Abstract
Objective
The endocannabinoid system hypertonicity features obesity. Excess circulating 2-arachidonoylglycerol was variously associated with obesity-related metabolic impairment; however, unstandardized experimental and analytical settings have clouded its usefulness as a dysmetabolism biomarker. We aimed at assessing the influence of body mass index (BMI), menopause in women, and aging in men on 2-arachidonoylglycerol relationship with metabolic parameters.
Methods
Adult, unmedicated women (premenopausal (preMW): n = 103; menopausal (MW): n = 81) and men (n = 144) were stratified in normal weight (NW; BMI: 18.5–24.9 kg/m2), overweight (OW; BMI: 25.0–29.9 kg/m2), and obese (OB; BMI ≥ 30.0 kg/m2) classes. Anthropometric and metabolic parameters were determined. Plasma 2-arachidonoylglycerol was measured by a validated liquid chromatography-mass spectrometry assay.
Results
2-arachidonoylglycerol level was raised by menopause (P < 0.001) and by obesity in preMW (P < 0.001) and in men (P = 0.019). In the overall cohorts, 2-arachidonoylglycerol displayed BMI-independent relationships with dyslipidemia (preMW, MW and men), insulin resistance (MW and men), and hypertension (men), but not with waist circumference. Within preMW BMI classes, 2-arachidonoylglycerol correlations were found with triglycerides (P = 0.020) and total cholesterol (TC; P = 0.040) in OB women. In MW, 2-arachidonoylglycerol correlation with triglycerides was found in NW (P = 0.001) and OW (P = 0.034), but not in OB class. Moreover, we found 2-arachidonoylglycerol correlations with TC (P = 0.003), glucose (P < 0.001), and HOMA-IR (P = 0.035) specific for NW MW class. In men, 2-arachidonoylglycerol correlated with triglycerides in NW, OW (both P < 0.001), and OB (P = 0.029), with SBP (P = 0.023) and diastolic BP (DBP; P = 0.048) in OB, and with TC (P < 0.001) in OW class. In NW class 2-arachidonoylglycerol correlations were found with insulin (P = 0.003) and HOMA-IR (P = 0.001), both enhanced by aging (both P = 0.004), and with glucose (P = 0.015) and HDL (P = 0.004).
Conclusions
Plasma 2AG is a biomarker of clustering metabolic dysfunctions, especially in lean men and menopausal women, and could be of help in identifying subjects with elevated cardiometabolic risk despite a healthy anthropometric appearance.
Keywords: 2-Arachidonoylglycerol, Endocannabinoid system, Obesity, Menopause, Aging, Dysmetabolism
Abbreviations: 1AG, 1-arachidonoylglycerol; 2AG, 2-arachidonoylglycerol; BMI, body mass index; DBP, diastolic blood pressure; ECS, endocannabinoid system; EC, endocannabinoid; HDL, high density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; Int, interaction; LC-MS/MS, liquid chromatography-tandem mass spectrometry; NW, normal weight; OB, obese; OW, overweight; SBP, systolic blood pressure; SD, standard deviation
Highlights
-
•
Plasma 2AG is a biomarker of dysmetabolism rather than obesity.
-
•
Menopause is a major determinant of plasma 2AG levels in females.
-
•
Increased plasma 2AG level features obese premenopausal females and obese males.
-
•
2AG is a biomarker of dyslipidemia and insulin resistance in lean menopausal women.
-
•
2AG is a biomarker of dyslipidemia and age-related insulin resistance in lean men.
1. Introduction
The lipid mediators of the endocannabinoid system (ECS), N-arachidonoyl-ethanolamine and 2-arachidonoylglycerol (2AG), act on specific cannabinoid receptors in an autocrine/paracrine fashion according to an on-demand synthesis from cell membrane phospholipids and to rapid degradation. The presence of endocannabinoids (ECs) in the bloodstream has been attributed to their release from peripheral tissues and blood cells [1], and their level is presumed to reflect the overall ECS tone throughout the body. The ECS positively regulates energy balance by acting on food intake and energy storage and expenditure [2], [3]. Increased circulating ECs have often been reported in human obesity, witnessing the link between ECS hyperactivation and excess weight. In particular, 2AG was presumed to be spilled-over from expanded and deregulated visceral adipose tissue [4], [5], [6], [7], [8]. EC levels have been associated with a worse metabolic profile and defined as biomarkers of cardiometabolic risk [7], but such observations were confined within the obesity context. Whether ECs in the bloodstream actively contribute to metabolic processes and whether they accomplish endocrine functions independently of excess weight has not been clarified.
Plasma concentrations and association with anthropometric and metabolic parameters reported to date do not appear to be sufficiently homogeneous for circulating 2AG to become useful for clinical purposes. The scenario depicted by previous human studies is clouded by several confounding factors hiding in selection criteria, such as gender, age, obesity related-comorbidities, and medications, and in concurrent physiopathological conditions known to impact the ECS, such as immunological, psychological, or sleep disturbances. Moreover, studies have mostly been performed on small cohorts and using a variety of experimental and statistical approaches. The usefulness of 2AG testing is further undermined by its dramatic chemical and biological instability, causing ex vivo increase in plasma, degradation during extraction, particularly by traditional methanol/chloroform protocols, and massive acyl-migration leading to the formation of 1-arachidonoylglycerol (1AG) isomer in plasma and protic solvents [9], [10], [11], [12], [13], [14]. For this reason, the sum of 2AG and 1AG was often used in spite of 2AG in studies involving plasma material. To complicate the scenario, 1AG was reported to have an agonist activity on the cannabinoid receptor 1 lower than 2AG [15]; however, the relationship among the rate of formation of this isomer, the rate of degradation of both 2AG and 1AG, and the half-life of the 2AG signaling largely vary according to the physiological environment [16].
Though efforts have been made to solve pre- and analytical pitfalls, the absence of procedural standardization has limited 2AG evaluation to the research field, and clouded the contribute of 1AG to the pool of compounds able to participate in ECS involvement in metabolic regulation.
In our previous study, we reported a thorough validation of EC measurement by liquid chromatography-tandem mass spectrometry (LC-MS/MS). We also provided gender-specific reference intervals estimated on a strictly selected population of metabolically-healthy, normal weight adults, reporting that 2AG as well as 1AG levels are higher in males than in females and are influenced by age and metabolic status even in the reference cohort [12].
Given the remarkable sex influence on 2AG levels as well as on fat distribution and metabolic comorbidities featuring obesity, the present study focused on separate female and male cohorts to investigate the relevance of 2AG as a biomarker of dysmetabolism independent of obesity and whether its relevance is modified by conditions known to exacerbate the metabolic status, such as an increasing body mass index (BMI), menopause in females and aging in males. In addition, we aimed at assessing whether the sum of 2AG and 1AG values may convey different information on the metabolic status as compared to 2AG.
2. Materials and methods
2.1. Subjects
Subjects were recruited from a general population of the town of Massa Lombarda, Italy [12]. The study was approved by University Ethics Committee. Volunteers aged ≥18 years were invited by telephone interview to attend the local health unit between 8 and 10 am after overnight fasting. Anamnestic and anthropometric data and systolic (SBP) and diastolic (DBP) blood pressures were recorded before blood withdrawal in volunteers providing written informed consent.
Inclusion criteria were: a BMI ≥18.5 kg/m2 stable over the previous three months, a normal wake–sleep cycle and complete sexual development. Exclusion criteria were shift work, perimenopausal status for females, drug consumption in the previous three months (including estro-progestogens and drugs for type II diabetes, hypertension, hypercholesterolemia or hypertriglyceridemia), and previous or present endocrine (except hypothyroidism and obesity), hepatic, renal, tumoral, autoimmune, cardiovascular, hematological, neurological, or psychiatric diseases and allergies. Antipyretic and anti-inflammatory compounds taken before the last month and stable thyroxine replacement were tolerated. Blood processing inadequate for EC measurement caused exclusion from the study [12].
Two cohorts composed of 184 females and 144 males, respectively, were defined. Among females, 103 were premenopausal (age <53 years; regular menses), and 81 were menopausal (age 42–89 years; absence of menses in the previous 12 months). Subjects were further classified as normal weight (NW; BMI: 18.5–24.9 kg/m2), overweight (OW; BMI: 25.0–29.9 kg/m2), and obese (OB; BMI ≥30.0 kg/m2) (Table 1, Table 2; Supplemental Tables 1 and 2).
Table 1.
Mean ± SD anthropometric and metabolic parameters of the female cohort according to the menopausal status and to body mass index classes (two-way ANOVA). Italic data represent the P values referring to the BMI and the menopause effects as well as to their interaction.
| Anthropometric and metabolic parameters | Cohort | Overall | BMI classes |
Effect of BMIa |
||
|---|---|---|---|---|---|---|
| NW | OW | OB | P value | |||
| No. of cases | Premenopausal | 103 (100%) | 60 (58.2%) | 17 (16.5%) | 26 (25.2%) | – |
| Menopausal | 81 (100%) | 29 (35.8%) | 33 (40.7%) | 19 (23.5%) | – | |
| Age (years) | Premenopausal | 39.6 ± 8.0 | 39.0 ± 8.6 | 41.1 ± 6.7 | 39.9 ± 7.5 | 0.653 (+) |
| Menopausal | 61.7 ± 9.1 | 60.4 ± 8.9 | 62.6 ± 9.4 | 62.3 ± 9.1 | 0.436 (+) | |
| Effect of MPb | <0.001 (+) | <0.001 (+) | <0.001 (+) | <0.001 (+) | 0.741 (+)c | |
| BMI (kg/m2) | Premenopausal | 26.0 ± 6.1 | 21.8 ± 1.8 | 27.0 ± 1.2 | 35.1 ± 3.7 | <0.001 (+) |
| Menopausal | 26.9 ± 5.1 | 22.3 ± 1.8 | 26.9 ± 1.3 | 33.9 ± 4.9 | <0.001 (+) | |
| Effect of MPb | 0.996 (+) | 0.136 (+) | 0.921 (−) | 0.311 (−) | 0.089 (−)c | |
| Waist circumference (cm) | Premenopausal | 86.1 ± 15.2 | 76.5 ± 7.4 | 88.7 ± 9.7 | 106.5 ± 9.5 | <0.001 (+) |
| Menopausal | 90.0 ± 11.8 | 80.9 ± 8.7 | 89.8 ± 5.7 | 104.1 ± 10.2 | <0.001 (+) | |
| Effect of MPb | 0.287 (+) | 0.009 (+) | 0.566 (+) | 0.404 (−) | 0.026 (−)c | |
| SBP (mmHg) | Premenopausal | 120 ± 13 | 116 ± 10 | 125 ± 15 | 127 ± 15 | <0.001 (+) |
| Menopausal | 134 ± 19 | 131 ± 18 | 132 ± 19 | 142 ± 20 | 0.047 (+) | |
| Effect of MPb | <0.001 (+) | <0.001 (+) | 0.157 (+) | 0.006 (+) | 0.580 (−)c | |
| DBP (mmHg) | Premenopausal | 78 ± 8 | 76 ± 7 | 81 ± 7 | 81 ± 9 | 0.014 (+) |
| Menopausal | 82 ± 9 | 81 ± 7 | 81 ± 9 | 87 ± 10 | 0.009 (+) | |
| Effect of MPb | 0.006 (+) | 0.016 (+) | 0.894 (+) | 0.015 (+) | 0.614 (+)c | |
| Glucose (mg/dL) | Premenopausal | 88.6 ± 10.3 | 86.4 ± 8.4 | 89.5 ± 9.5 | 92.9 ± 13.3 | 0.039 (+) |
| Menopausal | 92.9 ± 14.8 | 90.6 ± 13.5 | 93.8 ± 16.1 | 95.1 ± 14.5 | 0.265 (+) | |
| Effect of MPb | 0.147 (+) | 0.189 (+) | 0.377 (+) | 0.649 (+) | 0.666 (−)c | |
| Insulin (μU/mL) | Premenopausal | 7.29 ± 4.21 | 5.53 ± 2.27 | 6.93 ± 2.81 | 11.74 ± 5.29 | <0.001 (+) |
| Menopausal | 7.88 ± 3.97 | 6.28 ± 2.72 | 7.84 ± 3.81 | 10.38 ± 4.69 | <0.001 (+) | |
| Effect of MPb | 0.446 (+) | 0.128 (+) | 0.341 (+) | 0.390 (−) | 0.111 (−)c | |
| HOMA-IR | Premenopausal | 1.62 ± 1.00 | 1.18 ± 0.54 | 1.56 ± 0.72 | 2.67 ± 1.21 | <0.001 (+) |
| Menopausal | 1.85 ± 1.12 | 1.45 ± 0.79 | 1.85 ± 1.09 | 2.49 ± 1.34 | <0.001 (+) | |
| Effect of MPb | 0.235 (+) | 0.067 (+) | 0.266 (+) | 0.559 (−) | 0.117 (−)c | |
| Total cholesterol (mg/dL) | Premenopausal | 182.9 ± 32.4 | 180.8 ± 32.7 | 191.7 ± 36.5 | 181.8 ± 29.2 | 0.810 (+) |
| Menopausal | 208.7 ± 25.6 | 209.8 ± 29.9 | 208.3 ± 22.3 | 207.5 ± 25.1 | 0.850 (−) | |
| Effect of MPb | <0.001 (+) | <0.001 (+) | 0.045 (+) | 0.004 (+) | 0.766 (−)c | |
| HDL-cholesterol (mg/dL) | Premenopausal | 59.4 ± 14.2 | 61.9 ± 13.0 | 61.0 ± 9.6 | 52.8 ± 17.5 | 0.002 (−) |
| Menopausal | 59.8 ± 14.2 | 63.3 ± 11.8 | 59.9 ± 16.3 | 54.1 ± 12.6 | 0.015 (−) | |
| Effect of MPb | 0.820 (+) | 0.592 (+) | 0.605 (−) | 0.637 (+) | 0.955 (+)c | |
| Triglycerides (mg/dL) | Premenopausal | 71.1 ± 32.0 | 63.0 ± 21.5 | 73.5 ± 26.7 | 88.3 ± 46.4 | 0.003 (+) |
| Menopausal | 101.5 ± 46.9 | 87.0 ± 41.9 | 117.2 ± 55.3 | 96.5 ± 28.1 | 0.111 (+) | |
| Effect of MPb | <0.001 (+) | 0.003 (+) | 0.001 (+) | 0.147 (+) | 0.523 (−)c | |
BMI: body mass index; NW: normal weight; OW: overweight; OB: obese; MP: menopause; SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostatic model assessment – insulin resistance; HDL: high density lipoprotein.
Effect of BMI on anthropometric and metabolic parameters. Positive effect (+): values increased with increasing BMI classes; negative effect (−): values decreased with increasing BMI classes.
Effect of MP on anthropometric and metabolic parameters. Positive effect (+): higher values in menopausal than in premenopausal females; negative effect (−): lower values in menopausal than in premenopausal females.
Interaction between BMI and MP effects on anthropometric and metabolic parameters. Positive interaction (+): the positive effect of BMI in menopausal is higher than in premenopausal females (i.e., the higher (or lower) values in menopausal than in premenopausal females increased (decreased) with BMI); negative interaction (−): the positive effect of BMI in menopausal is lower than in premenopausal females (i.e., the higher (or lower) values in menopausal than in premenopausal females decreased (increased) with BMI).
Table 2.
Mean ± SD anthropometric and metabolic parameters of the male cohort according to body mass index classes. The P values of age effect are also reported (two-way ANOVA). Italic data represent the P values referring to the BMI and the age effects as well as to their interaction.
| Anthropometric and metabolic parameters | Overall | BMI classes |
Effect of BMIb |
||
|---|---|---|---|---|---|
| NW | OW | OB | P value | ||
| No. of cases | 144 (100%) | 61 (42.4%) | 61 (42.4%) | 22 (15.3%) | |
| Age (years)a | 49.0 ± 14.9 | 45.2 ± 15.4 | 50.8 ± 14.5 | 54.8 ± 12.0 | 0.009 (+) |
| BMI (kg/m2) | 26.3 ± 3.9 | 23.1 ± 1.5 | 27.0 ± 1.4 | 33.3 ± 3.3 | <0.001 (+) |
| Effect of agec | 0.178 (−) | <0.001 (+) | 0.718 (−) | 0.003 (−) | <0.001 (−)d |
| Waist circumference (cm) | 94.0 ± 11.7 | 85.2 ± 8.1 | 96.7 ± 7.2 | 111.1 ± 7.0 | <0.001 (+) |
| Effect of agec | 0.879 (+) | <0.001 (+) | 0.228 (+) | 0.054 (−) | 0.002 (−)d |
| SBP (mmHg) | 131 ± 17 | 126 ± 15 | 130 ± 14 | 145 ± 18 | <0.001 (+) |
| Effect of agec | <0.001 (+) | <0.001 (+) | <0.001 (+) | 0.268 (+) | 0.436 (−)d |
| DBP (mmHg) | 83 ± 7 | 80 ± 7 | 83 ± 6 | 88 ± 7 | <0.001 (+) |
| Effect of agec | 0.371 (+) | 0.072 (+) | 0.029 (+) | 0.418 (−) | 0.140 (−)d |
| Glucose (mg/dL) | 92.9 ± 11.8 | 92.1 ± 8.1 | 92.2 ± 14.2 | 97.4 ± 12.4 | 0.177 (+) |
| Effect of agec | 0.054 (+) | 0.516 (−) | 0.003 (+) | 0.253 (+) | 0.191 (+)d |
| Insulin (μU/mL) | 7.95 ± 4.77 | 6.11 ± 3.37 | 7.71 ± 3.22 | 13.75 ± 6.89 | <0.001 (+) |
| Effect of agec | 0.091 (−) | 0.464 (−) | 0.592 (−) | 0.150 (−) | 0.311 (−)d |
| HOMA-IR | 1.85 ± 1.20 | 1.40 ± 0.79 | 1.77 ± 0.88 | 3.31 ± 1.74 | <0.001 (+) |
| Effect of agec | 0.247 (−) | 0.405 (−) | 0.886 (+) | 0.278 (−) | 0.518 (−)d |
| Total cholesterol (mg/dL) | 195.2 ± 33.9 | 187.5 ± 33.2 | 203.9 ± 33.5 | 192.9 ± 32.8 | 0.648 (+) |
| Effect of agec | 0.135 (+) | <0.001 (+) | 0.024 (+) | 0.403 (−) | 0.032 (−)d |
| HDL-cholesterol (mg/dL) | 50.2 ± 11.3 | 52.7 ± 11.0 | 50.1 ± 10.9 | 43.3 ± 10.8 | <0.001 (−) |
| Effect of agec | 0.165 (+) | 0.135 (+) | 0.051 (+) | 0.972 (+) | 0.558 (−)d |
| Triglycerides (mg/dL) | 104.3 ± 57.9 | 91.8 ± 54.3 | 105.3 ± 57.8 | 136.0 ± 57.5 | <0.001 (+) |
| Effect of agec | 0.542 (−) | 0.694 (−) | 0.784 (+) | 0.492 (−) | 0.642 (−)d |
BMI: body mass index; NW: normal weight; OW: overweight; OB: obese; SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostatic model assessment – insulin resistance; HDL: high density lipoprotein.
One-way ANOVA.
Effect of BMI on anthropometric and metabolic parameters. Positive effect (+): values increased with increasing BMI classes; negative effect (−): values decreased with increasing BMI classes.
Effect of age on anthropometric and metabolic parameters. Positive effect (+): values increased with age; negative effect (−): values decreased with age.
Interaction between BMI and age effects on metabolic parameters. Positive interaction (+): the positive effect of age increased with BMI; negative interaction (−): the positive (or negative) effect of age decreased (or increased) with BMI.
2.2. Biochemical and AG measurements
Following 10 min saline infusion, blood was withdrawn and immediately processed as reported elsewhere [12]. Plasma and serum aliquots were stored at −80 °C and −20 °C, respectively, until analysis. Glucose was measured by Breeze-2 glucometer (Bayer, Leverkusen, Germany, CV: 2–4.5%). Serum parameters (intra-, inter-assay CVs) were measured by the Roche Modular Analyzer (Mannheim, Germany): triglycerides (<1.5, 1.8%), total cholesterol (<1.0, 2.7%), high density lipoprotein (HDL)-cholesterol (<0.95, 1.3%), and insulin (1.5, 4.9%). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to the formula (glucose x insulin)/405 [17].
As previously described [12], 2AG and 1AG were extracted from 0.5 mL of plasma by toluene-based liquid–liquid extraction. Samples were injected into the LC-MS/MS platform (HPLC Series200, PerkinElmer, Waltham, Massachusetts; API4000 QTrap, AB-Sciex, Toronto, Canada). Baseline separation between 2AG and 1AG isomers was achieved in 22 min run. Analytes were detected by quantitative and confirmation transitions with 0.078 pmol/mL functional sensitivity. Results were obtained in fourteen runs over two months, each including calibrators, fifty-to-seventy samples, and three replicates of two-level quality controls. Inter-assay imprecision was <7.2 and <8.0% for 2AG and 1AG, respectively.
2.3. Statistics
Mean, standard deviation (SD), range, and absolute and relative frequencies were used as descriptive statistics. Females and males were analyzed separately by considering BMI and menopause, and BMI and age as the main factors, respectively, while metabolic parameters were considered as covariates in both populations. In the male cohort, age was stratified in six classes (18–29, 30–39, 40–49, 50–59, 60–69, ≥70 years). Two-way ANOVA was used to test anthropometric and metabolic differences among BMI classes and according to menopause/age in females/males, respectively, with the exception of age which was tested among BMI classes by one-way ANOVA in males.
Concerning 2AG analysis, two-way ANOVA was applied to evaluate the unadjusted effects of BMI and menopause/age in females/males, while two-way ANCOVAs were applied to evaluate the same effects adjusted for each metabolic parameter. The linear trend of the polynomial contrast was computed for testing the BMI effect in both populations and for testing the age effect in the male population; to avoid multiple comparisons, the simple contrast was applied for testing differences between pairs of BMI classes. Results are reported as P values, while the coefficients estimated by ANOVAs and ANCOVAs, indicating the sign and size of the evaluated effects, are reported in the Supplemental Tables. All the main effects of the explanatory variables (i.e., factors (BMI and menopause/age) and covariates (metabolic parameters)), as well as all possible interactions, were considered in the analyses according to a saturated model. Significant interaction between two explanatory variables indicated that they did not act independently on the response variable (i.e., the effect of the two explanatory variables together was different from the sum of the two separate effects; e.g., a significant interaction between BMI and menopause/age indicated that the BMI effect impacted 2AG levels with a significant difference over classes in different fertility statuses/ages; this can also be interpreted as the effect of menopause/age impacting 2AG levels differently over classes at different BMI levels). All the analyses performed on 2AG were also performed on the variable given by the sum of 2AG and 1AG values (2AG + 1AG). Results are reported as P values in the Supplemental Tables.
All significantly skewed variables showed a positive skewness and were transformed according to the formula log10 (x + k); k values that zeroed the skewness after transformations were chosen. All transformed and untransformed variables showed normal distribution at the Kolmogorov–Smirnov test except for SBP in females and DBP in females and males due to the discrete values used in recording these data.
Data were managed and analyzed by IBM SPSS Statistics package (Version 23; IBM Co., Armonk, NY, USA); two-tailed P values < 0.050 were considered statistically significant.
3. Results
3.1. Females
3.1.1. Descriptive features of the female cohort (Table 1 and Supplemental Table 1)
Waist circumference was similar between menopausal and premenopausal women and, as expected, increased with BMI in both cohorts (both P < 0.001). A significant negative interaction between BMI and menopause (P = 0.026) effects indicated that NW menopausal women had higher waist circumferences than their premenopausal (P = 0.009) counterparts, but this difference was nullified in classes at increasing BMI.
Compared to premenopausal subjects, menopausal women displayed higher SBP (P < 0.001), DBP (P = 0.006), total cholesterol (P < 0.001) and triglycerides (P < 0.001). Differences were confirmed for SBP and DBP within NW (P < 0.001 and P = 0.016, respectively) and OB (P = 0.006 and P=0.015, respectively), for triglycerides within NW (P = 0.003) and OW (P = 0.001), and for total cholesterol within NW (P < 0.001), OW (P = 0.045) and OB (P = 0.004) classes.
SBP (P < 0.001), DBP (P = 0.014), glucose (P = 0.039), insulin (P < 0.001), HOMA-IR (P < 0.001), and triglycerides (P = 0.003) increased, and HDL-cholesterol decreased (P = 0.002) with BMI in premenopausal women. In menopausal females, BMI positively influenced SBP (P = 0.047), DBP (P = 0.009), insulin and HOMA-IR (both P < 0.001) and negatively influenced HDL-cholesterol (P = 0.015).
3.1.2. Effect of menopause and BMI on circulating 2AG (Table 3 and Supplemental Table 3)
Table 3.
Results of the two-way ANCOVA evaluating the effects of body mass index and menopause, as well as of the correlation with metabolic parameters, on 2-arachidonoylglycerol (2AG) circulating levels in the female cohort. The effects of interactions are also reported. Data are shown as P values of the evaluated effects: non italic data show first order effects; italic data show the interactions between first order effects (coefficients are reported in Supplemental Table 3).
| Factors | Cohort | Unadjusteda | Waist circumference | SBP | DBP | Glucose | Insulin | HOMA-IR | Total cholesterol | HDL-cholesterol | Triglycerides |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect of MP | Overallc | <0.001 (+) | 0.124 (−) | 0.280 (+) | 0.747 (+) | 0.144 (−) | 0.525 (+) | 0.017 (+) | 0.744 (−) | 0.714 (−) | 0.894 (−) |
| NWc | 0.001 (+) | 0.616 (−) | 0.967 (+) | 0.489 (−) | 0.007 (−) | 0.546 (−) | 0.030 (+) | 0.041 (−) | 0.853 (−) | 0.320 (−) | |
| OWc | <0.001 (+) | 0.156 (−) | 0.162 (+) | 0.629 (+) | 0.588 (−) | 0.270 (+) | 0.007 (+) | 0.356 (−) | 0.769 (−) | 0.921 (+) | |
| OBc | 0.770 (−) | 0.518 (−) | 0.703 (+) | 0.530 (+) | 0.638 (+) | 0.755 (+) | 0.700 (+) | 0.092 (+) | 0.892 (−) | 0.787 (+) | |
| Effect of BMI | Premenopausalb | <0.001 (+) | 0.471 (+) | 0.700 (+) | 0.769 (−) | 0.976 (+) | 0.738 (−) | 0.134 (+) | 0.164 (−) | 0.360 (+) | 0.831 (−) |
| Menopausalb | 0.504 (+) | 0.815 (+) | 0.493 (+) | 0.340 (+) | 0.002 (+) | 0.644 (+) | 0.456 (+) | 0.035 (+) | 0.511 (+) | 0.495 (+) | |
| Int MP x BMId | 0.027 (−) | 0.772 (−) | 0.774 (+) | 0.353 (+) | 0.028 (+) | 0.569 (+) | 0.640 (−) | 0.012 (+) | 0.972 (+) | 0.484 (+) |
SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostatic model assessment – insulin resistance; HDL: high density lipoprotein; MP: menopause; NW: normal weight; OW: overweight; OB: obese; BMI: body mass index; Int.: interaction.
Two-way ANOVA (analysis unadjusted for the metabolic parameters).
Effect of BMI on 2AG levels. Positive effect (+): values increased with increasing BMI classes. Negative effect (−): values decreased with increasing BMI classes.
Effect of MP on 2AG levels. Positive effect (+): higher values in menopausal than in premenopausal females; negative effect (−): lower values in menopausal than in premenopausal females.
Interaction between BMI and MP effects on 2AG levels. Positive interaction (+): the positive effect of BMI in menopausal is higher than in premenopausal females (i.e., the higher (or lower) values in menopausal than in premenopausal females increased (decreased) with BMI); negative interaction (−): the positive effect of BMI in menopausal is lower than in premenopausal females (i.e., the higher (or lower) values in menopausal than in premenopausal females decreased (increased) with BMI).
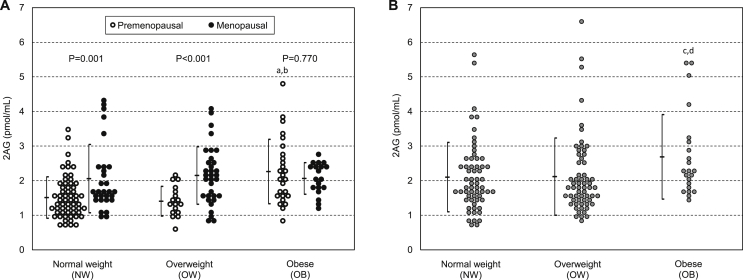
Circulating 2AG was significantly higher in the menopausal compared to the premenopausal cohort (2.10 ± 0.82 vs. 1.68 ± 0.75 pmol/mL, respectively; P < 0.001). As indicated by the significant negative interaction between BMI and menopause effects (P = 0.027; Table 3), the 2AG difference between menopausal and premenopausal females diminished at increasing BMI, being significant within NW (2.06 ± 0.99 vs. 1.51 ± 0.60 pmol/ml, respectively; P = 0.001) and within OW (2.15 ± 0.83 vs. 1.41 ± 0.43 pmol/ml, respectively; P < 0.001) but not within OB (2.06 ± 0.46 vs. 2.26 ± 0.93 pmol/ml, respectively; P = 0.770) (Figure 1A). Indeed, the two cohorts were differently influenced by BMI, as 2AG increased with BMI in premenopausal (P < 0.001) but not in menopausal females (Table 3). Paired comparisons in premenopausal females highlighted that 2AG levels were similar between OW and NW but higher in OB compared to both NW and OW (both P < 0.001) (Figure 1A). Supplemental Figure 1A shows the distribution of 2AG + 1AG values in the six BMI and fertility classes: the analysis of BMI and menopausal effect and of their interaction on the 2AG + 1AG level, as well as the paired comparisons, revealed results totally overlapping those reported for 2AG (Supplemental Table 4).
Figure 1.
2-Arachidonoylglycerol (2AG) levels (Mean ± SD) according to body mass index in premenopausal and menopausal females (A) and in males (B) (Two-way ANOVA). aP < 0.001: OB vs. NW premenopausal women; bP < 0.001: OB vs. OW premenopausal women; cP = 0.019: OB vs. NW men; dP = 0.031: OB vs. OW men. No significant differences were found by comparing pairs of BMI classes within menopausal women (OW vs. NW: P = 0.551; OB vs. NW: P = 0.504; OB vs. OW: P = 0.874) and by comparing OW vs. NW premenopausal women (P = 0.626), as well as no significant difference was found in OW vs. NW men (P = 0.747).
When metabolic parameters were introduced one at a time as adjusting covariates in the analysis, the positive menopause effect observed in the overall cohort and within NW and OW classes was lost, with the exception of adjusting for HOMA-IR (P = 0.017, P = 0.030 and P = 0.007, respectively). Moreover, when analyzed within the NW class, the effect of menopause became negative after adjustment for glucose (P = 0.007) and total cholesterol (P = 0.041). The adjustment for metabolic parameters caused the loss of BMI effect on 2AG observed in premenopausal females. Conversely, in menopausal women, the effect of BMI became significantly positive after adjustment for both glucose (P = 0.002) and total cholesterol (P = 0.035). Finally, the negative interaction between BMI and menopause became positive after adjustment for glucose (P = 0.028) and total cholesterol (P = 0.012), indicating that the adjusted BMI effect on 2AG levels was stronger in menopausal compared to premenopausal females, and was lost after adjustment for other parameters. The analysis of MP and BMI effects and of their interaction on 2AG + 1AG level after adjusting for metabolic covariates, revealed results overlapping those above reported for 2AG, except for the MP effect within the NW class and for the BMI effect within the menopausal cohort, which were preserved only after adjusting for glucose (P = 0.004 and P = 0.002, respectively) but not after adjusting for total cholesterol (P = 0.063 and P = 0.075, respectively) (Supplemental Table 4).
3.1.3. Association between 2AG and metabolic parameters in the female cohort (Table 4 and Supplemental Table 5)
Table 4.
Correlation between 2-arachidonoylglycerol (2AG) and metabolic parameters within overall premenopausal and menopausal cohorts and within body mass index classes. Data are shown as P values of the evaluated effects: non italic data show the effects of the metabolic parameters on 2AG levels; italic data show the BMI and menopause effects as well as their interactions (two-way ANCOVA; coefficients are reported in Supplemental Table 5).
| Metabolic parameter | Cohort | Overall | BMI classes |
Effect of BMIb |
||
|---|---|---|---|---|---|---|
| NW | OW | OB | P value | |||
| Waist circumference | Premenopausal | 0.253 (−) | 0.753 (−) | 0.508 (−) | 0.363 (−) | 0.541 (−) |
| Menopausal | 0.249 (+) | 0.624 (+) | 0.171 (+) | 0.957 (+) | 0.817 (−) | |
| Effect of MPa | 0.106 (+) | 0.563 (+) | 0.138 (+) | 0.522 (+) | 0.826 (+)c | |
| SBP | Premenopausal | 0.079 (+) | 0.174 (+) | 0.366 (+) | 0.360 (+) | 0.906 (−) |
| Menopausal | 0.546 (+) | 0.152 (+) | 0.517 (−) | 0.827 (+) | 0.507 (−) | |
| Effect of MPa | 0.386 (−) | 0.899 (+) | 0.267 (−) | 0.667 (−) | 0.674 (−)c | |
| DBP | Premenopausal | 0.179 (+) | 0.420 (+) | 0.706 (+) | 0.144 (+) | 0.493 (+) |
| Menopausal | 0.135 (+) | 0.094 (+) | 0.758 (+) | 0.646 (+) | 0.344 (−) | |
| Effect of MPa | 0.989 (−) | 0.346 (+) | 0.865 (−) | 0.476 (−) | 0.244 (−)c | |
| Glucose | Premenopausal | 0.624 (−) | 0.916 (−) | 0.538 (−) | 0.938 (+) | 0.897 (+) |
| Menopausal | 0.036 (+) | <0.001 (+) | 0.804 (+) | 0.538 (−) | 0.002 (−) | |
| Effect of MPa | 0.109 (+) | 0.005 (+) | 0.507 (+) | 0.628 (−) | 0.022 (−)c | |
| Insulin | Premenopausal | 0.285 (+) | 0.280 (−) | 0.225 (+) | 0.347 (+) | 0.172 (+) |
| Menopausal | 0.197 (+) | 0.274 (+) | 0.357 (+) | 0.753 (+) | 0.637 (−) | |
| Effect of MPa | 0.815 (+) | 0.138 (+) | 0.685 (−) | 0.735 (−) | 0.241 (−)c | |
| HOMA-IR | Premenopausal | 0.377 (+) | 0.410 (−) | 0.354 (+) | 0.377 (+) | 0.236 (+) |
| Menopausal | 0.099 (+) | 0.035 (+) | 0.381 (+) | 0.953 (+) | 0.189 (−) | |
| Effect of MPa | 0.581 (+) | 0.027 (+) | 0.821 (−) | 0.584 (−) | 0.078 (−)c | |
| Total cholesterol | Premenopausal | 0.084 (+) | 0.530 (+) | 0.807 (+) | 0.040 (+) | 0.135 (+) |
| Menopausal | 0.087 (+) | 0.006 (+) | 0.137 (+) | 0.523 (−) | 0.037 (−) | |
| Effect of MPa | 0.707 (+) | 0.036 (+) | 0.323 (+) | 0.089 (−) | 0.011 (−)c | |
| HDL-cholesterol | Premenopausal | 0.887 (−) | 0.484 (+) | 0.772 (−) | 0.721 (−) | 0.466 (−) |
| Menopausal | 0.565 (+) | 0.439 (+) | 0.736 (+) | 0.915 (−) | 0.530 (−) | |
| Effect of MPa | 0.627 (+) | 0.773 (+) | 0.689 (+) | 0.901 (+) | 0.911 (−)c | |
| Triglycerides | Premenopausal | 0.006 (+) | 0.117 (+) | 0.265 (+) | 0.020 (+) | 0.415 (+) |
| Menopausal | 0.005 (+) | 0.001 (+) | 0.034 (+) | 0.415 (+) | 0.486 (−) | |
| Effect of MPa | 0.631 (+) | 0.161 (+) | 0.762 (+) | 0.710 (−) | 0.308 (−)c | |
BMI: body mass index; NW: normal weight; OW: overweight; OB: obese; MP: menopause; SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostatic model assessment – insulin resistance; HDL: high density lipoprotein.
Effect of MP on the association between 2AG levels and metabolic parameters within BMI classes. Positive effect (+): the positive (or negative) association found in menopausal women is higher (or lower) than in premenopausal women; negative effect (−): the positive (negative) association found in menopausal women is lower (or higher) than in premenopausal women.
Effect of BMI on the association between 2AG levels and metabolic parameters within premenopausal and menopausal cohorts. Positive effect (+): the positive (or negative) correlation found within the premenopausal/menopausal cohort increased (or decreased) with increasing BMI classes; negative effect (−): the positive (or negative) correlation within the premenopausal/menopausal cohort decreased (or increased) with increasing BMI classes.
Interaction between BMI and MP effects on the correlation between 2AG levels and metabolic parameters. $ Positive interaction (+): the negative effect of BMI in menopausal is lower than in premenopausal females (i.e., the higher values in menopausal than in premenopausal females increased with BMI); negative interaction (−): the negative effect of BMI in menopausal is lower than in premenopausal females (i.e., the higher values in menopausal than in premenopausal females decreased with BMI).
Menopausal and premenopausal females did not exhibit significant differences in the association between 2AG and metabolic parameters. A BMI-independent 2AG association with triglycerides (P = 0.006 and P = 0.005, respectively) and a non-significant trend with total cholesterol (P = 0.084 and P = 0.087, respectively) were seen in both premenopausal and menopausal cohorts. In menopausal females, 2AG was also positively associated with glucose (P = 0.036). Non-significant 2AG trends were observed with HOMA-IR (P = 0.099) in menopausal and with SBP (P = 0.079) in premenopausal females. BMI-independent associations of 2AG + 1AG with metabolic parameters paralleled those reported for 2AG, with the addition of a significant correlation with total cholesterol in menopausal females (P = 0.033) but not in premenopausal females (P = 0.083) (Supplemental Table 6).
The interaction between BMI and menopause effects negatively influenced 2AG correlation with glucose (P = 0.022) and total cholesterol (P = 0.011). To better characterize the role of these interactions, the correlations within the six BMI/fertility classes were compared. In the premenopausal cohort, 2AG was not correlated with glucose in any BMI class and was correlated with total cholesterol within the OB class (P = 0.040) with non-significant BMI effect. Conversely, in menopausal females, the 2AG association with glucose and total cholesterol was significantly affected by increasing BMI (P = 0.002 and P = 0.037, respectively), indicating that the positive correlations observed within the NW class (P < 0.001 and P = 0.006, respectively) were diminished to non-significant levels within OW and OB classes. Furthermore, the aforementioned 2AG correlations with glucose and total cholesterol, together with the correlation with HOMA-IR (P = 0.035) reported for NW menopausal women, significantly distinguished this class from its premenopausal counterpart (P = 0.005, P = 0.036 and P = 0.027, respectively). No significant menopause-dependent differences were present within OW and OB classes. Finally, significant correlations between 2AG and triglycerides were shown within premenopausal OB class (P = 0.020) and within menopausal NW (P < 0.001) and OW (P = 0.034) classes.
A significant negative interaction between BMI and MP effects was confirmed to influence the association of 2AG + 1AG with glucose (P = 0.023), total cholesterol (P = 0.033), and HOMA-IR (P = 0.047). However, both the BMI effect and the menopausal effect acting on the correlation between 2AG + 1AG and total cholesterol, distinguishing menopausal NW class from menopausal OW and OB classes (P = 0.078) and from fertile NW women (P = 0.056) did not achieve significance. Furthermore, the association between 2AG + 1AG and total cholesterol analyzed within the premenopausal OB class did not achieve significance (P = 0.052) (Supplemental Table 6).
3.2. Males
3.2.1. Descriptive features of the male cohort (Table 2 and Supplemental Table 2)
Age (P = 0.009), waist circumference, SBP, DBP, insulin, HOMA-IR, and triglycerides increased, while HDL-cholesterol decreased, among classes at increasing BMI (all P < 0.001). Though BMI and waist circumference were not influenced by age when analyzed in the overall cohort, a negative interaction between BMI and age effects impacted both adiposity parameters (P < 0.001 and P = 0.002, respectively), revealing that they were positively correlated with age in NW subjects (both P < 0.001), and that these correlations diminished to a non-significant level in OW subjects, and to negative significant (P = 0.003) and non-significant (P = 0.054) trends within the OB class, respectively.
In the overall cohort, age had a positive influence on SBP, further confirmed within the NW and OW classes (all P < 0.001). Moreover, age positively influenced DBP (P = 0.029) and glucose (P = 0.003) within the OW class.
A significant negative interaction between BMI and age effects (P = 0.032) impacted total cholesterol, indicating that the positive association with age observed in NW subjects (P < 0.001) is diminished to a lower but still significant level in OW subjects (P = 0.024) and to a non-significant level within the OB class.
3.2.2. Effect of BMI and age on circulating 2AG (Table 5 and Supplemental Table 7)
Table 5.
Results of the two-way ANCOVA evaluating the effects of body mass index and age, as well as of the correlation with metabolic parameters, on 2-arachidonoylglycerol (2AG) circulating levels in the male cohort. The effects of interactions are also reported. Data are shown as P values of the evaluated effects: non italic data show first order effects, italic data show the interactions between first order effects (coefficients are reported in Supplemental Table 7).
| Factors | Cohort | Unadjusteda | Waist circumference | SBP | DBP | Glucose | Insulin | HOMA-IR | Total cholesterol | HDL-cholesterol | Triglycerides |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect of BMI | Overallb | 0.019 (+) | 0.661 (−) | 0.083 (−) | 0.123 (−) | 0.095 (+) | 0.894 (+) | 0.861 (+) | 0.767 (−) | 0.655 (−) | 0.368 (+) |
| Effect of age | Overallc | 0.751 (−) | 0.230 (−) | 0.483 (−) | 0.778 (+) | 0.100 (−) | 0.182 (−) | 0.409 (−) | 0.586 (−) | 0.694 (+) | 0.353 (−) |
| NWc | 0.362 (−) | 0.545 (−) | 0.470 (+) | 0.350 (+) | 0.311 (−) | 0.003 (−) | 0.291 (−) | 0.174 (−) | 0.061 (+) | 0.146 (−) | |
| OWc | 0.842 (−) | 0.955 (+) | 0.029 (−) | 0.269 (−) | 0.189 (−) | 0.361 (+) | 0.799 (+) | 0.729 (−) | 0.689 (+) | 0.685 (−) | |
| OBc | 0.897 (+) | 0.257 (−) | 0.982 (+) | 0.549 (+) | 0.272 (−) | 0.352 (−) | 0.451 (−) | 0.931 (−) | 0.608 (−) | 0.698 (−) | |
| Int. with BMId | 0.622 (+) | 0.330 (−) | 0.767 (−) | 0.786 (+) | 0.497 (−) | 0.989 (−) | 0.568 (−) | 0.775 (+) | 0.226 (−) | 0.907 (+) | |
| Correlation with metabolic parameter | Overalle | – | 0.091 (+) | 0.020 (+) | 0.052 (+) | 0.434 (+) | 0.011 (+) | 0.006 (+) | 0.001 (+) | 0.046 (−) | <0.001 (+) |
| NWe | – | 0.164 (+) | 0.884 (+) | 0.873 (+) | 0.015 (+) | 0.003 (+) | 0.001 (+) | 0.072 (+) | 0.004 (−) | <0.001 (+) | |
| OWe | – | 0.801 (+) | 0.226 (+) | 0.476 (+) | 0.208 (+) | 0.590 (+) | 0.327 (+) | <0.001 (+) | 0.150 (−) | <0.001 (+) | |
| OBe | – | 0.214 (+) | 0.023 (+) | 0.048 (+) | 0.535 (−) | 0.168 (+) | 0.216 (+) | 0.192 (+) | 0.548 (−) | 0.029 (+) | |
| Int. with BMIg | – | 0.651 (+) | 0.065 (+) | 0.095 (+) | 0.116 (−) | 0.868 (−) | 0.664 (−) | 0.729 (+) | 0.637 (+) | 0.366 (−) | |
| Effect of age on the correlation | Overallf | – | 0.228 (+) | 0.532 (+) | 0.771 (−) | 0.102 (+) | 0.097 (+) | 0.073 (+) | 0.595 (+) | 0.735 (−) | 0.312 (+) |
| NWf | – | 0.628 (+) | 0.426 (−) | 0.308 (−) | 0.328 (+) | 0.004 (+) | 0.004 (+) | 0.186 (+) | 0.059 (−) | 0.175 (+) | |
| OWf | – | 0.937 (−) | 0.032 (+) | 0.278 (+) | 0.205 (+) | 0.333 (−) | 0.573 (−) | 0.749 (+) | 0.698 (−) | 0.727 (+) | |
| OBf | – | 0.242 (+) | 0.961 (−) | 0.565 (−) | 0.271 (+) | 0.291 (+) | 0.308 (+) | 0.925 (+) | 0.604 (+) | 0.650 (+) | |
| Int. Age x BMIh | – | 0.323 (+) | 0.754 (+) | 0.851 (−) | 0.493 (+) | 0.684 (−) | 0.636 (−) | 0.789 (−) | 0.238 (+) | 0.908 (−) |
SBP: systolic blood pressure; DBP: diastolic blood pressure; HOMA-IR: homeostatic model assessment – insulin resistance; HDL: high density lipoprotein; BMI: body mass index; NW: normal weight; OW: overweight; OB: obese; Int.: interaction.
Two-way ANOVA (analysis unadjusted for the metabolic parameters).
Effect of BMI on 2AG levels. Positive effect (+): values increased with increasing BMI classes; negative effect (−): values decreased with increasing BMI classes.
Effect of age on 2AG levels. Positive effect (+): values increased with age; negative effect (−): values decreased with age.
Interaction between BMI and age effects on 2AG levels. Positive interaction (+): the positive (or negative) effect of age in the overall cohort increased (or decreased) with increasing BMI classes; negative interaction (−): the positive (or negative) effect of age in the overall cohort decreased (or increased) with increasing BMI classes.
Effect of metabolic parameters on 2AG levels (i.e., correlation of 2AG vs. metabolic parameter). Positive effect (+): 2AG level increased with increasing parameter; negative effect (−): 2AG level decreased with increasing parameter.
Effect of age over the association between 2AG levels and metabolic parameters. Positive effect (+): the positive (or negative) association found in the overall cohort or within BMI classes increased (or decreased) with age; negative effect (−): the positive (or negative) association found in the overall cohort or in the BMI classes decreased (or increased) with age.
Effect of BMI on the association between 2AG levels and metabolic parameters. Positive effect (+): the positive (or negative) association found in the overall cohort increased (or decreased) with increasing BMI classes; negative effect (−): the positive association found in the overall cohort decreased with increasing BMI classes.
Interaction between BMI and age effects on the association between 2AG levels and metabolic parameters. Positive interaction (+): the positive (or negative) effect of age in the overall cohort increased (or decreased) with increasing BMI classes; negative interaction (−): the positive (or negative) effect of age in the overall cohort decreased (or increased) with increasing BMI classes.
As shown in Figure 1B, plasma 2AG was positively influenced by BMI (P = 0.019; Table 5); in particular, 2AG was significantly higher in OB (2.69 ± 1.22 pmol/mL) compared to both NW (2.10 ± 1.00 pmol/mL; P = 0.019) and OW (2.12 ± 1.12 pmol/mL; P = 0.031) males, but similar between OW and NW classes (Figure 1B). The significant effect of BMI on 2AG was lost after adjustment for each metabolic parameter. No significant age effect or any significant interaction between age and BMI on 2AG levels were observed in the overall cohort, even after adjusting for metabolic parameters. Nonetheless, age displayed a significant negative effect on 2AG after adjusting for insulin within the NW (P = 0.003) and for SBP within the OW (P = 0.029) class (Table 5). Overlapping results were obtained when BMI and age effects were tested on 2AG + 1AG plasma level as well as after adjustment for metabolic parameters (Supplemental Figure 1B and Supplemental Table 8).
3.2.3. Association between 2AG and metabolic parameters in the male cohort (Table 5 and Supplemental Table 7)
In the overall cohort, 2AG showed BMI- and age-independent positive correlations with SBP (P = 0.020), insulin (P = 0.011), HOMA-IR (P = 0.006), total cholesterol (P = 0.001), and triglycerides (P < 0.001) and a negative correlation with HDL-cholesterol (P = 0.046); non-significant positive trends were found with DBP (P = 0.052) and waist circumference (P = 0.091). All BMI- and age-independent associations were confirmed when 2AG + 1AG was evaluated, with the addition of a significant association of 2AG + 1AG with waist circumference (P = 0.035) and DBP (P = 0.034) (Supplemental Table 8).
BMI, age, and their interactions had no significant influence on 2AG association with metabolic parameters in the overall cohort. A non-significant positive BMI effect on 2AG correlation with SBP (P = 0.065) and DPB (P = 0.095) was found, in line with the significant 2AG correlation with both SBP (P = 0.023) and DBP (P = 0.048) observed in OB males, but not within the NW and OW classes. However, such associations in OB males were not confirmed when 2AG + 1AG was tested (2AG + 1AG vs. SBP: P = 0.059; 2AG + 1AG vs. DBP: P = 0.060) (Supplemental Table 8). A significant association was found between 2AG and total cholesterol within OW males (P < 0.001), while, within the NW class, 2AG associated positively with insulin (P = 0.003) and glucose (P = 0.015), HOMA-IR (P = 0.001) and negatively with HDL-cholesterol (P = 0.004). Finally, the correlation between 2AG and triglycerides was confirmed in each BMI class (NW and OW: P < 0.001; OB: P = 0.029).
Age positively influenced 2AG correlation with insulin and HOMA-IR within NW subjects (both P = 0.004), but significance was not achieved in the overall cohort (P = 0.097 and P = 0.073, respectively). A non-significant age effect also reinforced the negative association between 2AG and HDL-cholesterol within the NW class (P = 0.059).
All significant associations with biochemical parameters and age effects were confirmed also by testing 2AG + 1AG (Supplemental Table 8).
4. Discussion
In the attempt to standardize the relevance of plasma 2AG as a biomarker of dysmetabolism, we investigated whether its association with parameters of visceral obesity, hypertension, insulin resistance, and dyslipidemia was mainly detectable in obesity, as the current state of the art would suggest, and whether its levels are influenced by events, such as menopause or aging, usually associated with metabolic worsening. In our previous study [12], we showed that our pre-analytical and analytical procedure was able to reduce 2AG-to-1AG isomerization to a minimum. Nonetheless, a measurable amount of 1AG was present in our samples, which can only partly be explained by a processing artifact. Though to a lower extent as compared to 2AG, 1AG is able to activate the cannabinoid receptor 1 and to stabilize 2AG mediated signaling [15], [16]. Therefore, we decided to evaluate its contribution in terms of metabolic associations by performing a parallel analysis on the sum of the two isomers.
Our results revealed menopause as a major determinant of circulating 2AG levels. Indeed, while increased 2AG levels were a specific feature of the obese females in premenopausal status, levels in lean and overweight menopausal women were as high as those observed in obese menopausal women. Moreover, we showed that the relevance of plasma 2AG as a BMI-independent biomarker of metabolic impairment is prevalent in the menopausal context. Although menopausal and premenopausal cohorts exhibited a similar glucose metabolism profile, 2AG appeared to be a biomarker of glycemic variation only in menopausal women. Furthermore, the value of 2AG as a glycemic biomarker is specific for the lean but not for the overweight/obese menopausal condition. Plasma 2AG's role as a biomarker of triglyceride levels was revealed to a similar extent in premenopausal and menopausal women. However, 2AG's association with both triglycerides and total cholesterol was confirmed only in obese premenopausal condition; by contrast, the same association was confirmed in menopausal non-obese females. Results obtained by replacing 2AG + 1AG to 2AG largely paralleled those reported for 2AG. Minor variations were observed in the 2AG + 1AG correlation with total cholesterol, causing the loss of the association within premenopausal obese women, and the gain of the association in the overall menopausal cohort.
Menopause is essentially characterized by the relative depletion of sex steroids, particularly estradiol. At variance with what has been described for other ECs [18], [19], a relationship between sex steroids and 2AG release in the bloodstream has not yet been described, and merits future investigations.
In males, plasma 2AG relevance as a BMI-independent biomarker involved a wider spectrum of dysmetabolic features in respect to females, including hypertension, insulin resistance, and dyslipidemia. A further significant association with visceral obesity, as determined by waist circumference, was observed when 2AG + 1AG was valued. When BMI classes were considered, we found that 2AG's association with hypertension was a feature of the obese condition, while in lean males 2AG appeared to be a biomarker for both dyslipidemia and insulin resistance, with its role being enhanced by aging, paralleling what was observed in menopausal women. In line with results by Quercioli et al., describing an association between plasma ECs and coronary circulatory dysfunction in obese individuals, our results on 2AG's association with hypertension in male obesity supported its role in conveying metabolic derangement toward overt cardiometabolic impairment [20]. However, such association with hypertension within the obese class was not confirmed when 2AG + 1AG was considered. Conversely, the sum of the AGs perfectly parallels 2AG for what concerns the association with insulin resistance and dyslipidemia within BMI classes.
Taken together, our results indicate that, with the progressive reduction of sex hormone action on metabolic homeostasis determined by menopause and aging, a rise in the involvement of plasma 2AG in glucose and lipid metabolism occurs and is detectable in the lean condition, thus supporting the concept that an enhancement in ECS tone precedes obesity. This hypothesis is supported by findings in animals showing that increased 2AG levels in lean mice, obtained by the inhibition of its degradation, caused impaired triglyceride clearance [21] and hepatic insulin resistance [22]. The fading of 2AG's association with lipid and glycemic status observed with increasing BMI in male and menopausal cohorts indicate that, once metabolic derangement results in obesity, circulating 2AG is no longer a biomarker, and possibly no longer contribute to metabolic worsening. So far, the ECS has been described as an autocrine/paracrine system. Nonetheless, it is improbable for a circulating compound not to carry a message. The 2AG plasma concentration reported in our study was approximately 100 fold lower with respect to the EC50 at 150 nM reported by Sugiura et al., in 1996 [23]. Therefore, levels we reported are not expected to be able to result in a relevant activation on the cannabinoid receptor 1. Nonetheless, the minimal 2AG concentration able to elicit a signal was estimated at 0.3 nM by the same group [15]. Our results in the lean condition could suggest that plasma 2AG reflects the metabolic status as long as the ECS acts in a highly integrated endocrine fashion. Once obesity occurs, the ECS starts acting in a self-sustained autocrine/paracrine fashion, i.e. when 2AG in the bloodstream loses its biomarker value. Data on the association of 2AG with triglycerides and total cholesterol obtained in premenopausal obesity seem to be in contrast with what was observed in lean males and menopausal females; however, these findings could be explained by the possible dominant role played by sex hormones, masking 2AG's relevance in lean and overweight conditions.
Early reports postulated visceral obesity as a major determinant of circulating 2AG [5], [7]. Our data do not fully support such a concept. Indeed, we could not find any association between 2AG or the sum of AGs and waist circumference in females, while such an association was detected for 2AG + 1AG, but not for 2AG, in the overall male cohort. Moreover, even though we confirmed elevated 2AG levels in association with the obese status in men and in premenopausal women, we revealed that such an increase is related to the clustering of dysmetabolic features rather than adiposity as measured by BMI and waist circumference. This is in clear contrast to the current view identifying adipose depots as the major source of circulating ECs and the primum movens in the hyperactivation of ECS. In searching for alternative sources of circulating 2AG, one could postulate a role for diacyglycerol lipase-independent lipid catabolism [14], explaining the strong increase in 2/1AG levels in plasma ex vivo [12], [24]; however, whether such a mechanism has a relevance in vivo is not known. Alternatively, recent findings strongly suggested that composition and balance of dietary fatty acid can influence EC levels in the bloodstream by determining the availability of their substrate arachidonic acid [25]. In this regard, a limitation of our study is represented by the lack of data on dietary habits.
To the best of our knowledge, this is the first study addressing in a gender-specific fashion the involvement of circulating 2AG in dysmetabolisms involved in obesity comorbidities. Previous studies were performed on small cohorts, including single gender or both indistinctly and prevalently overweight/obese subjects [4], [5], [8], [26], [27]. The relevance of menopause, together with the previously described gender effect [12], on 2AG levels reinforces the concept that studies on the ECS should be strictly contextualized not only as regards gender, but also as regards the fertility status.
A major strength of this study is represented by the large sample size and by the rigorous methodological approach used in restraining pre-analytical and analytical variability and in defining strict selection criteria. The decision to exclude medicated subjects to avoid possible pharmacological determinants of circulating 2AG limited the presence of severe obesity, probably explaining the inverse association between age and BMI observed in obese males, and might have caused the underestimation of the effect of severe dysmetabolism requiring treatment. Nonetheless, our obese subjects, considered overall, exhibited an impaired metabolic profile.
5. Conclusions
In conclusion, we provide evidence that our overall pre-analytical and analytical procedure assures adequate robustness for using either 2AG or the sum of 2AG and 1AG measurements in investigations concerning the metabolic health involving plasma material.
Our findings in lean subjects may suggest a possible causative role for circulating 2AG in driving menopause- and aging-related insulin resistance and dyslipidemia.
Plasma 2AG appears to be a relevant biomarker of clustering metabolic dysfunctions especially in lean men and menopausal women, thus helping in identifying subjects with elevated cardiometabolic risk despite a healthy anthropometric appearance. However, the distribution of circulating 2AG levels in healthy and dysmetabolic subjects show a high degree of overlap. Therefore, the 2AG upper reference limit established in our previous work [12] as the 97.5° centile of distribution in a reference population composed by normal weight metabolically healthy females (3.12 pmol/mL) and males (4.23 pmol/mL), is not expected to provide optimal predictive values. Further studies are needed to define a risk threshold for plasma 2AG and to prospectively assess whether an elevated ECS tone, as described by plasma 2AG, in lean subjects can favor the development of obesity and of cardiometabolic events later in life.
Acknowledgements
Financial support: the study was supported by the European Union (REPROBESITY, FPVII-223713 and NEUROFAST, FPVII-KBBE-2009-3-245009) and by the Emilia-Romagna Region – University Program 2007–2009 (“The unifying inflammatory background of the metabolic syndrome: identification of genetic and metabolic biomarkers profiling tool for patient classification and clinical assessment”, PRUa1a-2007-006).
Author contributions: FF designed the study, performed 2AG measurements, data collection and management and statistical analyses and wrote the manuscript. MM1, IB and VDDL performed 2AG measurements. MB, DIG and EC recruited and examined participants. MM6 contributed to the statistical design and analyses. AMML performed the statistical design and contributed to manuscript writing. VV, AG and RP contributed to the study concept and design. UP designed the population study of Massa Lombarda and wrote the manuscript.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.03.005.
Contributor Information
Flaminia Fanelli, Email: flaminia.fanelli@gmail.com.
Marco Mezzullo, Email: marco.mezzullo@gmail.com.
Ilaria Belluomo, Email: ilaria.belluomo@gmail.com.
Valentina Diana Di Lallo, Email: valedilallo@gmail.com.
Margherita Baccini, Email: margherita.baccini@gmail.com.
Daniela Ibarra Gasparini, Email: danielita.ig@gmail.com.
Elena Casadio, Email: elena.casadio82@gmail.com.
Marianna Mastroroberto, Email: marianna.mastroroberto@gmail.com.
Valentina Vicennati, Email: vicennati@aosp.bo.it.
Alessandra Gambineri, Email: alessandra.gambineri@aosp.bo.it.
Antonio Maria Morselli-Labate, Email: antonio.morselli@unibo.it.
Renato Pasquali, Email: renato.pasquali@unibo.it.
Uberto Pagotto, Email: uberto.pagotto@unibo.it.
Conflict of interest
The authors report no conflict of interest in this work.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Gasperi V., Evangelista D., Savini I., Del Principe D., Avigliano L., Maccarrone M. Downstream effects of endocannabinoid on blood cells: implications for health and disease. Cellular and Molecular Life Sciences. 2015;72:3235–3252. doi: 10.1007/s00018-015-1924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quarta C., Mazza R., Obici S., Pasquali R., Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends in Molecular Medicine. 2011;17:518–526. doi: 10.1016/j.molmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Gatta-Cherifi B., Cota D. New insights on the role of the endocannabinoid system in the regulation of energy balance. International Journal of Obesity. 2016;40:210–219. doi: 10.1038/ijo.2015.179. [DOI] [PubMed] [Google Scholar]
- 4.Engeli S., Böhnke J., Feldpausch M., Gorzelniak K., Janke J., Bátkai S. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blüher M., Engeli S., Klöting N., Berndt J., Fasshauer M., Bátkai S. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matias I., Gonthier M.P., Orlando P., Martiadis V., De Petrocellis L., Cervino C. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. The Journal of Clinical Endocrinology and Metabolism. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 7.Côté M., Matias I., Lemieux I., Petrosino S., Alméras N., Després J.P. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. International Journal of Obesity (London) 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- 8.Sipe J.C., Scott T.M., Murray S., Harismendy O., Simon G.M., Cravatt B.F. Biomarkers of endocannabinoid system activation in severe obesity. PLoS One. 2010;5:e8792. doi: 10.1371/journal.pone.0008792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouzer C.A., Ghebreselasie K., Marnett L.J. Chemical stability of 2-arachidonylglycerol under biological conditions. Chemistry and Physics of Lipids. 2002;119:69–82. doi: 10.1016/s0009-3084(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 10.Vogeser M., Schelling G. Pitfalls in measuring the endocannabinoid 2-arachidonoyl glycerol in biological samples. Clinical Chemistry and Laboratory Medicine. 2007;45:1023–1025. doi: 10.1515/CCLM.2007.197. [DOI] [PubMed] [Google Scholar]
- 11.Wood J.T., Williams J.S., Pandarinathan L., Courville A., Keplinger M.R., Janero D.R. Comprehensive profiling of the human circulating endocannabinoid metabolome: clinical sampling and sample storage parameters. Clinical Chemistry and Laboratory Medicine. 2008;46:1289–1295. doi: 10.1515/CCLM.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanelli F., Di Lallo V.D., Belluomo I., De Iasio R., Baccini M., Casadio E. Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS. Journal of Lipid Research. 2012;53:481–493. doi: 10.1194/jlr.M021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoerner A.A., Batkai S., Suchy M.T., Gutzki F.M., Engeli S., Jordan J. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2012;883–884:161–171. doi: 10.1016/j.jchromb.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Pastor A., Farré M., Fitó M., Fernandez-Aranda F., de la Torre R. Analysis of ECs and related compounds in plasma: artifactual isomerization and ex vivo enzymatic generation of 2-MGs. Journal of Lipid Research. 2014;55:966–977. doi: 10.1194/jlr.D043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiura T., Kodaka T., Nakane S., Miyashita T., Kondo S., Suhara Y. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. Journal of Biological Chemistry. 1999;29(274):2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 16.Dócs K., Mészár Z., Gonda S., Kiss-Szikszai A., Holló K., Antal M. The ratio of 2-AG to its isomer 1-AG as an intrinsic fine tuning mechanism of CB1 receptor activation. Frontiers in Cellular Neuroscience. 2017;20(11):39. doi: 10.3389/fncel.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.El-Talatini M.R., Taylor A.H., Konje J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertility and Sterility. 2010;93:1989–1996. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Rapino C., Battista N., Bari M., Maccarrone M. Endocannabinoids as biomarkers of human reproduction. Human Reproduction Update. 2014;20:501–516. doi: 10.1093/humupd/dmu004. [DOI] [PubMed] [Google Scholar]
- 20.Quercioli A., Pataky Z., Vincenti G., Makoundou V., Di Marzo V., Montecucco F. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. European Heart Journal. 2011;32:1369–1378. doi: 10.1093/eurheartj/ehr029. [DOI] [PubMed] [Google Scholar]
- 21.Ruby M.A., Nomura D.K., Hudak C.S., Mangravite L.M., Chiu S., Casida J.E. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14561–14566. doi: 10.1073/pnas.0807232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruby M.A., Nomura D.K., Hudak C.S., Barber A., Casida J.E., Krauss R.M. Acute overactive endocannabinoid signaling induces glucose intolerance, hepatic steatosis, and novel cannabinoid receptor 1 responsive genes. PLoS One. 2011;6:e26415. doi: 10.1371/journal.pone.0026415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiura T., Kodaka T., Kondo S., Tonegawa T., Nakane S., Kishimoto S. 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma x glioma hybrid NG108-15 cells. Biochemical and Biophysical Research Communications. 1996;229:58–64. doi: 10.1006/bbrc.1996.1757. [DOI] [PubMed] [Google Scholar]
- 24.Ueda N., Tsuboi K., Uyama T., Ohnishi T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors. 2011;37:1–7. doi: 10.1002/biof.131. [DOI] [PubMed] [Google Scholar]
- 25.Bisogno T., Maccarrone M. Endocannabinoid signaling and its regulation by nutrients. Biofactors. 2014;40:373–380. doi: 10.1002/biof.1167. [DOI] [PubMed] [Google Scholar]
- 26.Di Marzo V., Verrijken A., Hakkarainen A., Petrosino S., Mertens I., Lundbom N. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. European Journal of Endocrinology. 2009;161:715–722. doi: 10.1530/EJE-09-0643. [DOI] [PubMed] [Google Scholar]
- 27.Jumpertz R., Guijarro A., Pratley R.E., Piomelli D., Krakoff J. Central and peripheral endocannabinoids and cognate acylethanolamides in humans: association with race, adiposity, and energy expenditure. The Journal of Clinical Endocrinology and Metabolism. 2011;96:787–791. doi: 10.1210/jc.2010-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.