Abstract
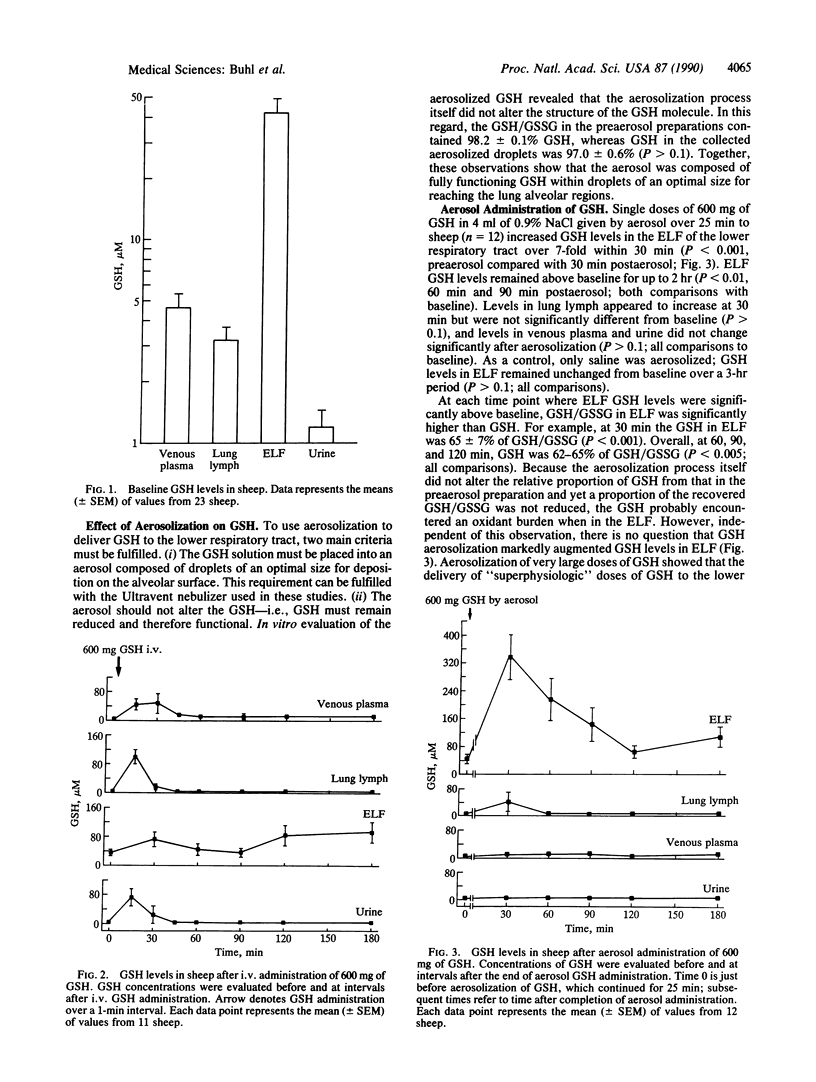
Glutathione (GSH), a cysteine-containing tripeptide, functions as an antioxidant, provides cells with cysteine, and is required for optimal function of the immune system. Because the epithelial-lining fluid (ELF) of the lower respiratory tract normally contains high GSH levels and lung ELF GSH deficiency states can exist, we evaluated the feasibility of augmenting lung ELF GSH levels by (i) administering GSH to sheep i.v. and by direct aerosolization and then (ii) measuring the GSH levels in lung ELF, lung lymph, venous plasma, and urine. When GSH (600 mg) was administered i.v. (n = 11), GSH levels in venous plasma, lung lymph, and ELF rose, but only transiently, suggesting the i.v. route would not deliver adequate GSH to the alveolar epithelial surface. For directly administering GSH to the lung by the aerosol route, in vitro studies were first conducted to show that greater than 50% of a GSH solution could be converted to droplets less than 3 microns in aerodynamic diameter without oxidizing the GSH. To target functional GSH to the lower respiratory tract, an aerosolized solution of GSH (600 mg) was administered to sheep (n = 12). Significantly, the GSH level in ELF increased 7-fold at 30 min (preaerosol, 45.7 +/- 10 microM; 30-min postaerosol, 337 +/- 64 microM; P less than 0.001). The ELF GSH levels remained above baseline at 1 hr (P less than 0.01), returning toward baseline over a 2-hr period. In contrast, GSH levels in lung lymph, venous plasma, and urine were not significantly increased during the period--i.e., aerosol therapy selectively augmented the GSH levels only at the lung epithelial surface. Thus, functional GSH can be delivered by aerosol to directly augment the ELF GSH levels of the lower respiratory tract. Such an approach may prove useful in treating a variety of lung disorders.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. D., Jr, Lauterburg B. H., Mitchell J. R. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983 Dec;227(3):749–754. [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F., Griffith O. W., Cohn Z. A. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982 Feb 10;257(3):1231–1237. [PubMed] [Google Scholar]
- Cantin A. M., Hubbard R. C., Crystal R. G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989 Feb;139(2):370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Fells G. A., Hubbard R. C., Crystal R. G. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest. 1987 Jun;79(6):1665–1673. doi: 10.1172/JCI113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987 Jul;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J. R., Vähäkangas K., Jernström B., Moldéus P. Glutathione conjugation by isolated lung cells and the isolated, perfused lung. Effect of extracellular glutathione. Eur J Biochem. 1984 Feb 1;138(3):439–443. doi: 10.1111/j.1432-1033.1984.tb07935.x. [DOI] [PubMed] [Google Scholar]
- Fischman C. M., Udey M. C., Kurtz M., Wedner H. J. Inhibition of lectin-induced lymphocyte activation by 2-cyclohexene-1-one: decreased intracellular glutathione inhibits an early event in the activation sequence. J Immunol. 1981 Dec;127(6):2257–2262. [PubMed] [Google Scholar]
- Fridovich I., Freeman B. Antioxidant defenses in the lung. Annu Rev Physiol. 1986;48:693–702. doi: 10.1146/annurev.ph.48.030186.003401. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Brown L. A., Jones D. P. Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells. Biochem Pharmacol. 1986 Dec 15;35(24):4537–4542. doi: 10.1016/0006-2952(86)90776-8. [DOI] [PubMed] [Google Scholar]
- Hamilos D. L., Wedner H. J. The role of glutathione in lymphocyte activation. I. Comparison of inhibitory effects of buthionine sulfoximine and 2-cyclohexene-1-one by nuclear size transformation. J Immunol. 1985 Oct;135(4):2740–2747. [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner J. E., Repine J. E. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989 Aug;140(2):531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Hubbard R. C., Brantly M. L., Sellers S. E., Mitchell M. E., Crystal R. G. Anti-neutrophil-elastase defenses of the lower respiratory tract in alpha 1-antitrypsin deficiency directly augmented with an aerosol of alpha 1-antitrypsin. Ann Intern Med. 1989 Aug 1;111(3):206–212. doi: 10.7326/0003-4819-111-3-206. [DOI] [PubMed] [Google Scholar]
- Hubbard R. C., Casolaro M. A., Mitchell M., Sellers S. E., Arabia F., Matthay M. A., Crystal R. G. Fate of aerosolized recombinant DNA-produced alpha 1-antitrypsin: use of the epithelial surface of the lower respiratory tract to administer proteins of therapeutic importance. Proc Natl Acad Sci U S A. 1989 Jan;86(2):680–684. doi: 10.1073/pnas.86.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. C., McElvaney N. G., Sellers S. E., Healy J. T., Czerski D. B., Crystal R. G. Recombinant DNA-produced alpha 1-antitrypsin administered by aerosol augments lower respiratory tract antineutrophil elastase defenses in individuals with alpha 1-antitrypsin deficiency. J Clin Invest. 1989 Oct;84(4):1349–1354. doi: 10.1172/JCI114305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C. M., Lee N., Cattell D., Liang S. M. Glutathione regulates interleukin-2 activity on cytotoxic T-cells. J Biol Chem. 1989 Aug 15;264(23):13519–13523. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Arrick B. A., Murray H. W., DeSantis N. M., Cohn Z. A. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981 Apr 1;153(4):766–782. doi: 10.1084/jem.153.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yamamoto I. Mechanism of augmentation of the antibody response in vitro by 2-mercaptoethanol in murine lymphocytes. I. 2-Mercaptoethanol-induced stimulation of the uptake of cystine, an essential amino acid. J Exp Med. 1982 May 1;155(5):1277–1290. doi: 10.1084/jem.155.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller M. S. Hypothyroidism protects against free radical damage in ischemic acute renal failure. Kidney Int. 1986 Jun;29(6):1162–1166. doi: 10.1038/ki.1986.122. [DOI] [PubMed] [Google Scholar]
- Patterson C. E., Rhoades R. A. Protective role of sulfhydryl reagents in oxidant lung injury. Exp Lung Res. 1988;14 (Suppl):1005–1019. doi: 10.3109/01902148809064189. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y. Lung inflammation: normal host defense or a complication of some diseases? Annu Rev Med. 1987;38:295–323. doi: 10.1146/annurev.me.38.020187.001455. [DOI] [PubMed] [Google Scholar]
- Rom W. N., Bitterman P. B., Rennard S. I., Cantin A., Crystal R. G. Characterization of the lower respiratory tract inflammation of nonsmoking individuals with interstitial lung disease associated with chronic inhalation of inorganic dusts. Am Rev Respir Dis. 1987 Dec;136(6):1429–1434. doi: 10.1164/ajrccm/136.6.1429. [DOI] [PubMed] [Google Scholar]
- Schraufstätter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Glutathione cycle activity and pyridine nucleotide levels in oxidant-induced injury of cells. J Clin Invest. 1985 Sep;76(3):1131–1139. doi: 10.1172/JCI112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Akerboom T. P. Glutathione disulfide (GSSG) efflux from cells and tissues. Methods Enzymol. 1984;105:445–451. doi: 10.1016/s0076-6879(84)05062-x. [DOI] [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Travis J. Oxidants and antioxidants in the lung. Am Rev Respir Dis. 1987 Apr;135(4):773–774. doi: 10.1164/arrd.1987.135.4.773. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Rosano C. L. Modulation of endothelial GSH concentrations: effect of exogenous GSH and GSH monoethyl ester. J Appl Physiol (1985) 1989 Mar;66(3):1029–1034. doi: 10.1152/jappl.1989.66.3.1029. [DOI] [PubMed] [Google Scholar]
- Wedner H. J., Simchowitz L., Stenson W. F., Fischman C. M. Inhibition of human polymorphonuclear leukocyte function by 2-cyclohexene-1-one. A role for glutathione in cell activation. J Clin Invest. 1981 Aug;68(2):535–543. doi: 10.1172/JCI110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel A., Cikryt P. The level and half-life of glutathione in human plasma. FEBS Lett. 1980 Nov 3;120(2):209–211. doi: 10.1016/0014-5793(80)80299-7. [DOI] [PubMed] [Google Scholar]
- White C. W., Repine J. E. Pulmonary antioxidant defense mechanisms. Exp Lung Res. 1985;8(2-3):81–96. doi: 10.3109/01902148509057515. [DOI] [PubMed] [Google Scholar]
- Yoda Y., Nakazawa M., Abe T., Kawakami Z. Prevention of doxorubicin myocardial toxicity in mice by reduced glutathione. Cancer Res. 1986 May;46(5):2551–2556. [PubMed] [Google Scholar]
- van Asbeck B. S., Hoidal J., Vercellotti G. M., Schwartz B. A., Moldow C. F., Jacob H. S. Protection against lethal hyperoxia by tracheal insufflation of erythrocytes: role of red cell glutathione. Science. 1985 Feb 15;227(4688):756–759. doi: 10.1126/science.2982213. [DOI] [PubMed] [Google Scholar]


