Abstract
The prokaryotic peptidyl-prolyl cis-trans-isomerase called "rotamase", a homolog of the human cyclophilin, has been identified in Escherichia coli. The E. coli rotamase, a product of the gene we suggest be called "rot," has been purified to homogeneity after cloning of the gene by the polymerase chain reaction and its overexpression in E. coli. Based on the chymotrypsin-coupled assay using the tetrapeptide substrate succinyl-Ala-Ala-Pro-Phe-p-nitroanilide, the purified protein has rotamase activity identical to human cyclophilin with a catalytic efficiency close to the upper diffusional limit (kcat/Km approximately 1.0 x 10(7) M-1 x S-1 at 10 degrees C). Unlike the human cyclophilins, however, the E. coli rotamase is not significantly inhibited by the immunosuppressant drug cyclosporin A. By spheroplast fractionation of cells harboring the expression vector for the complete rot gene, the rotamase is located in the periplasm, where it could function in refolding of secreted proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Halvorson H. R., Brennan M. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry. 1975 Nov 4;14(22):4953–4963. doi: 10.1021/bi00693a026. [DOI] [PubMed] [Google Scholar]
- Briggs M. S., Gierasch L. M. Molecular mechanisms of protein secretion: the role of the signal sequence. Adv Protein Chem. 1986;38:109–180. doi: 10.1016/s0065-3233(08)60527-6. [DOI] [PubMed] [Google Scholar]
- Brown R. D., 3rd, Brewer C. F., Koenig S. H. Conformation states of concanavalin A: kinetics of transitions induced by interaction with Mn2+ and Ca2+ ions. Biochemistry. 1977 Aug 23;16(17):3883–3896. doi: 10.1021/bi00636a026. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P. The influence of peptidyl-prolyl cis-trans isomerase on the in vitro folding of type III collagen. J Biol Chem. 1987 Dec 15;262(35):17144–17148. [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Dalgarno D. C., Harding M. W., Lazarides A., Handschumacher R. E., Armitage I. M. 1H NMR studies on bovine cyclophilin: preliminary structural characterization of this specific cyclosporin A binding protein. Biochemistry. 1986 Nov 4;25(22):6778–6784. doi: 10.1021/bi00370a008. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Boswell B. A., Bächinger H. P. Thermal stability and folding of type IV procollagen and effect of peptidyl-prolyl cis-trans-isomerase on the folding of the triple helix. J Biol Chem. 1989 May 25;264(15):8956–8962. [PubMed] [Google Scholar]
- Dietmeier K., Tropschug M. Nucleotide sequence of a full-length cDNA coding for cyclophilin (peptidyl-prolyl cis-trans isomerase) of Saccharomyces cerevisiae. Nucleic Acids Res. 1990 Jan 25;18(2):373–373. doi: 10.1093/nar/18.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Fischer G., Bang H., Mech C. Nachweis einer Enzymkatalyse für die cis-trans-Isomerisierung der Peptidbindung in prolinhaltigen Peptiden. Biomed Biochim Acta. 1984;43(10):1101–1111. [PubMed] [Google Scholar]
- Fischer G., Berger E., Bang H. Kinetic beta-deuterium isotope effects suggest a covalent mechanism for the protein folding enzyme peptidylprolyl cis/trans-isomerase. FEBS Lett. 1989 Jul 3;250(2):267–270. doi: 10.1016/0014-5793(89)80735-5. [DOI] [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Haendler B., Hofer-Warbinek R., Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987 Apr;6(4):947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendler B., Keller R., Hiestand P. C., Kocher H. P., Wegmann G., Movva N. R. Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene. 1989 Nov 15;83(1):39–46. doi: 10.1016/0378-1119(89)90401-0. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harrison R. K., Stein R. L. Mechanistic studies of peptidyl prolyl cis-trans isomerase: evidence for catalysis by distortion. Biochemistry. 1990 Feb 20;29(7):1684–1689. doi: 10.1021/bi00459a003. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamukai M., Matsuda H., Fujii W., Utsumi R., Komano T. Nucleotide sequences of fic and fic-1 genes involved in cell filamentation induced by cyclic AMP in Escherichia coli. J Bacteriol. 1989 Aug;171(8):4525–4529. doi: 10.1128/jb.171.8.4525-4529.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang K., Schmid F. X., Fischer G. Catalysis of protein folding by prolyl isomerase. Nature. 1987 Sep 17;329(6136):268–270. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- Lang K., Schmid F. X. Protein-disulphide isomerase and prolyl isomerase act differently and independently as catalysts of protein folding. Nature. 1988 Feb 4;331(6155):453–455. doi: 10.1038/331453a0. [DOI] [PubMed] [Google Scholar]
- Lightowlers M. W., Haralambous A., Rickard M. D. Amino acid sequence homology between cyclophilin and a cDNA-cloned antigen of Echinococcus granulosus. Mol Biochem Parasitol. 1989 Oct;36(3):287–289. doi: 10.1016/0166-6851(89)90177-1. [DOI] [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Hasumi H., Brandts J. F. Catalysis of proline isomerization during protein-folding reactions. Biochim Biophys Acta. 1988 Oct 12;956(3):256–266. doi: 10.1016/0167-4838(88)90142-2. [DOI] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Chen C. M., Schreiber S. L., Walsh C. T. Cloning, expression, and purification of human cyclophilin in Escherichia coli and assessment of the catalytic role of cysteines by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2304–2308. doi: 10.1073/pnas.87.6.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
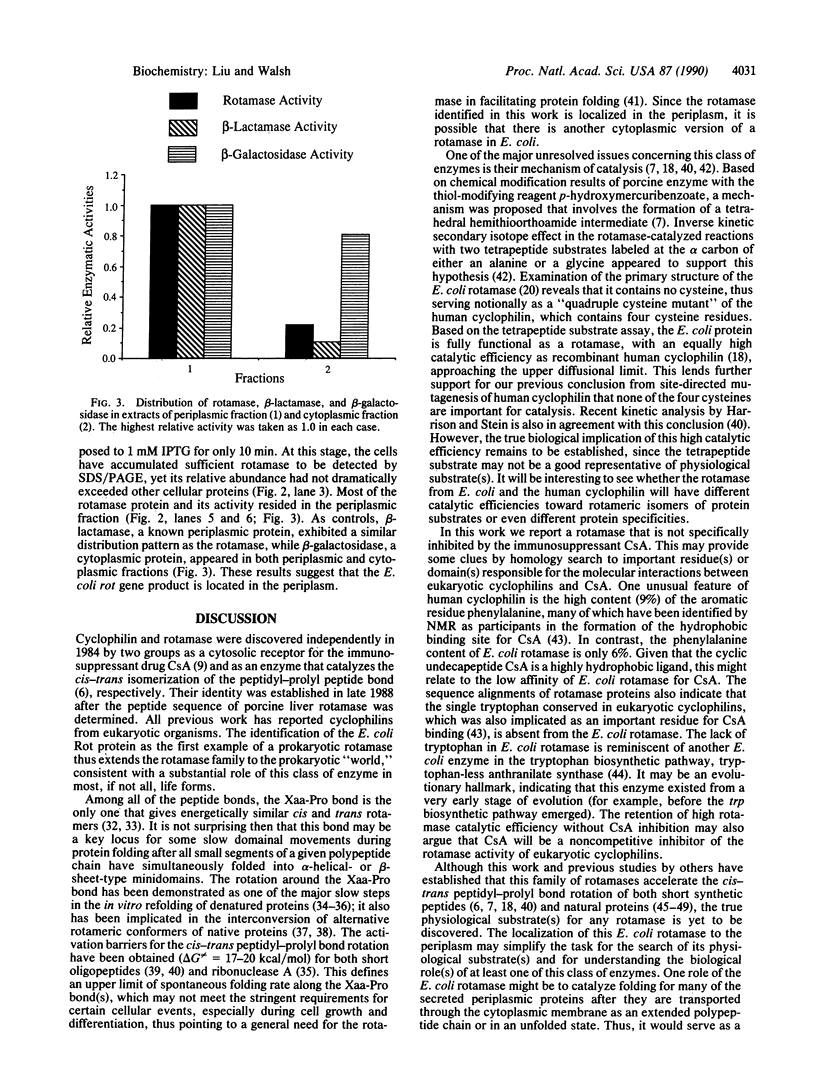
- MacFerrin K. D., Terranova M. P., Schreiber S. L., Verdine G. L. Overproduction and dissection of proteins by the expression-cassette polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1937–1941. doi: 10.1073/pnas.87.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Marsh H. C., Scott M. E., Hiskey R. G., Koehler K. A. The nature of the slow metal ion-dependent conformational transition in bovine prothrombin. Biochem J. 1979 Dec 1;183(3):513–517. doi: 10.1042/bj1830513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., van Cleemput M., Yanofsky C. Nucleotide sequence of Escherichia coli trpE. Anthranilate synthetase component I contains no tryptophan residues. J Mol Biol. 1981 Feb 15;146(1):45–54. doi: 10.1016/0022-2836(81)90365-x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Mitra A. K. An explanation for the rare occurrence of cis peptide units in proteins and polypeptides. J Mol Biol. 1976 Oct 15;107(1):85–92. doi: 10.1016/s0022-2836(76)80019-8. [DOI] [PubMed] [Google Scholar]
- Ross G. W., O'Callaghan C. H. Beta-lactamase assays. Methods Enzymol. 1975;43:69–85. doi: 10.1016/0076-6879(75)43081-6. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid F. X., Baldwin R. L. Acid catalysis of the formation of the slow-folding species of RNase A: evidence that the reaction is proline isomerization. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4764–4768. doi: 10.1073/pnas.75.10.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid F. X., Grafl R., Wrba A., Beintema J. J. Role of proline peptide bond isomerization in unfolding and refolding of ribonuclease. Proc Natl Acad Sci U S A. 1986 Feb;83(4):872–876. doi: 10.1073/pnas.83.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Shortridge R. D., Larrivee D. C., Ono T., Ozaki M., Pak W. L. Drosophila ninaA gene encodes an eye-specific cyclophilin (cyclosporine A binding protein). Proc Natl Acad Sci U S A. 1989 Jul;86(14):5390–5394. doi: 10.1073/pnas.86.14.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh B. H., Stamnes M. A., Seavello S., Harris G. L., Zuker C. S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989 Mar 2;338(6210):67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tran P. V., Bannor T. A., Doktor S. Z., Nichols B. P. Chromosomal organization and expression of Escherichia coli pabA. J Bacteriol. 1990 Jan;172(1):397–410. doi: 10.1128/jb.172.1.397-410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropschug M., Barthelmess I. B., Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989 Dec 21;342(6252):953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Nicholson D. W., Hartl F. U., Köhler H., Pfanner N., Wachter E., Neupert W. Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. One gene codes for both the cytosolic and mitochondrial forms. J Biol Chem. 1988 Oct 5;263(28):14433–14440. [PubMed] [Google Scholar]
- Watanabe M., Blobel G. SecB functions as a cytosolic signal recognition factor for protein export in E. coli. Cell. 1989 Aug 25;58(4):695–705. doi: 10.1016/0092-8674(89)90104-9. [DOI] [PubMed] [Google Scholar]



