Abstract
Objective:
To evaluate the pharmacokinetics of TX-004HR vaginal estradiol softgel capsules when used for treating moderate-to-severe dyspareunia in postmenopausal women with vulvar and vaginal atrophy.
Methods:
A substudy of the REJOICE trial (multicenter, double-blind, placebo-controlled, phase 3) evaluated the pharmacokinetics of 4, 10, and 25-μg TX-004HR doses once/d for 2 weeks, followed by twice/wk for 10 weeks. Serum samples obtained at 2, 4, 6, 10, and 24 hours postdose on days 1 and 14, and once on day 84, were analyzed for area under the serum concentration-time curve, tmax, Cmin, Cavg, and Cmax for estradiol, estrone, and estrone conjugates.
Results:
Seventy-two women (mean 59 y) participated. TX-004HR 4 μg showed no statistical differences from placebo in estradiol pharmacokinetic (PK) parameters. At 10 μg, estradiol Cmax was statistically higher than placebo on day 1, but was not different from placebo on day 14. With 25 μg, estradiol PK parameters were statistically higher than placebo. Estradiol Cavg values for 25 μg were 9.1 pg/mL on day 1 and 7.1 pg/mL on day 14. Estrone and estrone conjugate PK parameters with TX-004HR were lower than or similar to placebo across all doses. No drug accumulation was observed.
Conclusions:
Vaginal TX-004HR resulted in negligible to very low systemic absorption of estradiol. No statistical differences in estradiol PK parameters were observed on day 14 with 4 and 10 μg, and only minor increases were observed with 25 μg (within the normal postmenopausal range). This PK substudy, in conjunction with the primary efficacy results, demonstrated that TX-004HR provided local benefits of estradiol with limited systemic exposure.
Keywords: Estradiol, Estrogen therapy, Menopause, Pharmacokinetics, TX-004HR, Vaginal atrophy
Low-dose vaginal estrogen is a safe and effective treatment for symptoms associated with vulvar and vaginal atrophy (VVA), such as vaginal dryness, irritation, and pain with intercourse, which occur as a result of declining estrogen associated with menopause.1,2 The rationale for using a vaginal delivery system includes the ability to provide low doses of estrogen with local administration to eliminate or minimize levels of circulating estrogens,1,3 and bypass hepatic circulation, thus reducing potential side effects.4
The Working Group on Women's Health and Well-Being in Menopause petitioned the US Food and Drug Administration (FDA) to modify the labeling of low-dose vaginal estrogens, which do not significantly increase systemic levels of estradiol. The US FDA held a workshop on November 10, 2015, in which they invited a panel of scientific experts to discuss the topic of product labeling for local estrogen therapies.5 Some concerns remain regarding elevations in circulating estrogen concentrations with some vaginal products,6 despite the well-characterized and positive benefit/risk ratio of low-dose vaginal estrogen products.
TX-004HR (TherapeuticsMD, Inc., Boca Raton, FL) is a new 17β-estradiol vaginal product developed to rapidly and effectively treat menopausal VVA signs and symptoms with no systemic exposure. Its novel design does not require an applicator, intended to make it easy to use and increase user acceptability and overall satisfaction.
Phase 1 pharmacokinetic (PK) studies have shown that women using 10 or 25-μg doses of TX-004HR have very low levels of serum estradiol after administration, which were within the postmenopausal range.7 Furthermore, these same women had estrogen levels that were approximately 30% of the serum estrogen concentrations observed in women who received identical doses of an approved vaginal estradiol tablet after 24 hours.7
The REJOICE trial demonstrated that TX-004HR (4, 10, and 25 μg) was safe, well-tolerated, and effective for treating symptomatic VVA. Women who were treated with any TX-004HR dose had significant improvements in percentages of vaginal superficial and parabasal cells, vaginal pH, and severity of dyspareunia as early as 2 weeks, and vaginal dryness as early as 6 weeks, relative to women treated with placebo, with improvements continuing to 12 weeks.8
The objective of this study was to evaluate the pharmacokinetic profile after the daily vaginal administration of 3 doses of TX-004HR (4, 10, and 25 μg) for 2 weeks and then at day 84 to assess systemic exposure of estradiol and its metabolites.
METHODS
Study design
The PK substudy was part of the REJOICE trial—a large, multicenter, double-blind, randomized, placebo-controlled phase 3 trial evaluating the efficacy and safety of the investigational drug, TX-004HR (4, 10, and 25 μg), for the treatment of dyspareunia associated with VVA in postmenopausal women (NCT02253173). The REJOICE trial was conducted at 89 sites in the United States and Canada from October 2014 to October 2015, and assessed the four co-primary endpoints of changes from baseline to week 12 in the percentages of vaginal superficial and parabasal cells, vaginal pH, and the severity of dyspareunia compared with placebo.8
Women were randomized to the PK substudy separately from the main phase 3 study participants using a computer-generated system based on block sizes of four, and a ratio of 1:1:1:1 to either 4, 10, or 25 μg TX-004HR, or matching placebo. Women self-administered one vaginal capsule daily for 2 weeks, followed by 10 weeks of twice-weekly dosing. The appearance and packaging of TX-004HR and placebo were identical to facilitate double-blinding of the study participants and all those involved in the conduct of the study (site staff, clinical research associates, sponsor representatives). Double-blinding was to be broken only in emergency situations and was to be sustained through database lock. The protocol and informed consent form were approved by an independent institutional review board. The trial was conducted in accordance with applicable laws and regulations including the International Conference on Harmonization Guideline for Good Clinical Practice and the Declaration of Helsinki.
Study participants
Participants provided written informed consent before any study-related activities. Healthy postmenopausal women 40 to 75 years of age were included if they had ≤5% superficial cells on vaginal cytological smear; vaginal pH >5.0; a most bothersome symptom of moderate-to-severe vaginal pain associated with sexual activity (dyspareunia); onset of moderate-to-severe dyspareunia during postmenopause; body mass index (BMI) ≤38 kg/m2; and anticipated having sexual activity (with vaginal penetration) during the trial period. Main exclusion criteria included use of oral estrogen, progestin, androgen, or selective estrogen receptor modulator-containing products within 8 weeks; transdermal hormones within 4 weeks; vaginal hormones (rings, creams, gels) within 4 weeks; intrauterine progestins within 8 weeks; progestin implants/injectables or estrogen pellets/injectables within 6 months; vaginal lubricants or moisturizers within 7 days before vaginal pH assessment during screening; investigational drugs within 60 days; or an intrauterine device within 12 weeks. Concomitant medications were allowed, with the exception of the above excluded medications, and recorded in diaries. Participants who had used hormone therapies underwent a washout period.
Study endpoints
The PK substudy of the REJOICE trial was designed to assess hormone concentrations of estradiol, estrone, and estrone conjugates at screening, and at days 1, 14, and 84 of treatment in a subset of participants. Serum samples were analyzed to characterize the following PK parameters from 0 to 24 hours: area under the serum concentration-time curve (AUC, by the trapezoidal rule), average concentration (Cavg, AUC/dosing interval), peak concentration (Cmax), minimum concentration (Cmin), and time to maximum concentration (tmax) for each hormone on days 1 and 14. Accumulation ratios (day 14/day 1) were calculated for AUC and Cmax. Mean serum estradiol concentrations were calculated for samples collected on day 84, approximately 4 days after the last insertion of TX-004HR, to determine the trough value of estradiol after chronic treatment where the product was given twice weekly.
Time 0 hour serum samples were obtained at screening, and at day 1 and day 14 before dosing. Baseline values were characterized by averaging the two pretreatment samples (screening and day 1). After women self-administered treatments, serum samples were taken at five postdose time points (2, 4, 6, 10, and 24 h) on days 1 and 14, and one final sample at approximately day 84. Sex hormone binding globulin (SHBG) measurements were obtained before treatment on days 1 and 14, and at the final hormone blood draw on day 84.
Analytical methods
The bioanalysis was performed at InVentiv Clinical Laboratories, Inc. (Princeton, NJ). Serum samples were analyzed for estradiol, estrone, and estrone conjugates using gas chromatography/tandem mass spectrometry (GC/MS/MS). Estradiol and estrone were extracted from 0.400 mL of human serum by a solid-phase extraction procedure. Separately, estrone conjugates were also extracted from 0.400 mL of human serum by a solid-phase extraction procedure that selectively eluted the conjugates and retained estrone. The eluates were then subjected to a hydrolysis step to convert the estrone conjugates to estrone. All compounds were detected and quantified by tandem mass spectrometry in negative ion mode on a Finnigan TSQ-700 mass spectrometer equipped with a Varian GC and Leap CTC A200S autosampler. Calibration curves were obtained by performing linear regression (weighted 1/x2) on the calibration standards. Within-run and between-run coefficients of variation for accuracy and precision for estradiol, estrone, and estrone conjugates were within the US FDA-allowable limits, which were 20% for the lower limit of quantification (LLOQ) and 15% for all other quality control (QC) samples. Accuracy (bias), as reported below, is defined as the closeness of the mean test results obtained by the assay to the actual value (concentration) of the analyte, and precision is defined as the closeness of individual measure of an analyte when the procedure is applied repeatedly to multiple aliquots of a single homogeneous serum sample.
Estradiol
Calibration standards for estradiol were prepared in water fresh each day. Validation QC samples were prepared for estradiol at five target concentrations (2.00, 6.52, 51.5, 441, and 3216 pg/mL), which spanned the calibration range of the assay. The LLOQ QC samples were prepared in water. All other QC samples were prepared in charcoal-stripped human serum by spiking above the endogenous levels to approximately the target nominal concentrations. The true concentrations of the serum samples were calculated from the mean value of all valid measurements obtained for each level.
The validated concentration range for estradiol was 2.00 to 500 pg/mL. The coefficient of variation for estradiol standards was ≤7.30% for between-run precision and the bias was −2.00% to 3.80% for between-run accuracy. Sensitivities for estradiol were 4.83% for precision and −8.00% for accuracy for the LLOQ samples. The within-run and between-run coefficients of variation for estradiol for other QC samples were within allowable limits for accuracy (−9.97% to 5.45%) and precision (≤8.46%). The specificity and selectivity of the method were also verified against endogenous matrix components. The average recovery of estradiol from charcoal-stripped human serum was 75.3%, and the average recovery of internal standard was 73.9%.
Estrone
Calibration standards for estrone were prepared in water fresh each day. Validation QC samples were prepared for estrone at five target concentrations (5.00, 17.1, 120, 865, and 6176 pg/mL), which spanned the calibration range of the assay. The LLOQ QC samples were prepared in water. All other QC samples were prepared in charcoal-stripped human serum by spiking above the endogenous levels to approximately the target nominal concentrations. The true concentrations of the serum samples were calculated from the mean value of all valid measurements obtained for each level.
The validated concentration range for estrone was 5.00 to 1000 pg/mL. The coefficient of variation for estrone standards was ≤6.37% for between-run precision and the bias was −3.20% to 4.00% for between-run accuracy. Sensitivities for estrone were 13.2% for precision and 20.0% for accuracy for the LLOQ samples. The within-run and between-run coefficients of variation for estrone for other QC samples were within allowable limits for accuracy (−7.41% to 14.1%) and precision (≤9.33%). The specificity and selectivity of the method were also verified against endogenous matrix components. The average recovery of estrone from charcoal-stripped human serum was 73.4%, and the average recovery of internal standard was 73.5%.
Estrone conjugates
The bioanalytical method measured total estrone conjugates via a hydrolysis step; however, the standard curve was prepared with estrone sulfate, the only conjugate that is commercially available. Final concentrations included all hydrolyzed estrone conjugates (glucuronides and sulfates), which were quantified against hydrolyzed estrone sulfate standards. Calibration standards were prepared in water fresh each day. Validation QC samples were prepared at six target concentrations (25.0, 75.0, 525, 3750, and 5000 pg/mL), which spanned the calibration range of the assay, and 25,000 pg/mL, which was used to verify dilution samples with concentrations above the calibration range. The LLOQ QC samples were prepared in water due to high endogenous levels observed in the matrix. All other QC samples were prepared in unaltered or noncharcoal-stripped human serum by spiking above the endogenous levels to approximately the target nominal concentrations. The true concentrations of the serum samples were calculated from the mean value of all valid measurements obtained for each level.
The validated concentration range for estrone conjugates was 25.0 to 5000 pg/mL. The coefficients of variation for estrone sulfate standards were ≤6.62% for between-run precision and the bias was −3.20% to 3.20% for between-run accuracy. Sensitivities for estrone conjugates were 5.88% for precision and −4.00% for accuracy for the LLOQ samples. The within-run and between-run coefficients of variation for estrone conjugates for other QC samples were within allowable limits for accuracy (−13.1% to 3.54%) and precision (≤4.51%).
Statistical analyses
For pharmacokinetic analyses, data from participants were included if they had completed the study, were at least 80% compliant with the treatment, had measurements for all co-primary efficacy endpoints, had no significant protocol violations, and had sufficient blood samples for PK analyses. Descriptive and inferential statistical analyses were performed using SAS v.9.2. All statistical tests were two-sided. Baseline and demographic variables were descriptively summarized by treatment group. T tests were used to determine significant differences between TX-004HR doses and placebo for PK parameters.
Because estradiol, estrone, and estrone conjugates are present in women as endogenous compounds, the PK analyses determined PK parameters as “unadjusted for baseline” and “baseline-adjusted” values. Baseline estrogen values for each participant were determined. Sample values estimated as below the limit of quantification were replaced by the LLOQ for that analyte, as determined during the method validation (estradiol 2.0 pg/mL, estrone 5.0 pg/mL, and estrone conjugates 25.0 pg/mL). For unadjusted AUC and Cavg calculations, the concentration at time 0 was set to 0.0 pg/mL.
RESULTS
Participant disposition and baseline characteristics
Of the 764 women randomized to treatment in the main trial, 72 participants at 11 centers qualified for and agreed to participate in the PK substudy. These 72 women were randomized to TX-004HR 4 μg (n = 18), 10 μg (n = 19), 25 μg (n = 18), or placebo (n = 17). One participant in the placebo group discontinued due to an adverse event (emotional liability). Overall, 97.2% of the women were compliant (≥80% vaginal tablets) with dosing based on diary data.
Participants had a mean age of 59 years and a mean BMI of 28 kg/m2; the majority (94%) were white (Table 1).
TABLE 1.
Participant demographics and baseline characteristics
| Characteristics | TX-004HR 4 μg (n = 18) | TX-004HR 10 μg (n = 19) | TX-004HR 25 μg (n = 18) | Placebo (n = 17) |
| Age, y | 57.4 ± 7.1 | 58.5 ± 7.1 | 59.2 ± 6.4 | 58.9 ± 6.1 |
| Race, n (%) | ||||
| White | 18 (100) | 18 (94.7) | 16 (88.9) | 16 (94.1) |
| Black or African American | 0 (0) | 1 (5.3) | 2 (11.1) | 1 (5.9) |
| BMI, kg/m2 | 28.3 ± 5.6 | 28.2 ± 4.5 | 28.9 ± 5.3 | 27.2 ± 5.5 |
| Smoking history, n (%) | ||||
| No | 12 (66.7) | 6 (31.6) | 12 (66.7) | 10 (58.8) |
| Yes | 6 (33.3) | 13 (68.4) | 6 (33.3) | 7 (41.2) |
| Years of smoking | 33.2 ± 12.0 | 21.8 ± 11.2 | 23.8 ± 15.8 | 21.9 ± 12.4 |
| Baseline hormone values, pg/mL | ||||
| Estradiol | 3.9 ± 2.4 | 4.9 ± 3.4 | 3.6 ± 1.7 | 4.5 ± 2.6 |
| Estrone | 15.3 ± 4.8 | 20.3 ± 8.6 | 16.7 ± 7.8 | 19.4 ± 8.8 |
| Estrone conjugates | 237.7 ± 180.8 | 239.2 ± 174.2 | 343.4 ± 421.1 | 275.9 ± 152.6 |
Data represented as mean ± SD unless stated otherwise.
BMI, body mass index.
Pharmacokinetics results
Estradiol
Mean serum estradiol concentrations by time for each dose are shown in Figures 1 to 3. Mean baseline values were ≤5.0 pg/mL for all groups and ranged from 2.0 to 15.7 pg/mL for individuals. Estradiol concentrations on day 84 were similar to baseline and placebo for TX-004HR 4, 10, and 25-μg dose groups.
FIG. 1.
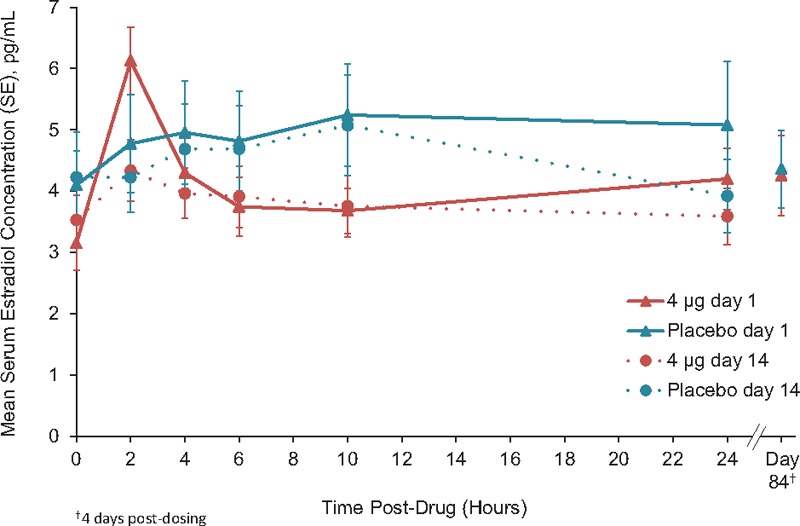
Unadjusted mean serum estradiol concentrations TX-004HR 4 μg (n = 18) versus placebo (n = 17).
FIG. 3.
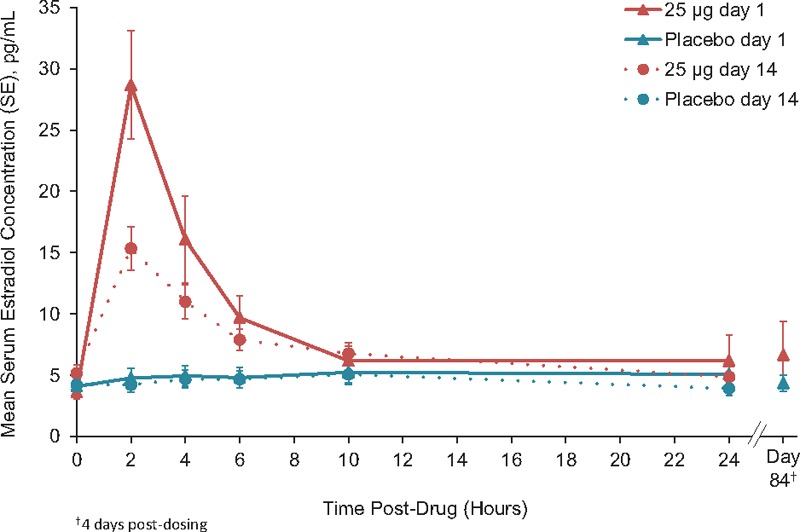
Unadjusted mean serum estradiol concentrations TX-004HR 25 μg (n = 18) versus placebo (n = 17).
FIG. 2.
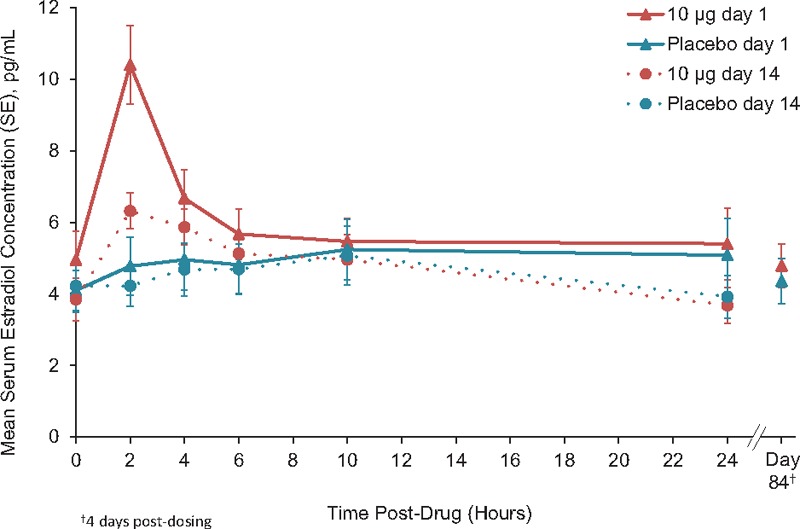
Unadjusted mean serum estradiol concentrations TX-004HR 10 μg (n = 19) versus placebo (n = 17).
No statistical differences were observed between TX-004HR 4 μg and placebo in any of the unadjusted estradiol measures of AUC, Cmax, Cavg, Cmin, and tmax (Table 2).
TABLE 2.
Unadjusted pharmacokinetic parameters (mean ± SD)
| TX-004HR | Placebo (n = 17) | |||
| 4 μg (n = 18) | 10 μg (n = 19) | 25 μg (n = 18) | ||
| Estradiol | ||||
| Day 1 | ||||
| AUC, pg×h/mL | 91.7 ± 37.9 | 138.2 ± 75.2 | 217.4 ± 99.0a | 116.6 ± 77.3 |
| Cmax, pg/mL | 6.5 ± 2.1 | 10.9 ± 5.0b | 29.8 ± 17.5c | 6.6 ± 4.9 |
| Cavg, pg/mL | 3.9 ± 1.5 | 5.8 ± 3.1 | 9.1 ± 4.1a | 4.9 ± 3.2 |
| Cmin, pg/mL | 3.3 ± 1.3 | 4.4 ± 2.7 | 3.5 ± 1.7 | 3.9 ± 2.6 |
| tmax, h | 7.0 ± 9.4 | 6.1 ± 8.0 | 4.6 ± 7.1 | 8.6 ± 6.7 |
| Day 14 | ||||
| AUC, pg×h/mL | 87.2 ± 42.8 | 110.1 ± 54.6 | 171.6 ± 80.1b | 104.2 ± 66.4 |
| Cmax, pg/mL | 4.8 ± 2.3 | 7.3 ± 2.4 | 15.7 ± 7.6c | 5.5 ± 3.4 |
| Cavg, pg/mL | 3.6 ± 1.8 | 4.6 ± 2.3 | 7.1 ± 3.3b | 4.3 ± 2.8 |
| Cmin, pg/mL | 3.2 ± 1.5 | 3.6 ± 1.9 | 4.3 ± 2.5 | 3. 4 ± 2.1 |
| tmax, h | 9.3 ± 8.9 | 4.0 ± 2.6a | 2.7 ± 1.9c | 7.2 ± 3.0 |
| Estrone | ||||
| Day 1 | ||||
| AUC, pg×h/mL | 290.2 ± 123.7b | 462.7 ± 195.6 | 419.1 ± 147.9 | 467.9 ± 278.8 |
| Cmax, pg/mL | 15.8 ± 6.1b | 23.5 ± 9.9 | 21.9 ± 7.7 | 25.7 ± 18.4 |
| Cavg, pg/mL | 13.0 ± 4.7b | 19.3 ± 8.2 | 17.5 ± 6.2 | 19.5 ± 11.6 |
| Cmin, pg/mL | 11.6 ± 4.3 | 17.1 ± 7.5 | 15.0 ± 5.8 | 16.5 ± 10.0 |
| tmax, h | 14.1 ± 9.4 | 11.9 ± 9.8 | 9.1 ± 7.4 | 12.1 ± 9.4 |
| Day 14 | ||||
| AUC, pg×h/mL | 326.6 ± 114.1 | 464.1 ± 243.9 | 428.7 ± 161.7 | 426.8 ± 180.7 |
| Cmax, pg/mL | 16.0 ± 5.5b | 23.9 ± 13.5 | 22.4 ± 8.9 | 22.8 ± 10.9 |
| Cavg, pg/mL | 13.6 ± 4.8 | 19.3 ± 10.2 | 17.9 ± 6.7 | 17.8 ± 7.5 |
| Cmin, pg/mL | 12.2 ± 4.4 | 17.4 ± 8.5 | 16.0 ± 6.1 | 14.3 ± 7.1 |
| tmax, h | 10.9 ± 9.0 | 10.4 ± 8.9 | 6.3 ± 6.9b | 12.2 ± 9.2 |
| Estrone conjugates | ||||
| Day 1 | ||||
| AUC, pg×h/mL | 5078 ± 3798 | 5932 ± 4210 | 9126 ± 9186 | 5638 ± 3151 |
| Cmax, pg/mL | 273.1 ± 196.4 | 329.4 ± 226.6 | 542.1 ± 475.5 | 309.8 ± 146.1 |
| Cavg, pg/mL | 215.9 ± 154.8 | 247.2 ± 175.4 | 380.3 ± 382.8 | 244.6 ± 128.1 |
| Cmin, pg/mL | 178.3 ± 128.7 | 208.7 ± 137.0 | 288.0 ± 311.3 | 207.6 ± 146.0 |
| tmax, h | 10.9 ± 8.7 | 9.2 ± 9.3 | 5.4 ± 2.6a | 13.1 ± 9.7 |
| Day 14 | ||||
| AUC, pg×h/mL | 5173 ± 3383 | 8978 ± 9811 | 9930 ± 11710 | 6275 ± 3398 |
| Cmax, pg/mL | 289.0 ± 183.8 | 511.7 ± 568.8 | 579.5 ± 610.1 | 343.6 ± 182.2 |
| Cavg, pg/mL | 215.5 ± 141.0 | 374.1 ± 408.8 | 413.8 ± 488.0 | 261.5 ± 141.6 |
| Cmin, pg/mL | 174.3 ± 121.8 | 271.6 ± 285.0 | 303.7 ± 380.6 | 207. 8 ± 111.5 |
| t, h | 8.4 ± 7.8 | 9.0 ± 8.6 | 5.9 ± 2.9 | 8.1 ± 6.8 |
Data are unadjusted for baseline and represented as mean ± SD.
AUC, area under the serum concentration-time curve.
aP < 0.01, bP < 0.05, cP < 0.0001 versus placebo using t tests.
TX-004HR 10-μg dose, AUC, and Cavg were not different than with placebo. Cmax values were higher than placebo (mean of 10.9 pg/mL for 10 μg vs 6.6 pg/mL for placebo) on day 1, but were not significantly different from placebo on day 14 (Table 2).
With the 25-μg dose, estradiol AUC, Cmax, and Cavg were statistically different from placebo at days 1 and 14 (Table 2). Estradiol average concentrations for the 25-μg dose were 9.1 and 7.1 pg/mL on days 1 and 14, respectively. Overall, AUC values were lower at day 14 than day 1 for all groups. No significant differences were observed in overall mean AUC and Cmax, ratios (day 14-day 1) between each group and placebo. Baseline-adjusted results for estradiol were similar to the unadjusted results (Supplemental Table S1, Supplemental Digital Content 1).
Estrone and estrone conjugates
Mean baseline estrone concentrations ranged from 15.3 to 20.3 pg/mL for all groups. No values were significantly higher for TX-004HR 4, 10, and 25-μg doses versus placebo for any of the unadjusted estrone AUC, Cmax, Cavg, Cmin, and tmax on days 1 and 14, except on day 14 tmax for the 25-μg dose (Table 2).
Similar mean estrone conjugates baseline concentrations were observed across all groups, ranging from 237.7 to 343.4 pg/mL. At days 1 and 14, the estrone conjugate concentrations ranged between approximately 100 to 400 pg/mL in most women. No differences from placebo in any estrone conjugate parameters were observed for any of the TX-004HR doses, except for day 1 tmax for the 25-μg dose (Table 2). Baseline-adjusted results for estrone and estrone conjugates were similar to the unadjusted results (Supplemental Table S1, Supplemental Digital Content 1).
Sex hormone binding globulin
Least square mean changes in SHBG from baseline to week 2 were: 3.0, 1.9, 2.2, and −2.1 nmol/L; and to week 12 were: 1.9, 4.4, −0.6, and 10.2 nmol/L, respectively, for TX-004HR 4, 10, and 25 μg, and placebo.
DISCUSSION
Vaginal administration of 4, 10, and 25 μg of TX-004HR resulted in negligible to very low systemic absorption of estradiol in healthy postmenopausal women with VVA while providing VVA efficacy and symptom relief as early as week 2. In the primary REJOICE study, TX-004HR at 4, 10, and 25 μg demonstrated robust efficacy for improvement in the percentage of superficial and parabasal cells, vaginal pH, and severity of dyspareunia as early as 2 weeks, with continued efficacy up to 12 weeks.8 Also, 4, 10, and 25 μg TX-004HR, when compared with placebo, significantly improved vaginal dryness and vulvar and/or vaginal itching or irritation.8
The most relevant pharmacokinetic parameters in this study are Cavg, which measures the average plasma concentration of TX-004HR during a dosing interval, and AUC, which measures the overall systemic exposure of TX-004HR. Both estradiol Cavg and AUC were not statistically different for 4 and 10 μg TX-004HR compared with placebo. Estradiol AUC and Cavg for the 25-μg dose were significantly higher than with the placebo on days 1 and 14, but average levels of estradiol were very low and remained within the normal postmenopausal range (≤30 pg/mL).9 An initial increase in estradiol absorption followed by a decrease over time has been commonly observed in PK studies of vaginal estrogens,10-12 and may be due to higher absorption through thin, atrophic vaginal tissues early in treatment, and then less absorption with improvement in vaginal maturation later in treatment.13 These data demonstrated that TX-004HR provided a novel delivery system with negligible systemic estradiol absorption.
Overall, there did not appear to be any estradiol accumulation with any doses of TX-004HR as endogenous values were observed at day 84. In addition, no notable changes in estrone conjugates or SHBG were observed with TX-004HR, consistent with the negligible to very low absorption of estradiol with its administration. Vaginal, low-dose estrogen therapies are intended to effectively treat symptoms of VVA with negligible systemic absorption of estrogen.1 However, whereas current products limit systemic estrogen absorption, they do not completely eliminate it, as elevated circulating estrogen levels with some vaginal products have been shown in PK studies.12,14 A rapid rise in estradiol concentrations was found with a 25-μg vaginal estradiol tablet within 8 hours of administration, which remained above normal postmenopausal levels for 2 weeks.12 Vaginal creams have also been associated with adverse effects such as breast pain and uterine bleeding, which are indicative of systemic exposure to estradiol.15 Similarly, a low-dose vaginal estrogen ring, which releases 7.5 μg/d, showed a peak estradiol concentration of 60 pg/mL at 2 hours; although estradiol rapidly declined to be within normal postmenopausal estrogen levels, decreases in the rate of bone resorption and in low-density lipoprotein cholesterol levels were observed, suggesting systemic estrogenic effects.16 Further, a PK study using a validated, accurate, and sensitive MS assay showed that serum estradiol increased by at least five-fold with a vaginal estradiol tablet and a conjugated estrogens cream after 1 week of daily treatment.14 Advantages of limiting estrogen exposure to the vagina as opposed to systemic estrogen include decreased risk of systemic adverse effects and endometrial stimulation.17 In the current study, TX-004HR administration resulted in estradiol levels that remained well within the normal postmenopausal range, and the reported adverse events were not indicative of estrogen systemic exposure.
The PK profile for TX-004HR (ie, negligible to very low systemic absorption) could allow clinicians to follow the recommendation of The North American Menopause Society to use low-dose vaginal estrogens without a concomitant progestin.1 Furthermore, the lack of systemic absorption with the use of TX-004HR is aligned with the American Congress of Obstetricians and Gynecologists’ recommendation for the use of low-dose vaginal estrogens for treating vaginal symptoms in survivors of estrogen-dependent breast cancer.3
Although any woman meeting entry criteria was eligible for enrollment into the clinical trial, the study population was predominately white, which may limit the generalizability of the study results to the general population.
CONCLUSIONS
Vaginal administration of TX-004HR resulted in negligible to very low systemic absorption of estradiol with no drug accumulation. PK parameters with TX-004HR at 4 and 10 μg were similar to placebo for key estradiol parameters. Whereas there were minor increases in these estradiol parameters with 25 μg TX-004HR, serum concentrations remained within the normal postmenopausal range. This PK substudy, in conjunction with the efficacy results of the main study, demonstrates that TX-004HR provides local estrogenic benefits with minimal increases in estrogen systemic exposure.
Supplementary Material
Acknowledgments
The authors would like to thank the investigators of the REJOICE Study Group who participated in the PK substudy: David F. Archer, MD, Eastern Virginia Medical School, Norfolk, VA; Manon Gélinas, MD, Diex Research Montreal, Inc, Montreal, Quebec, Canada; Ginette Girard, MD, CPI, Diex Research Sherbrooke Inc, Sherbrooke, Quebec, Canada; Irwin Goldstein, MD, San Diego Sexual Medicine, San Diego, CA; David A. Grainger, MD, MPH, Cypress Medical Research Center, LLC, Wichita, KS; John M. Hill, MD, RRT, CPI, Avail Clinical Research, LLC, Deland, FL; Robin Kroll, MD, Seattle Women's: Health, Research, and Gynecology, Seattle, WA; Barry Lubin, MD, National Clinical Research – Norfolk, Inc, Norfolk, VA; Andrea S. Lukes, MD, Carolina Women's Research & Wellness Center, Durham, NC; R. Garn Mabey, MD, Office of R. Garn Mabey, MD, Las Vegas, NV; Andrée Vachon, MD, Centre de Recherche Saint-Louis, Quebec, Quebec, Canada; Debra Walland, MD, FACOG, CPI, Fellows Research Alliance, Inc, Savannah, GA. The authors would also like to acknowledge the medical writing assistance of Jolene Mason, PhD and Dominique Verlaan, PhD of Precise Publications, LLC, which was supported by TherapeuticsMD.
Footnotes
Funding/support: TherapeuticsMD sponsored the study and funded the medical writing support provided by Jolene Mason, PhD and Dominique Verlaan, PhD of Precise Publications, LLC.
Financial disclosure/conflicts of interest: Dr Archer (within the past 3 years) has received research support from Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Bayer Healthcare, Endoceutics, Glenmark, Merck (previously Schering Plough, Organon), Radius Health, Shionogi Inc, and TherapeuticsMD; has served as consultant to Abbvie (previously Abbott Laboratories), Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis (previously CHEMO), Ferring Pharmaceuticals, InnovaGyn, Merck (previously Schering Plough, Organon), Pfizer, Radius Health, Sermonix Pharmaceuticals, Shionogi, Inc, Teva Women's Healthcare, and TherapeuticsMD; and has received Speakers’ Bureau honorarium for Ascend Therapeutics, Merck (previously Schering Plough, Organon), Noven, and Pfizer. Dr Constantine, Dr Kushner, and Dr Mayer consult to pharmaceutical companies, including, but not limited to, TherapeuticsMD. Dr Simon has served (within the past year) or is currently serving as a consultant to or on the advisory boards of: AbbVie, Inc, AMAG Pharmaceuticals, Inc, Amgen Inc, Apotex, Inc, Ascend Therapeutics, JDS Therapeutics, LLC, Merck & Co, Inc, Noven Pharmaceuticals, Inc, Novo Nordisk, Nuelle, Inc, Perrigo Company, PLC, Radius Health, Inc, Regeneron Pharmaceuticals, Inc, Roivant Sciences, Inc, Sanofi S.A., Sermonix Pharmaceuticals, Inc, Shionogi Inc, Sprout Pharmaceuticals, Symbiotec Pharmalab, TherapeuticsMD; and has also served (within the past year) or is currently serving on the speaker's bureaus of: Amgen Inc, Eisai, Inc, Merck, Noven Pharmaceuticals, Inc (New York, NY), Novo Nordisk, Shionogi Inc; and in the last year has received or is currently receiving grant/research support from: AbbVie, Inc, Actavis, PLC, Agile Therapeutics, Bayer Healthcare LLC, New England Research Institute, Inc, Novo Nordisk, Palatin Technologies, Symbio Research, Inc, TherapeuticsMD; and is a stockholder (direct purchase) in Sermonix Pharmaceuticals. Dr Bernick is a board member and an employee of TherapeuticsMD with stock/stock options. Dr Graham and Dr Mirkin are employees of TherapeuticsMD with stock/stock options.
REFERENCES
- 1.North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20:888–902. [DOI] [PubMed] [Google Scholar]
- 2.Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women's Health Initiative. Maturitas 2004; 49:292–303. [DOI] [PubMed] [Google Scholar]
- 3.The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Committee Opinion No. 659. American College of Obstetricians and Gynecologists. Obstet Gynecol 2016; 127:e93–e96. [DOI] [PubMed] [Google Scholar]
- 4.Ballagh SA. Vaginal hormone therapy for urogenital and menopausal symptoms. Semin Reprod Med 2005; 23:126–140. [DOI] [PubMed] [Google Scholar]
- 5.NAMS Leaders Request Labeling Change for Low-dose Vaginal Estrogen for Vulvovaginal Atrophy. 2016. Available at: https://speakingofwomenshealth.com/news/nams-leaders-request-labeling-change-for-low-dose-vaginal-estrogen-for-vulvovaginal-atrophy Accessed January 6, 2016. [Google Scholar]
- 6.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (Real Women's Views of Treatment Options for Menopausal Vaginal Changes) survey. J Sex Med 2013; 10:1790–1799. [DOI] [PubMed] [Google Scholar]
- 7.Pickar JH, Amadio JM, Bernick BA, Mirkin S. Pharmacokinetic studies of solubilized estradiol given vaginally in a novel softgel capsule. Climacteric 2016; 19:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantine GD, Simon JA, Pickar JH, et al. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause 2016; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo RA. Strauss JF, Barbieri RL. Menopause and aging. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 6th ed.Philadelphia, PA: Saunders Elsevier; 2009. 325–355. [Google Scholar]
- 10.Gupta P, Ozel B, Stanczyk FZ, Felix JC, Mishell DR., Jr The effect of transdermal and vaginal estrogen therapy on markers of postmenopausal estrogen status. Menopause 2007; 15:94–97. [DOI] [PubMed] [Google Scholar]
- 11.Rioux JE, Devlin C, Gelfand MM, Steinberg WM, Hepburn DS. 17β-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause 2000; 7:156–161. [DOI] [PubMed] [Google Scholar]
- 12.Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 microg 17beta-estradiol vaginal tablets. Climacteric 2010; 13:219–227. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson K, Heimer G. Low-dose oestradiol in the treatment of urogenital oestrogen deficiency: a pharmacokinetic and pharmacodynamic study. Maturitas 1992; 15:121–127. [DOI] [PubMed] [Google Scholar]
- 14.Labrie F, Cusan L, Gomez JL, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause 2009; 16:30–36. [DOI] [PubMed] [Google Scholar]
- 15.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006; 4: CD001500. [DOI] [PubMed] [Google Scholar]
- 16.Holmgren PA, Lindskog M, von Schoultz B. Vaginal rings for continuous low-dose release of oestradiol in the treatment of urogenital atrophy. Maturitas 1989; 11:55–63. [DOI] [PubMed] [Google Scholar]
- 17.Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000-2009. Menopause 2011; 18:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


