Supplemental Digital Content is available in the text.
Keywords: bariatric surgery; diet, reducing; gastric bypass; heart failure; weight loss
Abstract
Background:
Associations of obesity with incidence of heart failure have been observed, but the causality is uncertain. We hypothesized that gastric bypass surgery leads to a lower incidence of heart failure compared with intensive lifestyle modification in obese people.
Methods:
We included obese people without previous heart failure from a Swedish nationwide registry of people treated with a structured intensive lifestyle program and the Scandinavian Obesity Surgery Registry. All analyses used inverse probability weights based on baseline body mass index and a propensity score estimated from baseline variables. Treatment groups were well balanced in terms of weight, body mass index, and most potential confounders. Associations of treatment with heart failure incidence, as defined in the National Patient Register, were analyzed with Cox regression.
Results:
The 25 804 gastric bypass surgery patients had on average lost 18.8 kg more weight after 1 year and 22.6 kg more after 2 years than the 13 701 lifestyle modification patients. During a median of 4.1 years, surgery patients had lower heart failure incidence than lifestyle modification patients (hazard ratio, 0.54; 95% confidence interval, 0.36–0.82). A 10-kg achieved weight loss after 1 year was related to a hazard ratio for heart failure of 0.77 (95% confidence interval, 0.60–0.97) in both treatment groups combined. Results were robust in sensitivity analyses.
Conclusions:
Gastric bypass surgery was associated with approximately one half the incidence of heart failure compared with intensive lifestyle modification in this study of 2 large nationwide registries. We also observed a graded association between increasing weight loss and decreasing risk of heart failure.
One billion people worldwide are overweight and another half-billion are obese, and these numbers are increasing.1 Obesity may lead to heart failure, the leading cause of hospitalization in Western societies.2 The association was observed in the Framingham Heart Study,3 has since been confirmed by multiple other observational studies,4 and is backed by experimental evidence.5 In support of a causal association are mendelian randomization study findings6 and observational findings of beneficial effects of bariatric surgery on cardiac function in obese patients.7
The effect of substantial intentional weight reduction on heart failure incidence among obese people is unknown. Such evidence is unlikely to be produced by randomized trials because heart failure is rare in middle-aged individuals, requiring long follow-up in large samples.
We hypothesized that gastric bypass surgery leads to a lower incidence of heart failure compared with intensive lifestyle modification among obese people because of its larger effect on weight loss. We hence aimed to compare the incidence of heart failure in a nationwide registry of obese individuals treated with a structured intensive lifestyle modification program versus those in a nationwide quality of care registry of bariatric surgery who underwent gastric bypass surgery.
Methods
Study Design and Participants
The cohorts used in the present study were the Scandinavian Obesity Surgery Registry (SOReg)8 and the Itrim Health Database.9,10
SOReg is a nationwide, prospective, electronically captured quality-of-care registry for bariatric surgery8 that was started in 2007 and estimated to cover 98.5% of all bariatric surgery procedures in Sweden. For this study, data were available between 2007 and 2012.
The Itrim Health Database is a registry of individuals treated with a low-calorie diet (LCD) or very-low-calorie diet (VLCD) and lifestyle modification.9,10 The registry prospectively collects data on individuals participating in the commercial weight loss programs at 38 Itrim franchise centers across Sweden, using a common electronic platform for quarterly follow-up of a standardized set of clinical variables. For this study, data were available from 2006 to 2013.
In the present study, people ≥18 years of age with a baseline body mass index (BMI) between 30 and 49.9 kg/m2 were included from SOReg and Itrim (Figure I in the online-only Data Supplement). We excluded people who crossed over from Itrim to SOReg (n=811) and people with missing data on education or marital status (n=254). No participant had a history of heart failure before baseline, as determined in the same way as the outcome, described below.
The study was approved by the regional ethics committee in Stockholm, Sweden. All analyses were performed on deidentified data.
Interventions
All SOReg participants underwent primary gastric bypass for weight loss purposes, 96.0% of which were conducted laparoscopically.
All Itrim participants started with a 3-month weight-loss phase with either LCD or VLCD treatment, determined from baseline BMI, personal preference, and contraindications.
The structured weight loss program is presented in detail in Methods in the online-only Data Supplement. In short, VLCD treatment was a liquid-based formula diet of 500 kcal/d for 3 to 10 weeks followed by a 2- to 8-week gradual introduction of normal food. In June 2009, the program was changed to 600 kcal/d for 3 weeks followed by 800 kcal/d for up to 9 weeks. The LCD treatment included 2 calorie-restricted normal food meals and 2 formula diet meal replacement sachets per day, providing a total caloric intake of ≈1200 to 1500 kcal.
After the weight-loss phase, patients entered a 9-month weight maintenance program of exercise, dietary advice, and behavioral therapy.
Covariables
Participants were linked to nationwide health registers with their unique Swedish personal identity number. From these registries, data were obtained on socioeconomic variables, diseases, and drug treatments. Data sources and definitions using the International Classification of Diseases and Anatomic Therapeutic Chemical classification system codes used are given in Table I in the online-only Data Supplement. In SOReg, blood pressure was measured as part of routine health care; in Itrim, blood pressure was measured with a standardized protocol (Table I in the online-only Data Supplement). We imputed missing data for smoking and systolic blood pressure (each missing in ≈25% of participants) using multiple imputation from all other complete baseline data. Baseline characteristics in individuals with complete data compared with those with any missing data are presented in Table II in the online-only Data Supplement.
Follow-Up and Outcome
Participants were followed up in the National Patient Register and the Causes of Death Register until December 31, 2014. Patients were followed up from the treatment date until the first instance of the outcome, death, emigration, or end of follow-up. Itrim participants who during follow-up crossed over to bariatric surgery were censored at the crossover date. During follow-up, 135 participants emigrated, making register-based follow-up complete for 99.5%.
The primary outcome was the first hospitalization for heart failure, International Classification of Diseases, 10th Revision code I50 as main reason for hospitalization, retrieved from the National Patient Register. We have previously shown this outcome to have 95% positive predictive value in a validation study.11 The secondary outcome was nonischemic heart failure, defined as the first hospitalization of heart failure that was not preceded by a myocardial infarction (censoring patients at time of myocardial infarction).
In mediation analyses, interim instances of myocardial infarction and atrial fibrillation were obtained from the National Patient Register, and diabetes mellitus and hypertension were defined on the basis of drug treatment at the 1-year follow-up.
Statistical Analysis
We estimated a propensity score for treatment (surgery/lifestyle). Methods in the online-only Data Supplement gives variables included in the analysis. Common support was observed over the majority of the propensity score distribution, but 988 individuals (2.3%) had to be dropped at the lower end and 3291 (7.5%) at the upper end of the distribution. We checked for balance in covariables between treatment groups using weighted analyses described below and using standardized differences in means.
We then used coarsened exact matching12 to assign inverse probability weights to the participants based on baseline BMI and the propensity score. This procedure assigns an inverse probability weight to each participant based on the number of participants in the 2 treatment groups in strata of the variables chosen for the matching. This weight was used in all further analyses. We excluded 11 individuals who could not be matched. The resulting study sample consisted of 39 505 individuals.
Associations between treatment and weight loss were described with mixed models with body weight at baseline and 1- and 2-year follow-up visits as dependent variables, fixed effects for time and treatment, and a random intercept for participant identification.
Associations of treatment with heart failure incidence were estimated with Cox proportional hazard models. Proportionality of hazards was investigated with Schoenfeld tests.
We analyzed whether the association of treatment with heart failure incidence was mediated by weight loss per se in 2 ways. Both analyses made use of a weight measurement at the 1-year visit, and start of follow-up was reset to the 1-year visit. First, we studied whether there was a graded association between achieved weight loss at 1 year and the incidence of heart failure. To the total sample including both treatment groups, we fit a model that included the 3 independent variables of baseline weight, treatment group, and achieved weight loss at 1 year (modeled with the use of a linear term because of the limited number of cases). Second, we reweighted the sample and included BMI at 1 year, in addition to the other weighting variables (baseline characteristics of that sample are provided in Table III in the online-only Data Supplement), to investigate whether achieved weight loss was a major mediator of the effect.
We used generalized structural equation modeling to investigate whether parts of the associations between weight loss and incident heart failure were mediated by myocardial infarction, atrial fibrillation (both as time-updated variables), diabetes mellitus, or hypertension (both at the 1-year visit), each in a separate analysis, assuming sequential ignorability in the associations of weight loss with mediators and heart failure and no post–weight loss confounders.
To investigate potential competing risk, we constructed a composite outcome consisting of the first instance of heart failure, myocardial infarction, or cardiovascular death.
In a sensitivity analysis, we also adjusted the main model for variables that differed between the weighted groups (standardized difference in means ≥0.1). Inverse probability weighting is an effective way of minimizing selection bias but relies on a correctly specified propensity score. Therefore, as sensitivity analyses, we also investigated associations of treatment with heart failure incidence using Cox analyses stratified by levels of a combination of the propensity score and baseline BMI and using Cox analyses adjusted for restricted cubic splines of the propensity score and BMI. We also investigated the multiplicative interaction term treatment group by sex.
In a secondary analysis, we restricted the sample to patients usually determined to be eligible for bariatric surgery (BMI >40 kg/m2 or BMI between 35 and 40 kg/m2 with significant obesity-related comorbidity defined as hypertension, diabetes mellitus, and previous myocardial infarction).
For comparisons of heart failure rates in the obese samples with a general population sample, we matched up to 10 comparators from the Swedish Total Population Registry13 to each patient who had gastric bypass surgery on birth year, intervention year, sex, and place of residence (county, commune, parish).
Two-sided tests with a 5% α level were considered statistically significant. Stata 14 was used for all analyses.
Results
Participant Characteristics
In this study, 25 804 individuals were treated with gastric bypass surgery and 13 701 with lifestyle modification. Baseline characteristics of the study sample are outlined in Table 1.
Weight and BMI were perfectly balanced between the weighted treatment groups. Minor differences in systolic blood pressure, income, and use of antihypertensive, antidiabetic, and lipid-lowering drugs were still apparent between the weighted treatment groups, all favoring the lifestyle participants (Table 1).
Table 1.
Baseline Characteristics
Effects of Treatment on Weight Loss and Heart Failure
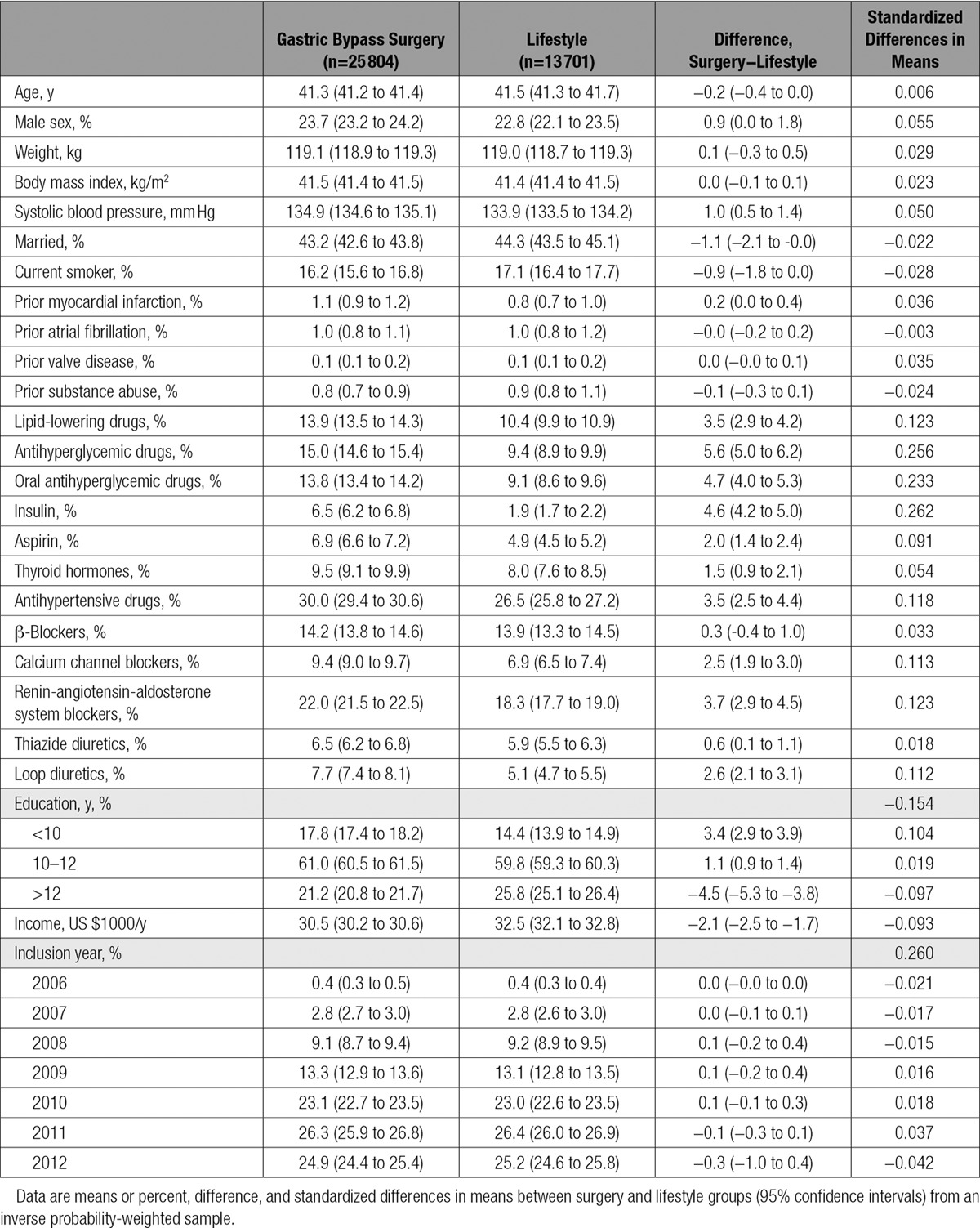
On average, surgery led to 18.8 kg more weight loss than lifestyle treatment at the 1-year follow-up and 22.6 kg more weight loss at the 2-year follow-up visit. Figure 1 shows the development of weight, waist circumference, and body fat during the first 2 years of the study.
Figure 1.
Changes in weight, waist circumference, and body fat during the first 2 years of the study. Data are from an inverse probability-weighted sample. Error bars are 95% confidence intervals. Body fat data were not available for the gastric bypass surgery patients.
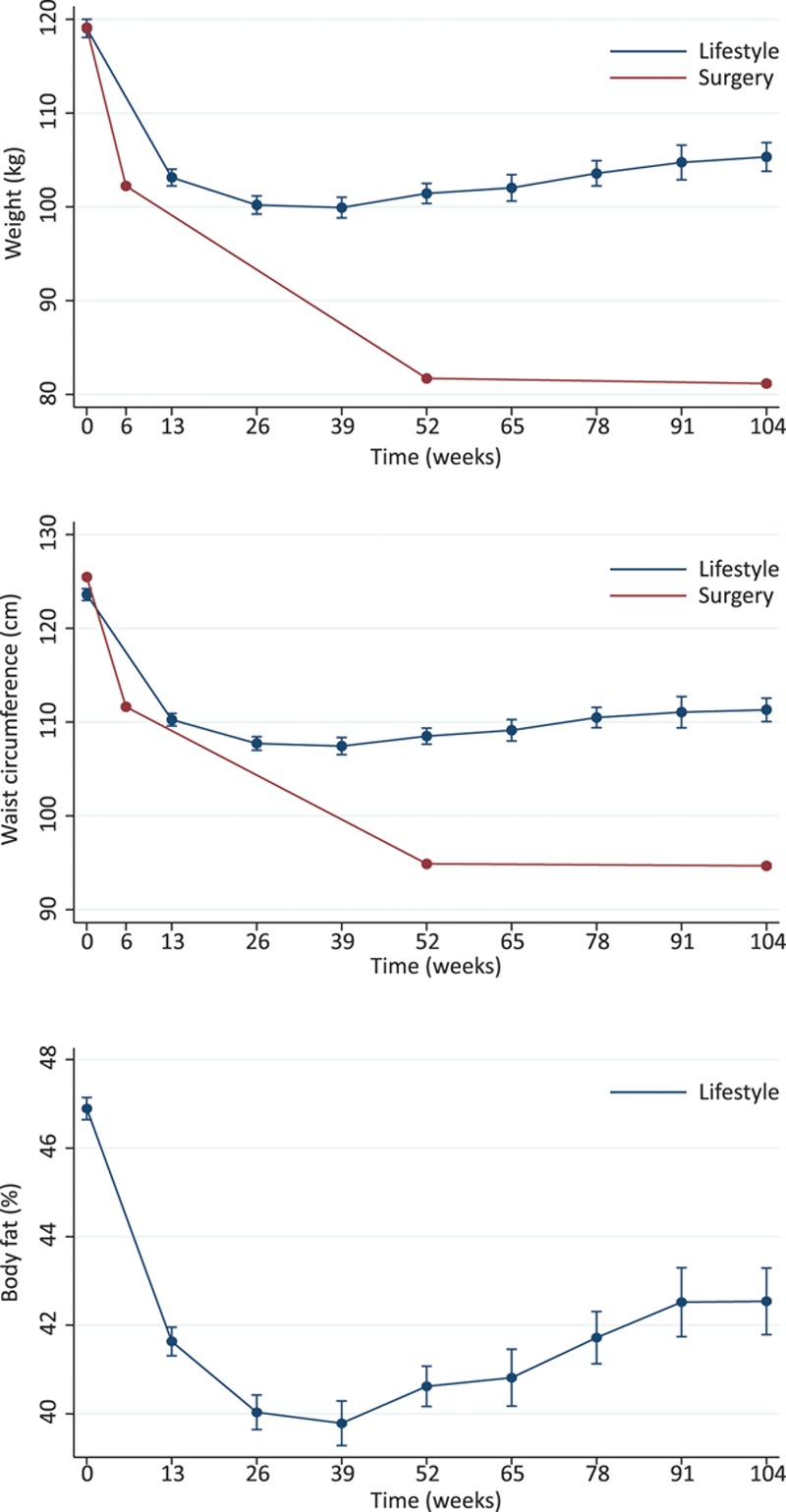
During a median follow-up of 4.1 years (range, 0.0–9.0 years), 73 new heart failure cases occurred with an incidence rate of 5.4 per 10 000 person-years at risk (95% confidence interval [CI], 3.5–8.7). Of these, 29 occurred in the lifestyle group and 44 in the surgery group, with incidence rates of 7.6 (95% CI, 3.4–21.3) and 4.1 (95% CI, 3.1–5.6) per 10 000 person-years at risk, respectively. Hence, surgical treatment was associated with lower incidence of heart failure than lifestyle treatment (hazard ratio, 0.54; 95% CI, 0.36–0.82). Cumulative hazards are presented in Figure 2. The absolute 5-year risk of heart failure was 0.4% among the lifestyle modification patients and 0.2% among surgery patients.
Figure 2.
Cumulative hazard of heart failure in individuals treated with lifestyle or gastric bypass surgery. Data are from an inverse probability-weighted sample.
The incidence of heart failure in the age-, sex-, and place of residence–matched general population sample was 3.0 (95% CI, 2.7–3.4) per 10 000 person-years at risk.
Mediation Analyses
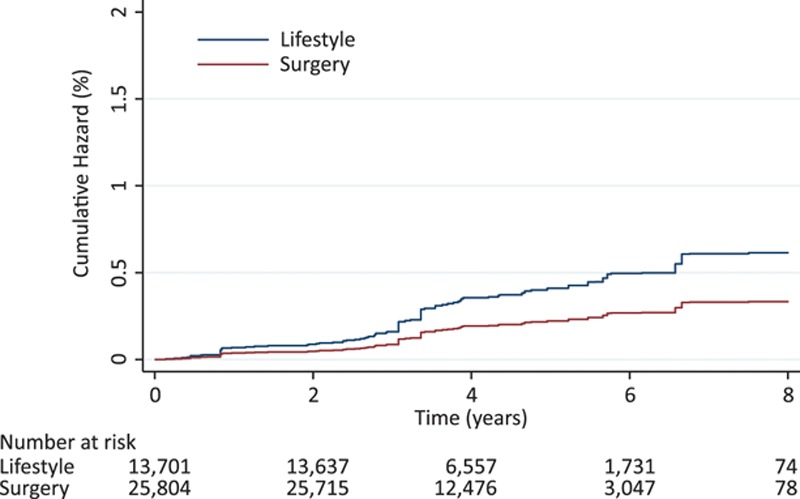
In analyses using 1-year achieved weight loss and setting start of follow-up to the 1-year visit, 31 347 individuals participated and 50 cases of heart failure occurred. We found a dose-response relationship between 1-year weight loss and risk of heart failure with a hazard ratio for a 10 kg weight loss of 0.77 (95% CI, 0.60–0.97) in a model adjusted for baseline weight and treatment fitted to the 2 treatment groups combined (Figure 3). In an analysis weighted also for BMI at 1 year, no association of treatment group with risk of heart failure was seen (hazard ratio, 1.09; 95% CI, 0.62–1.92), supporting that the achieved weight loss is a major mediator of the effect.
Figure 3.
Hazard ratio of heart failure in relation to achieved weight loss at 1 year. Data are from an inverse probability-weighted sample of both lifestyle and gastric bypass surgery patients combined. The model included the 3 variables of baseline weight, treatment group, and achieved weight loss at 1 year. Shaded area is 95% confidence interval.
Among 39 114 individuals without myocardial infarction before baseline, 67 heart failure cases occurred without an interim myocardial infarction. Surgery also was associated with a lower incidence of this nonischemic heart failure (hazard ratio, 0.48; 95% CI, 0.31–0.74).
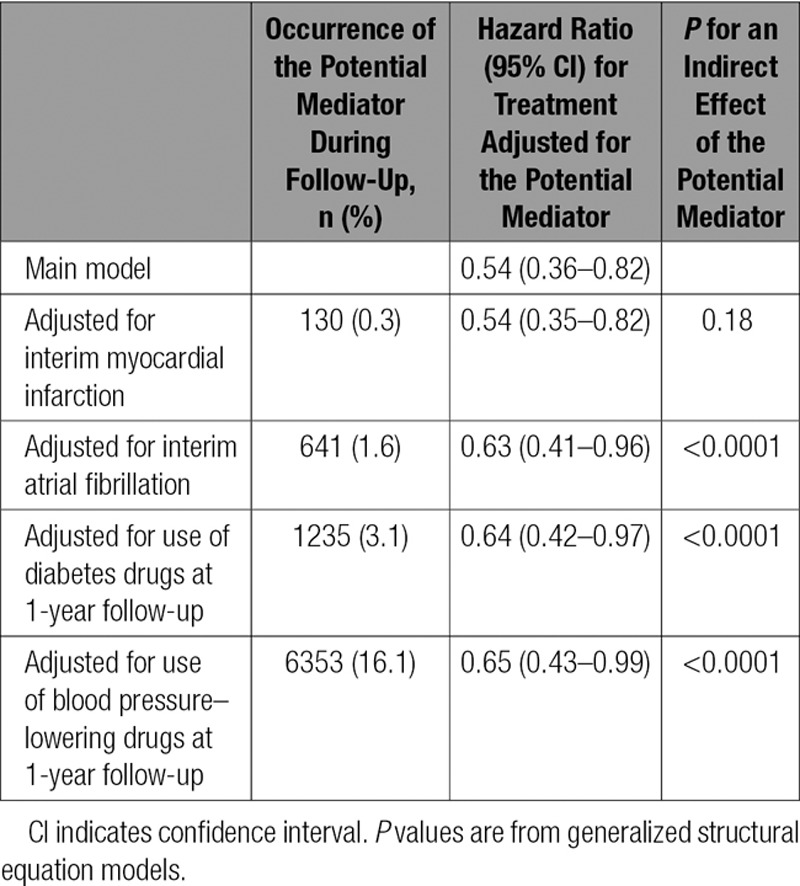
The association of treatment with heart failure in the total sample was not mediated by the effects of treatment on myocardial infarction to any substantial extent but appeared to be partly mediated by effects of treatment on interim atrial fibrillation, diabetes mellitus, and hypertension (Table 2).
Table 2.
Mediation Analysis
Sensitivity Analyses
In a sensitivity analysis investigating potential competing risk, we included 39 114 individuals without prior heart failure or myocardial infarction. Of these, 244 suffered a composite end point of a first instance of heart failure, myocardial infarction, or cardiovascular death (69 experienced heart failure, 113 had a myocardial infarction, and 72 died of cardiovascular disease; patients could experience >1 outcome). The surgery group also experienced a lower rate of this composite end point (hazard ratio, 0.58; 95% CI, 0.46–0.74). Of the 113 myocardial infarctions, 51 occurred in the lifestyle modification group (rate, 9.2 per 10 000 person-years at risk; 95% CI, 4.2–23.7) and 62 in the surgery group (rate, 5.9 per 10 000 person-years at risk; 95% CI, 4.6–7.7).
In a sensitivity analysis adjusting the main model for variables that differed between the weighted groups (use of antihypertensive, antidiabetic, and lipid-lowering drugs, use of loop diuretics, education level, and inclusion year), results were similar to the main model (hazard ratio, 0.45; 95% CI, 0.27–0.76). In sensitivity analyses, we substituted the weighted analyses for models stratified according to matching weights, with essentially the same results (hazard ratio, 0.46; 95% CI, 0.23–0.92). We also performed analyses adjusting for splines of baseline BMI and the propensity score instead. This again produced similar results (hazard ratio, 0.47; 95% CI, 0.24–0.93). No deviation from proportionality of hazards was observed (all P>0.9).
In a model including the multiplicative interaction term treatment group by sex, similar main results were observed (hazard ratio, 0.37; 95% CI, 0.19–0.72), and no treatment by sex interaction was seen (P=0.17).
In a sample of 21 620 individuals typically determined to be eligible for bariatric surgery (BMI >40 kg/m2 or BMI between 35 and 40 kg/m2 with significant obesity-related comorbidity), 57 cases of heart failure occurred. In this sample, surgery was associated with a hazard ratio of 0.53 (95% CI, 0.35–0.82) for heart failure.
Discussion
Principal Observations
In this study of a large sample of obese people without previous heart failure from 2 Swedish nationwide registries, gastric bypass surgery was associated with a nearly halved incidence of heart failure compared with intensive lifestyle treatment including LCD/VLCD. The associations were to a large extent mediated by weight loss; partly mediated by effects of treatment on interim atrial fibrillation, diabetes mellitus, and hypertension; but likely not mediated by the effects of treatment on myocardial infarction. Results were robust in multiple sensitivity analyses.
Comparisons With Previous Studies
Associations of obesity with incident heart failure are well established,3,4,6 but the effect of substantial weight loss on incident heart failure is unknown. Patient series have suggested beneficial effects on cardiac function7 and prognosis14 of bariatric surgery in patients with heart failure, but other studies have shown an inverse association of BMI with mortality among people with heart failure.15,16 The present study included only people without heart failure at baseline and hence gives no implications for treatment of obese people with heart failure.
Potential Mechanisms
The association of treatment with heart failure was mediated mainly by weight loss, as supported by the strong dose-response relationship and the absence of a treatment effect when 1-year weight loss was accounted for in the models.
Bariatric surgery, compared with conventional treatment, has been associated with a lower incidence of myocardial infarctions17 and an improvement in several other risk factors for heart failure, including diabetes mellitus, hypertriglyceridemia, and high blood pressure,18 in the nonrandomized prospective Swedish Obese Subjects study. All of these may contribute to the associations observed in the present study,19,20 but in our data, the effects appeared less likely to be mediated by the effects of treatment on myocardial infarction (although myocardial infarction rate was lower among surgery patients) and more likely to be mediated by the effects of treatment on interim atrial fibrillation, diabetes mellitus, and blood pressure. This is in line with the known substantial effects of bariatric surgery on diabetes prevention21 and remission.22–24 It should, however, be noted that the mediation analyses had low and differential statistical power.
Another potential mode of action is a direct effect of weight loss on cardiac stress such as hemodynamic load. Cardiac troponin-I is a biomarker of cardiac stress and an important risk marker for heart failure.25 Recent data have shown that gastric bypass reduces cardiac troponin-I concentration compared with intensive lifestyle intervention.26
In addition to the mediation by effects on risk factors, there may be effects of weight loss on obesity cardiomyopathy per se. Fatty acids are the major oxidative fuel for the heart,27 and myocardial fatty acid β-oxidation is regulated mainly by peroxisome proliferator-activated receptor-α.28 In experimental models, obesity impairs myocardial fatty acid β-oxidation regulation.29 This leads to lipid deposition in cardiomyocytes, contractile dysfunction,29 myocardial fibrosis,28 and cardiomyocyte apoptosis.5,30 In experimental models, weight loss leads to an upregulation of myocardial peroxisome proliferator-activated receptors, improved lipid metabolism, and increased ejection fraction.31
In the recent EMPA-REG OUTCOME study [BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients], empagliflozin treatment led to a modest weight loss and a strong preventive effect against heart failure in individuals with diabetes mellitus.32 In light of the present study, it should be considered whether it is the energy loss and consequent weight loss with the sodium/glucose cotransporter-2 inhibitors that underpin their protection against heart failure. In the present study, surgically treated patients lost on average 20 kg more than patients in the lifestyle program. It will be important to follow the full effect of this on the incidence of heart failure over longer time periods.
Strengths and Limitations
Strengths include 2 large prospectively designed nationwide registries of bariatric surgery and intensive lifestyle treatment with a program that is a relevant comparator to gastric bypass surgery33 and a validated outcome ascertained with nationwide health registries with virtually complete coverage and negligible loss to follow-up.
Weaknesses include the observational nature of the study. It is unlikely that randomized trials of bariatric surgery will provide evidence for a relatively rare event such as heart failure. Furthermore, the low number of outcomes is a limitation. Despite balance in most of the available baseline factors between the treatment groups after weighting, residual confounding may have affected our observations. This would likely result in a conservative bias because participants in the lifestyle program had higher educational level and income and can be assumed to be more health conscious. They are also likely more motivated because they paid out of pocket for their treatment, whereas the government paid for 90% of the bariatric surgery procedures. Furthermore, the gastric bypass surgery group had a worse clinical profile in terms of diabetes mellitus, dyslipidemia, and hypertension. The statistical modeling accounted well for differences between groups, and results were robust in sensitivity analyses further adjusting for group differences and using other statistical techniques. We did not have data on ethnicity, and it is reasonable to assume that the participants were predominantly white. Generalizability to other ethnic groups is unknown. We also lacked data on exercise and dietary advice and compliance to such advice in the surgery group, as well as measurements of physical activity in either group. Furthermore, generalizability to other bariatric surgery methods than gastric bypass is unknown, and it should be noted that the preferred type of bariatric surgery may differ over time and between geographic regions.
Unsurprisingly, the incidence of heart failure was higher in both of the obese samples than in the age-, sex-, and place of residence–matched general population, likely explained by a higher prevalence of obesity-related risk factors. Nevertheless, the low heart failure rate in this sample led to a mere 0.2% 5-year risk difference (corresponding to a number needed to treat of 534) in this comparison of 2 groups who both had an effective weight loss treatment.
Conclusions
Gastric bypass surgery was associated with a nearly halved incidence of heart failure compared with an intensive lifestyle modification program including LCD/VLCD in this study of nationwide registries including almost 40 000 individuals. We also observed a dose-response relationship between weight loss and risk of heart failure. Taken together with mendelian randomization findings, these findings support a causal effect of obesity on heart failure, the leading cause of hospitalization in Western societies.
Sources of Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award R01DK105948. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Authors of this study were also supported by the Swedish Research Council (Dr Neovius, 2013-3770; Dr Sundström, 2010-1078). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Supplementary Material
Disclosures
Drs Marcus, Neovius, and Sundström report receiving consulting fees (modest) for participation in the scientific advisory committee of Itrim. Dr Näslund is the previous director of the Scandinavian Obesity Surgery Registry. Dr Ottosson is its current director. Dr Bruze reports no conflicts.
Footnotes
The online-only Data Supplement, podcast, and transcript are available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.116.025629/-/DC1.
Circulation is available at http://circ.ahajournals.org.
Clinical Perspective
What Is New?
In this study of nearly 40 000 obese people without previous heart failure from 2 Swedish nationwide registries, gastric bypass surgery was associated with approximately one half the incidence of heart failure compared with intensive lifestyle treatment, including low- and very-low-calorie diets.
The associations were to a large extent mediated by weight loss, partly mediated by the effects of treatment on interim atrial fibrillation, diabetes mellitus, and hypertension, but likely not mediated by the effects of treatment on myocardial infarction.
Results were robust in multiple sensitivity analyses.
What Are the Clinical Implications?
Taken together with previous mendelian randomization findings, this study supports a causal effect of obesity on heart failure.
With increasing obesity prevalence (currently affecting half a billion people worldwide) and heart failure being the leading cause of hospitalization and hospital costs in Western societies, the public health implication of these observations is a strong incentive for the prevention and aggressive treatment of obesity.
References
- 1.Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, Bixby H, Cowan MJ, Riley LM, Hajifathalian K, Fortunato L, Taddei C, Bennett JE, Ikeda N, Khang YH, Kyobutungi C, Laxmaiah A, Li Y, Lin HH, Miranda JJ, Mostafa A, Turley ML, Paciorek CJ, Gunter M, Ezzati M NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 4.Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133:639–649. doi: 10.1161/CIRCULATIONAHA.115.016801. doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- 5.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fall T, Hägg S, Mägi R, Ploner A, Fischer K, Horikoshi M, Sarin AP, Thorleifsson G, Ladenvall C, Kals M, Kuningas M, Draisma HH, Ried JS, van Zuydam NR, Huikari V, Mangino M, Sonestedt E, Benyamin B, Nelson CP, Rivera NV, Kristiansson K, Shen HY, Havulinna AS, Dehghan A, Donnelly LA, Kaakinen M, Nuotio ML, Robertson N, de Bruijn RF, Ikram MA, Amin N, Balmforth AJ, Braund PS, Doney AS, Döring A, Elliott P, Esko T, Franco OH, Gretarsdottir S, Hartikainen AL, Heikkilä K, Herzig KH, Holm H, Hottenga JJ, Hyppönen E, Illig T, Isaacs A, Isomaa B, Karssen LC, Kettunen J, Koenig W, Kuulasmaa K, Laatikainen T, Laitinen J, Lindgren C, Lyssenko V, Läärä E, Rayner NW, Männistö S, Pouta A, Rathmann W, Rivadeneira F, Ruokonen A, Savolainen MJ, Sijbrands EJ, Small KS, Smit JH, Steinthorsdottir V, Syvänen AC, Taanila A, Tobin MD, Uitterlinden AG, Willems SM, Willemsen G, Witteman J, Perola M, Evans A, Ferrières J, Virtamo J, Kee F, Tregouet DA, Arveiler D, Amouyel P, Ferrario MM, Brambilla P, Hall AS, Heath AC, Madden PA, Martin NG, Montgomery GW, Whitfield JB, Jula A, Knekt P, Oostra B, van Duijn CM, Penninx BW, Smith GD, Kaprio J, Samani NJ, Gieger C, Peters A, Wichmann HE, Boomsma DI, de Geus EJ, Tuomi T, Power C, Hammond CJ, Spector TD, Lind L, Orho-Melander M, Palmer CN, Morris AD, Groop L, Järvelin MR, Salomaa V, Vartiainen E, Hofman A, Ripatti S, Metspalu A, Thorsteinsdottir U, Stefansson K, Pedersen NL, McCarthy MI, Ingelsson E, Prokopenko I European Network for Genetic and Genomic Epidemiology (ENGAGE) Consortium. The role of adiposity in cardiometabolic traits: a mendelian randomization analysis. PLoS Med. 2013;10:e1001474. doi: 10.1371/journal.pmed.1001474. doi: 10.1371/journal.pmed.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal R, Harling L, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. The effects of bariatric surgery on cardiac structure and function: a systematic review of cardiac imaging outcomes. Obes Surg. 2016;26:1030–1040. doi: 10.1007/s11695-015-1866-5. doi: 10.1007/s11695-015-1866-5. [DOI] [PubMed] [Google Scholar]
- 8.Hedenbro JL, Näslund E, Boman L, Lundegårdh G, Bylund A, Ekelund M, Laurenius A, Möller P, Olbers T, Sundbom M, Ottosson J, Näslund I. Formation of the Scandinavian Obesity Surgery Registry, SOReg. Obes Surg. 2015;25:1893–1900. doi: 10.1007/s11695-015-1619-5. doi: 10.1007/s11695-015-1619-5. [DOI] [PubMed] [Google Scholar]
- 9.Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014;38:279–284. doi: 10.1038/ijo.2013.83. doi: 10.1038/ijo.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr. 2012;96:953–961. doi: 10.3945/ajcn.112.038265. doi: 10.3945/ajcn.112.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingelsson E, Arnlöv J, Sundström J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. doi: 10.1016/j.ejheart.2004.12.007. doi: 10.1016/j.ejheart.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell M, Iacus S, King G, Porro G. CEM: coarsened exact matching in Stata. Stata J. 2009;9:524–546. [Google Scholar]
- 13.Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaëlsson K, Neovius M, Stephansson O, Ye W. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31:125–136. doi: 10.1007/s10654-016-0117-y. doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 14.Lim CP, Fisher OM, Falkenback D, Boyd D, Hayward CS, Keogh A, Samaras K, MacDonald P, Lord RV. Bariatric surgery provides a “bridge to transplant” for morbidly obese patients with advanced heart failure and may obviate the need for transplantation. Obes Surg. 2016;26:486–493. doi: 10.1007/s11695-015-1789-1. doi: 10.1007/s11695-015-1789-1. [DOI] [PubMed] [Google Scholar]
- 15.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D, Lazar JM. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428–1434. doi: 10.1016/j.amjcard.2015.02.024. doi: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lönroth H, Narbro K, Näslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 18.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 19.Ingelsson E, Björklund-Bodegård K, Lind L, Arnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 20.Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 21.Carlsson LM, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, Jacobson P, Lönroth H, Maglio C, Näslund I, Pirazzi C, Romeo S, Sjöholm K, Sjöström E, Wedel H, Svensson PA, Sjöström L. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 22.Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, Bouchard C, Carlsson B, Karason K, Lönroth H, Näslund I, Sjöström E, Taube M, Wedel H, Svensson PA, Sjöholm K, Carlsson LM. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–2304. doi: 10.1001/jama.2014.5988. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 23.Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes. N Engl J Med. 2014;371:682. doi: 10.1056/NEJMc1407393. doi: 10.1056/NEJMc1407393. [DOI] [PubMed] [Google Scholar]
- 24.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 25.Sundström J, Ingelsson E, Berglund L, Zethelius B, Lind L, Venge P, Arnlöv J. Cardiac troponin-I and risk of heart failure: a community-based cohort study. Eur Heart J. 2009;30:773–781. doi: 10.1093/eurheartj/ehp047. doi: 10.1093/eurheartj/ehp047. [DOI] [PubMed] [Google Scholar]
- 26.Lyngbakken MN, Omland T, Nordstrand N, Norseth J, Hjelmesæth J, Hofsø D. Effect of weight loss on subclinical myocardial injury: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Prev Cardiol. 2016;23:874–880. doi: 10.1177/2047487315618796. doi: 10.1177/2047487315618796. [DOI] [PubMed] [Google Scholar]
- 27.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions: potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe K, Fujii H, Takahashi T, Kodama M, Aizawa Y, Ohta Y, Ono T, Hasegawa G, Naito M, Nakajima T, Kamijo Y, Gonzalez FJ, Aoyama T. Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor alpha associated with age-dependent cardiac toxicity. J Biol Chem. 2000;275:22293–22299. doi: 10.1074/jbc.M000248200. doi: 10.1074/jbc.M000248200. [DOI] [PubMed] [Google Scholar]
- 29.Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–2595. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- 30.Lu MC, Tzang BS, Kuo WW, Wu FL, Chen YS, Tsai CH, Huang CY, Lee SD. More activated cardiac mitochondrial-dependent apoptotic pathway in obese Zucker rats. Obesity (Silver Spring) 2007;15:2634–2642. doi: 10.1038/oby.2007.315. doi: 10.1038/oby.2007.315. [DOI] [PubMed] [Google Scholar]
- 31.Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK, Mertens A, Quarck R, Benhabilès N, Marguerie G, Mackness B, Mackness M, Ninio E, Herregods MC, Balligand JL, Holvoet P. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 2004;110:3259–3269. doi: 10.1161/01.CIR.0000147614.85888.7A. doi: 10.1161/01.CIR.0000147614.85888.7A. [DOI] [PubMed] [Google Scholar]
- 32.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME® Trial Investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludwig DS, Ebbeling CB, Livingston EH. Surgical vs lifestyle treatment for type 2 diabetes. JAMA. 2012;308:981–982. doi: 10.1001/2012.jama.10156. doi: 10.1001/2012.jama.10156. [DOI] [PubMed] [Google Scholar]


