Abstract
Isoprostanes (IsoPs) are prostaglandin-like molecules generated independent of the cyclooxygenase (COX) by the free radical-induced peroxidation of arachidonic acid. The first isoprostane species discovered were isomeric to prostaglandin F2α and were thus termed F2-IsoPs. Since the initial discovery of the F2-IsoPs, IsoPs with differing ring structures have been identified as well as IsoPs from different polyunsaturated fatty acids, including eicosapentaenoic acid and docosahexanenoic acid. The discovery of these molecules in vivo in humans has been a major contribution to the field of lipid oxidation and free radical research over the course of the past 25 years. These molecules have been determined to be both biomarkers and mediators of oxidative stress in numerous disease settings. This review focuses on recent developments in the field with an emphasis on clinical research. Special focus is given to the use of IsoPs as biomarkers in obesity, ischemia-reperfusion injury, the central nervous system, cancer, and genetic disorders. Additionally, attention is paid to diet and lifestyle factors that can affect endogenous levels of IsoPs. This article is part of a Special Issue entitled “Oxygenated metabolism of PUFA: analysis and biological relevance”.
Keywords: Isoprostane, Oxidative stress, Biomarker, Lipid peroxidation, Mass spectrometry
1. Introduction
January 2015 marks the 25th anniversary of the first report by Morrow and Roberts on the discovery of the isoprostanes (IsoPs), prostaglandin-like molecules generated in vivo in humans independent of the cyclooxygenase (COX) by the free radical-induced peroxidation of arachidonic acid [1]. Over the course of the past 25 years, more than 3800 articles have been published in the field of IsoP research by numerous investigators around the world. Numerous excellent reviews have been written describing the formation, chemical synthesis, and biological activities of the IsoPs as well as their potential use as biomarkers of disease. This review will provide a brief historical perspective on the discovery of the IsoPs with the primary focus on recent clinical research in the field.
2. Historical perspective
2.1. Discovery and formation of isoprostanes
Oxidative stress is characterized by the inability of the body’s natural antioxidant defenses to detoxify and protect against pro-oxidant, and often pro-inflammatory, species. Production of reactive oxygen species (ROS), typically free radicals, is a hallmark of oxidative stress. Overproduction of ROS has been implicated in a variety of diseases yet much remains to be understood about mechanisms of oxidant injury in humans. Polyunsaturated fatty acids (PUFAs), such as arachidonic acid, are one target of free radical insult during oxidative stress. In the mid-1970s, it was shown that prostaglandin (PG)-like compounds could be formed in vitro by the non-enzymatic peroxidation of purified PUFA; however, this work had never been carried out beyond in vitro studies [2].
In the 1980s, a study showed that PGD2 derived from COX is primarily metabolized in vivo in humans to 9α,11β-PGF2α by the enzyme 11-ketoreductase [3]. In aqueous solutions, however, PGD2 is an unstable compound that undergoes isomerization of the lower side chain and these isomers can also be reduced by 11-ketoreductase to yield isomers of 9α,11β-PGF2α [4]. In studies undertaken to further characterize these compounds utilizing mass spectrometry, it was found that when plasma samples from normal volunteers were processed and analyzed immediately, a series of peaks were detected possessing characteristics of F-ring PGs. Interestingly, however, when plasma samples that had been stored at −20 °C for several months were reanalyzed, identical chromatographic peaks were detected but levels of putative PGF2-like compounds were up to 100-fold higher [1]. In addition, base-catalyzed hydrolysis of plasma lipids also yielded significant amounts of the PGF2-like compounds. Antioxidants and reducing agents suppressed the formation of these compounds and CCl4, a toxic agent known to generate free radicals in the liver through metabolism to the CCl3•, dramatically increased the formation of these compounds in rats [1,6]. These experiments confirmed that the observed PGF2-like compounds were generated in vivo, not by a COX-derived mechanism, but rather non-enzymatically by autoxidation of arachidonic acid. Morrow and Roberts termed this new class of compounds F2-isoprostanes (IsoPs) because they were isomeric to PGF2α [5,6].
A mechanism to explain the formation of the F2-IsoPs from arachidonic acid is outlined in Fig. 1 [7]. Following abstraction of a bisallylic hydrogen atom and the addition of molecular oxygen to arachidonic acid to form a peroxyl radical, the peroxyl radical undergoes 5-exo cyclization and a second molecule of oxygen adds to the backbone of the compound to form PGG2-like compounds. These unstable bicycloendoperoxide intermediates are then reduced to the F2-IsoPs. Based on this mechanism of formation, four F2-IsoP regioisomers, each of which is comprised of eight racemic diastereomers, a total of 64 compounds, are generated. The four regioisomer classes are named according to the carbon number on which the side chain hydroxyl group is attached (Fig. 1). This nomenclature system has been approved by the Eicosanoid Nomenclature Committee, which is sanctioned by JCBN of IUPAC [8]. An alternative nomenclature system for the IsoPs has been proposed by FitzGerald and colleagues in which the abbreviation iP is used for isoprostane, and the regioisomers are denoted as III–VI (Fig. 1) [9]. It is important to note that 5- and 15-series regioisomers are formed in significantly greater abundance than the 8- and 12-series regioisomers as 8- and 12-series regioisomers readily undergo further oxidation [10].
Fig. 1.
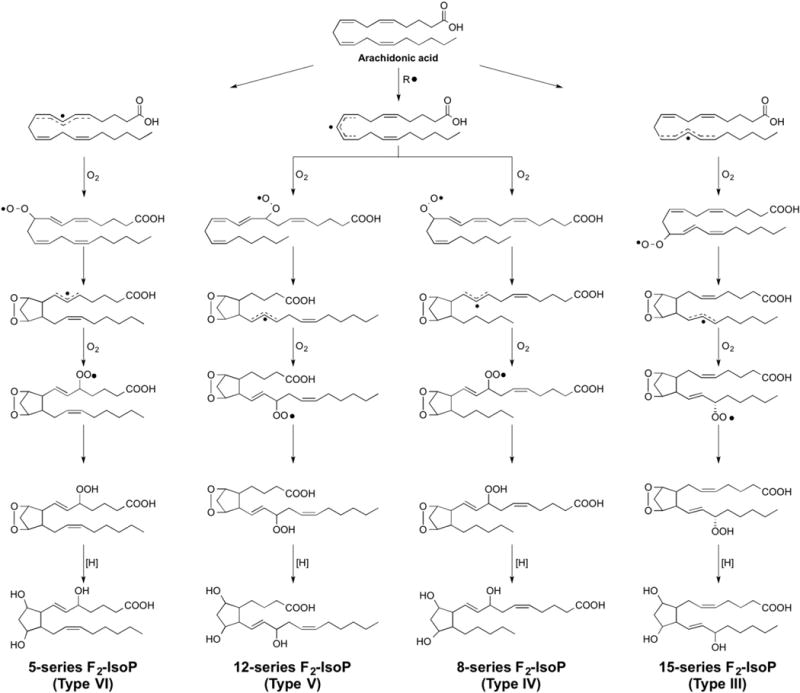
Mechanism of formation of F2-isoprostanes from the free radical-cataylzed peroxidation of arachidonic acid. Two primary nomenclature systems have been developed to classify isoprostanes [8,9]. In the nomenclature system used throughout this manuscript, IsoP is used as the abbreviation for isoprostane and the four regioisomer classes are named according to the carbon number on which the side chain hydroxyl group is attached, with the carboxyl carbon being 1 [8]. This nomenclature system has been approved by the Eicosanoid Nomenclature Committee, which is sanctioned by JCBN of IUPAC. The alternative nomenclature system uses the abbreviation iP for isoprostane and the regioisomers are denoted as III–VI based upon the number of carbons between the omega carbon and the first double bond [9].
In addition to F2-IsoPs, various classes of IsoPs that differ in regards to the functional groups on the prostane ring have been discovered; the structures of these different compounds are summarized in Fig. 2. The compounds are named based upon the structure of the functional groups on the cyclopentane ring in a manner analogous to the prostaglandins. Like F2-IsoPs, multiple isomers of these compounds are formed. E2-/D2-IsoPs that are isomeric to PGE2 and PGD2, are formed competitively with F2-IsoPs from the isomerization of the arachidonyl endoperoxide intermediate 1 (Fig. 2) [11]. Depletion of cellular reducing agents, such as glutathione (GSH) or α-tocopherol, favors the formation of E2/D2-IsoPs over that of F2-IsoPs, which is formed via reduction of the endoperoxide 1 (Fig. 2) [12]. E2/D2-IsoPs, however, are not terminal products of the IsoP pathway. These compounds readily dehydrate in vivo to yield A2/J2-IsoPs, which are also known as cyclopentenone IsoPs because they contain an α,β-unsaturated cyclopentenone ring structure [13,14]. A2/J2-IsoPs can be further metabolized, through rearrangement and dehydration, to yield deoxy-A2 and deoxy-J2-IsoPs [15]. Hardy and colleagues have shown that 15-deoxy-Δ12,14-PGJ2 (15-d-PGJ2), a highly studied molecule originally identified as a metabolite of PGD2, can also be generated non-enzymatically during free radical-induced oxidation and is one of the deoxy-J2-IsoP isomers [15]. Finally, in addition to the molecules described above, compounds isomeric to the COX-derived thromboxane B2, termed isothromboxanes are products of the free radical-catalyzed peroxidation of arachidonic acid [16].
Fig. 2.
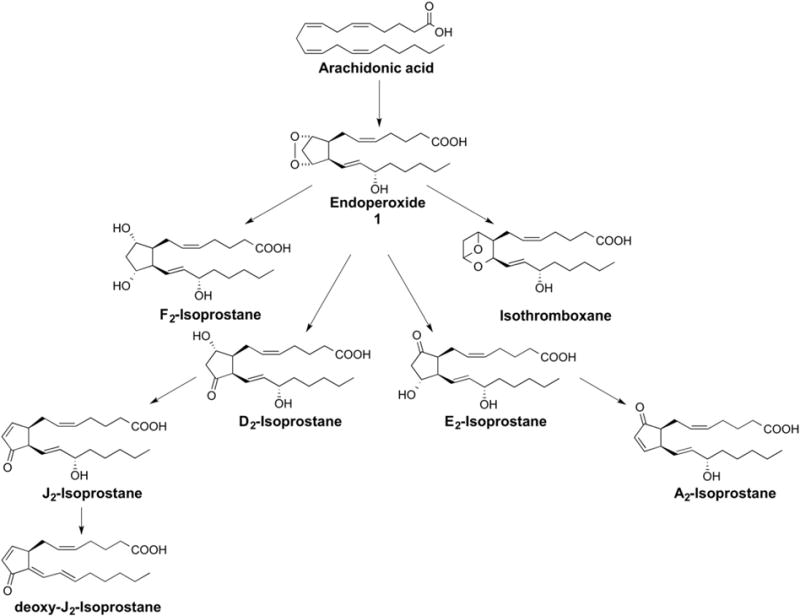
Arachidonic acid can be oxidized to many different classes of isoprostanes. F2-isoprostanes are most commonly used as biomarkers of oxidative stress in human disease but E2/D2-isoprostanes have also been used as biomarkers of oxidative stress under certain conditions. Isothromboxanes as well as A2/J2-isoprostanes and deoxy-J2-isoprostanes are also formed.
Besides IsoPs, another group of related compounds are also generated from the peroxidation of arachidonic acid via a similar mechanism. IsoPs are formed when the indicated carbon-centered radical undergoes 5-exo-cyclization to form the cyclopentane ring, as shown in Fig. 3. Alternatively, this carbon-centered radical could react with a molecule of oxygen to generate oxidized products of a different structure (Fig. 3) [17]. These products have a substituted tetrahydrofuran ring and thus have been named isofurans (IsoFs). A total of eight regioisomers are formed that are comprised of 16 racemic diastereomers for a total of 256 compounds [18].
Fig. 3.
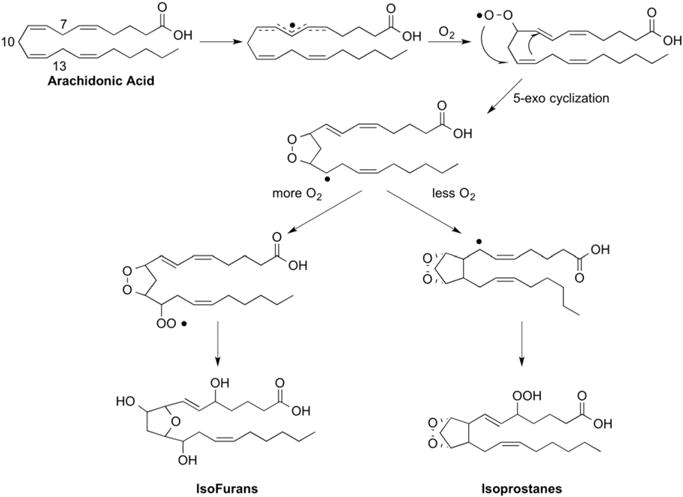
Under settings of increased oxygen tension isofurans are formed preferentially to isoprostanes from a common radical intermediate.
2.2. Isoprostane metabolism
In contrast to COX-derived PGs that are generated from free arachidonic acid, IsoPs are initially formed from arachidonic acid in situ on lipids [5,19]. Molecular modeling of IsoP-containing phospholipids reveals them to be remarkably distorted molecules. IsoPs can be released from the phospholipid backbone as free acids by phospholipase action. Platelet activating factor-acetylhydrolase (PAF-AH) has also been shown to release F2-IsoPs from phospholipids [20].
Free F2-IsoPs are found circulating in plasma, are further filtered in the kidney, and appear in the urine [21,22]. F2-IsoPs can also undergo metabolism in the liver to yield a variety of metabolites (Fig. 4) [23–29]. In humans, two major urinary metabolites of 15-F2t-IsoP (also referred to as 8-iso-PGF2α or iPF2α-III), one of the most abundant endogenous F2-IsoPs, have been identified to be 2,3-dinor-15-F2t-IsoP and 2,3-dinor-5,6-dihydro-15-F2t-IsoP [24,26,27]. Metabolism studies of eight different 15-series F2-IsoP stereoisomers have also been conducted in isolated rat hepatocytes, an accepted model of metabolism [28]. In addition to 2,3-dinor- and 2,3-dinor-5,6-dihydro metabolites, 13,14-dihydro-15-keto- and 2,3,4,5-tetranor- derivatives of 15-F2t-IsoP were identified (Fig. 4) [28]. Taurine conjugates of 15-F2t-IsoP and its various metabolites were also observed (Fig. 4) [28]. In human clinical trials, different F2-IsoP stereoisomers, including 5- and 15-F2t-IsoP, as well as 2,3-dinor-15-F2t-IsoP and 2,3-dinor-5,6-dihydro-15-F2t-IsoP have been used as biomarkers of IsoP formation in vivo.
Fig. 4.
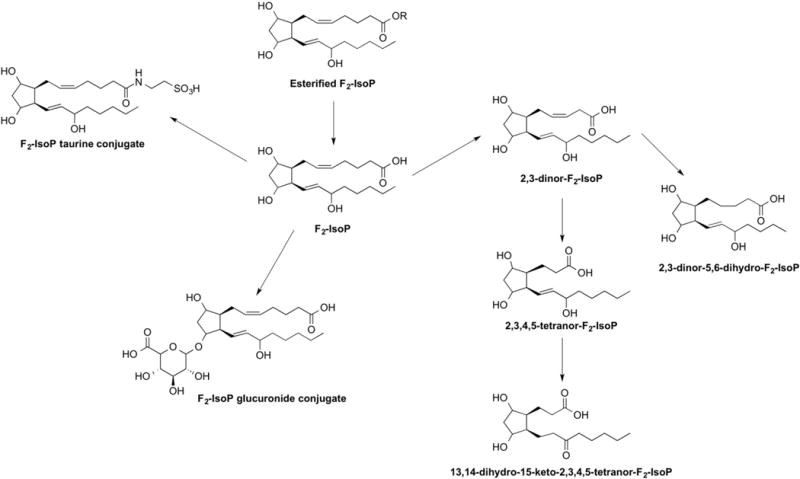
Urinary metabolites of F2-isoprostanes.
Importantly, Yan and colleagues have proposed that F2-IsoPs are excreted in the urine conjugated with glucuronide [29]. The structure of the glucuronide metabolite(s) has not been directly confirmed (proposed structure in Fig. 4), but the authors noted that levels of urinary F2-IsoPs were significantly increased after treatment of the urine with β-glucuronidase (0.43 ± 0.02 vs. 0.61 ± 0.03 nmol/mmol Cr) [29]. Typically, glucuronide conjugates are formed by reaction with free hydroxyl groups or carboxylic acid groups. Due to the similarity in structure between 15-F2t-IsoP and the 2,3-dinor- and 2,3-dinor-5,6-dihydro- metabolites, it is possible that glucuronide conjugates of 2,3-dinor-15-F2t-IsoP and 2,3-dinor-5,6-dihydro-15-F2t-IsoP could exist but these compounds have not been studied to date. Glucuronidation thus represents another route of F2-IsoP metabolism that needs to be considered when measuring these compounds in urine.
The metabolism of D2/E2-IsoPs is less well studied. As discussed above, it is known that these compounds dehydrate in vivo to yield A2/J2-IsoPs [13,14] and that A2/J2-IsoPs can be further metabolized, through rearrangement and dehydration, to yield deoxy-A2 and deoxy-J2-IsoPs [15]. The metabolism of cyclopentenone IsoPs has been studied in HepG2 cells, a cell line derived from human hepatocytes, as well as in the rat [30,31]. These molecules are rapidly metabolized by glutathione transferase enzymes yielding water-soluble modified glutathione conjugates. The metabolism of isothromboxanes and isofurans has not been studied.
3. Isoprostanes as biomarkers of oxidative stress in human disease
A major impediment in the field of free radical research has historically been the lack of specific and sensitive methods to assess endogenous oxidative damage. Over the course of the past 25 years, F2-IsoPs have been shown to be a reliable biomarker of endogenous lipid peroxidation because they are ubiquitous in the body and are chemically stable in biological fluids when stored correctly. Both animal and clinical studies have tested the reliability of this biomarker. An NIH-sponsored study, termed the Biomarkers of Oxidative Stress Study (BOSS), found that plasma F2-IsoPs are increased in a time- and dose-dependent manner in rats administered carbon tetrachloride, a toxic agent known to systematically generate free radicals in the liver [32]. Il’yasova and coworkers then conducted a follow-up study to the BOSS trial in humans using doxorubicin-based chemotherapy as the model because this drug is known to generate hydroxyl radicals at pharmacological doses [33]. Several commonly measured indices of oxidative stress were examined in 23 women newly diagnosed with breast cancer undergoing standard chemotherapy. F2-IsoPs, as well as the F2-IsoP metabolite 2,3-dinor-15-F2t-IsoP, were significantly increased in the urine 1 h following treatment and returned to baseline levels 24 h following treatment. Together these findings confirm that F2-IsoPs are reflective of endogenous lipid peroxidation following free radical production.
A multitude of papers have been published describing different analytical methods for the quantification of F2-IsoPs in biological fluids [34–58]. Three primary techniques are used to measure these compounds: (1) gas chromatography–mass spectrometry, (2) liquid chromatography–mass spectrometry, and (3) immunoassays (ELISAs). Mass spectrometric-based assays are widely accepted as the most accurate methodologies as the results of immunoassays could be confounded due to the structural similarities between F2-IsoPs and COX-derived prostaglandins as well as other related molecules [59–62]. F2-IsoPs can be measured in plasma, urine, any tissue, cerebral spinal fluid, exhaled breath condensate, amniotic fluid, and saliva. Measurement in plasma and urine is most common in humans as these fluids are the least invasive. Caution must be taken, however, when collecting and storing plasma for F2-IsoP analysis as these molecules can be generated from ex vivo oxidation of arachidonic acid in the plasma. In a recent commentary by Barden and colleagues, it is recommended to collect blood into tubes containing the anticoagulant EDTA and the antioxidants butylated hydroxytoluene (BHT) and GSH to min-imize artifactual elevation of F2-IsoPs [63]. All samples should be stored at −80 °C, not −20 °C, upon collection because autoxidation can occur at −20 °C leading to artifactual generation of F2-IsoPs ex vivo.
In addition to the collection and storage of the samples, there are several factors that are important to consider when measuring F2-IsoPs as a biomarker of oxidative stress in disease. These factors include choosing the appropriate sample matrix, the timing of sample collection, and considerations regarding the hydrolysis, metabolism, and excretion of these compounds [64]. In plasma, most clinical studies have quantified F2-IsoPs as free fatty acids; however, as mentioned previously, F2-IsoPs are found esterified in phospholipids in the plasma. Base hydrolysis of plasma lipids prior to analysis provides a measure of total F2-IsoPs in the plasma. In urine, the use of F2-IsoPs as a biomarker of systemic oxidative stress is even more complex as these molecules can be directly excreted or, as discussed above, metabolized to yield a variety of different compounds, the formation of which is regulated by numerous enzymes. In a recent commentary, Halliwell and Lee propose that in order to obtain a clear picture of F2-IsoP formation in vivo it is best to measure total F2-IsoPs (both molecules esterified in phospho-lipids and those which are unesterified) in plasma along with free and metabolized F2-IsoPs in urine [64].
Numerous excellent reviews have been written on F2-IsoPs as biomarkers of oxidative stress in human health and disease [65–94]. In the coming sections, we will highlight several recent human studies that have examined the formation of endogenous F2-IsoPs and explored their applications as biomarkers of oxidative stress.
3.1. Isoprostanes and obesity
The marked increase in the incidence of overweight and obese persons is recognized as perhaps the most serious public health issue in the United States. It is estimated that 69% of American adults over age 20 are either overweight or obese. Additionally, the incidence of overweight and obesity in children and adolescents is rising; in 2013, it was estimated that 18% of children 6–19 are either overweight or obese [95]. Both morbidity and mortality increase with excessive body weight. Multiple studies have clearly shown that levels of F2-IsoPs, as measured in the plasma or urine, increase in obese adults [74,96–101]. F2-IsoPs have been positively correlated with body mass index (BMI), waist circumference (WC), visceral fat area, and percent body fat [102–118]. Interestingly, recent epidemiological studies have implicated possible racial differences in the association of F2-IsoPs with obesity. In the Insulin Resistance Atherosclerosis Study (IRAS) three different F2-IsoP isomers along with the metabolite 2,3-dinor-15-F2t-IsoP were measured in the urine of 237 African Americans, 342 non-Hispanic whites, and 275 Hispanic whites [115]. Levels of the urinary F2-IsoPs and the F2-IsoP metabolite increased with BMI in non-Hispanic whites and His-panic whites but there was no increase with BMI observed in African Americans. A study by Warolin et al. in African American (n = 82) and white (n = 76) youth, ages 8–17 years, found plasma F2-IsoPs correlated with percent body fat and truncal fat but no correlation was found with BMI nor were racial differences observed [119]. In a population of 845 Chinese women living in Shanghai, urinary 2,3-dinor-5,6-dihydro-15-F2t-IsoP but not urinary F2-IsoPs correlated with increased in BMI [120]. Finally, in a study of 294 Canadian Artic Inuits with metabolic syndrome, plasma F2-IsoPs and IsoFs correlated positively with BMI and abdominal obesity while IsoFs alone correlated with WC [116]. Together these studies indicate that lipid peroxidation associated with oxidative stress is increased in obese populations. These studies also highlight the importance of assessing multiple IsoP entities including plasma and urine F2-IsoPs as well as their urinary metabolites and other molecular species such as IsoFs to detect pathophysiological associations with oxidative stress.
3.2. Isoprostanes and ischemia-reperfusion
It is well understood that ischemia/reperfusion (I/R) elicits formation of reactive oxygen species (ROS). F2-IsoPs have been shown to increase post-I/R in humans in disease settings [121]. Levels of F2-IsoPs have been shown to increase in patients with chronic lower limb ischemia [122], following ischemic stroke [123,124], and after aneurysm rupture [125]. Model studies have also been conducted to better understand the human response to I/R in different populations. For example, in a human model using suprasystolic inflation of an arm blood pressure cuff to safely induce localized forearm I/R, Davies and colleagues showed that levels of F2-IsoPs in the plasma increase 15 min post-I/R and remain elevated for at least 3 h [126]. Interestingly, the observed increase in F2-IsoPs is more dramatic in healthy older adults (ages 62–81) compared to healthy young adults (ages 20–33) and levels remain elevated in the older population for a longer period of time. In a follow-up study comparing physically fit older adults with unfit age-matched controls, classified based upon VO2max and maximal leg power, the F2-IsoP response to forearm I/R was lower in the physically fit group [127]. This observation is postulated to result from differences in the activity of antioxidant enzymes.
I/R injury is a major concern in certain surgical settings, such as coronary artery bypass grafting (CABG) surgery and organ transplantation, when blood flow is restored following a period of blood vessel occlusion. Multiple studies have shown increases in F2-IsoPs during CABG or cardiopulmonary bypass (CPB) surgery [121,128–131]. Importantly, this observed increase in oxidative stress is thought to contribute to postoperative complications. One such complication is acute kidney injury (AKI). AKI occurs in up to 30% of all patients undergoing CPB surgery and independently predicts associated morbidity and mortality. In a case–control study of 10 cardiac surgery AKI patients and 10 cardiac surgery risk-matched controls, Billings and colleagues determined that the increase in plasma F2-IsoPs, and IsoFs, during surgery were greater in the AKI patients in comparison to controls [132]. In a follow-up study of 445 patients undergoing cardiac surgery, it was determined that baseline F2-IsoPs as well as intraoperative levels of F2-IsoPs independently predicted AKI [114]. Pulmonary dysfunction is another common complication associated with cardiac surgery. It is particularly problematic in infants and children having surgical procedures performed to repair congenital heart defects. In a study of 20 infants (ages 3–12 months) undergoing elective stage 2 palliation on CPB to repair univentricular physiology, plasma F2-IsoPs were quantified at baseline (prior to surgical incision), 30 min after initiation of CPB, immediately after separation from CPB, and 24 h post-operatively [133]. Like adults on CPB, levels of F2-IsoPs increased significantly during surgery and returned to baseline levels 24 h following the procedure. Interestingly, higher levels of plasma F2-IsoP levels immediately after separation from CPB were correlated with decreased dynamic lung compliance. Due to the small size of this study, it was not possible to determine if F2-IsoPs can predict long-term outcomes in this population but the findings, together with the adult studies presented herein, suggest that further study of F2-IsoPs, and potentially IsoFs, as predictive biomarkers of poor outcome following CPB is warranted.
In follow-up to the finding that lipid peroxidation is increased during CABG and CPB surgeries, multiple studies have monitored treatments that could reduce levels of surgery-induced oxidative stress. For example, in a study of 20 patients scheduled for CABG surgery, Berg and colleagues showed that subjects treated with aspirin (160 mg daily) for 1 week prior to surgery experienced a less robust elevation of F2-IsoPs during surgery compared to subjects not taking aspirin [134]. Interestingly, the aspirin treated group also showed lower baseline levels of F2-IsoPs at initiation of surgery. Others have studied the effect of acetaminophen treatment on the day of CPB surgery [135]. No effect of acetaminophen on levels of urinary F2-IsoPs was noted but the drug significantly lowered the magnitude of increase in urinary IsoFs during surgery. Finally, it has been suggested that certain anesthesia agents can exert antioxidant activity. Ballester et al. recently reported a study comparing the effect of the anaesthetics sevoflurane and propofol on F2-IsoP levels in blood samples taken from the coronary sinus of 38 patients undergoing elective off-pump CABG surgery [136]. Interestingly, F2-IsoPs did not increase in the patients on sevoflurane while signifi-cant increases were noted in the group who received propofol. No other studies that we are aware of have compared the effect of the chosen an-esthesia in cardiac surgery patients. In a study of patients undergoing total knee arthroplasty (TKA), a surgery that also represents a setting of I/R, Mas and co-workers compared levels of F2-IsoPs in patients receiving either general anesthesia or spinal anesthesia [137]. All patients showed a significant increase in F2-IsoPs following TKA; however, patients receiving general anesthesia had more significant increases in F2-IsoPs than those receiving spinal anesthesia. Together, these studies indicate that procedures can be taken to help lower the resulting oxidative stress from surgery-associated I/R, but further work needs to be done in clinical populations to determine the most effective treatments to help prevent adverse clinical outcomes following surgery.
3.3. Isoprostanes and the central nervous system
Due to the high concentration of PUFA in the brain compared to other organs, lipid peroxidation is a primary outcome of free radical-induced brain injury. To that end, F2-IsoPs have been shown to increase in a number of chronic neurodegenerative diseases including Alzheimer’s disease (AD) [138–141], Huntington’s disease [142], Parkinson’s disease [143,144], and amyotrophic lateral sclerosis (ALS) [145]. Importantly, measurement of F2-IsoPs in the plasma or urine does not consistently show increases in these diseases while their measurement in cerebral spinal fluid (CSF) is more indicative of the changes in oxidative stress in the brain [146,147]. A very recent study by Montine and colleagues measured F2-IsoPs, along with biomarkers of AD, in the CSF of more than 400 healthy adults (ages 21–100) to examine the association between these biomarkers and cognitive function in relation to aging. Higher levels of F2-IsoPs in the CSF were found to be associated with poorer executive function [148]. Additionally, Guest et al. also recently determined that F2-IsoPs in the CSF also increase with age [149].
F2-IsoPs in the CSF are increased in acute brain injury as well. Corcoran et al. quantified F2-IsoPs, IsoFs, and F4-neuroprostanes (NPs), F2-IsoP-like compounds generated from the free radical-catalyzed oxidation of docosahexaenoic acid (DHA), in the CSF of patients within 24 h following aneurysmal subarachnoid hemorrhage (aSAH) or traumatic brain injury (TBI) and compared them to age-matched controls [150]. All three classes of lipid peroxidation products were increased in the CSF of aSAH patients while only IsoFs and F4-NPs were increased following TBI. Interestingly, in a separate study monitoring the production of lipid mediators in the CSF following TBI, Farius and colleagues tentatively identified levels of D2/E2-IsoPs to be elevated compared to controls [151]. This same group previously reported a dramatic increase in the levels of D2/E2-IsoPs compared to F2-IsoPs in a rat model of TBI [38]. Further, Reich et al. determined that D2/E2-IsoPs, and not F2-IsoPs, were the favored products of the IsoP pathway post-mortem brain samples from humans with AD [152,153]. As discussed at the beginning of this review, D2/E2-IsoPs are formed competitively with F2-IsoPs; lower levels of cellular reducing agents, such as GSH or α-tocopherol, may favor the formation of E2/D2-IsoPs over F2-IsoPs in the brain [154].
3.4. Isoprostanes and cancer risk
Few studies have investigated the associations between levels of F2-IsoPs and risk of cancer. One case–control study reported that the level of F2-IsoPs was increased among subjects with breast cancer compared to controls [155]. Compared to the lowest quartile, the increasing quartiles of F2-IsoPs were associated with odds ratios (OR) (95% confidence interval (95% CI)) of 1.25 (0.81–1.94), 1.53 (0.99–2.35) and 1.88 (1.23–2.88) for the 2nd, 3rd, and 4th quartile (p for trend, 0.002). In a subsequent cohort study conducted in Taiwan, high urinary excretion rates of F2-IsoPs was significantly linked to an increased risk of hepatocellular carcinoma with an OR of 2.53 (95% CI: 1.30–4.93) [156]. Two nested case–control studies were conducted within the multiethnic cohort [157,158]. One study found, when compared to the lowest tertile of urinary F2-IsoPs, the second and third tertiles of F2-IsoPs were associated with two-fold elevated risk of lung cancer among men, but not women [157]. The other study found that high urinary F2-IsoPs was not related to risk of prostate cancer [158]. In contrast, two subsequent case–control studies found that urinary levels of F2-IsoPs significantly increased in prostate cancer patients compared to controls [159,160]. A recent case–control study determined that urinary levels of F2-IsoPs were significantly higher in gastric cancer patients compared to controls [161]. In a prospective study, occurrence of adenoma was not significantly associated with urinary levels of F2-IsoPs and other three metabolites in the pathway [162]. In a very recent case–control study, the investigators found that an oxidative stress balance score which took into account of pro-oxidant and antioxidant exposure was significantly lower in colorectal adenoma patients compared to controls and this score was inversely correlated with plasma concentrations of F2-IsoPs [163].
Taken together, these case–control studies conducted so far consistently found higher levels of F2-IsoPs were associated with risk of cancer, including cancers of the breast, prostate, and stomach as well as colorectal adenoma. On the other hand, the findings from prospective studies have been few and inconsistent. The possible explanation for the inconsistent findings between case–control studies and prospective nested case–control studies is that it is not clear in case–control studies if oxidative stress leads to cancer or results from cancer.
In recent years, Dai and colleagues conducted the first study to prospectively examine both urinary F2-IsoP and the metabolite 2,3-dinor-5,6-dihydro-15-F2t-IsoP as biomarkers of breast cancer risk in a large-scale nested case–control study within the Shanghai Women’s Health Study (SWHS), a population-based cohort study of 74,942 Chinese women between 40 and 70 years of age [164]. In this study, F2-IsoPs nor 2,3-dinor-5,6-dihydro-15-F2t-IsoP [165] significantly differed by breast cancer status. Levels of F2-IsoPs and 2,3-dinor-5,6-dihydro-15-F2t-IsoP were actually related to a reduced risk of breast cancer among women with a BMI of <25. Among women with a BMI of <23, high F2-IsoPs was associated with a reduced risk of breast cancer in a dose–response manner (p for trend, 0.006) with an OR of 0.46 (95% CI: 0.26–0.80) for the highest tertile vs. the lowest (p for interaction, 0.006). This reduction in risk appeared in both pre- and postmenopausal women. In contrast, however, F2-IsoPs and 2,3-dinor-5,6-dihydro-15-F2t-IsoP were associated with an increased risk of breast cancer among women with a BMI of ≥25. The associations became stronger with increasing levels of BMI and were more significant for 2,3-dinor-5,6-dihydro-15-F2t-IsoP compared to F2-IsoPs. These findings indicate that the role of ROS in the development of breast cancer is different by BMI status. As discussed previously, numerous studies have consistently observed that overweight or obese men and women have a significantly elevated level of F2-IsoPs, indicating women with a high level of BMI have an excessive production of ROS and, thus, are at high risk of oxidative stress [97–99]. Therefore, among overweight/obese women, high levels of F2-IsoP-M and/or F2-IsoPs may be related to an increased risk of breast cancer [166]. Conversely, among women with normal BMI, basal levels of ROS are necessary to trigger p53 activation, directly mediate apoptosis and induce senescence [167]. Additionally, F2-IsoPs was found to increase the glucose-induced synthesis of TGF-β1, a critical tumor suppressor at initial stage [168]. Several protective factors for breast cancer risk, such as physical activity, parity (normal pregnancy) and preeclampsia, are also linked to significantly elevated levels of lipid peroxidation [169]. Thus, the role of F2-IsoPs, lipid peroxidation, and oxidative stress are complex in the pathophysiology of cancer.
3.5. Isoprostanes, diet, and lifestyle
In general, increased levels of F2-IsoPs are considered to be detrimental to human health. For that reason, numerous studies have focused on identifying dietary or lifestyle modifications that could alter levels of endogenous F2-IsoPs. Dietary antioxidants have naturally been an area of intense research in the field. Unfortunately, the results of published studies often vary due to inherent differences in study design including factors such as antioxidant dose, duration of dietary supplementation, matrix analyzed (ie. urine, plasma, other), and IsoP analyzed (i.e. F2-IsoPs or its urinary metabolite) [64,170]. This is particularly true for studies involving supplementation with Vitamin E (α-tocopherol), a commonly used antioxidant [170]. A dose-finding study of vitamin E actually helped to clarify the optimal parameters for studying vitamin E as it relates to the lowering of lipid peroxidation associated with oxidative stress [171]. This study determined that daily doses of 1600 IU or greater for at least 8 weeks were required to statistically reduce plasma levels of F2-IsoPs. Lower doses have also been effective at lowering F2-IsoPs in settings where levels of lipid peroxidation are expected to be elevated. For example, Block and colleagues have shown that 1000 mg/day vitamin C or 800 IU/day vitamin E for 2 months can lower levels of plasma F2-IsoPs by 22% (p = 0.01) or 9.8% (p = 0.46), respectively, when baseline levels of F2-IsoPs are high (>50 mg/ml), such as in obese populations [172]. Importantly, neither treatment decreased levels of F2-IsoPs in individuals with normal baseline levels. In other studies, pre-treatment of skin with topical vitamin E reduced levels of F2-IsoPs generated post-UV irradiation [173]. Also, supplementation with vitamin E acetate decreased both baseline and allergen-induced F2-IsoPs in the brochoalveolar lavage fluid of atopic asthmatics [174]. Dorjgochoo et al. reported that urinary excretion rates of 2,3-dinor-5,6-dihydro-15-F2t-IsoP, but not F2-IsoPs, were lower in Chinese women who used a vitamin E supplement, as determined from diet and food questionnaire responses, compared to those who did not [120]. In that same population, plasma concentrations of several antioxidants (i.e., β-carotenes, both trans and cis β-carotenes, lycopene other than trans, 5-cis and 7-cis isomers, cis anhydrolutein, and cis β-cryptoxanthin) were inversely associated with 2,3-dinor-5,6-dihydro-15-F2t-IsoP but not with F2-IsoPs, whereas β-, γ-, and δ-tocopherols were positively associated with 2,3-dinor-5,6-dihydro-15-F2t-IsoP but not with F2-IsoPs.
A variety of antioxidants other than Vitamin E have been studied in relationship to F2-IsoPs. Similarly to Vitamin E, other antioxidants tend to have the greatest effects in populations with an increased level of basal oxidative stress (ie. obese, asthmatics, etc.) than in normal healthy populations. For example, vitamin C has been shown to decrease pancreatic cancer tumor progression in pre-clinical models [175]. Results of a phase I clinical study of twice-weekly intravenous pharmacological doses of vitamin C, given in combination with gemcitabine, showed that levels of F2-IsoPs decreased post-infusion in all patients tested (n = 5) [176]. Anthocyanins, polyphenolic antioxidants typically found in dark fruits such as blackberries, strawberries, blueberries, blackcurrants, and cherries, also lower levels of F2-IsoPs [177–180]. Consumption of tart cherry juice, and not placebo, lowers the observed increase in plasma F2-IsoP levels following forearm I/R induced by blood pressure cuff inflation [177]. In another study of well-trained distance runners, subjects who consumed 250 g of blueberries per day for 6 weeks had reduced increases in plasma F2-IsoPs following a 2.5 h run compared to controls [178]. Consumption of a blackcurrant juice drink (20% juice) also reduced basal levels of F2-IsoPs in plasma in a population of healthy adults who typically consume <2 servings of fruit and vegetables per day [179].
Acetaminophen, similar to vitamin E and anthocyanins, is an electron donor capable of reducing radicals. Commonly, acetaminophen is known to modulate the activity of the COX enzyme by reducing the radical cation in the peroxidase site of the enzyme that is necessary for the synthesis of PGs. In recent studies, acetaminophen has been shown to modulate non-enzymatic lipid peroxidation [181,182]. The antioxidant properties of acetaminophen were recently studied in a human population with sepsis [183]. Levels of F2-IsoPs were significantly lower in subjects who received acetaminophen during treatment to patients who did not.
Recently, the association between diet, typically reported through the use of food questionnaires, and F2-IsoPs has been examined. In a large study of more than 5000 young and middle-aged Caucasian and African Americans followed for over the course of 20 years, diets high in fruits and vegetables and low in red meat were associated with lower levels of plasma F2-IsoPs [184]. Diets with a higher intake of red meat showed increased levels of F2-IsoPs. In a smaller study of premenopausal women (n = 258) participating in the 5 A Day for Better Health Program, women who met the daily requirement of at least five servings of fruits and vegetables had lower plasma levels of F2-IsoPs [185]. In a study of 1005 pre- and post-menopausal Chinese women, however, consumption of cruciferous vegetables was not associated with lower urinary F2-IsoPs or F2-IsoP-M [186]. Other dietary factors that reduce levels of endogenous F2-IsoPs include marine fish oil [101,187–197]. Marine fish oil is rich in the omega-3 PUFAs eicosapentaenoic acid (EPA) and DHA. Importantly, IsoP-like oxidation products can be generated from the oxidation of EPA and DHA. F3-, E3/D3-, and A3/J3-IsoPs are generated from the peroxidation of EPA; the related products produced from DHA have been identified and are termed neuroprostanes, as DHA is enriched in the brain [198–202]. Interestingly, while arachidonic acid-derived F2-IsoPs induce vasoconstriction and platelet aggregation and increase mean systemic arterial pressure, EPA-derived F3-IsoPs are less potent vasoconstrictors and do not cause platelet aggregation or an increase in arterial pressure [193,203]. The biological activities of the neuroprostanes are less well studied.
The studies highlighted herein represent the many complex factors in the diet that can affect levels of lipid peroxidation in vivo in humans. It is important to note that even caloric restriction alone has been shown to decrease systemic F2-IsoPs [113]. Thus, while quantifying levels of F2-IsoPs and/or related compounds has given great insight into the physiological and pathophysiological roles of oxidative stress in human disease, care must be taken when interpreting results from any given study.
3.6. Isoprostanes and genetic disorders
Human genetic disorders are characterized by an abnormality in an individual’s DNA. Genetic disorders can involve a mutation in a single gene or involve an entire chromosome. Oxidative stress has been implicated in the pathophysiology of multiple human genetic disorders. F2-IsoPs levels are increased in patients with autism-spectrum disorders [204,205], Smith-Lemli-Opitz Syndrome (SLOS) [206], sickle cell anemia [207], cystic fibrosis [208–210], and in various inborn errors of metabolism [211]. Interestingly, levels of F2-IsoPs are increased in the amniotic fluid of pregnancies carrying fetuses with Down syndrome [212]. Oxida-tive stress and IsoPs are well-studied in patients with Rett syndrome (RTT) by De Felice and colleagues [213–216]. RTT is a severe autism spectrum disorder caused by a mutation in the X-linked MECP2 gene that encodes the methyl-CpG-binding protein-2 in most cases. The disease primarily affects girls and is characterized by loss of acquired cognitive, social, and motor skills together with development of autistic behavior; symptoms typically appear between 6 and 18 months of age. Levels of F2-IsoPs as well as levels of F4-NPs and F2-dihomo-IsoPs, derived from adrenic acid, are increased in plasma of RTT patients [213–216]. F4-NPs correlate with disease severity and F2-dihomo-IsoPs are elevated in early disease stages. Notably, in a cohort of 21 typical RTT patients supplemented with 20–40 mg/kg marine fish oil twice daily, levels of plasma F4-NPs decreased by ~80% over the course of 12 months, with more than one-third of those patients reaching levels in the normal range compared to age-matched controls [214]. This finding that marine fish oil can reduced levels of oxidative stress biomakers in RTT patients has led to additional clinical trials in which supplementation with EPA and DHA at early stages in the disease has led to a partial clinical rescue of RTT [193].
4. Biological functions of the isoprostanes
The chemical synthesis of specific IsoPs by many different investigators around the globe has allowed the exploration of the biological activity of these molecules. Most studies have focused on the analysis of IsoPs in the form of the free fatty acid. Among the most studied of the IsoPs is 15-F2t-IsoP. Much of the work surrounding the biological activity of this molecule has focused on the cardiovascular system. Recently, Bauer and colleagues published an elegant review regarding IsoPs in the cardiovascular system [217]. Briefly, 15-F2t-IsoP is a potent vasoconstrictor in most vascular beds. Additionally, this molecule can modulate platelet activity, inhibit angiogenesis, and promote atherosclerosis by stimulating adhesion of monocytes and neutrophils to endothelial cells. These biological activities of 15-F2t-IsoP are primarily mediated through interaction with the thromboxane (TP) receptor. Similarly, 15-E2t-IsoP (also referred to as 8-iso-PGE2 or iPE2-III) mediates its functions through the TP receptor.
15-F2t-IsoP and 15-E2t-IsoP have been shown to exert biological activity through other PG receptors as well, including the PGE2 (EP2-4) receptors and PGF2α (FP) receptors [218–227]. Interestingly, the activity of the molecules can vary depending upon the receptor through which it acts. For example, while 15-F2t-IsoP and 15-E2t-IsoP are vaso-constrictors through the TP receptors, both molecules can induce vaso-dilation through the EP receptor. These opposing effects have been particularly well studied in the lung [219]. Additionally, Chen and coworkers have observed both the vasoconstrictive and vasodilatory effects of 15-F2t-IsoP and 15-E2t-IsoP in murine ductus arteriosus (DA), a central vascular shunt that constricts soon after birth, allowing redirection of blood flow from the placenta to the lungs [228]. These authors showed that isolated term murine DA constricts in response to both 15-F2t-IsoP and 15-E2t-IsoP in a concentration-dependent manner and that this effect could be reversed by administration of a TP receptor antagonist. Interestingly, when TP receptors were blocked prior to IsoP exposure, both 15-F2t-IsoP and 15-E2t-IsoP produced potent vasorelaxation of the term DA. This action was determined to be due to activation of the EP4 receptor by the IsoPs. Further, the authors noted a difference between the actions of 15-F2t-IsoP and 15-E2t-IsoP in term and preterm isolated DA. Whereas 15-F2t-IsoP induced vasoconstriction in both the term and preterm DA via the TP receptor, 15-E2t-IsoP induced a dose-dependent vasodilation in the preterm DA and an EP4 receptor antagonist could reverse this effect. The authors explained that the response of the term and preterm vessels could be explained by a gestational shift in expression of the target receptors as EP4 receptors expression is upregulated in the preterm DA and maintained through birth while expression of the TP receptor is low in the preterm DA. In addition to these mouse studies, 15-F2t-IsoP and 15-E2t-IsoP were shown to be potent dose-dependent constrictors of the DA in chicken embryos, again acting through the TP receptor, and, interestingly, the presence of H2O2 can modify this response [229,230]. Historically, COX-derived PGs have been thought to play a major role in postnatal DA homeostasis, however, these findings suggest that IsoPs could play a role in the mediation of DA constriction. In related work, Comporti and colleagues found that F2-IsoPs levels are significantly higher in neonates than in older infants and further are inversely correlated with gestational age [231]. More recently, this group has shown that levels of F2-IsoPs were significantly decreased in a study of 43 preterm infants (gestational age <33 weeks) being treated with ibuprofen to induce DA closure. The authors suggest that ibuprofen is dual-acting as both an antioxidant and COX inhibitor and could be beneficial in the treatment of this at-risk population. Further studies are required to confirm these results and explore the pathophysiology of IsoPs in newborns where the DA fails to close.
In addition to the studies described above, 15-F2t-IsoP and 15-E2t-IsoP have been shown to produce hyperalgesia, an increased sensitivity to pain. 15-E2t-IsoP, when injected into the hindpaw of rats, was shown to significantly reduce the withdrawal threshold in response to both mechanical and thermal stimuli, while 15-F2t-IsoP reduced the withdrawal threshold to mechanical stimuli alone [232]. Further, 15-E2t-IsoP enhanced the release of transmitters from isolated rodent sensory neurons; this effect could not be reversed by the treatment of COX inhibitors. Additionally, 15-F2t-IsoP and 15-E2t-IsoP enhanced the firing of C-nociceptors in pentobarbital-anesthetized rats [233]. Together these findings broaden our understanding of IsoP biology and pave the way for new areas of research regarding potential consequences of oxidative stress and lipid peroxidation.
The biological activities of other IsoPs have been explored as well. A2/J2-IsoPs, dehydration products of D2/E2-IsoPs, are reactive electro-philes that readily form Michael adducts with cellular thiols due to the presence of an α,β-unsaturated cyclopentenone ring structure. Current knowledge regarding the biological actions of these molecules has been reviewed elsewhere [70,87,234–238]. The biological activity of isothromboxanes and isofurans has not been studied.
5. A look towards the future
The discovery of the IsoPs as products of non-enzymatic lipid peroxidation has been a major contribution to the field of lipid oxidation and free radical research. Twenty-five years after their initial discovery in human plasma, our knowledge regarding the formation of these potent molecules continues to expand, providing new insights into the nature of lipid peroxidation in vivo and revealing new biological activities. Basic research into the biochemistry, pharmacology, and metabolism of the IsoPs, coupled with clinical studies employing these molecules as biomarkers, will continue to provide important insights into the role of oxidant stress in human physiology and pathophysiology.
Acknowledgments
The authors would like to acknowledge support from GM15431 (L.J.R. and G.L.M.), ES000267 (L.J.R. and G.L.M), DK020593 (G.L.M.), and CA106591 (Q.D.).
Abbreviations
- AD
Alzheimer’s disease
- AKI
Acute kidney injury
- aSAH
Aneurysmal subarachnoid hemorrhage
- BMI
Body mass index
- CAPG
Coronary artery bypass grafting
- COX
Cyclooxygenase
- CPB
Cardiopulmonary bypass
- CSF
Cerebral spinal fluid
- DA
Ductus arteriosus
- DHA
Docosahexaenoic acid
- EP
Prostaglandin E2 receptor
- EPA
Eicosapentaenoic acid
- GSH
Glutathione
- I/R
Ischemic-reperfusion
- IsoF
Isofuran
- IsoP
Isoprostane
- NP
Neuroprostane
- PAF
Platelet activating factor
- PG
Prostaglandin
- PUFA
Polyunsaturated fatty acid
- ROS
Reactive oxygen species
- RTT
Rett syndrome
- TBI
Traumatic brain injury
- TP
Thromboxane receptor
- WC
Waist circumference
Footnotes
This article is part of a Special Issue entitled “Oxygenated metabolism of PUFA: analysis and biological relevance”.
References
- 1.Morrow JD, Harris TM, Roberts LJ., II Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 2.Pryor WA, Stanley JP. Letter: a suggested mechanism for the production of mal-onaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40:3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 3.Liston TE, Oates JA, Roberts LJ., II Prostaglandin D2 is metabolized in humans to 9 alpha,11 beta-prostaglandin F2, a novel biologically active prostaglandin. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:365–367. [PubMed] [Google Scholar]
- 4.Wendelborn DF, Seibert K, Roberts LJ., II Isomeric prostaglandin F2 compounds arising from prostaglandin D2: a family of icosanoids produced in vivo in humans. Proc Natl Acad Sci U S A. 1988;85:304–308. doi: 10.1073/pnas.85.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., II A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrow JD, Awad JA, Kato T, Takahashi K, Badr KF, Roberts LJ, II, Burk RF. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992;90:2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow JD, Roberts LJ., II Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Methods Enzymol. 1994;233:163–174. doi: 10.1016/s0076-6879(94)33019-0. [DOI] [PubMed] [Google Scholar]
- 8.Taber DF, Morrow JD, Roberts LJ., II A nomenclature system for the isoprostanes. Prostaglandins. 1997;53:63–67. doi: 10.1016/s0090-6980(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 9.Rokach J, Khanapure SP, Hwang SW, Adiyaman M, Lawson JA, FitzGerald GA. Nomenclature of isoprostanes: a proposal. Prostaglandins. 1997;54:853–873. doi: 10.1016/s0090-6980(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 10.Yin H, Morrow JD, Porter NA. Identification of a novel class of endoperoxides from arachidonate autoxidation. J Biol Chem. 2004;279:3766–3776. doi: 10.1074/jbc.M307137200. [DOI] [PubMed] [Google Scholar]
- 11.Morrow JD, Minton TA, Mukundan CR, Campbell MD, Zackert WE, Daniel VC, Badr KF, Blair IA, Roberts LJ., II Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J Biol Chem. 1994;269:4317–4326. [PubMed] [Google Scholar]
- 12.Morrow JD, Roberts LJ, Daniel VC, Awad JA, Mirochnitchenko O, Swift LL, Burk RF. Comparison of formation of D2/E2-isoprostanes and F2-isoprostanes in vitro and in vivo–effects of oxygen tension and glutathione. Arch Biochem Biophys. 1998;353:160–171. doi: 10.1006/abbi.1998.0645. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Zackert WE, Roberts LJ, II, Morrow JD. Evidence for the formation of a novel cyclopentenone isoprostane, 15-A2t-isoprostane (8-iso-prostaglandin A2) in vivo. Biochim Biophys Acta. 1999;1436:550–556. doi: 10.1016/s0005-2760(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Morrow JD, Roberts LJ., II Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J Biol Chem. 1999;274:10863–10868. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- 15.Hardy KD, Cox BE, Milne GL, Yin H, Roberts LJ., II Nonenzymatic free radical-catalyzed generation of 15-deoxy-Δ(12,14)-prostaglandin J2-like compounds (deoxy-J2-isoprostanes) in vivo. J Lipid Res. 2011;52:113–124. doi: 10.1194/jlr.M010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow JD, Awad JA, Wu A, Zackert WE, Daniel VC, Roberts LJ., II Nonen-zymatic free radical-catalyzed generation of thromboxane-like compounds (isothromboxanes) in vivo. J Biol Chem. 1996;271:23185–23190. doi: 10.1074/jbc.271.38.23185. [DOI] [PubMed] [Google Scholar]
- 17.Taber DF, Fessel JP, Roberts LJ., II A nomenclature system for isofurans. Prostaglandins Lipid Mediat. 2004;73(1–2):47–50. doi: 10.1016/j.prostaglandins.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., II Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayganich-Harrison KA, Rose DM, Murphy RC, Morrow JD, Roberts LJ., II Collision-induced dissociation of F2-isoprostane-containing phospholipids. J Lipid Res. 1993;34:1229–1235. [PubMed] [Google Scholar]
- 20.Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, Bonventre JV, Prescott SM, Roberts LJ., II Release of free F2-isoprostanes from ester-ified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J Biol Chem. 2006;281:4616–4623. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 21.Praticò D, Barry OP, Lawson JA, Adiyaman M, Hwang SW, Khanapure SP, Iuliano L, Rokach J, FitzGerald GA. iPF2alpha-I: an index of lipid peroxidation in humans. Proc Natl Acad Sci U S A. 1998;95:3449–3454. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Lawson JA, Reilly M, Adiyaman M, Hwang SW, Rokach J, FitzGerald GA. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F2-isoprostanes in human urine. Proc Natl Acad Sci U S A. 1999;96:13381–13386. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awad JA, Morrow JD, Takahashi K, Roberts LJ., II Identification of non-cyclooxygenase-derived prostanoid (F2-isoprostane) metabolites in human urine and plasma. J Biol Chem. 1993;268:4161–4169. [PubMed] [Google Scholar]
- 24.Roberts LJ, II, Moore KP, Zackert WE, Oates JA, Morrow JD. Identification of the major urinary metabolite of the F2-isoprostane 8-iso-prostaglandin F2alpha in humans. J Biol Chem. 1996;271:20617–20620. doi: 10.1074/jbc.271.34.20617. [DOI] [PubMed] [Google Scholar]
- 25.Basu S. Metabolism of 8-iso-prostaglandin F2alpha. FEBS Lett. 1998;428:32–36. doi: 10.1016/s0014-5793(98)00481-5. [DOI] [PubMed] [Google Scholar]
- 26.Schwedhelm E, Tsikas D, Durand T, Gutzki FM, Guy A, Rossi JC, Frölich JC. Tandem mass spectrometric quantification of 8-iso-prostaglandin F2alpha and its metabolite 2,3-dinor-5,6-dihydro-8-iso-prostaglandin F2alpha in human urine. J Chromatogr B Biomed Sci Appl. 2000;744:99–112. doi: 10.1016/s0378-4347(00)00236-x. [DOI] [PubMed] [Google Scholar]
- 27.Nourooz-Zadeh J, Cooper MB, Ziegler D, Betteridge DJ. Urinary 8-epi-PGF2alpha and its endogenous beta-oxidation products (2,3-dinor and 2,3-dinor-5,6-dihydro) as biomarkers of total body oxidative stress. Biochem Biophys Res Commun. 2005;330:731–736. doi: 10.1016/j.bbrc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Chiabrando C, Rivalta C, Bagnati R, Valagussa A, Durand T, Guy A, Villa P, Rossi JC, Fanelli R. Identification of metabolites from type III F2-isoprostane diaste-reoisomers by mass spectrometry. J Lipid Res. 2002;43:495–509. [PubMed] [Google Scholar]
- 29.Yan Z, Mas E, Mori TA, Croft KD, Barden AE. A significant proportion of F2-isoprostanes in human urine are excreted as glucuronide conjugates. Anal Biochem. 2010;403:126–128. doi: 10.1016/j.ab.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Milne GL, Zanoni G, Porta A, Sasi S, Vidari G, Musiek ES, Freeman ML, Morrow JD. The cyclopentenone product of lipid peroxidation, 15-A2t-isoprostane, is efficiently metabolized by HepG2 cells via conjugation with glutathione. Chem Res Toxicol. 2004;17:17–25. doi: 10.1021/tx034213o. [DOI] [PubMed] [Google Scholar]
- 31.Milne GL, Gao L, Porta A, Zanoni G, Vidari G, Morrow JD. Identification of the major urinary metabolite of the highly reactive cyclopentenone isoprostane 15-A(2 t)-isoprostane in vivo. J Biol Chem. 2005;280:25178–25184. doi: 10.1074/jbc.M502891200. [DOI] [PubMed] [Google Scholar]
- 32.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, II, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Thiel DHVan, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Il’yasova D, Spasojevic I, Wang F, Tolun AA, Base K, Young SP, Marcom PK, Marks J, Mixon G, DiGiulio R, Millington DS. Urinary biomarkers of oxidative status in a clinical model of oxidative assault. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1506–1510. doi: 10.1158/1055-9965.EPI-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masoodi M, Nicolaou A. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3023–3029. doi: 10.1002/rcm.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sircar D, Subbaiah PV. Isoprostane measurement in plasma and urine by liquid chromatography-mass spectrometry with one-step sample preparation. Clin Chem. 2007;53:251–258. doi: 10.1373/clinchem.2006.074989. [DOI] [PubMed] [Google Scholar]
- 36.Mas E, Michel F, Guy A, Bultel V, Falquet Y, Chardon P, Rossi JC, Cristol JP, Durand T. Quantification of urinary F2-isoprostanes with 4(RS)-F4t-neuroprostane as an internal standard using gas chromatography–mass spectrometry Application to polytraumatized patients. J Chromatogr B Anal Technol Biomed Life Sci. 2008;872:133–140. doi: 10.1016/j.jchromb.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Taylor AW, Bruno RS, Traber MG. Women and smokers have elevated urinary F2-isoprostane metabolites: a novel extraction and LC-MS methodology. Lipids. 2008;43:925–936. doi: 10.1007/s11745-008-3222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC. Formation of eicosanoids, E2/D2-isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J Lipid Res. 2008;49:1990–2000. doi: 10.1194/jlr.M800200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesaros C, Lee SH, Blair IA. Targeted quantitative analysis of eicosanoid lipids in biological samples using liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877:2736–2745. doi: 10.1016/j.jchromb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiswedel I. F2-isoprostanes: sensitive biomarkers of oxidative stress in vitro and in vivo: a gas chromatography–mass spectrometric approach. Methods Mol Biol. 2009;580:3–16. doi: 10.1007/978-1-60761-325-1_1. [DOI] [PubMed] [Google Scholar]
- 41.Taylor AW, Traber MG. Quantitation of plasma total 15-series F2-isoprostanes by sequential solid phase and liquid-liquid extraction. Anal Biochem. 2010;396:319–321. doi: 10.1016/j.ab.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 42.Cavalca V, Minardi F, Scurati S, Guidugli F, Squellerio I, Veglia F, Dainese L, Guarino A, Tremoli E, Caruso D. Simultaneous quantification of 8-iso-prostaglandin-F(2alpha) and 11-dehydro thromboxane B(2) in human urine by liquid chromatography-tandem mass spectrometry. Anal Biochem. 2010;397:168–174. doi: 10.1016/j.ab.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Mas E, Barden A, Durand T, Galano JM, Croft KD, Mori TA. Measurement of urinary F2-isoprostanes by gas chromatography–mass spectrometry is confounded by interfering substances. Free Radic Res. 2010;44:191–198. doi: 10.3109/10715760903390838. [DOI] [PubMed] [Google Scholar]
- 44.Callewaert DM, Sloan C. Enzyme immunoassay of isoprostanes. Methods Mol Biol. 2010;610:435–449. doi: 10.1007/978-1-60327-029-8_26. [DOI] [PubMed] [Google Scholar]
- 45.Langhorst ML, Hastings MJ, Yokoyama WH, Hung SC, Cellar N, Kuppannan K, Young SA. Determination of F2-isoprostanes in urine by online solid phase extraction coupled to liquid chromatography with tandem mass spectrometry. J Agric Food Chem. 2010;58:6614–6620. doi: 10.1021/jf101146q. [DOI] [PubMed] [Google Scholar]
- 46.Korecka M, Clark CM, Lee VM, Trojanowski JQ, Shaw LM. Simultaneous HPLC-MS-MS quantification of 8-iso-PGF(2alpha) and 8,12-iso-iPF(2alpha) in CSF and brain tissue samples with on-line cleanup. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878:2209–2216. doi: 10.1016/j.jchromb.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastani NE, Gundersen TE, Blomhoff R. Dried blood spot (DBS) sample collection for determination of the oxidative stress biomarker 8-epi-PGF(2α) in humans using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2012;26:645–652. doi: 10.1002/rcm.6149. [DOI] [PubMed] [Google Scholar]
- 48.Casetta B, Longini M, Proietti F, Perrone S, Buonocore G. Development of a fast and simple LC-MS/MS method for measuring the F2-isoprostanes in newborns. J Matern Fetal Neonatal Med. 2012;25(Suppl. 1):114–118. doi: 10.3109/14767058.2012.664856. [DOI] [PubMed] [Google Scholar]
- 49.Janicka M, Kubica P, Kot-Wasik A, Kot J, Namieśnik J. Sensitive determination of isoprostanes in exhaled breath condensate samples with use of liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2012;893–894:144–149. doi: 10.1016/j.jchromb.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Measurement of F2-isoprostanes and isofurans using gas chromatography–mass spectrometry. Free Radic Biol Med. 2013;59:36–44. doi: 10.1016/j.freeradbiomed.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasain JK, Arabshahi A, Taub PR, Sweeney S, Moore R, Sharer JD, Barnes S. Simultaneous quantification of F2-isoprostanes and prostaglandins in human urine by liquid chromatography tandem-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2013;913–914:161–168. doi: 10.1016/j.jchromb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brose SA, Baker AG, Golovko MY. A fast one-step extraction and UPLC-MS/MS analysis for E2/D2 series prostaglandins and isoprostanes. Lipids. 2013;48:411–419. doi: 10.1007/s11745-013-3767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larose J, Julien P, Bilodeau JF. Analysis of F2-isoprostanes in plasma of pregnant women by HPLC-MS/MS using a column packed with core-shell particles. J Lipid Res. 2013;54:1505–1511. doi: 10.1194/jlr.D034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balgoma D, Larsson J, Rokach J, Lawson JA, Daham K, Dahlén B, Dahlén SE, Wheelock CE. Quantification of lipid mediator metabolites in human urine from asthma patients by electrospray ionization mass spectrometry: controlling matrix effects. Anal Chem. 2013;85:7866–7874. doi: 10.1021/ac401461b. [DOI] [PubMed] [Google Scholar]
- 55.Briskey DR, Wilson GR, Fassett RG, Coombes JS. Optimized method for quantification of total F2-isoprostanes using gas chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2014;90:161–166. doi: 10.1016/j.jpba.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y, Zhu M, Li Z, Sa R, Chu Q, Zhang Q, Zhang H, Tang W, Zhang M, Yin H. Mass spectrometry-based metabolomic profiling identifies alterations in salivary redox status and fatty acid metabolism in response to inflammation and oxidative stress in periodontal disease. Free Radic Biol Med. 2014;70:223–232. doi: 10.1016/j.freeradbiomed.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 57.Kuligowski J, Escobar J, Quintás G, Lliso I, Torres-Cuevas I, Nuñez A, Cubells E, Rook D, van Goudoever JB, Vento M. Analysis of lipid peroxidation biomarkers in extremely low gestational age neonate urines by UPLC-MS/MS. Anal Bioanal Chem. 2014 May 11;18:4345–4356. doi: 10.1007/s00216-014-7824-6. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, Il’yasova D, Sztaray J, Young SP, Wang F, Millington DS. Quantification of the oxidative damage biomarker 2,3-dinor-8-isoprostaglandin-F(2alpha) in human urine using liquid chromatography-tandem mass spectrometry. Anal Biochem. 2010;399:302–304. doi: 10.1016/j.ab.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Il’yasova D, Morrow JD, Ivanova A, Wagenknecht LE. Epidemiological marker for oxidant status: comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann Epidemiol. 2004;14:793–797. doi: 10.1016/j.annepidem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Tsikas D, Suchy MT. Assessment of urinary F2-isoprostanes in experimental and clinical studies: mass spectrometry versus ELISA. Hypertension. 2012;60:e14. doi: 10.1161/HYPERTENSIONAHA.112.199315. author reply e15. [DOI] [PubMed] [Google Scholar]
- 61.Klawitter J, Haschke M, Shokati T, Klawitter J, Christians U. Quantification of 15-F2t-isoprostane in human plasma and urine: results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun Mass Spectrom. 2011;25:463–468. doi: 10.1002/rcm.4871. [DOI] [PubMed] [Google Scholar]
- 62.Soffler C, Campbell VL, Hassel DM. Measurement of urinary F2-isoprostanes as markers of in vivo lipid peroxidation: a comparison of enzyme immunoassays with gas chromatography–mass spectrometry in domestic animal species. J Vet Diagn Invest. 2010;22:200–209. doi: 10.1177/104063871002200205. [DOI] [PubMed] [Google Scholar]
- 63.Barden AE, Mas E, Croft KD, Phillips M, Mori TA. Minimizing artifactual elevation of lipid peroxidation products (F2-isoprostanes) in plasma during collection and storage. Anal Biochem. 2014;449:129–131. doi: 10.1016/j.ab.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 64.Halliwell B, Lee CY. Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues. Antioxid Redox Signal. 2010;13:145–156. doi: 10.1089/ars.2009.2934. [DOI] [PubMed] [Google Scholar]
- 65.Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxi-dation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol Sci. 2002;23:360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 66.Cracowski JL, Durand T. Cardiovascular pharmacology and physiology of the isoprostanes, Fundam. Clin Pharmacol. 2006;20:417–427. doi: 10.1111/j.1472-8206.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 67.Montuschi P, Barnes P, Roberts LJ., II Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–717. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 68.Montine TJ, Quinn J, Kaye J, Morrow JD. F2-isoprostanes as biomarkers of late-onset Alzheimer’s disease. J Mol Neurosci. 2007;33:114–119. doi: 10.1007/s12031-007-0044-1. [DOI] [PubMed] [Google Scholar]
- 69.Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L, II, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 70.Comporti M, Signorini C, Arezzini B, Vecchio D, Monaco B, Gardi C. F2-isoprostanes are not just markers of oxidative stress. Free Radic Biol Med. 2008;44:247–256. doi: 10.1016/j.freeradbiomed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Yin H. New techniques to detect oxidative stress markers: mass spectrometry-based methods to detect isoprostanes as the gold standard for oxidative stress in vivo. Biofactors. 2008;34:109–124. doi: 10.1002/biof.5520340203. [DOI] [PubMed] [Google Scholar]
- 72.Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janssen LJ. Isoprostanes and lung vascular pathology. Am J Respir Cell Mol Biol. 2008;39:383–389. doi: 10.1165/rcmb.2008-0109TR. [DOI] [PubMed] [Google Scholar]
- 74.Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal. 2008;10:1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- 75.Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202:321–329. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Nourooz-Zadeh J. Key issues in F2-isoprostane analysis. Biochem Soc Trans. 2009;36:1060–1065. doi: 10.1042/BST0361060. [DOI] [PubMed] [Google Scholar]
- 77.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 78.Belik J, González-Luis GE, Perez-Vizcaino F, Villamor E. Isoprostanes in fetal and neonatal health and disease. Free Radic Biol Med. 2010;48:177–188. doi: 10.1016/j.freeradbiomed.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 79.Yin H, Gao F, An Y. The application of LC-MS/MS in measurement of oxidative stress parameters. Wei Sheng Yan Jiu. 2009;38:757–761. [PubMed] [Google Scholar]
- 80.Basu S. Fatty acid oxidation and isoprostanes: oxidative strain and oxidative stress. Prostaglandins Leukot Essent Fat Acids. 2010;82:219–225. doi: 10.1016/j.plefa.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 81.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Durand T, Bultel-Poncé V, Guy A, Fangour SEl, Rossi JC, Galano JM. Isoprostanes and phytoprostanes: bioactive lipids. Biochimie. 2011;93:52–60. doi: 10.1016/j.biochi.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Basu S. Bioactive eicosanoids: role of prostaglandin F(2α) and F2-isoprostanes in inflammation and oxidative stress related pathology. Mol Cells. 2010;30:383–391. doi: 10.1007/s10059-010-0157-1. [DOI] [PubMed] [Google Scholar]
- 84.Davies SS, Roberts LJ., II F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med. 2011;50:559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Voynow JA, Kummarapurugu A. Isoprostanes and asthma. Biochim Biophys Acta. 2011;1810:1091–1095. doi: 10.1016/j.bbagen.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 87.Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ., II Isoprostane generation and function. Chem Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Il’yasova D, Scarbrough P, Spasojevic I. Urinary biomarkers of oxidative status. Clin Chim Acta. 2012;413:1446–1453. doi: 10.1016/j.cca.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang ZJ. Systematic review on the association between F2-isoprostanes and cardiovascular disease. Ann Clin Biochem. 2013;50(Pt 2):108–114. doi: 10.1258/acb.2012.011263. [DOI] [PubMed] [Google Scholar]
- 90.Jacob KD, Hooten NNoren, Trzeciak AR, Evans MK. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech Ageing Dev. 2013;134:139–157. doi: 10.1016/j.mad.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lowe FJ, Luettich K, Gregg EO. Lung cancer biomarkers for the assessment of modified risk tobacco products: an oxidative stress perspective. Biomarkers. 2013;18:183–195. doi: 10.3109/1354750X.2013.777116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niki E. Biomarkers of lipid peroxidation in clinical material. Biochim Biophys Acta. 2014;1840:809–817. doi: 10.1016/j.bbagen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 93.Signorini C, De Felice C, Durand T, Oger C, Galano JM, Leoncini S, Pecorelli A, Valacchi G, Ciccoli L, Hayek J. Isoprostanes and 4-hydroxy-2-nonenal: markers or mediators of disease? Focus on Rett syndrome as a model of autism spectrum disorder. Oxidative Med Cell Longev. 2013;2013:343824. doi: 10.1155/2013/343824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of ox-idative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.http://www.cdc.gov/nchs/fastats/obesity-overweight.htm.
- 96.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 97.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes. 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 98.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 99.Dow CA, Wertheim BC, Patil BS, Thomson CA. Daily consumption of grapefruit for 6 weeks reduces urine F2-isoprostanes in overweight adults with high baseline values but has no effect on plasma high-sensitivity C-reactive protein or soluble vascular cellular adhesion molecule 1. J Nutr. 2013;143:1586–1592. doi: 10.3945/jn.113.175166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Framingham study. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 101.Kaikkonen JE, Vilppo T, Asikainen J, Voutilainen S, Kurl S, Salonen JT. Fatty acids as determinants of in-vivo lipid peroxidation: the EFFGE study in Eastern Finnish hypertensive and non-hypertensive subjects. Ann Med. 2013;45:455–464. doi: 10.3109/07853890.2013.809915. [DOI] [PubMed] [Google Scholar]
- 102.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxida-tive stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 103.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, Brown L, Teague GW, Holguin F. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framing-ham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 105.Redhage LA, Shintani A, Haas DW, Emeagwali N, Markovic M, Oboho I, Mwenya C, Erdem H, Acosta EP, Morrow JD, Hulgan T. Clinical factors associated with plasma F2-isoprostane levels in HIV-infected adults. HIV Clin Trials. 2009;10:181–192. doi: 10.1310/hct1003-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dai Q, Gao YT, Shu XO, Yang G, Milne G, Cai Q, Wen W, Rothman N, Cai H, Li H, Xiang Y, Chow WH, Zheng W. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women’s Health Study. J Clin Oncol. 2009;27:2482–2488. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crist BL, Alekel DL, Ritland LM, Hanson LN, Genschel U, Reddy MB. Association of oxidative stress, iron, and centralized fat mass in healthy postmenopausal women. J Women’s Health (Larchmt) 2009;18:795–801. doi: 10.1089/jwh.2008.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tadros TM, Massaro JM, Rosito GA, Hoffmann U, Vasan RS, Larson MG, Keaney JF, Jr, Lipinska I, Meigs JB, Kathiresan S, O’Donnell CJ, Fox CS, Benjamin EJ. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010;18:1039–1045. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Araki S, Dobashi K, Yamamoto Y, Asayama K, Kusuhara K. Increased plasma isoprostane is associated with visceral fat, high molecular weight adiponectin, and metabolic complications in obese children. Eur J Pediatr. 2010;169:965–970. doi: 10.1007/s00431-010-1157-z. [DOI] [PubMed] [Google Scholar]
- 110.Pfeuffer M, Fielitz K, Laue C, Winkler P, Rubin D, Helwig U, Giller K, Kammann J, Schwedhelm E, Böger RH, Bub A, Bell D, Schrezenmeir J. CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J Am Coll Nutr. 2011;30:19–28. doi: 10.1080/07315724.2011.10719940. [DOI] [PubMed] [Google Scholar]
- 111.Kanaya AM, Wassel CL, Stoddard PJ, Harris TB, Cummings SR, Kritchevsky SB, Goodpaster BH, Green C, Satterfield S, Gross MD. F2-isoprostanes and adiposity in older adults. Obesity (Silver Spring) 2011;19:861–867. doi: 10.1038/oby.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dorjgochoo T, Gao YT, Chow WH, Shu XO, Yang G, Cai Q, Rothman N, Cai H, Li H, Deng X, Shrubsole MJ, Murff H, Milne G, Zheng W, Dai Q. Obesity, age, and oxidative stress in middle-aged and older women. Antioxid Redox Signal. 2011;14:2453–2460. doi: 10.1089/ars.2010.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buchowski MS, Hongu N, Acra S, Wang L, Warolin J, Roberts LJ., II Effect of modest caloric restriction on oxidative stress in women, a randomized trial. PLoS One. 2012;7:e47079. doi: 10.1371/journal.pone.0047079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Billings FT, IV, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, Brown NJ. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. 2012;23:1221–1228. doi: 10.1681/ASN.2011090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB, Jr, Wagenknecht LE. Racial differences in urinary F2-isoprostane levels and the cross-sectional association with BMI. Obesity (Silver Spring) 2012;20:2147–2150. doi: 10.1038/oby.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alkazemi D, Egeland GM, Roberts LJ, II, Kubow S. Isoprostanes and isofurans as non-traditional risk factors for cardiovascular disease among Canadian Inuit. Free Radic Res. 2012;46:1258–1266. doi: 10.3109/10715762.2012.702900. [DOI] [PubMed] [Google Scholar]
- 117.Dennis BA, Ergul A, Gower BA, Allison JD, Davis CL. Oxidative stress and cardiovascular risk in overweight children in an exercise intervention program. Child Obes. 2013;9:15–21. doi: 10.1089/chi.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crist MB, Melekhin VV, Bian A, Shintani A, Milne GL, Kallianpur AR, Dageforde LA, Haas DW, Hulgan T. Higher serum iron is associated with increased oxidant stress in HIV-infected men. J Acquir Immune Defic Syndr. 2013;64:367–373. doi: 10.1097/QAI.0b013e3182a60f36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Warolin J, Coenen KR, Kantor JL, Whitaker LE, Wang L, Acra SA, Roberts LJ, II, Buchowski MS. The relationship of oxidative stress, adiposity and metabolic risk factors in healthy Black and White American youth. Pediatr Obes. 2014;9:43–52. doi: 10.1111/j.2047-6310.2012.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dorjgochoo T, Gao YT, Chow WH, Shu XO, Yang G, Cai Q, Rothman N, Cai H, Li H, Deng X, Franke A, Roberts LJ, Milne G, Zheng W, Dai Q. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am J Clin Nutr. 2012;96:405–414. doi: 10.3945/ajcn.112.034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sakamoto H, Corcoran TB, Laffey JG, Shorten GD. Isoprostanes–markers of ischaemia reperfusion injury. Eur J Anaesthesiol. 2002;19:550–559. doi: 10.1017/s0265021502000893. [DOI] [PubMed] [Google Scholar]
- 122.Rossi P, Riutta A, Kuukasjärvi P, Vehmas T, Mucha I, Salenius JP. Revascularization decreases 8-isoprostaglandin F2alpha excretion in chronic lower limb ische-mia, Prostaglandins Leukot. Essent Fat Acids. 2004;71(2):97–101. doi: 10.1016/j.plefa.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 123.Kelly PJ, Morrow JD, Ning M, Koroshetz W, Lo EH, Terry E, Milne GL, Hubbard J, Lee H, Stevenson E, Lederer M, Furie KL. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. 2008;39(1):100–104. doi: 10.1161/STROKEAHA.107.488189. [DOI] [PubMed] [Google Scholar]
- 124.Seet RC, Lee CY, Chan BP, Sharma VK, Teoh HL, Venketasubramanian N, Lim EC, Chong WL, Looi WF, Huang SH, Ong BK, Halliwell B. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke. 2011;42(8):2326–2329. doi: 10.1161/STROKEAHA.111.618835. [DOI] [PubMed] [Google Scholar]
- 125.Lindsay TF, Luo XP, Lehotay DC, Rubin BB, Anderson M, Walker PM, Romaschin AD. Ruptured abdominal aortic aneurysm, a “two-hit” ischemia/reperfusion injury: evidence from an analysis of oxidative products. J Vasc Surg. 1999;30(2):219–228. doi: 10.1016/s0741-5214(99)70131-x. [DOI] [PubMed] [Google Scholar]
- 126.Davies SS, Traustadóttir T, Stock AA, Ye F, Shyr Y, Harman SM, Roberts LJ., II Ischemia/reperfusion unveils impaired capacity of older adults to restrain oxidative insult. Free Radic Biol Med. 2009;47(7):1014–1018. doi: 10.1016/j.freeradbiomed.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Traustadóttir T, Davies SS, Su Y, Choi L, Brown-Borg HM, Roberts LJ, II, Harman SM. Oxidative stress in older adults: effects of physical fitness. Age (Dordr) 2012;34:969–982. doi: 10.1007/s11357-011-9277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ulus AT, Aksoyek A, Ozkan M, Katircioglu SF, Basu S. Cardiopulmonary bypass as a cause of free radical-induced oxidative stress and enhanced blood-borne isoprostanes in humans. Free Radic Biol Med. 2003;34:911–917. doi: 10.1016/s0891-5849(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 129.Zhang SH, Wang SY, Yao SL. Antioxidative effect of propofol during cardiopulmonary bypass in adults. Acta Pharmacol Sin. 2004;25:334–340. [PubMed] [Google Scholar]
- 130.Caputo M, Mokhtari A, Rogers CA, Panayiotou N, Chen Q, Ghorbel MT, Angelini GD, Parry AJ. The effects of normoxic versus hyperoxic cardiopulmonary bypass on oxidative stress and inflammatory response in cyanotic pediatric patients undergoing open cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2009;138:206–214. doi: 10.1016/j.jtcvs.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Veglia F, Werba JP, Tremoli E, Squellerio I, Sisillo E, Parolari A, Minardi F, Cavalca V. Assessment of oxidative stress in coronary artery bypass surgery: comparison between the global index OXY-SCORE and individual biomarkers. Biomarkers. 2009;14:465–472. doi: 10.3109/13547500903134395. [DOI] [PubMed] [Google Scholar]
- 132.Billings FT, IV, Ball SK, Roberts LJ, II, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50:1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Albers E, Donahue BS, Milne G, Saville BR, Wang W, Bichell D, McLaughlin B. Perioperative plasma F2-Isoprostane levels correlate with markers of impaired ventilation in infants with single-ventricle physiology undergoing stage 2 surgical palliation on the cardiopulmonary bypass. Pediatr Cardiol. 2012;33:562–568. doi: 10.1007/s00246-012-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berg K, Langaas M, Ericsson M, Pleym H, Basu S, Nordrum IS, Vitale N, Haaverstad R. Acetylsalicylic acid treatment until surgery reduces oxidative stress and inflammation in patients undergoing coronary artery bypass grafting. Eur J Cardiothorac Surg. 2013;43:1154–1163. doi: 10.1093/ejcts/ezs591. [DOI] [PubMed] [Google Scholar]
- 135.Simpson SA, Zaccagni H, Bichell DP, Christian KG, Mettler BA, Donahue BS, Roberts LJ, II, Pretorius M. Acetaminophen attenuates lipid peroxidation in children undergoing cardiopulmonary bypass. Pediatr Crit Care Med. 2014 Apr 11;15:503–510. doi: 10.1097/PCC.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ballester M, Llorens J, Garcia-de-la-Asuncion J, Perez-Griera J, Tebar E, Martinez-Leon J, Belda J, Juez M. Myocardial oxidative stress protection by sevoflurane vs. propofol: a randomised controlled study in patients undergoing off-pump coronary artery bypass graft surgery. Eur J Anaesthesiol. 2011;28:874–881. doi: 10.1097/EJA.0b013e32834bea2a. [DOI] [PubMed] [Google Scholar]
- 137.Mas E, Barden AE, Corcoran TB, Phillips M, Roberts LJ, II, Mori TA. Effects of spinal or general anesthesia on F2-isoprostanes and isofurans during ischemia/reperfusion of the leg in patients undergoing knee replacement surgery. Free Radic Biol Med. 2011;50:1171–1176. doi: 10.1016/j.freeradbiomed.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 138.Montine TJ, Markesberry WR, Morrow JD, Roberts LJ., II Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann Neurol. 1998;44:410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- 139.Praticò D, MY Lee V, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 140.Montine TJ, Beal MF, Cudkowicz ME, O’Donnell H, Margolin RA, McFarland L, Bachrach AF, Zackert WE, Roberts LJ, Morrow JD. Increased CSF F2-isoprostane concentration in probable AD. Neurology. 1999 Feb;52(3):562–565. doi: 10.1212/wnl.52.3.562. [DOI] [PubMed] [Google Scholar]
- 141.Praticò D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48(5):809–812. [PubMed] [Google Scholar]
- 142.Montine TJ, Beal MF, Robertson D, Cudkowicz ME, Biaggioni I, O’Donnell H, Zackert WE, Roberts LJ, Morrow JD. Cerebrospinal fluid F2-isoprostanes are elevated in Huntington’s disease. Neurology. 1999;52(5):1104–1105. doi: 10.1212/wnl.52.5.1104. [DOI] [PubMed] [Google Scholar]
- 143.Seet RC, Lee CY, Lim EC, Tan JJ, Quek AM, Chong WL, Looi WF, Huang SH, Wang H, Chan YH, Halliwell B. Oxidative damage in Parkinson disease: measurement using accurate biomarkers. Free Radic Biol Med. 2010;48(4):560–566. doi: 10.1016/j.freeradbiomed.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 144.Fessel JP, Hulette C, Powell S, Roberts LJ, II, Zhang J. Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson’s disease and with dementia with Lewy body disease. J Neurochem. 2003;85(3):645–650. doi: 10.1046/j.1471-4159.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 145.D’Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509–527. doi: 10.1016/j.freeradbiomed.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Montine TJ, Shinobu L, Montine KS, Roberts LJ, II, Kowall NW, Beal MF, Morrow JD. No difference in plasma or urinary F2-isoprostanes among patients with Huntington’s disease or Alzheimer’s disease and controls. Ann Neurol. 2000;48(6):950. [PubMed] [Google Scholar]
- 147.Montine TJ, Quinn JF, Milatovic D, Silbert LC, Dang T, Sanchez S, Terry E, Roberts LJ, II, Kaye JA, Morrow JD. Peripheral F2-isoprostanes and F4-neuroprostanes are not increased in Alzheimer’s disease. Ann Neurol. 2002;52(2):175–179. doi: 10.1002/ana.10272. [DOI] [PubMed] [Google Scholar]
- 148.Li G, Millard SP, Peskind ER, Zhang J, Yu CE, Leverenz JB, Mayer C, Shofer JS, Raskind MA, Quinn JF, Galasko DR, Montine TJ. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA Neurol. 2014;6(71):742–751. doi: 10.1001/jamaneurol.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guest J, Grant R, Mori TA, Croft KD. Changes in oxidative damage, inflammation and [NAD(H)] with age in cerebrospinal fluid. PLoS One. 2014;9(1):e85335. doi: 10.1371/journal.pone.0085335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Corcoran TB, Mas E, Barden AE, Durand T, Galano JM, Roberts LJ, Phillips M, Ho KM, Mori TA. Are isofurans and neuroprostanes increased after subarachnoid hemorrhage and traumatic brain injury? Antioxid Redox Signal. 2011;15(10):2663–2667. doi: 10.1089/ars.2011.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Farias SE, Heidenreich KA, Wohlauer MV, Murphy RC, Moore EE. Lipid mediators in cerebral spinal fluid of traumatic brain injured patients. J Trauma. 2011;71(5):1211–1218. doi: 10.1097/TA.0b013e3182092c62. [DOI] [PubMed] [Google Scholar]
- 152.Reich EE, Markesbery WR, Roberts LJ, II, Swift LL, Morrow JD, Montine TJ. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Am J Pathol. 2001;158(1):293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reich EE, Markesbery WR, Roberts LJ, II, Swift LL, Morrow JD, Montine TJ. Quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Adv Exp Med Biol. 2001;500:253–256. doi: 10.1007/978-1-4615-0667-6_39. [DOI] [PubMed] [Google Scholar]
- 154.Montine TJ, Montine KS, Reich EE, Terry ES, Porter NA, Morrow JD. Antioxidants significantly affect the formation of different classes of isoprostanes and neuroprostanes in rat cerebral synaptosomes. Biochem Pharmacol. 2003;65(4):611–617. doi: 10.1016/s0006-2952(02)01607-6. [DOI] [PubMed] [Google Scholar]
- 155.Rossner P, Jr, Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL, Eng SM, Gaudet MM, Neugut AI, Santella RM. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006 Apr;15(4):639–644. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- 156.Wu HC, Wang Q, Yang HI, Ahsan H, Tsai WY, Wang LY, Chen SY, Chen CJ, Santella RM. Urinary 15-F2t-isoprostane, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis. 2008 May;29(5):971–976. doi: 10.1093/carcin/bgn057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Epplein M, Franke AA, Cooney RV, Morris JS, Wilkens LR, Goodman MT, Murphy SP, Henderson BE, Kolonel LN, Le ML. Association of plasma micronutrient levels and urinary isoprostane with risk of lung cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009 Jul;18(7):1962–1970. doi: 10.1158/1055-9965.EPI-09-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gill JK, Franke AA, Steven MJ, Cooney RV, Wilkens LR, Le ML, Goodman MT, Henderson BE, Kolonel LN. Association of selenium, tocopherols, carotenoids, retinol, and 15-isoprostane F(2 t) in serum or urine with prostate cancer risk: the multiethnic cohort. Cancer Causes Control. 2009 Sep;20(7):1161–1171. doi: 10.1007/s10552-009-9304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barocas DA, Motley S, Cookson MS, Chang SS, Penson DF, Dai Q, Milne G, Roberts LJ, Morrow J, Concepcion RS, et al. Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J Urol. 2011 Jun;185(6):2102–2107. doi: 10.1016/j.juro.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brys M, Morel A, Forma E, Krzeslak A, Wilkosz J, Rozanski W, Olas B. Relationship of urinary isoprostanes to prostate cancer occurrence. Mol Cell Biochem. 2013 Jan;372(1–2):149–153. doi: 10.1007/s11010-012-1455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Asombang AW, Kayamba V, Mwanza-Lisulo M, Colditz G, Mudenda V, Yarasheski K, Chott R, Rubin DC, Gyawali CP, Sinkala E, et al. Gastric cancer in Zambian adults: a prospective case–control study that assessed dietary intake and antioxidant status by using urinary isoprostane excretion. Am J Clin Nutr. 2013 May;97(5):1029–1035. doi: 10.3945/ajcn.112.051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Siamakpour-Reihani S, Scarbrough PM, Wang F, Spasojevic I, Base K, Sedjo R, D’Agostino RB, Jr, Il’yasova D. Systemic markers of oxidative status and colorectal adenomatous polyps. Ann Epidemiol. 2012 Aug;22(8):587–591. doi: 10.1016/j.annepidem.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kong SY, Bostick RM, Flanders WD, McClellan WM, Thyagarajan B, Gross MD, Judd S, Goodman M. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammation. Cancer Epidemiol Biomarkers Prev. 2014 Mar;23(3):545–554. doi: 10.1158/1055-9965.EPI-13-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dai Q, Gao YT, Shu XO, Yang G, Milne G, Cai Q, Wen W, Rothman N, Cai H, Li H, et al. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women’s health study. J Clin Oncol. 2009 Apr 20;27:2482–2488. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morrow JD, Zackert WE, Yang JP, Kurhts EH, Callewaert D, Dworski R, Kanai K, Taber D, Moore K, Oates JA, et al. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999 May 1;269(2):326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 166.Thompson PA, Ambrosone C. Molecular epidemiology of genetic polymorphisms in estrogen metabolizing enzymes in human breast cancer. J Natl Cancer Inst Monogr. 2000;27:125–134. doi: 10.1093/oxfordjournals.jncimonographs.a024235. [DOI] [PubMed] [Google Scholar]
- 167.Montero A, Munger KA, Khan RZ, Valdivielso JM, Morrow JD, Guasch A, Ziyadeh FN, Badr KF. F2-isoprostanes mediate high glucose-induced TGF-beta synthesis and glomerular proteinuria in experimental type I diabetes. Kidney Int. 2000 Nov;58(5):1963–1972. doi: 10.1111/j.1523-1755.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- 168.McGowan TA, Dunn SR, Falkner B, Sharma K. Stimulation of urinary TGF-beta and isoprostanes in response to hyperglycemia in humans. Clin J Am Soc Nephrol. 2006;1:263–268. doi: 10.2215/CJN.00990905. [DOI] [PubMed] [Google Scholar]
- 169.Chang CF, Westbrook R, Ma J, Cao D. Transforming growth factor-beta signaling in breast cancer. Front Biosci. 2007;12:4393–4401. doi: 10.2741/2396. [DOI] [PubMed] [Google Scholar]
- 170.Davì G, Falco A, Patrono C. Determinants of F2-isoprostane biosynthesis and inhibition in man. Chem Phys Lipids. 2004;128:149–163. doi: 10.1016/j.chemphyslip.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 171.Roberts LJ, II, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Block G, Jensen CD, Morrow JD, Holland N, Norkus EP, Milne GL, Hudes M, Dalvi TB, Crawford PB, Fung EB, Schumacher L, Harmatz P. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic Biol Med. 2008;45:377–384. doi: 10.1016/j.freeradbiomed.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Belli R, Amerio P, Brunetti L, Orlando G, Toto P, Proietto G, Vacca M, Tulli A. Elevated 8-isoprostane levels in basal cell carcinoma and in UVA irradiated skin. Int J Immunopathol Pharmacol. 2005;18:497–502. doi: 10.1177/039463200501800309. [DOI] [PubMed] [Google Scholar]
- 174.Hoskins A, Roberts JL, II, Milne G, Choi L, Dworski R. Natural-source d-α-tocopheryl acetate inhibits oxidant stress and modulates atopic asthma in humans in vivo. Allergy. 2012;67:676–682. doi: 10.1111/j.1398-9995.2012.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, Chen Q. Pharmaco-logic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med. 2011;50:1610–1619. doi: 10.1016/j.freeradbiomed.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Welsh JL, Wagner BA, Erve TJvan’t, Zehr PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts LJ, II, Drisko J, Levine M, Buettner GR, Cullen JJ. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013;71:765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Traustadóttir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ, II, Harman SM. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. 2009;139:1896–1900. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McAnulty LS, Nieman DC, Dumke CL, Shooter LA, Henson DA, Utter AC, Milne G, McAnulty SR. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl Physiol Nutr Metab. 2011;36:976–984. doi: 10.1139/h11-120. [DOI] [PubMed] [Google Scholar]
- 179.Khan F, Ray S, Craigie AM, Kennedy G, Hill A, Barton KL, Broughton J, Belch JJ. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: a randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic Biol Med. 2014;72C:232–237. doi: 10.1016/j.freeradbiomed.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 180.Alvarez-Suarez JM, Giampieri F, Tulipani S, Casoli T, Stefano GDi, González-Paramás AM, Santos-Buelga C, Busco F, Quiles JL, Cordero MD, Bompadre S, Mezzetti B, Battino M. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J Nutr Biochem. 2014;25:289–294. doi: 10.1016/j.jnutbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 181.Nam TG, Nara SJ, Zagol-Ikapitte I, Cooper T, Valgimigli L, Oates JA, Porter NA, Boutaud O, Pratt DA. Pyridine and pyrimidine analogs of acetaminophen as inhibitors of lipid peroxidation and cyclooxygenase and lipoxygenase catalysis. Org Biomol Chem. 2009;7:5103–5112. doi: 10.1039/b912528k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shchepin RV, Liu W, Yin H, Zagol-Ikapitte I, Amin T, Jeong BS, Roberts LJ, II, Oates JA, Porter NA, Boutaud O. Rational Design Of Novel Pyridinol-Fused Ring Acetaminophen Analogues. ACS Med Chem Lett. 2013;4:710–714. doi: 10.1021/ml4000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Janz DR, Bastarache JA, Peterson JF, Sills G, Wickersham N, May AK, Roberts LJ, II, Ware LB. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41:784–790. doi: 10.1097/CCM.0b013e3182741a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Meyer KA, Sijtsma FP, Nettleton JA, Steffen LM, Van Horn L, Shikany JM, Gross MD, Mursu J, Traber MG, Jacobs DR., Jr Dietary patterns are associated with plasma F2-isoprostanes in an observational cohort study of adults. Free Radic Biol Med. 2013;57:201–209. doi: 10.1016/j.freeradbiomed.2012.08.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rink SM, Mendola P, Mumford SL, Poudrier JK, Browne RW, Wactawski-Wende J, Perkins NJ, Schisterman EF. Self-report of fruit and vegetable intake that meets the 5 a day recommendation is associated with reduced levels of oxidative stress biomarkers and increased levels of antioxidant defense in premen-opausal women. J Acad Nutr Diet. 2013;113:776–785. doi: 10.1016/j.jand.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jiang Y, Wu SH, Shu XO, Xiang YB, Ji BT, Milne GL, Cai Q, Zhang X, Gao YT, Zheng W, Yang G. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. J Acad Nutr Diet. 2014;114:700–708. doi: 10.1016/j.jand.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Barden AE, Mori TA, Dunstan JA, Taylor AL, Thornton CA, Croft KD, Beilin LJ, Prescott SL. Fish oil supplementation in pregnancy lowers F2-isoprostanes in neonates at high risk of atopy. Free Radic Res. 2004;38:233–239. doi: 10.1080/10715760310001656722. [DOI] [PubMed] [Google Scholar]
- 188.Mori TA. Effect of fish and fish oil-derived omega-3 fatty acids on lipid oxidation. Redox Rep. 2004;9:193–197. doi: 10.1179/135100004225005200. [DOI] [PubMed] [Google Scholar]
- 189.Nälsén C, Vessby B, Berglund L, Uusitupa M, Hermansen K, Riccardi G, Rivellese A, Storlien L, Erkkilä A, Ylä-Herttuala S, Tapsell L, Basu S. Dietary (n-3) fatty acids reduce plasma F2-isoprostanes but not prostaglandin F2alpha in healthy humans. J Nutr. 2006;136:1222–1228. doi: 10.1093/jn/136.5.1222. [DOI] [PubMed] [Google Scholar]
- 190.Mas E, Woodman RJ, Burke V, Puddey IB, Beilin LJ, Durand T, Mori TA. The omega-3 fatty acids EPA and DHA decrease plasma F2-isoprostanes: results from two placebo-controlled interventions. Free Radic Res. 2010;44:983–990. doi: 10.3109/10715762.2010.492830. [DOI] [PubMed] [Google Scholar]
- 191.McAnulty SR, Nieman DC, McAnulty LS, Lynch WS, Jin F, Henson DA. Effect of mixed flavonoids, n-3 fatty acids, and vitamin C on oxidative stress and antioxidant capacity before and after intense cycling. Int J Sport Nutr Exerc Metab. 2011;21:328–337. doi: 10.1123/ijsnem.21.4.328. [DOI] [PubMed] [Google Scholar]
- 192.De Felice C, Signorini C, Durand T, Ciccoli L, Leoncini S, D’Esposito M, Filosa S, Oger C, Guy A, Bultel-Poncé V, Galano JM, Pecorelli A, Felice LDe, Valacchi G, Hayek J. Partial rescue of Rett syndrome by ω-3 polyunsaturated fatty acids (PUFAs) oil. Genes Nutr. 2012;7:447–458. doi: 10.1007/s12263-012-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Barden A, Mas E, Henry P, Durand T, Galano JM, Roberts LJ, Croft KD, Mori TA. The effects of oxidation products of arachidonic acid and n3 fatty acids on vascular and platelet function. Free Radic Res. 2011;45:469–476. doi: 10.3109/10715762.2010.544730. [DOI] [PubMed] [Google Scholar]
- 194.Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, Malarkey WB, Hwang BS, Blackburn E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav Immun. 2013;28:16–24. doi: 10.1016/j.bbi.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jones ML, Mark PJ, Mori TA, Keelan JA, Waddell BJ. Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol Reprod. 2013;88:37. doi: 10.1095/biolreprod.112.103754. [DOI] [PubMed] [Google Scholar]
- 196.Signorini C, Felice CDe, Leoncini S, Durand T, Galano JM, Cortelazzo A, Zollo G, Guerranti R, Gonnelli S, Caffarelli C, Rossi M, Pecorelli A, Valacchi G, Ciccoli L, Hayek J. Altered erythrocyte membrane fatty acid profile in typical Rett syndrome: effects of omega-3 polyunsaturated fatty acid supplementation. Prostaglandins Leukot Essent Fatty Acids. 2014 Sep 6; doi: 10.1016/j.plefa.2014.08.002. pii: S0952-3278(14)00129-X. http://dx.doi.org/10.1016/j.plefa.2014.08.002 (Epub ahead of print) [DOI] [PubMed]
- 197.Deshpande G, Simmer K, Deshmukh M, Mori TA, Croft KD, Kristensen J. Fish oil (SMOFlipid) and olive oil lipid (clinoleic) in very preterm neonates. J Pediatr Gastroenterol Nutr. 2014;58:179–184. doi: 10.1097/MPG.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 198.Gao L, Yin H, Milne GL, Porter NA, Morrow JD. Formation of F-ring isoprostane-like compounds (F3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2006;281(20):14092–14099. doi: 10.1074/jbc.M601035200. [DOI] [PubMed] [Google Scholar]
- 199.Brooks JD, Milne GL, Yin H, Sanchez SC, Porter NA, Morrow JD. Formation of highly reactive cyclopentenone isoprostane compounds (A3/J3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2008;283(18):12043–12055. doi: 10.1074/jbc.M800122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Roberts LJ, II, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273(22):13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 201.Reich EE, Montine TJ, Morrow JD. Formation of novel D-ring and E-ring isoprostane-like compounds (D4/E4-neuroprostanes) in vivo from docosahexaenoic acid. Adv Exp Med Biol. 2002;507:519–524. doi: 10.1007/978-1-4615-0193-0_79. [DOI] [PubMed] [Google Scholar]
- 202.Fam SS, Murphey LJ, Terry ES, Zackert WE, Chen Y, Gao L, Pandalai S, Milne GL, Roberts LJ, Porter NA, Montine TJ, Morrow JD. Formation of highly reactive A-ring and J-ring isoprostane-like compounds (A4/J4-neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 2002;277(39):36076–36084. doi: 10.1074/jbc.M205638200. [DOI] [PubMed] [Google Scholar]
- 203.Song WL, Paschos G, Fries S, Reilly MP, Yu Y, Rokach J, Chang CT, Patel P, Lawson JA, Fitzgerald GA. Novel eicosapentaenoic acid-derived F3-isoprostanes as biomarkers of lipid peroxidation. J Biol Chem. 2009;284:23636–23643. doi: 10.1074/jbc.M109.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gorrindo P, Lane CJ, Lee EB, McLaughlin B, Levitt P. Enrichment of elevated plasma F2t-isoprostane levels in individuals with autism who are stratified by presence of gastrointestinal dysfunction. PLoS One. 2013;8(7):e68444. doi: 10.1371/journal.pone.0068444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ming X, Stein TP, Brimacombe M, Johnson WG, Lambert GH, Wagner GC. Increased excretion of a lipid peroxidation biomarker in autism, Prostaglandins Leukot. Essent Fat Acids. 2005;73(5):379–384. doi: 10.1016/j.plefa.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 206.Korade Z, Xu L, Mirnics K, Porter NA. Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2013;36(1):113–122. doi: 10.1007/s10545-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Akohoue SA, Shankar S, Milne GL, Morrow J, Chen KY, Ajayi WU, Buchowski MS. Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatr Res. 2007;61(2):233–238. doi: 10.1203/pdr.0b013e31802d7754. [DOI] [PubMed] [Google Scholar]
- 208.Collins CE, Quaggiotto P, Wood L, O’Loughlin EV, Henry RL, Garg ML. Elevated plasma levels of F2 alpha isoprostane in cystic fibrosis. Lipids. 1999;34(6):551–556. doi: 10.1007/s11745-999-0397-1. [DOI] [PubMed] [Google Scholar]
- 209.Montuschi P, Kharitonov SA, Ciabattoni G, Corradi M, van Rensen L, Geddes DM, Hodson ME, Barnes PJ. Exhaled 8-isoprostane as a new non-invasive biomarker of oxidative stress in cystic fibrosis. Thorax. 2000;55(3):205–209. doi: 10.1136/thorax.55.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ciabattoni G, Davì G, Collura M, Iapichino L, Pardo F, Ganci A, Romagnoli R, Maclouf J, Patrono C. In vivo lipid peroxidation and platelet activation in cystic fibrosis. Am J Respir Crit Care Med. 2000;162:1195–1201. doi: 10.1164/ajrccm.162.4.9911071. [DOI] [PubMed] [Google Scholar]
- 211.McGuire PJ, Parikh A, Diaz GA. Profiling of oxidative stress in patients with inborn errors of metabolism. Mol Genet Metab. 2009;98(1–2):173–180. doi: 10.1016/j.ymgme.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Perrone S, Longini M, Bellieni CV, Centini G, Kenanidis A, De Marco L, Petraglia F, Buonocore G. Early oxidative stress in amniotic fluid of pregnancies with Down syndrome. Clin Biochem. 2007;40(3–4):177–180. doi: 10.1016/j.clinbiochem.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 213.De Felice C, Ciccoli L, Leoncini S, Signorini C, Rossi M, Vannuccini L, Guazzi G, Latini G, Comporti M, Valacchi G, Hayek J. Systemic oxidative stress in classic Rett syndrome. Free Radic Biol Med. 2009;47(4):440–448. doi: 10.1016/j.freeradbiomed.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 214.Signorini C, Felice CDe, Leoncini S, Giardini A, D’Esposito M, Filosa S, Della Ragione F, Rossi M, Pecorelli A, Valacchi G, Ciccoli L, Hayek J. F4-neuroprostanes mediate neurological severity in Rett syndrome. Clin Chim Acta. 2011;412(15–16):1399–1406. doi: 10.1016/j.cca.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 215.De Felice C, Signorini C, Durand T, Oger C, Guy A, Bultel-Poncé V, Galano JM, Ciccoli L, Leoncini S, D’Esposito M, Filosa S, Pecorelli A, Valacchi G, Hayek J. F2-dihomo-isoprostanes as potential early biomarkers of lipid oxidative damage in Rett syndrome. J Lipid Res. 2011;52(12):2287–2297. doi: 10.1194/jlr.P017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Durand T, De Felice C, Signorini C, Oger C, Bultel-Poncé V, Guy A, Galano JM, Leoncini S, Ciccoli L, Pecorelli A, Valacchi G, Hayek J. F2-Dihomo-isoprostanes and brain white matter damage in stage 1 Rett syndrome. Biochimie. 2013;95(1):86–90. doi: 10.1016/j.biochi.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 217.Bauer J, Ripperger A, Frantz S, Ergün S, Schwedhelm E, Benndorf RA. Pathophys-iology of isoprostanes in the cardiovascular system: implications of isoprostane-mediated thromboxane A2 receptor activation. Br J Pharmacol. 2014 Mar 20;171:3115–3131. doi: 10.1111/bph.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Elmhurst JL, Betti PA, Rangachari PK. Intestinal effects of isoprostanes: evidence for the involvement of prostanoid EP and TP receptors. J Pharmacol Exp Ther. 1997;282:1198–1205. [PubMed] [Google Scholar]
- 219.Janssen LJ, Tazzeo T. Involvement of TP and EP3 receptors in vasoconstrictor responses to isoprostanes in pulmonary vasculature. J Pharmacol Exp Ther. 2002;301:1060–1066. doi: 10.1124/jpet.301.3.1060. [DOI] [PubMed] [Google Scholar]
- 220.Sametz W, Hennerbichler S, Glaser S, Wintersteiger R, Juan H. Characterization of prostanoid receptors mediating actions of the isoprostanes, 8-iso-PGE(2) and 8-iso-PGF(2alpha), in some isolated smooth muscle preparations. Br J Pharmacol. 2000;130:1903–1910. doi: 10.1038/sj.bjp.0703522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Weber TJ, Markillie LM. Regulation of activator protein-1 by 8-iso-prostaglandin E2 in a thromboxane A2 receptor-dependent and independent manner. Mol Pharmacol. 2003;63:1075–1081. doi: 10.1124/mol.63.5.1075. [DOI] [PubMed] [Google Scholar]
- 222.Clarke DL, Giembycz MA, Patel HJ, Belvisi MG. E-ring 8-isoprostanes inhibit ACh release from parasympathetic nerves innervating guinea-pig trachea through agonism of prostanoid receptors of the EP3-subtype. Br J Pharmacol. 2004;141:600–609. doi: 10.1038/sj.bjp.0705648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Clarke DL, Belvisi MG, Hardaker E, Newton R, Giembycz MA. E-ring 8-isoprostanes are agonists at EP2- and EP4-prostanoid receptors on human airway smooth muscle cells and regulate the release of colony-stimulating factors by activating cAMP-dependent protein kinase. Mol Pharmacol. 2005;67:383–393. doi: 10.1124/mol.104.006486. [DOI] [PubMed] [Google Scholar]
- 224.Paredes C, Tazzeo T, Janssen LJ. E-ring isoprostane augments cholinergic neuro-transmission in bovine trachealis via FP prostanoid receptors. Am J Respir Cell Mol Biol. 2007;37:739–747. doi: 10.1165/rcmb.2007-0022OC. [DOI] [PubMed] [Google Scholar]
- 225.Seto V, Hirota C, Hirota S, Janssen LJ. E-Ring isoprostanes stimulate a Cl conductance in airway epithelium via prostaglandin E2-selective prostanoid receptors. Am J Respir Cell Mol Biol. 2008;38:88–94. doi: 10.1165/rcmb.2007-0117OC. [DOI] [PubMed] [Google Scholar]
- 226.Joy AP, Cowley EA. 8-iso-PGE2 stimulates anion efflux from airway epithelial cells via the EP4 prostanoid receptor. Am J Respir Cell Mol Biol. 2008;38:143–152. doi: 10.1165/rcmb.2006-0295OC. [DOI] [PubMed] [Google Scholar]
- 227.Zhao M, Destache CJ, Ohia SE, Opere CA. Role of prostanoid production and receptors in the regulation of retinal endogenous amino acid neurotransmitters by 8-isoprostaglandin E2, ex vivo. Neurochem Res. 2009;34:2170–2180. doi: 10.1007/s11064-009-0013-x. [DOI] [PubMed] [Google Scholar]
- 228.Chen JX, O’Mara PW, Poole SD, Brown N, Ehinger NJ, Slaughter JC, Paria BC, Aschner JL, Reese J. Isoprostanes as physiological mediators of transition to newborn life: novel mechanisms regulating patency of the term and preterm ductus arteriosus. Pediatr Res. 2012;72:122–128. doi: 10.1038/pr.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.van der Sterren S, Villamor E. Contractile effects of 15-E2t-isoprostane and 15-F2t-isoprostane on chicken embryo ductus arteriosus. Comp Biochem Physiol A Mol Integr Physiol. 2011;159:436–444. doi: 10.1016/j.cbpa.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 230.van der Sterren S, Kessels L, Perez-Vizcaino F, Cogolludo AL, Villamor E. Prenatal exposure to hyperoxia modifies the thromboxane prostanoid receptor-mediated response to H2O2 in the ductus arteriosus of the chicken embryo. J Physiol Pharmacol. 2014;65:283–293. [PubMed] [Google Scholar]
- 231.Comporti M, Signorini C, Leoncini S, Buonocore G, Rossi V, Ciccoli L. Plasma F2-isoprostanes are elevated in newborns and inversely correlated to gestational age. Free Radic Biol Med. 2004;37:724–732. doi: 10.1016/j.freeradbiomed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 232.Evans AR, Junger H, Southall MD, Nicol GD, Sorkin LS, Broome JT, Bailey TW, Vasko MR. Isoprostanes, novel eicosanoids that produce nociception and sensitize rat sensory neurons. J Pharmacol Exp Ther. 2000;293:912–920. [PubMed] [Google Scholar]
- 233.Junger H, Sorkin LS. C-nociceptor sensitization by isoprostanes is cyclooxygenase dependent. Brain Res. 2000;867:255–258. doi: 10.1016/s0006-8993(00)02306-4. [DOI] [PubMed] [Google Scholar]
- 234.Musiek ES, Yin H, Milne GL, Morrow JD. Recent advances in the biochemistry and clinical relevance of the isoprostane pathway. Lipids. 2005;40:987–994. doi: 10.1007/s11745-005-1460-7. [DOI] [PubMed] [Google Scholar]
- 235.Musiek ES, Milne GL, McLaughlin B, Morrow JD. Cyclopentenone eicosanoids as mediators of neurodegeneration: a pathogenic mechanism of oxidative stress-mediated and cyclooxygenase-mediated neurotoxicity. Brain Pathol. 2005;15:149–158. doi: 10.1111/j.1750-3639.2005.tb00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Milne GL, Musiek ES, Morrow JD. The cyclopentenone (A2/J2) isoprostanes– unique, highly reactive products of arachidonate peroxidation. Antioxid Redox Signal. 2005;7:210–220. doi: 10.1089/ars.2005.7.210. [DOI] [PubMed] [Google Scholar]
- 237.Stamatakis K, Pérez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic lipid-protein interactomes. Ann N Y Acad Sci. 2006;1091:548–570. doi: 10.1196/annals.1378.096. [DOI] [PubMed] [Google Scholar]
- 238.Musiek ES, McLaughlin B, Morrow JD. Electrophilic cyclopentenone isoprostanes in neurodegeneration. J Mol Neurosci. 2007;33:80–86. doi: 10.1007/s12031-007-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


