic DNA to the cytoplasm. Both cytosolic DNA and immature RNA can be recognized by pattern recognition receptors (PRRs) and illicit an immune response. D. Damage to NPCs in old neurons can lead to a breakdown of the permeability barrier and the free exchange of nuclear and cytosolic contents, leading to the accumulation of cytosolic proteins in the nucleus and nuclear-restricted nucleic acids (like immature mRNAs) in the cytosol. Cytoplasmic DNases (like TREX1) could drive DNA damage when they access the nucleoplasm.
Several recent studies suggest that the nuclear transport apparatus could be the evolutionarily conserved machinery targeted by HRE expression [21–24]. First, the overexpression of multiple NTRs leads to a decline in HRE toxicity in yeast, suggesting a capacity to overcome a block in nuclear transport [23]. These results were mirrored by several studies in Drosophila in which genetic perturbation of both the soluble phase (i.e. NTRs and Ran/Ran binding proteins) and the stationary phase (i.e. the NPC) of the nuclear transport machinery (Fig 1) leads to the exacerbation or suppression of HRE toxicity in ways that confound a straightforward mechanistic interpretation [21,22,25]. This is likely because the HRE transcripts and the DPR proteins influence cell physiology in distinct manners, perhaps by directly inhibiting unique factors involved in nuclear transport. For example, the HRE transcripts can directly bind to the Ran GTPase activating protein (RanGAP), which might lead to its mislocalization into cytosolic foci [21]. The appearance of these foci in both brain sections and iPS-derived neurons from ALS patients suggests that mislocalized RanGAP is a pathological feature of the disease [21]. It remains unclear, however, whether these interactions perturb the ability of RanGAP to promote conversion of RanGTP to RanGDP in the cytoplasm. Moreover, as additional components of the nuclear transport apparatus are also mislocalized under these conditions, including some nups, it may be that a more general effect on the nuclear transport system is responsible.
In order to gain a broader picture of the potential mechanisms by which HREs influence cellular function, unbiased proteomic approaches sought to identify DPR interacting proteins [25,26]; both nups and NTRs were identified [25]. Interestingly, there was a clear propensity for DPRs to interact with low complexity, often intrinsically disordered proteins (IDPs) that associate with membrane-less organelles [9]. Indeed, the infiltration of DPRs into phase-separated domains both in vivo and in vitro influences their biophysical properties and function [25,26]. Interestingly, there are many similarities between IDPs that form membrane-less organelles and the FG-nups [9]. In fact, it has long been understood that FG-nups can undergo a phase-transition into a hydrogel in vitro [27], which might be reinforced by intermolecular β-sheet interfaces that also contribute to amyloid formation [28]. While it remains debated whether such a hydrogel exists in the central channel of the NPC, recent work demonstrates that arginine-rich DPR repeat proteins can interact directly with a polymeric, amyloid-like form of the FG-nups, Nup54 and Nup98 [24].
The binding of DPRs to polymers of FG-nups is reflected in vivo by the accumulation of DPRs at NPCs [24]. Interestingly, the nanoscale distribution of DPRs suggest an enrichment along the NPC channel walls in contrast to other FG-nup epitopes, such as those in Nup62 [29] or wheat germ agglutinin (which binds to GlcNacylation in the FG domains) that reside at the center of the channel [24]. This distribution could reflect the restriction of β-sheet interactions that polymerize FG-nups in vitro to the channel walls. Alternatively, the DPRs may drive non-physiological interactions among the FG-nups that contribute to the observed disruption of nuclear import and export [24] (Fig. 2B). A prediction of the latter model would be that the NPC diffusion barrier would also be affected by DPR binding, which has yet to be tested. Regardless, these data provide compelling molecular insight into a direct mechanism by which HREs could disrupt nuclear transport. Excitingly, the DPRs may also provide a tool to probe the properties of the NPC channel to help solve a long-standing question as to the native organization of the FG-nups.
Causes and consequences of nuclear rupture
While an imbalance in the nuclear transport machinery can contribute to disease progression, recent evidence supports that more acute and dramatic releases of nuclear components into the cytoplasm (and in-flow of cytoplasmic contents into the nucleus) is driven by nuclear rupture events (Fig 2C). In these cases, it is thought that both the inner and outer nuclear membranes undergo rupture, which is sometimes preceded by the formation of a NE bleb [30,31]. Recently, there have been two contexts in which nuclear rupture occurs at high frequency, which may be driven by distinct factors, and have distinct consequences.
Two types of nuclear rupture occur downstream of chromosome segregation errors. In the first, highly persistent chromatin bridges, such as those that arise in cells with dicentric chromosomes due to telomere-telomere fusion, eventually lead to NE rupture in the subsequent interphase, which allows for the chromosomes to be fully segregated at the cost of a high mutational load presumably in the DNA that resided in the chromatin bridge [32]. In the second, a lagging chromosome nucleates formation of its own “micronucleus”, which is physically separated from the remainder of the chromosomes in the “primary nucleus”. For reasons that remain enigmatic, the NEs that define micronuclei fail to accumulate a normal NE proteome, including alterations to the nuclear lamina [33]. Interestingly, although micronuclei often succeed in establishing the NE barrier at mitotic exit, in the subsequent interphase many micronuclei undergo a catastrophic rupture [33]. Such ruptures sometimes are “healed”, while others appear to be irreparable. The loss of the intact NE barrier is linked to massive genome rearrangement events in which the entire (usually single) chromosome within the micronucleus is shattered and pieced back together (termed chromothripsis) – an occurrence linked to transformed cells, and a potential driver of further losses of genome integrity [34,35]. While much remains to be understood about this process, and its prevalence, it provides strong evidence for links between maintenance of the nuclear compartment and genome integrity. Although loss of nuclear compartmentalization in micronuclei correlates with deficiencies in replication [34], an increase in DNA damage load [33–35], and an altered DNA damage response [36], much remains to be determined about the mechanisms at play.
The nuclei of certain cell types must balance deformability necessary for effective migration through complex environments with cell survival. Short-lived immune cells such as neutrophils down-regulate A-type lamins during differentiation, coincident with lobulation of the nucleus (potentially to increase nuclear deformability) [37]. By contrast, longer lived dendritic cells (DCs) express A-type lamins. DCs surveil for foreign antigens and, after activation, mature and migrate to the lymph nodes, ultimately presenting antigens to T-cells to support adaptive immune system function [38]. Two recent studies, using both DC and transformed cell line models, highlight how migration through constricted environments can lead to dramatic losses of nucleocytoplasmic compartmentalization, driven by herniation and rupture of the NE [30,31]. Remarkably, these events are often transient due to a NE repair mechanism catalyzed by the ESCRT machinery; a NE rupture would likely present a topology similar to the NE discontinuities sealed by the ESCRT machinery at mitotic exit [30,31,39,40]. Interestingly, NE ruptures that occur during migration through constricted environments occur coincidentally with loading of DNA repair factors onto chromatin [30,31], suggesting that such events could also be drivers of genome instability. While numerous questions remain about the source of the potential DNA damage that occurs in this context, simultaneously compromising the DNA damage response machinery and the ESCRT machinery necessary for “healing” NE ruptures leads to enhanced cell death [30,31]. Physical confinement of the nucleus induced by cytoskeletal contractility in non-migrating cells also appears to be sufficient to drive similar NE ruptures [41], suggesting that mechanisms capable of maintaining nuclear integrity in response to physical force are likely to be critical in a variety of cellular and tissue contexts.
What might be the consequences of such catastrophic loss of the nuclear barrier? Transient NE ruptures could explain the observation that large protein aggregates (or even organelles [42]) accumulate within the nucleoplasm of aged cells [43](Fig 2C). This suggests that large, non-dynamic proteinaceous structures may become irreversibly trapped within the nucleoplasm, particularly in post-mitotic cells that never have the ability to “purge” themselves through sequential processes of NE breakdown, chromosome segregation, and NE reformation [44]. The sheer size of large protein aggregates or organelles in the nucleus would present a serious challenge for the autophagy machinery that is mostly cytosolic, thereby driving loss of cellular function and viability.
Outlook: emerging themes for crosstalk between the nuclear barrier and immunosurveillance?
Although toxic nuclear aggregates can be a driver of disease, we argue that improperly compartmentalized nucleic acids arising from NE ruptures or dysfunctional NPCs is perhaps most likely to compromise homeostasis at the level of the organism (Fig 2). For example, the addition of the nucleus as a dedicated compartment that houses the DNA (and immature mRNA) allowed for the adoption of innate immunity pathways that take advantage of the sequestration of “self” DNA within the nucleus, making the nucleoplasm essentially “immune-privileged”. Innate immune pattern recognition receptors are poised to respond to cytoplasmic nucleic acids during interphase, which is taken as evidence of an ongoing infection [45]. Activation of this machinery can drive cell death, inflammation, and an immune response; inappropriate triggering of these pathways due to exposure of the chromatin to the cytoplasm with NE ruptures or “leaky” NPCs could therefore be a major driver of pathologies, from aging to autoimmune disease (Fig 2D). Indeed, a domain of the innate immunity factor cGAS has been used as an effective reporter for nuclear rupture [30,31], although future studies will be necessary to assess whether such losses of nuclear compartmentalization are sufficient to trigger the cGAS-STING pathway [46]. Moreover, nuclear rupture could lead to degradation of nuclear DNA by (normally cytosolic) DNases, for example TREX1 (DNase III), the 3′ nuclease that acts to resolve the DNA in chromatin bridges when the NE ruptures during failed chromosome segregation [32]. A defect in the NPC barrier could also allow immature RNA to circumvent the normal mRNA surveillance machinery that acts at the nuclear basket of the NPC [47]; such immature cytoplasmic RNA, sensed by defects in the 5′ RNA cap, for example, could also be perceived as viral RNA, again driving an immune response [48](Fig 2D). Although the potential for loss of nucleocytoplasmic compartmentalization to drive an immune response remains largely untested, it nonetheless presents a tantalizing possibility for future work to investigate the connection between the maintenance of the nuclear compartment and aging.
Acknowledgments
CPL is supported by the NIH (RO1GM105672 and R21GM109466). MCK is funded by the NSF (CMMI-1634988).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cavalier-Smith T. Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution. Biol Direct. 2010;5:7. doi: 10.1186/1745-6150-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2004;2:e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz T. Functional insights from studies on the structure of the nuclear pore and coat protein complexes. Cold Spring Harb Perspect Biol. 2013:5. doi: 10.1101/cshperspect.a013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field MC, Koreny L, Rout MP. Enriching the pore: splendid complexity from humble origins. Traffic. 2014;15:141–156. doi: 10.1111/tra.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Obado SO, Brillantes M, Uryu K, Zhang W, Ketaren NE, Chait BT, Field MC, Rout MP. Interactome Mapping Reveals the Evolutionary History of the Nuclear Pore Complex. PLoS Biol. 2016;14:e1002365. doi: 10.1371/journal.pbio.1002365. Provides insight into the evolutionary origins of the NE and NPCs by a comprehensive analysis of the constituents of the Trypansome NPC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohr D, Frey S, Fischer T, Guttler T, Gorlich D. Characterisation of the passive permeability barrier of nuclear pore complexes. EMBO J. 2009;28:2541–2553. doi: 10.1038/emboj.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin W, Koonin EV. Introns and the origin of nucleus-cytosol compartmentalization. Nature. 2006;440:41–45. doi: 10.1038/nature04531. [DOI] [PubMed] [Google Scholar]
- 8.Grunwald D, Singer RH, Rout M. Nuclear export dynamics of RNA-protein complexes. Nature. 2011;475:333–341. doi: 10.1038/nature10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt HB, Gorlich D. Transport Selectivity of Nuclear Pores, Phase Separation, and Membraneless Organelles. Trends Biochem Sci. 2016;41:46–61. doi: 10.1016/j.tibs.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 10*.Wuhr M, Guttler T, Peshkin L, McAlister GC, Sonnett M, Ishihara K, Groen AC, Presler M, Erickson BK, Mitchison TJ, et al. The Nuclear Proteome of a Vertebrate. Curr Biol. 2015;25:2663–2671. doi: 10.1016/j.cub.2015.08.047. Suggests a new perspective on the role of nuclear and cytosolic retention and complex-formation as major determinants of the steady-state distribution of components of the nucleus and cytoplasm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruenbaum Y, Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem. 2015;84:131–164. doi: 10.1146/annurev-biochem-060614-034115. [DOI] [PubMed] [Google Scholar]
- 12*.Shimi T, Kittisopikul M, Tran J, Goldman AE, Adam SA, Zheng Y, Jaqaman K, Goldman RD. Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol Biol Cell. 2015;26:4075–4086. doi: 10.1091/mbc.E15-07-0461. Along with reference 13, super-resolution microscopy provides new insights into the distinct but co-dependent organization of A- and B-type lamin networks in somatic cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Xie W, Chojnowski A, Boudier T, Lim JS, Ahmed S, Ser Z, Stewart C, Burke B. A-type Lamins Form Distinct Filamentous Networks with Differential Nuclear Pore Complex Associations. Curr Biol. 2016 doi: 10.1016/j.cub.2016.07.049. Along with reference 12, super-resolution microscopy provides new insights into the distinct but co-dependent organization of A- and B-type lamin networks in somatic cells. [DOI] [PubMed] [Google Scholar]
- 14.Daigle N, Beaudouin J, Hartnell L, Imreh G, Hallberg E, Lippincott-Schwartz J, Ellenberg J. Nuclear pore complexes form immobile networks and have a very low turnover in live mammalian cells. J Cell Biol. 2001;154:71–84. doi: 10.1083/jcb.200101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Hampoelz B, Mackmull MT, Machado P, Ronchi P, Bui KH, Schieber N, Santarella-Mellwig R, Necakov A, Andres-Pons A, Philippe JM, et al. Pre-assembled Nuclear Pores Insert into the Nuclear Envelope during Early Development. Cell. 2016;166:664–678. doi: 10.1016/j.cell.2016.06.015. Demonstrates a remarkable form of NE plasticity where immature nuclear pore complexes that reside in ER stores called annulate lamellae are incorporated into the NE during embryogenesis. Moreover, NPC dynamics are restricted as cell differentiate and upregulate lamin binding proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savas JN, Toyama BH, Xu T, Yates JR, 3rd, Hetzer MW. Extremely long-lived nuclear pore proteins in the rat brain. Science. 2012;335:942. doi: 10.1126/science.1217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, 3rd, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15:384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- 20*.Woerner AC, Frottin F, Hornburg D, Feng LR, Meissner F, Patra M, Tatzelt J, Mann M, Winklhofer KF, Hartl FU, et al. Cytoplasmic protein aggregates interfere with nucleocytoplasmic transport of protein and RNA. Science. 2016;351:173–176. doi: 10.1126/science.aad2033. This work pinpoints that cytoplasmic protein aggregates leads to defects in nuclear transport by binding to components of the nuclear export machinery. [DOI] [PubMed] [Google Scholar]
- 21**.Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, Daley EL, Miller SJ, Cunningham KM, Vidensky S, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525:56–61. doi: 10.1038/nature14973. With references 22 and 23, provides genetic, functional and biochemical evidence for how expression of the c9orf72 HRE could directly inhibit the nuclear transport machinery to contribute to neurodegenerative disease mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee KH, Badders N, Valentine M, Miller BL, Wong PC, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525:129–133. doi: 10.1038/nature14974. With references 21 and 23, provides genetic, functional and biochemical evidence for how expression of the c9orf72 HRE could directly inhibit the nuclear transport machinery to contribute to neurodegenerative disease mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Jovicic A, Mertens J, Boeynaems S, Bogaert E, Chai N, Yamada SB, Paul JW, 3rd, Sun S, Herdy JR, Bieri G, et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat Neurosci. 2015;18:1226–1229. doi: 10.1038/nn.4085. With references 21 and 22, provides genetic, functional and biochemical evidence for how expression of the c9orf72 HRE could directly inhibit the nuclear transport machinery to contribute to neurodegenerative disease mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Shi KY, Mori E, Nizami ZF, Lin Y, Kato M, Xiang S, Wu LC, Ding M, Yu Y, Gall JG, et al. Toxic PRn poly-dipeptides encoded by the C9orf72 repeat expansion block nuclear import and export. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1620293114. Provides compelling in vitro evidence that DPRs can directly bind to FG-nups in a polymeric form, and to the walls of the NPC channel in vivo to inhibit nuclear transport. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Lee KH, Zhang P, Kim HJ, Mitrea DM, Sarkar M, Freibaum BD, Cika J, Coughlin M, Messing J, Molliex A, et al. C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell. 2016;167:774–788. e717. doi: 10.1016/j.cell.2016.10.002. One of two (also reference 26) recent studies exploring the interactome of DPR proteins. Here, they identify components of membrane-less organelles (and NPCs) and show how DPRs can influence their organization and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Lin Y, Mori E, Kato M, Xiang S, Wu L, Kwon I, McKnight SL. Toxic PR Poly-Dipeptides Encoded by the C9orf72 Repeat Expansion Target LC Domain Polymers. Cell. 2016;167:789–802. e712. doi: 10.1016/j.cell.2016.10.003. One of two (also reference 25) recent studies exploring the interactome of DPR proteins. Here, they identify components of membrane-less organelles (and NPCs) and show how DPRs can influence their organization and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey S, Richter RP, Gorlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314:815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- 28.Ader C, Frey S, Maas W, Schmidt HB, Gorlich D, Baldus M. Amyloid-like interactions within nucleoporin FG hydrogels. Proc Natl Acad Sci U S A. 2010;107:6281–6285. doi: 10.1073/pnas.0910163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szymborska A, de Marco A, Daigle N, Cordes VC, Briggs JA, Ellenberg J. Nuclear pore scaffold structure analyzed by super-resolution microscopy and particle averaging. Science. 2013;341:655–658. doi: 10.1126/science.1240672. [DOI] [PubMed] [Google Scholar]
- 30**.Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–358. doi: 10.1126/science.aad7297. With reference 31, it is shown that migration through constricted environments leads to nuclear rupture and loading of DNA damage factors onto the chromatin. Further, nuclear ruptures are sealed by the ESCRT-III machinery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, Lautenschlaeger F, Voituriez R, Lennon-Dumenil AM, Manel N, et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–362. doi: 10.1126/science.aad7611. With reference 30, it is shown that migration through constricted environments leads to nuclear rupture and loading of DNA damage factors onto the chromatin. Further, nuclear ruptures are sealed by the ESCRT-III machinery. [DOI] [PubMed] [Google Scholar]
- 32**.Maciejowski J, Li Y, Bosco N, Campbell PJ, de Lange T. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell. 2015;163:1641–1654. doi: 10.1016/j.cell.2015.11.054. This study reveals that the cytoplasmic exonuclease TREX-1 gains access to the chromatin during nuclear envelope ruptures that occur during telomere fusion-induced delayed anaphase to drive loss of genome integrity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482:53–58. doi: 10.1038/nature10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terradas M, Martin M, Hernandez L, Tusell L, Genesca A. Nuclear envelope defects impede a proper response to micronuclear DNA lesions. Mutat Res. 2012;729:35–40. doi: 10.1016/j.mrfmmm.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Olins AL, Zwerger M, Herrmann H, Zentgraf H, Simon AJ, Monestier M, Olins DE. The human granulocyte nucleus: Unusual nuclear envelope and heterochromatin composition. Eur J Cell Biol. 2008;87:279–290. doi: 10.1016/j.ejcb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haniffa M, Collin M, Ginhoux F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv Immunol. 2013;120:1–49. doi: 10.1016/B978-0-12-417028-5.00001-6. [DOI] [PubMed] [Google Scholar]
- 39.Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG. ESCRT-III controls nuclear envelope reformation. Nature. 2015;522:236–239. doi: 10.1038/nature14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, Stenmark H. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature. 2015;522:231–235. doi: 10.1038/nature14408. [DOI] [PubMed] [Google Scholar]
- 41*.Hatch EM, Hetzer MW. Nuclear envelope rupture is induced by actin-based nucleus confinement. J Cell Biol. 2016 doi: 10.1083/jcb.201603053. This work demonstrates that nuclear envelope ruptures can also be driven in non-migrating cells due to forces from cytoskeletal contractility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takemura G, Takatsu Y, Sakaguchi H, Fujiwara H. Intranuclear mitochondria in human myocardial cells. Pathol Res Pract. 1997;193:305–311. doi: 10.1016/s0344-0338(97)80008-8. [DOI] [PubMed] [Google Scholar]
- 43.von Mikecz A. Pathology and function of nuclear amyloid. Protein homeostasis matters. Nucleus. 2014;5:311–317. doi: 10.4161/nucl.29404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rich T, Assier E, Skepper J, Segard HB, Allen RL, Charron D, Trowsdale J. Disassembly of nuclear inclusions in the dividing cell--a novel insight into neurodegeneration. Hum Mol Genet. 1999;8:2451–2459. doi: 10.1093/hmg/8.13.2451. [DOI] [PubMed] [Google Scholar]
- 45.Dempsey A, Bowie AG. Innate immune recognition of DNA: A recent history. Virology. 2015;479–480:146–152. doi: 10.1016/j.virol.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 47.Fasken MB, Corbett AH. Mechanisms of nuclear mRNA quality control. RNA Biol. 2009;6:237–241. doi: 10.4161/rna.6.3.8330. [DOI] [PubMed] [Google Scholar]
- 48.Leung DW, Amarasinghe GK. When your cap matters: structural insights into self vs non-self recognition of 5′ RNA by immunomodulatory host proteins. Curr Opin Struct Biol. 2016;36:133–141. doi: 10.1016/j.sbi.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]