Abstract
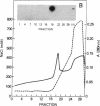
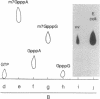
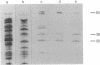
The genes D1 and D2, predicted to encode the 95- and 31-kDa subunits of the vaccinia virus mRNA capping enzyme, were coexpressed from the same plasmid in Escherichia coli. Induction with low concentrations of isopropyl beta-D-thiogalactoside was necessary to obtain soluble enzyme. The active heterodimer was purified by column chromatography and was shown to have both RNA guanylyltransferase and mRNA (guanine-N7-)-methyltransferase activities. Formation of the m7G(5')pppG cap structure was verified by enzyme digestion and thin-layer chromatography. Each subunit was also expressed individually in E. coli. Without the large subunit, the small one was very unstable in some bacterial strains and could only be detected by pulse labeling with radioactive amino acids. The individually expressed large subunit contained the guanylyltransferase domain, but the activity from E. coli was less than 2% of that obtained with both subunits. Two other products of the D1 open reading frame were formed: a 55-kDa subfragment with the GMP binding site and a 38-kDa C-terminal fragment that started at amino acid 498. Expression of this heterodimeric enzyme in E. coli may facilitate the analysis of its functional domains and provide a useful reagent for the specific 5' labeling of uncapped or capped RNA and for enhancing RNA translatability in eukaryotic systems.
Full text
PDF
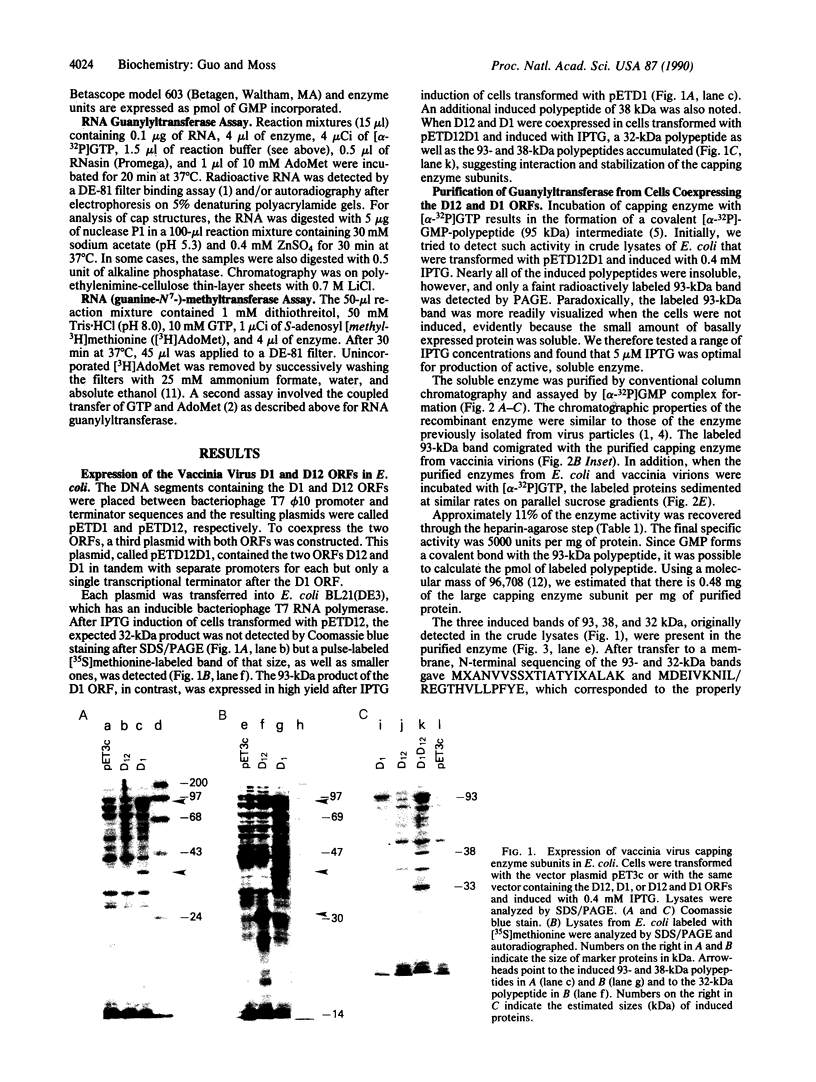



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa E., Moss B. mRNA(nucleoside-2'-)-methyltransferase from vaccinia virus. Purification and physical properties. J Biol Chem. 1978 Nov 10;253(21):7692–7697. [PubMed] [Google Scholar]
- Guo P. X., Bailey S., Bodley J. W., Anderson D. Characterization of the small RNA of the bacteriophage phi 29 DNA packaging machine. Nucleic Acids Res. 1987 Sep 11;15(17):7081–7090. doi: 10.1093/nar/15.17.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P. X., Erickson S., Anderson D. A small viral RNA is required for in vitro packaging of bacteriophage phi 29 DNA. Science. 1987 May 8;236(4802):690–694. doi: 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Martin S. A., Moss B. mRNA guanylyltransferase and mRNA (guanine-7-)-methyltransferase from vaccinia virions. Donor and acceptor substrate specificites. J Biol Chem. 1976 Dec 10;251(23):7313–7321. [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Morgan J. R., Cohen L. K., Roberts B. E. Identification of the DNA sequences encoding the large subunit of the mRNA-capping enzyme of vaccinia virus. J Virol. 1984 Oct;52(1):206–214. doi: 10.1128/jvi.52.1.206-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Condit R. C., Caro P., Davidson K., Matusick L., Seto J. Nucleotide sequence and genetic map of the 16-kb vaccinia virus HindIII D fragment. Virology. 1986 Aug;153(1):96–112. doi: 10.1016/0042-6822(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Lee-Chen G. J., Shuman S., Moss B., Broyles S. S. Vaccinia virus gene D12L encodes the small subunit of the viral mRNA capping enzyme. Virology. 1989 Oct;172(2):513–522. doi: 10.1016/0042-6822(89)90194-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Roth M. J., Hurwitz J. RNA capping by the vaccinia virus guanylyltransferase. Structure of enzyme-guanylate intermediate. J Biol Chem. 1984 Nov 10;259(21):13488–13494. [PubMed] [Google Scholar]
- Shuman S. Functional domains of vaccinia virus mRNA capping enzyme. Analysis by limited tryptic digestion. J Biol Chem. 1989 Jun 5;264(16):9690–9695. [PubMed] [Google Scholar]
- Shuman S., Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci U S A. 1981 Jan;78(1):187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Modification of the 5' end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-)methyltransferase complex from vaccinia virus. J Biol Chem. 1980 Feb 10;255(3):903–908. [PubMed] [Google Scholar]