Supplemental Digital Content is available in the text.
Keywords: Actovegin, post-stroke cognitive impairment, stroke, vascular dementia
Abstract
Background and Purpose—
Poststroke cognitive impairment is a debilitating consequence of stroke. The aim of this study was to assess whether Actovegin confers cognitive benefit in patients who have had an ischemic stroke.
Methods—
This was a 12-month, parallel-group, randomized, multicenter, double-blind, placebo-controlled study. Eligible patients were ≥60 years of age with a Montreal Cognitive Assessment test score of ≤25 points. Patients were randomized into 2 groups within 1 week of acute supratentorial ischemic stroke in a 1:1 ratio: Actovegin (a deproteinized hemoderivative of calf blood, 2000 mg/d for ≤20 intravenous infusions followed by 1200 mg/d orally) or placebo for 6 months. Patients were treated in accordance with standard clinical practice for a further 6 months. The primary end point was the change from baseline in Alzheimer’s Disease Assessment Scale, cognitive subscale, extended version at 6 months.
Results—
Two-hundred forty-eight patients were randomized to Actovegin and 255 patients to placebo. At month 6, the least squares mean change from baseline in Alzheimer’s Disease Assessment Scale, cognitive subscale, extended version was −6.8 for Actovegin and −4.6 for placebo; the estimated treatment difference was −2.3 (95% confidence interval, −3.9, −0.7; P=0.005). Recurrent ischemic stroke was the most frequently reported serious adverse event, with a nonsignificantly higher number for Actovegin versus placebo.
Conclusions—
Actovegin had a beneficial effect on cognitive outcomes in patients with poststroke cognitive impairment. The safety experience was consistent with the known safety and tolerability profile of the drug. These results warrant confirmation in additional robustly designed studies.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01582854.
Although the standard of stroke care has improved substantially and rates of stroke mortality have decreased in the past 2 decades, stroke remains one of the most common causes of death worldwide and the third leading cause of loss of disability-adjusted life years.1–3 Stroke significantly increases cognitive decline among stroke survivors.4 Poststroke cognitive impairment (PSCI) is associated with significant morbidity with ≤41% of patients becoming clinically demented in the first year after a stroke.5
Targets critical to the prevention of PSCI focus on acute treatment and the prevention of recurrence. However, there are no established therapeutic strategies, and candidate pharmacological therapies have yet to demonstrate efficacy in reducing or preventing cognitive decline after stroke, using randomized controlled trials in the acute stroke setting.6,7
Randomized controlled trials examining the use of Alzheimer’s disease (AD) symptomatic therapies such as acetylcholinesterase inhibitors (AChEIs) and memantine have shown some clinical benefits in vascular dementia (VaD) but have not been granted U.S. Food & Drug Administration approval for use in VaD primarily because of inconsistent efficacy with respect to activities of daily living and global function.8 Approaches targeting multiple pathogenetic mechanisms, such as biological therapies, have hinted at a breakthrough, but the available evidence has also been limited to date.9
Actovegin is a deproteinized pyrogen- and antigen-free hemodialysate manufactured from calf blood by ultrafiltration. It contains >200 bioactive constituents (with molecular weight <5 kDa) and exhibits a range of pleiotropic effects.10 Actovegin improves oxygen utilization and uptake, as well as energy metabolism and glucose uptake in mitochondria, thereby enhancing oxidative metabolism in the brain.11 Actovegin has been shown to possess neuroprotective potential; it ameliorated Aβ25–35-induced neuronal apoptosis by reducing caspase-3 levels in a dose-dependent manner and decreasing reactive oxygen species content in hippocampal neurons.12 The effects of Actovegin on cerebral metabolism, mortality, and cognitive performance have also been assessed in animal models of cerebral ischemia.13–15 Actovegin facilitated [14C] glucose uptake into the brain under hypoxic conditions and normalized metabolic parameters, measured as the concentrations of glucose, lactate, creatine phosphate, and adenosine triphosphate normalized in 2-year-old rats. Most recently, a study in a rat model of transient global cerebral ischemia found that Actovegin significantly decreased hippocampal CA1 cell death and improved spatial learning and memory.16
Actovegin received its market authorization in 1976 in Germany; in 1995, production was transferred from Germany to Linz, Austria. Actovegin is registered for clinical use in Austria, Russia, countries of The Commonwealth of Independent States, some Eastern European, and Asian countries. Actovegin was never introduced to the U.S. Food & Drug Administration; therefore, it is not marketed in North America. In the clinical setting, Actovegin has been used for around 40 years for the treatment of various neurological disorders, including cerebrovascular disease and cognitive decline of various origins. It is also prescribed for the treatment of peripheral arterial disease and diabetic polyneuropathy. In randomized, placebo-controlled trials, Actovegin has been shown to improve cognitive performance in patients with age-associated memory impairment.17,18 Two pilot studies in stroke patients have shown treatment benefits with Actovegin based on many cognitive and neurological deficit measures. It has provided some evidence on the effects of Actovegin in acute ischemic stroke, but these have never been tested in a multicenter randomized study.19,20 Apart from its clinical properties, it has been suggested that Actovegin also has ergogenic effects. This is, however, not based on scientific evidence; the speculation emerged because Actovegin has repeatedly been used as a performance-enhancing drug by professional cyclists and by Olympic athletes, possibly to accelerate muscle injury repair and improve endurance. Late in 2000, Actovegin was included on the World Anti-Doping Agency active list, but it was removed again after 2 months because of insufficient direct evidence demonstrating an ergogenic effect of Actovegin.21 The overall safety profile of Actovegin seems favorable, and its clinical use for >35 years has not identified any unacceptable safety concerns. Indeed, the Actovegin summary of product characteristics22 states that only in rare cases, patients prone to hypersensitivity may develop allergic reactions (medication fever and anaphylactic shock), urticaria, flush, and myalgia. In contrast, AChEIs and memantine have side effects which occur commonly in patients, including gastrointestinal effects (diarrhea or constipation), headache, and dizziness.23,24
The ARTEMIDA study (A Randomized Trial of Efficacy, 12 Months International Double-Blind Actovegin) was designed to test the hypothesis that Actovegin would confer cognitive benefits in patients with acute ischemic stroke.25 In addition, we wanted to explore whether the therapeutic effects are sustained after treatment cessation and provide evidence of efficacy and safety tolerability of Actovegin for the symptomatic effect on PSCI.
Methods
Study Design, Treatment Regimen, and Procedures
ARTEMIDA was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study assessing the effects of Actovegin on cognitive functioning in patients with PSCI. Patients were recruited from 33 tertiary hospitals in Russia, Belarus, and Kazakhstan (Table I in the online-only Data Supplement). The study consisted of a screening and randomization period (≤7 days post-stroke), a 6-month double-blind treatment period and a 6-month follow-up period (Figure I in the online-only Data Supplement). During double-blind treatment, patients were randomized to receive either intravenous Actovegin (0.9% sodium chloride; 2000 mg/250 mL daily for ≤20 infusions followed by 1200 mg/d orally [two 200 mg tablets 3× daily]) or placebo for 6 months. Actovegin and placebo were then discontinued, and patients were followed up for a subsequent 6 months. Patients were hospitalized in stroke units for first or recurrent stroke and received standard stroke care in accordance with local guidelines which included general supportive care, treatment of acute complications, rehabilitation, and antiplatelet therapy; nootropic agents were excluded. Visits were scheduled at baseline, at the end of the infusion period, then every 4 weeks until the end of 6 months treatment, and a single visit 6 months after the end of treatment. Adverse events (AEs), treatment compliance, and concomitant medication use were reported during each visit. The study protocol was approved by each respective institutional review board or ethics committee and followed established Good Clinical Practice guidelines. All patients gave written informed consent to participate in the study. An independent contract research organization, Pharm-Olam International Ltd (Houston), managed the administration, coordination, and monitoring of the study, including data management, statistical analysis, and the Interactive Voice Response System–Interactive Web Response System, with oversight by Takeda.
Inclusion and Exclusion Criteria
Patients were eligible for enrolment if they were ≥60 years of age, had a clinical diagnosis of acute supratentorial ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score of 3–18) confirmed by computed tomography or magnetic resonance imaging. Patients must have been conscious, able to complete the Montreal Cognitive Assessment (MoCA; score of ≤25 points with adjustment for level of education) and extended version of the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog+), and without evidence of dementia documented in medical records or according to concern of a knowledgeable informant. A detailed patient history was performed. Full lists of study inclusion and exclusion criteria and prohibited medications during double-blind treatment are provided in Tables II through IV in the online-only Data Supplement.
Randomization and Masking
Patients were randomized to receive either Actovegin or placebo in a 1:1 ratio, without stratification and using a block size of 4, by means of a computerized central randomization system, Interactive Voice Response System–Interactive Web Response System. During double-blind treatment and until end of follow-up, all investigators and patients were masked to treatment assignment (Randomization and Masking Full Methods in the online-only Data Supplement).
Efficacy and Safety Criteria
The primary end point, the ADAS-cog+ change from baseline, was performed at 6 months. The same measure was performed at 3 and 12 months as a secondary end point. Other secondary end points were performed as follows: the change from baseline in MoCA and the NIHSS during screening, 3, 6, 12 months, and at the end of the infusion period; the Beck Depression Inventory (BDI-II) at 3, 6, and 12 months; the Barthel Index at 6 months; the EuroQoL EQ-5D questionnaire at 6 and 12 months; and the International Statistical Classification and Related Health Problems, version 10 diagnosis of dementia at 6 and 12 months. A full list of all primary, secondary, and safety end points is given in Table V in the online-only Data Supplement. The presentation of safety data focused on treatment-emergent AEs (TEAEs). TEAEs were defined as AEs that first occurred or worsened (increase in severity) after the first dose of study drug. There were no prespecified safety end points. TEAEs were reported spontaneously by the patient in response to an open-ended question by the contact at each visit; abnormal laboratory values that constituted a serious adverse event or led to the discontinuation of Actovegin were reported as a TEAE.
Statistical Analysis
All reported efficacy analyses were predefined and based on the intent-to-treat (ITT) population (randomized and received any study medication). In addition, supportive analyses were performed in a predefined per protocol population which includes those ITT patients who did not have any key protocol deviations, including violation of inclusion and exclusion criteria (Per Protocol Analyses in the online-only Data Supplement).
For the analyses of change from baseline in ADAS-cog+, partially complete ADAS-cog+ assessments with missing individual item scores were imputed with the worst score on the test. Completely missing ADAS-cog+ scores at 6 months were imputed by last observation carried forward from 3 months, considered conservative as the condition under study was expected to improve spontaneously over this period.26 Change from baseline in ADAS-cog+ at 3, 6 (primary end point), and 12 months was analyzed using an ANCOVA model, including treatment, grouped center, baseline score, and the treatment by grouped center interaction (included as significant at the 10% significance level). The grouping of centers into 8 geographical groups was determined before unblinding. Changes in MoCA and NIHSS score were analyzed using ANCOVA models, including treatment, grouped center, baseline score, and in addition for MoCA, number of years of education. The least squares (LS [population margin mean]) means, mean differences between treatments, and associated 95% confidence intervals (CIs) were estimated from the ANCOVA models.
The ADAS-cog has been widely used as the primary efficacy outcome in AD clinical trials and has been used to assess cognitive impairment in stroke trials along with other instruments.27,28 The ADAS-cog+ is an expanded version of the ADAS-cog, which has increased sensitivity in detecting patients with mild cognitive impairment and may be particularly useful in vascular cognitive impairment29; however, it has never been used in clinical trials to assess the effects of treatments on cognition in stroke patients. Generally, in clinical trials, outcomes may be measured in different ways. We defined responders as having a ≥4-point improvement on the ADAS-cog+ as this is accepted to represent a clinically relevant change on an individual basis in patients with mild-to-moderate AD.30,31
The proportions of ADAS-cog+ responders at 3, 6, and 12 months were compared between treatments using a χ2 test, and the proportions of patients with a diagnosis of dementia (according to International Statistical Classification and Related Health Problems, version 10 criteria) by 6 and 12 months were compared using a Fisher exact test; 95% CI was calculated using the Normal approximation to the Binomial distribution with a continuity correction. The Barthel Index, BDI-II, and EuroQoL EQ-5D were summarized using descriptive statistics.
The sample size, based on a 2-sample t test with 5% 2-sided significance level, estimated that 200 patients per treatment arm would provide 90% power to detect a difference of at least 2.6 in the mean change from baseline to 6 months in ADAS-cog+ score between the groups, assuming a common SD of 8.0. After allowing for an estimated dropout rate of 20%, a total of 500 patients were planned to be randomized.
A more accurate power calculation would have been obtained if it had been based on the ANCOVA model, but at the time of planning the study, the information about correlation between outcome and covariate was not available. Therefore, a preexisting estimate of the SD from an ANOVA analysis was used as a suitable alternative.
No multiplicity adjustments were made to the primary and secondary end point results. SAS version 9.1.3 was used for all statistical analyses.
Results
Study Population
Patient recruitment began in June 2012, and the study ended in November 2014. Of 522 patients recruited, 503 patients were randomized (248 to Actovegin and 255 to placebo), received ≥1 dose of study medication, and were included in the ITT and safety analysis set (see Figure 1 for patient flow diagram). However, there were 2 patients who were randomized to the placebo group but received an incorrect kit, which after unblinding was determined to have contained Actovegin. Those patients were included as randomized for the ITT analyses (placebo group) and as treated for the safety analyses. Patients who had not prematurely discontinued the study were considered to have completed the study. Study discontinuation was similar in both groups, 36 (14.5%) in the Actovegin group and 34 (13.3%) in the placebo group. Key protocol deviations (eg, disallowed concomitant medication, ADAS-cog+ performed incorrectly; Table VI in the online-only Data Supplement) were similar between groups (placebo: n=35 [13.7%], Actovegin: n=36 [14.5%]). In total, 71 (14.1%) of 503 patients had at least 1 key protocol deviation. In terms of stroke care, overall, 81.1% of patients had received ≥1 of the following rehabilitation therapies: physiotherapy (86.3%), other rehabilitation therapy (40.0%), speech therapy (21.7%), cognitive rehabilitation therapy (18.9%), and occupational therapy (16.5%). The incidences of rehabilitation therapy were similar between the treatment groups.
Figure 1.
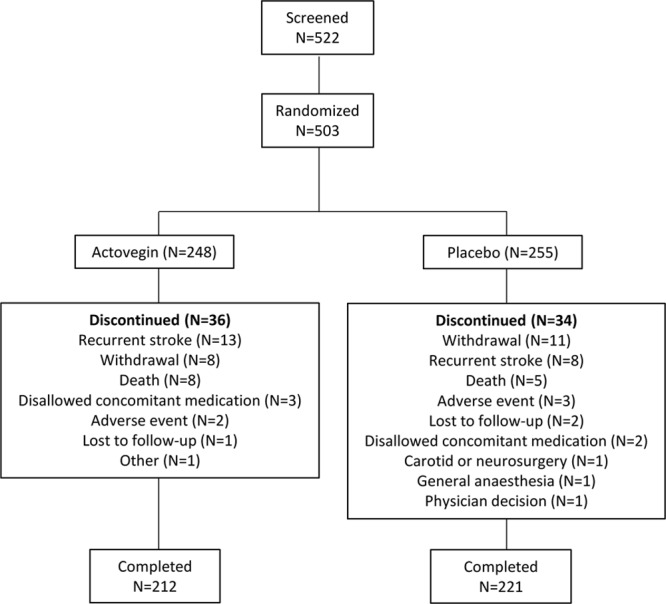
Patient flow diagram. The numbers presented are as treated. There were 2 patients who were randomized to the placebo group but received an incorrect kit, which after unblinding was determined to have contained Actovegin. Those patients are included as randomized for the intent-to-treat (ITT) analyses (placebo group) and as treated for the safety analyses (Actovegin group). More than 1 reason for discontinuation could have been selected.
All of the patients reported medical history and concomitant disease. The incidences of reported medical history and concomitant disease were similar between the treatment groups Table 1). Overall, the following were reported in ≥20.0% of patients: hypertension (87.1%), myocardial ischemia (28.8%), cerebral arteriosclerosis (23.5%), coronary artery disease (21.3%), and arteriosclerosis (24.1%). Table 1 shows the demographic and baseline characteristics of the ITT analysis set. Overall, there were no meaningful differences in demographic and baseline characteristics between the study groups. The mean (SD) NIHSS score at baseline was similar in both Actovegin and placebo groups (5.3 [2.24] versus 5.6 [2.37]). The mean total number of intravenous infusions was 12.2 (median of 12.0) in both groups. Adherence to treatment was high (a mean of 99.6% for the infusions and 93.3% for the tablets).
Table 1.
Baseline Characteristics of the Intent-to-Treat Population
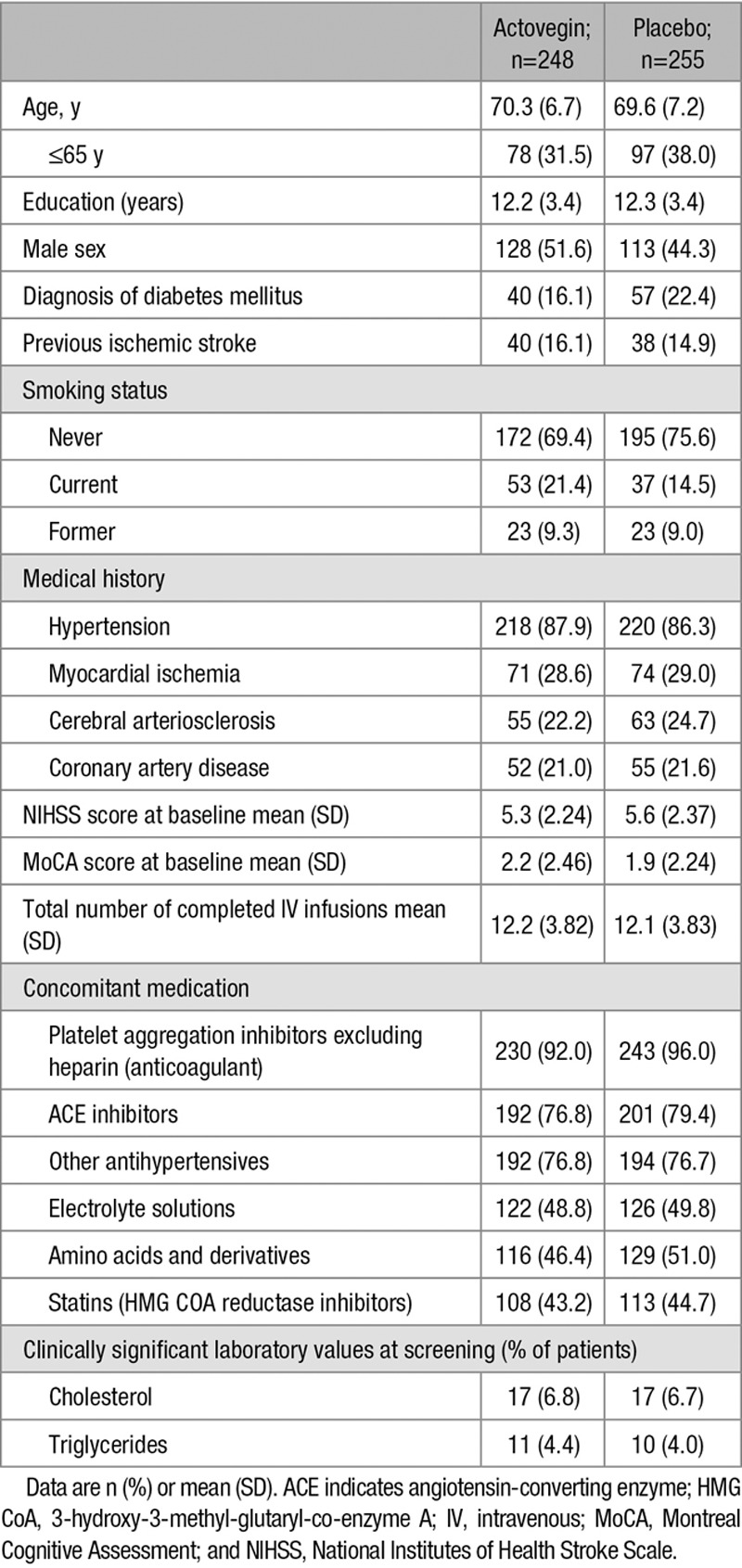
Clinically significant laboratory values (cholesterol and low-density lipoprotein cholesterol) were observed at screening (Table 1). These values remained significant at month 6 for cholesterol (14/253 patients [5.5%] in the placebo group and 12/250 patients (4.8%) in the Actovegin group) and low-density lipoprotein cholesterol (15/253 patients [5.9%] in the placebo group and 11/250 patients [4.4%] in the Actovegin group).
Efficacy
Primary End Point
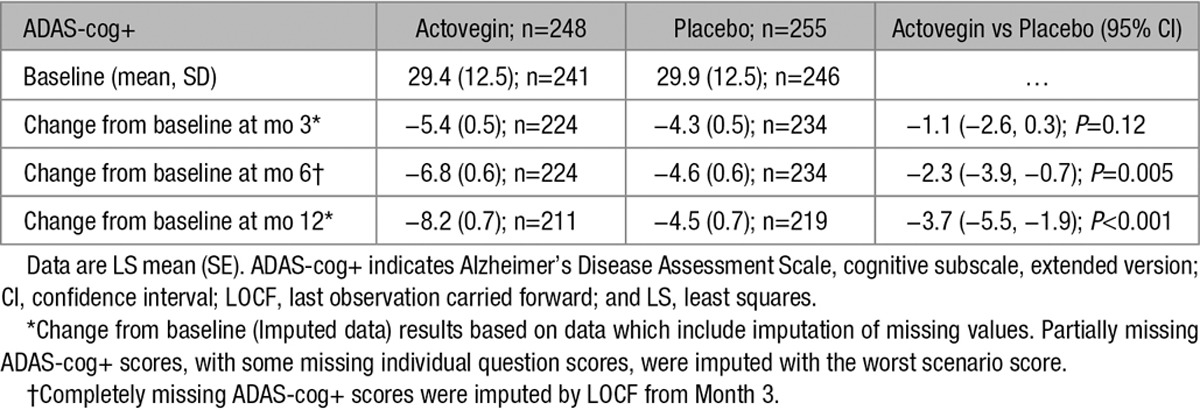
Figure 2A illustrates and Table 2 enumerates the effect of Actovegin and placebo on the change in ADAS-cog+ from baseline to months 3, 6 (primary end point), and 12. At baseline, the mean (SD) ADAS-cog+ score was similar between Actovegin and placebo groups (29.4 [12.45] and 29.9 [12.49], respectively). The LS mean change (SE) from baseline in ADAS-cog+ at month 6 was greater for Actovegin (−6.8 [0.58]) than for placebo (−4.6 [0.58]), and the estimated LS mean treatment difference from the model was statistically significant (−2.3; 95% CI, −3.9, −0.7; P=0.005). The results of the ANCOVA for the per protocol analysis set (196 and 202 patients in the Actovegin and placebo groups, respectively, at 6 months) were supportive of the results of the primary analysis on the ITT set described (Per Protocol Analyses in the online-only Data Supplement). Table 2 illustrates that missing scores at month 6 were imputed using the month 3 scores (therefore, the number of observations is the same for these 2 measurements), whereas imputation for months 3 and 12 missing scores was not planned because these were secondary end points. The reasons for differing patient numbers with scores between randomization, baseline, and each visit is because of patients dropping out of the study (Figure 1), patients not turning up for a visit, or incorrectly completing the ADAS-cog+ (Table VI in the online-only Data Supplement), including 3 patients who completed the study but did not have the necessary ADAS-cog+ data from which to calculate a change from baseline required in Table 2.
Figure 2.
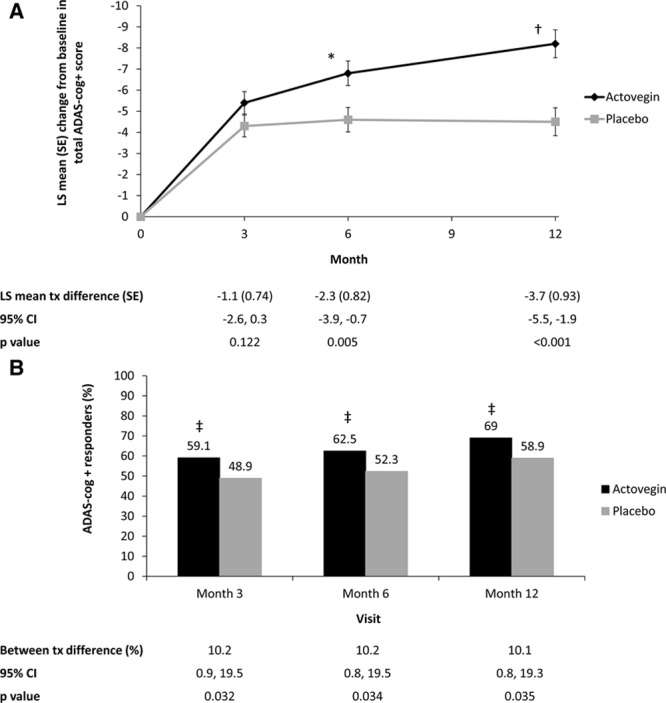
Analysis of the effect of Actovegin and placebo on Alzheimer’s Disease Assessment Scale, cognitive subscale, extended version (ADAS-cog+). A, Change in ADAS-cog+ score from baseline over the course of the study in the intent-to-treat (ITT) population. B, Analysis of ADAS-cog+ responders in the ITT population. A responder was defined as demonstrating an improvement of ≥4 points on the ADAS-cog+ scale using observed case data. *P=0.005; †P<0.001; ‡P<0.05 vs placebo. CI indicates confidence interval; LS, least squares; and tx, treatment.
Table 2.
Summary of Primary and Key Secondary Outcomes in the Intent-to-Treat Population
Secondary End Points
At month 3, the mean ADAS-cog+ score had improved in both groups (−5.4 [0.53] for Actovegin and −4.3 [0.52] for placebo), but the LS mean treatment difference did not reach statistical significance (−1.1; 95% CI, −2.6, 0.3; P=0.12). By month 12 (6 months after treatment cessation), the LS mean change had increased further for Actovegin (−8.2 [0.66]) than for placebo (−4.5 [0.66]), and the estimated LS mean treatment difference had increased to −3.7 (95% CI, −5.5, −1.9; P<0.001).
A summary of the ADAS-cog+ responder analysis is presented in Figure 2B and Supplemental Table VII in the online-only Data Supplement. At months 3, 6, and 12, statistically significantly more patients in the Actovegin group met the definition of responder (≥4-point improvement in ADAS-cog+ score from baseline, defined a priori in the protocol and an established measure30) than in the placebo group. By month 6, 62.5% of patients in the Actovegin group were considered responders versus 52.3% in placebo. The difference in rates for Actovegin–placebo was 10.2%, with an associated 95% CI (0.8%, 19.5%), which was statistically significant in favor of Actovegin (P=0.034).
Compared with placebo, Actovegin was also associated with statistically significant improvements in MoCA scores (Figure 3; Table VII in the online-only Data Supplement). At month 3, the LS mean difference between groups was 0.7 (95% CI, 0.1, 1.2; P=0.016), at month 6, the LS mean difference was 0.7 (95% CI, 0.2, 1.3; P=0.013), and at month 12, the LS mean difference increased to 1.0 (95% CI, 0.3, 1.7; P=0.003).
Figure 3.
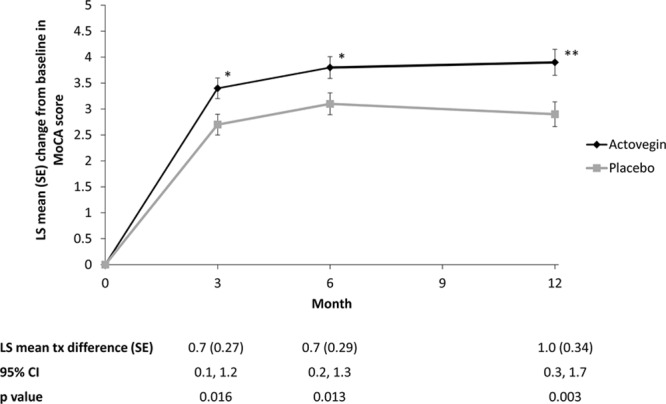
Change in Montreal Cognitive Assessment (MoCA) scores over the course of the study in the intent-to-treat (ITT) population. *P<0.05; **P<0.005 vs placebo. CI indicates confidence interval; LS, least squares; and tx, treatment.
At month 6, 10.5% of patients in the placebo group and 7.3% of patients in the Actovegin group had a diagnosis of dementia (according to International Statistical Classification and Related Health Problems, version 10 criteria). By month 12, the number of patients with a diagnosis of dementia had increased to 12.7% in the placebo group and 8.7% in the Actovegin group. Although the between-group differences were not statistically significant, the numeric differences showed fewer dementia diagnoses in the Actovegin group versus placebo at month 6 (−3.2; 95% CI, −8.5, 2.1; P=0.25) and month 12 (−4.0; 95% CI, −9.7, 1.7; P=0.22; Table VII in the online-only Data Supplement).
Both treatment groups showed similar results in NIHSS score with no statistically significant differences in scores between Actovegin and placebo at month 3 (−0.2; 95% CI, −0.5, 0.1; P=0.14), month 6 (0.0; 95% CI, −0.3, 0.2; P=0.89), and month 12 (−0.1; 95% CI, −0.4, 0.2; P=0.46; Table VII in the online-only Data Supplement).
Baseline scores for the Barthel Index, BDI-II, and EQ-5D were not collected; these parameters were only summarized using descriptive statistics. Health scores were similar between groups. At months 3 and 6, the median Barthel Index score was 100.0 for both groups. However, more patients in the Actovegin group had a score of ≥95 points compared with placebo at both months 3 (83.9% versus 76.6%) and 6 (84.0% versus 78.5%). At months 6 and 12, EuroQoL EQ-5D results were also similar between groups (Table VII in the online-only Data Supplement). In both treatment groups, most patients had no problem or slight problems with mobility, self-care, and usual activities; no pain or slight pain; and were not anxious or only slightly anxious. The mean general health scores were also similar between the treatment groups (Table VIII in the online-only Data Supplement). The majority of patients in both treatment groups had BDI-II scores between 0 and 13 (indicating minimal depression) at months 3, 6, and 12 (Actovegin: 59.7%, 60.9%, and 62.1%; placebo: 61.2%, 59.5%, and 55.3% respectively).
Safety
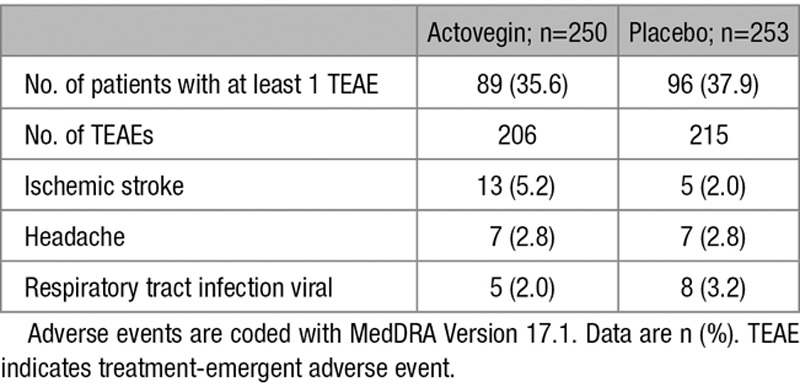
The safety analysis set includes all patients who received at least 1 dose of medication, 250 in the Actovegin group and 253 in the placebo group. The incidence of TEAE was similar between the 2 study groups. TEAEs were reported by 96 (37.9%) of 253 patients receiving placebo and by 89 (35.6%) of 250 patients receiving Actovegin.
Overall, 33 of 503 patients (6.6%) discontinued treatment because of a TEAE (12/253 [4.7%] in the placebo group and 21/250 patients [8.4%] in the Actovegin group). A total of 18 of 503 patients (3.6%) reported a TEAE that was considered related to the study medication, 9 (3.6%) in each treatment group. A summary of patients with clinically significant laboratory test results and a summary of TEAEs related to chemistry laboratory results are provided in Tables IX and X in the online-only Data Supplement, respectively.
The most frequently reported TEAE was recurrent ischemic stroke, followed by headache (Table 3). During double-blind treatment, 21 patients (14 in the Actovegin group and 7 in the placebo group) experienced cerebrovascular events (ischemic stroke, intracerebral hemorrhage, and transient ischemic attack). The odds ratio (95% CI) for cerebrovascular events for patients on Actovegin compared with placebo was 2.09 (0.83, 5.26), suggesting this was not statistically significant (post hoc analysis). During the 6-month follow-up, 3 patients in the placebo group and 2 patients in the Actovegin group discontinued because of recurrent ischemic stroke. Further safety results and an overview of TEAEs (Table XI in the online-only Data Supplement) and cerebrovascular events related to TEAEs are provided in Table XII in the online-only Data Supplement.
Table 3.
Summary of TEAEs (Occurring in ≥2% of Patients; Safety Analysis Set)
Discussion
In this study, Actovegin improved cognitive outcomes in patients with PSCI, compared with placebo. This is the first prospective randomized controlled trial to assess the effect of Actovegin on cognition in patients who had experienced a recent mild-to-moderate ischemic stroke.
Actovegin treatment was commenced 5 to 7 days after stroke onset to obtain credible cognitive function data at starting point, and PSCI diagnosed by MoCA32,33 was similar in the placebo and Actovegin groups at baseline. Patients with previously diagnosed dementia were excluded; however, it cannot be ruled out that patients may have had prestroke mild AD or vascular cognitive impairment-not dementia.34 PSCI represents vascular cognitive impairment by definition, but vascular and degenerative components often overlap and contribute to cognitive decline over the long term.35
Most patients in this study had multiple vascular risk factors, which could have triggered covert cerebrovascular disease. However, neither AD biomarker assessments nor specific magnetic resonance imaging protocols for detecting white matter abnormalities were included in the study design. This is a potential limitation of this study because patients with white matter lesions or AD changes could have been less susceptible to treatment or had a worse trajectory of cognitive decline after stroke.
The dosing regimen of Actovegin for this trial reflected current labeling, although oral treatment was for a longer period, thus the dosing regimen in the current study was exploratory to some extent. Our assumption that a 6-month treatment period would be sufficient to show benefits was based on data obtained in large-scale clinical trials on AChEIs for the treatment of vascular cognitive impairment.8
The primary efficacy outcome in our study was the change from baseline in ADAS-cog+ at 6 months using the last observation carried forward approach. A statistically significant LS mean treatment difference of 2.3 points in ADAS-cog+ score was achieved in the Actovegin group at 6 months and increased to 3.7 points at 12 months. The mean total ADAS-cog+ scores improved in both groups over 12 months; however, the change was more prominent in the Actovegin group at 3, 6, and 12 months and was supported by the secondary efficacy end point. It is noteworthy that a statistically significant improvement in Actovegin was sustained over 6 months after treatment withdrawal. Significantly more patients receiving Actovegin met the definition of responder comparing to placebo. The proportion of responders increased over time in both treatment groups, which can be partially explained by the spontaneous recovery observed in mild stroke survivors in the placebo group. However, the difference between treatments remained similar at all time points in favor of Actovegin.
It is debatable whether a statistically significant difference of 2.3 points achieved in ADAS-cog+ score reflects clinically meaningful change. In phase III trials, AChEIs have produced modest cognitive improvements (1–2 points on ADAS-cog), but AChEIs have not been granted U.S. Food & Drug Administration approval for use in VaD primarily because of inconsistent efficacy with respect to activities of daily living and global function.8 According to The European Medicines Agency guidelines, when choosing efficacy end points in clinical trials in patients with mild cognitive impairment and dementia, it is necessary to demonstrate the clinical relevance of results.36 However, it is also recognized that the inclusion of 2 coprimary end points addressing cognition and functional activities of daily living might be difficult. Currently used cognitive scales have demonstrated a ceiling effect which means that they are not sensitive enough to detect small changes in cognition, whereas complex neuropsychological batteries may be difficult to implement in large clinical trials. Also it is noteworthy that the VaD trials evaluated functional efficacy with the same measures used in AD trials. Stroke patients with cognitive impairment may have noticeable impairments in their daily functioning, not only because of affected cognitive domains, such as executive functions, but also because of motor or language deficits. It explains the fact that in VaD populations, demonstration of functional benefit is more difficult to achieve given the high prevalence of physical disability because of stroke.37 In addition, the extent to which individuals are capable to compensate for this deficit and adjust daily activities is highly variable. Therefore, clinical relevance, assessed by instrumental activities or health-related quality of life, may also be greatly confounded by differences in social status and occupational environment.
The MoCA was chosen as the other secondary supportive cognitive end point. Although the MoCA is considered a sensitive screening instrument to detect PSCI in clinical practice,33,38 further research is needed to validate its use as a tool to detect treatment effects and clinically meaningful longitudinal changes in cognitive function.39 The thresholds used to define test positivity on scales such as MoCA have been derived from community-dwelling older adults and are not well established in stroke survivor cohorts; it was also not prespecified in this study.33 Nevertheless, our results showed a similarity in cognition changes assessed by MoCA and ADAS-cog+ with statistically significant differences in favor of Actovegin sustained during the whole study, which was detected on MoCA scores with Actovegin treatment at month 3 onwards. However, the use of the MoCA as a secondary efficacy end point in our study and the related findings were exploratory, and its validity in testing cognitive changes over time should be tested in further trials.
A trend toward a reduction in the incidence of dementia was detected with Actovegin versus placebo, but this study was designed to determine symptomatic efficacy rather than dementia prevention, which requires large samples and long follow-up, and this study did not provide an opportunity to detect a clinical transformation from vascular mild cognitive impairment to overt dementia.
Other secondary end points assessed neurological deficits and functional recovery after stroke. The LS mean differences in NIHSS between groups did not reach statistical significance at any time point. At months 3 and 6, the median Barthel Index score was 100.0 for both groups, but more patients in the Actovegin group had a score of ≥95 points compared with the placebo group. Actovegin treatment was started ≤7 days after stroke onset, whereas an acute stroke management within the first 12 to 24 hours after the onset of symptoms is generally recommended. In addition, the study population consisted of patients with mild-to-moderate stroke; therefore, a high rate of spontaneous recovery under placebo might be expected and a ceiling effect cannot be ruled out. The aforementioned reasons might partially explain the similar neurological outcomes observed in both groups after 6 months of treatment. The study was also not primarily designed to assess stroke outcomes. Summary results from the EuroQoL EQ-5D and the BDI-II also showed similar responses in both groups. However, it is important to note that baseline scores for quality of life and depression were not collected because they were not easily accessible in the immediate poststroke state.
The incidence of TEAEs and deaths was similar between treatment groups. Although ischemic strokes occurred more frequently in the Actovegin group, the difference was not statistically significant (post hoc analysis) and they were not likely to occur at any given time point (eg, during infusion). The rate of recurrent ischemic stroke is not unexpected in this population; the risk of recurrence is higher within the first year (4.7%–15%) and is even higher in the first 3 months (9.5%–20%).40 It is noteworthy that the number of male patients with recurrent ischemic stroke was 73.3% in the Actovegin group and 40.0% in the placebo group; the same misbalance was found with regards to smokers: 53.3% in the Actovegin and 30.0% in the placebo group. The clinical use of Actovegin for 4 decades is supported by a favorable safety profile with a low incidence of AEs, and there have been no reports from previous studies or spontaneous reports of AEs associated with stroke.41 Therefore, a specific reason for this isolated finding, which we consider to be coincidental, has not yet been identified.
Limitations of this trial include missing data for the primary measure, ADAS-cog+ score, which consists of individual questionnaires that make up the primary efficacy end point. Missing data were recognized as a potential source of bias; however, this was addressed using a combination of 2 potentially conservative approaches (worst score imputation for partially complete ADAS-cog+ assessments, and last observation carried forward for completely missing ADAS-cog+ assessments). For data missing at baseline and month 3, it was not feasible to account for patients who did not have any baseline assessment or undertake imputation of missing month 3 ADAS-cog+ score based on baseline score as part of the planned analyses. As above, missing data for the individual questionnaire items were imputed using the worst score rather than the last nonmissing item score from the previous visit because this approach was considered to be a more conservative strategy, similar to that commonly adopted for binary end points where missing data are imputed as failure. The study showed an overall missing rate for the primary outcome in ≈9% of randomized patients, a rate that is comparable between treatment groups at baseline and month 3.
In conclusion, Actovegin has been shown to be effective in improving cognitive outcomes in a prospective randomized controlled trial which, to some extent, had an exploratory design and several limitations. Further studies with robust designs may help to establish the optimal dosing regimen and treatment duration for Actovegin in PSCI, and whether or not Actovegin improves neurological deficits and has an effect on activities of daily living and QoL in parallel with its effects on cognitive outcomes and disease progression.
Acknowledgments
We thank the participating centers’ staff and patients for their efforts and commitment during the trial; Ulf Vigonius, Martin Eeg, and Gediminas Puras for their contribution to the design of the study; Alexander Knyazev for scientific advice; and Alexander Kroll and Ella Palmer of Synergy Vision, London, United Kingdom for technical and medical writing assistance with the preparation of this report. See online-only Data Supplement for authors’ contributions and competing interests.
Sources of Funding
This study was organized and funded by Takeda Pharmaceuticals.
Disclosures
Drs Guekht, Skoog, and Zakharov were consultants for Takeda and have received honoraria for scientific clinical trial advice from Takeda. S. Edmundson is an employee of Takeda. The other author reports no conflicts.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.116.014321/-/DC1.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. doi: 10.1016/S0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Rajan KB, Aggarwal NT, Wilson RS, Everson-Rose SA, Evans DA. Association of cognitive functioning, incident stroke, and mortality in older adults. Stroke. 2014;45:2563–2567. doi: 10.1161/STROKEAHA.114.005143. doi: 10.1161/STROKEAHA.114.005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 6.Brainin M, Tuomilehto J, Heiss WD, Bornstein NM, Bath PM, Teuschl Y, et al. Post Stroke Cognition Study Group. Post-stroke cognitive decline: an update and perspectives for clinical research. Eur J Neurol. 2015;22:229–238, e13. doi: 10.1111/ene.12626. doi: 10.1111/ene.12626. [DOI] [PubMed] [Google Scholar]
- 7.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritter A, Pillai JA. Treatment of vascular cognitive impairment. Curr Treat Options Neurol. 2015;17:367. doi: 10.1007/s11940-015-0367-0. doi: 10.1007/s11940-015-0367-0. [DOI] [PubMed] [Google Scholar]
- 9.Guekht AB, Moessler H, Novak PH, Gusev EI Cerebrolysin Investigators. Cerebrolysin in vascular dementia: improvement of clinical outcome in a randomized, double-blind, placebo-controlled multicenter trial. J Stroke Cerebrovasc Dis. 2011;20:310–318. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.012. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Machicao F, Muresanu DF, Hundsberger H, Pflüger M, Guekht A. Pleiotropic neuroprotective and metabolic effects of Actovegin’s mode of action. J Neurol Sci. 2012;322:222–227. doi: 10.1016/j.jns.2012.07.069. doi: 10.1016/j.jns.2012.07.069. [DOI] [PubMed] [Google Scholar]
- 11.Kuninaka T, Senga Y, Senga H, Weiner M. Nature of enhanced mitochondrial oxidative metabolism by a calf blood extract. J Cell Physiol. 1991;146:148–155. doi: 10.1002/jcp.1041460119. doi: 10.1002/jcp.1041460119. [DOI] [PubMed] [Google Scholar]
- 12.Elmlinger MW, Kriebel M, Ziegler D. Neuroprotective and anti-oxidative effects of the hemodialysate Actovegin on primary rat neurons in vitro. Neuromolecular Med. 2011;13:266–274. doi: 10.1007/s12017-011-8157-7. doi: 10.1007/s12017-011-8157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyer S, Betz K. Elimination of the delayed postischemic energy deficit in cerebral cortex and hippocampus of aged rats with a dried, deproteinized blood extract (Actovegin). Arch Gerontol Geriatr. 1989;9:181–192. doi: 10.1016/0167-4943(89)90038-1. [DOI] [PubMed] [Google Scholar]
- 14.Krüger G, Quadbeck G. [The electroencephalogram of the rat in oxygen deficiency as an indicator of drug effects on cerebral metabolism]. Arzneimittelforschung. 1972;22:451–456. [PubMed] [Google Scholar]
- 15.Lanner G, Argyropoulos [Pharmacological effect of Solcoseryl on the metabolism of the brain. Animal experiments and clinical research]. Wien Med Wochenschr. 1975;125:681–685. [PubMed] [Google Scholar]
- 16.Meilin S, Machicao F, Elmlinger M. Treatment with Actovegin improves spatial learning and memory in rats following transient forebrain ischaemia. J Cell Mol Med. 2014;18:1623–1630. doi: 10.1111/jcmm.12297. doi: 10.1111/jcmm.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saletu B, Küfferle B, Anderer P, Grünberger J, Steinberger K. EEG-brain mapping in schizophrenics with predominantly positive and negative symptoms. Comparative studies with remoxipride/haloperidol. Eur Neuropsychopharmacol. 1990;1:27–36. doi: 10.1016/0924-977x(90)90007-w. [DOI] [PubMed] [Google Scholar]
- 18.Semlitsch HV, Anderer P, Saletu B, Hochmayer I. Topographic mapping of cognitive event-related potentials in a double-blind, placebo-controlled study with the hemoderivative Actovegin in age-associated memory impairment. Neuropsychobiology. 1990;24:49–56. doi: 10.1159/000119042. [DOI] [PubMed] [Google Scholar]
- 19.Derev’yannykh EA, Bel’skaya GN, Knoll EA, Krylova LG, Popov DV. Experience in the use of Actovegin in the treatment of patients with cognitive disorders in the acute period of stroke. Neurosci Behav Physiol. 2008;38:873–875. doi: 10.1007/s11055-008-9051-0. doi: 10.1007/s11055-008-9051-0. [DOI] [PubMed] [Google Scholar]
- 20.Shamalov NA, Stakhovskaia LV, Shetova IM, Efremova NM, Anisimov KV. [Efficacy and safety of the combined therapy with citicholine and actovegin in the acute period of ischemic stroke]. Zh Nevrol Psikhiatr Im S S Korsakova. 2010;110(9 p)(t 2):13–17. [PubMed] [Google Scholar]
- 21.Søndergård SD, Dela F, Helge JW, Larsen S. Actovegin, a non-prohibited drug increases oxidative capacity in human skeletal muscle. Eur J Sport Sci. 2016;16:801–807. doi: 10.1080/17461391.2015.1130750. doi: 10.1080/17461391.2015.1130750. [DOI] [PubMed] [Google Scholar]
- 22.Actovegin 80 mg Injection Solution - Summary of Product Characteristics (SPC) Http://www.Pharmazie.Com/graphic/a/17/2-00017.Pdf. Accessed November 15, 2016.
- 23.Ramipril 1.25 mg Capsules - Summary of Product Characteristics (SPC) Https://www.Medicines.Org.Uk/emc/medicine/24132. Accessed November 15, 2016.
- 24.Memantine Accord 20 mg Film-Coated Tablets - Summary of Product Characteristics (SPC) Https://www.Medicines.Org.Uk/emc/medicine/28669. Accessed November 15, 2016.
- 25.Guekht A, Skoog I, Korczyn AD, Zakharov V, Eeg M, Vigonius U. A randomised, double-blind, placebo-controlled trial of Actovegin in patients with post-stroke cognitive impairment: ARTEMIDA Study Design. Dement Geriatr Cogn Dis Extra. 2013;3:459–467. doi: 10.1159/000357122. doi: 10.1159/000357122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency. Guideline on Missing Data in Confirmatory Clinical Trials. Accessed April 11, 2017 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/09/WC500096793.pdf. [Google Scholar]
- 27.Ankolekar S, Geeganage C, Anderton P, Hogg C, Bath PM. Clinical trials for preventing post stroke cognitive impairment. J Neurol Sci. 2010;299:168–174. doi: 10.1016/j.jns.2010.08.052. doi: 10.1016/j.jns.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 28.Cano SJ, Posner HB, Moline ML, Hurt SW, Swartz J, Hsu T, et al. The ADAS-cog in Alzheimer’s disease clinical trials: psychometric evaluation of the sum and its parts. J Neurol Neurosurg Psychiatry. 2010;81:1363–1368. doi: 10.1136/jnnp.2009.204008. doi: 10.1136/jnnp.2009.204008. [DOI] [PubMed] [Google Scholar]
- 29.Skinner J, Carvalho JO, Potter GG, Thames A, Zelinski E, Crane PK, et al. Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Assessment Scale-Cognitive-Plus (ADAS-Cog-Plus): an expansion of the ADAS-Cog to improve responsiveness in MCI. Brain Imaging Behav. 2012;6:489–501. doi: 10.1007/s11682-012-9166-3. doi: 10.1007/s11682-012-9166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 31.Winblad B, Brodaty H, Gauthier S, Morris JC, Orgogozo JM, Rockwood K, et al. Pharmacotherapy of Alzheimer’s disease: is there a need to redefine treatment success? Int J Geriatr Psychiatry. 2001;16:653–666. doi: 10.1002/gps.496. [DOI] [PubMed] [Google Scholar]
- 32.Burton L, Tyson SF. Screening for cognitive impairment after stroke: a systematic review of psychometric properties and clinical utility. J Rehabil Med. 2015;47:193–203. doi: 10.2340/16501977-1930. doi: 10.2340/16501977-1930. [DOI] [PubMed] [Google Scholar]
- 33.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke. 2012;43:464–469. doi: 10.1161/STROKEAHA.111.633586. doi: 10.1161/STROKEAHA.111.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black SE. Vascular cognitive impairment: epidemiology, subtypes, diagnosis and management. J R Coll Physicians Edinb. 2011;41:49–56. doi: 10.4997/JRCPE.2011.121. doi: 10.4997/JRCPE.2011.121. [DOI] [PubMed] [Google Scholar]
- 35.Korczyn AD. Mixed dementia–the most common cause of dementia. Ann N Y Acad Sci. 2002;977:129–134. doi: 10.1111/j.1749-6632.2002.tb04807.x. [DOI] [PubMed] [Google Scholar]
- 36.European Medicines Agency. Guideline on Medicinal Products for the Treatment of Alzheimer’s Disease and Other Dementias. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003562.pdf. Accessed April 11, 2017. [Google Scholar]
- 37.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 39.Koski L. Validity and applications of the Montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovasc Dis. 2013;36:6–18. doi: 10.1159/000352051. doi: 10.1159/000352051. [DOI] [PubMed] [Google Scholar]
- 40.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke. 1994;25:333–337. doi: 10.1161/01.str.25.2.333. [DOI] [PubMed] [Google Scholar]
- 41.Buchmayer F, Pleiner J, Elmlinger MW, Lauer G, Nell G, Sitte HH. Actovegin®: a biological drug for more than 5 decades. Wien Med Wochenschr. 2011;161:80–88. doi: 10.1007/s10354-011-0865-y. doi: 10.1007/s10354-011-0865-y. [DOI] [PubMed] [Google Scholar]


