Abstract
Purpose
The aim of this study was to test whether patient medication adherence, a modifiable risk factor obtainable at hospital admission, predicts readmission within 30 days.
Patients and methods
We used a retrospective cohort study design to test whether patient medication adherence to all chronic medications, as determined by the 4-item Morisky Medication Adherence Scale (MMAS-4) administered by a pharmacist at the time of hospital admission, predicts 30-day readmissions. We compared readmission rates among 385 inpatients who had their adherence assessed from February 1, 2013, to January 31, 2014. Multiple logistic regression was used to examine the benefit of adding medication adherence to previously published variables that have been shown to predict 30-day readmissions.
Results
Patients with low and intermediate adherence (combined) had readmission rates of 20.0% compared to a readmission rate of 9.3% for patients with high adherence (P=0.005). By adding MMAS-4 data to previously published variables that have been shown to predict 30-day readmissions, we found that patients with low and intermediate medication adherence had an adjusted 2.54-fold higher odds of readmission compared to those in patients with high adherence (95% confidence interval [CI]: 1.32–4.90, P=0.005). The model’s predictive power, as measured by the c-statistic, improved from 0.65 to 0.70 after adding adherence.
Conclusion
Because medication adherence assessed at hospital admission was independently associated with 30-day readmission risk, it offers potential for targeting interventions to improve adherence.
Keywords: rehospitalization, predictive model, transition of care, care transitions, nonadherence, MMAS-4
Introduction
Multiple studies suggest that proper medication use by patients is one of the most important factors contributing to better outcomes and decreased utilization. Patients who are not adherent to their medication regimens have a poorer prognosis than patients who are adherent. Of all medication-related hospitalizations that occur in the USA, 33%–69% are the result of medication nonadherence, which translates into health care costs reaching $100 billion a year.1–3 In the face of growing readmission penalties, hospitals struggle with how to identify patients at risk for avoidable readmissions.4,5 Since transitional care interventions have the potential to reduce readmissions, interest in models that could identify patients at risk in real time has increased tremendously. Seven predictive models currently exist that have the potential to identify high-risk patients early during a hospitalization, and five of these can be utilized at the time of discharge. Unfortunately, most predictive models use medical comorbidity and prior utilization data, which in general yield poor predictive ability for hospital readmis-sions. Models that include functional and social variables produce greater predictive power, although data for these variables are more difficult to obtain.6
Researchers of the HOSPITAL study collected data on several types of variables from easily obtainable sources. These data included demographic information, previous health care utilization, primary care provider information, index admission characteristics, procedures and chronic medical conditions, and last known laboratory values before discharge. They then used multiple regression modeling to identify seven factors that independently predict 30-day hospital readmission: Hemoglobin at discharge, discharge from an Oncology service, Sodium level at discharge, Procedure during the index admission (any International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] coded procedure), Index Type of admission (nonelective vs elective), number of Admissions during the past 12 months, and Length of stay, which they referred to as the HOSPITAL score.7,8
Medication adherence is one type of social variable, and while it has been hypothesized that nonadherence might predict hospital readmission, this relationship has not been extensively studied because medication adherence measurements are not easily obtained.7 In contrast to most predictors of readmission, nonadherence is a “modifiable” risk factor.9 Furthermore, low baseline adherence has been found to be a predictor of low adherence postdischarge.10 As such, identifying nonadherent patients in real time could be helpful in developing interventions that could safely reduce hospitalizations.
There are several adherence tools available that identify medication behavior, barriers to adherence, or behaviors associated with nonadherence. However, they have either not been validated or not been proven to be reliable and/or are not sufficiently sensitive. Most of these tools are also time-consuming to administer and have complicated score calculations that make them impractical in day-to-day practice. Therefore, even after years of research, a gold standard scale has still not been identified.11–13 The 4-item Morisky Medication Adherence Scale (MMAS-4) has been shown to be reliable, has good validity, and is quick to administer and score.14–16 Many researchers have been using it to assess their new medication adherence scales.17,18 For this reason, we obtained baseline medication adherence data at hospital admission using the MMAS-4. We then used these data to test the hypothesis that medication nonadherence can predict 30-day hospital readmission.
Patients and methods
Setting and participants
This retrospective cohort study was conducted at the Cedars-Sinai Medical Center (CSMC), a large nonprofit, tertiary care teaching hospital in Los Angeles, California, USA. Our analysis included patients admitted by a large hospitalist service who received a pharmacist consultation as part of a quality improvement (QI) project from February 1, 2013, to January 31, 2014. The QI project included all patients who met at least one of the following inclusion criteria: age ≥65 years, on ≥10 chronic medications, therapeutic duplicates in patient’s most recent electronic health record (EHR) medication list, heart failure (defined as having an ejection fraction <40%, documented heart failure in the History & Physical examination note or in the EHR problem list), and if they were referred for a pharmacist consultation by the treating hospitalist based on clinical judgment. Chronic medications were defined as medications prescribed for chronic conditions that were to be taken on a scheduled basis. Short-term antimicrobial therapy, vitamins, herbal supplements, and medications prescribed on an as-needed basis were not considered to be chronic medications. Patients were excluded from the analysis if they would be excluded from Centers for Medicare & Medicaid (CMS) readmission measures: those who died before discharge, were discharged to another health care facility or hospice, or left against medical advice.19 Patients were also excluded if the consulting pharmacist was not able to assess medication adherence (ie, if the patient was intubated) or if they were intentionally readmitted for an elective procedure within 30 days of discharge of the index hospitalization. The CSMC Institutional Review Board (IRB) approved the study. All data collection and analysis were performed in compliance with IRB requirements. Written informed consent was not required by the CSMC IRB.
Outcome variable
Our outcome measure was nonelective hospital readmissions within 30 days of discharge. Any patient rehospitalized to CSMC via the Emergency Department was considered non-elective. Of note, unplanned direct admissions are very rare at CSMC due to bed shortages, so inpatient admissions via the Emergency Department accurately reflect nonelective rehospitalizations.
Independent variable
The independent variable of interest was medication adherence, used as a predictor of 30-day readmissions. Our null hypothesis was that this odds ratio would not differ from 1. We also evaluated the c-statistic for the multiple regression model predicting 30-day readmissions. Our null hypothesis was that adding adherence to the model would not change the c-statistic.
Other variables
For each patient included in the analysis, we extracted all seven covariates used in the HOSPITAL model.7 We used the CSMC EHR to extract demographic information, insurance information, previous health care utilization data (number of admissions in the previous year), and data from the index admission (hemoglobin and sodium levels before discharge, length of stay, and index admission type). We extracted information about procedures performed during the hospitalization from billing data. Because many CSMC oncologists use hospitalists, we checked if patients had received a consultation from an oncologist during the hospital stay to approximate discharge from an oncology service variable (Table 1).
Table 1.
Patient characteristics
| Patient characteristics | Medication adherencea
|
P-valueb | |||||
|---|---|---|---|---|---|---|---|
| Low (N=94) | Intermediate (N=141) | High (N=150) | |||||
| Unplanned readmission, n (%) | 0.020 | ||||||
| Not readmitted | 75 | (79.8%) | 113 | (80.1%) | 136 | (90.7%) | |
| Readmitted | 19 | (20.2%) | 28 | (19.9%) | 14 | (9.3%) | |
| Age, median (Q1, Q3) | 68.0 | (56, 78) | 67.0 | (56, 80) | 69.0 | (59, 81) | 0.66 |
| Female, n (%) | 50 | (53.2%) | 70 | (49.6%) | 70 | (46.7%) | 0.61 |
| Ethnicity, n (%) | 0.61 | ||||||
| Hispanic | 8 | (8.5%) | 15 | (10.6%) | 11 | (7.3%) | |
| Non-Hispanic | 86 | (91.5%) | 126 | (89.4%) | 139 | (92.7%) | |
| Race, n (%) | 0.047 | ||||||
| Asian | 6 | (6.4%) | 6 | (4.3%) | 13 | (8.7%) | |
| Black or African American | 30 | (31.9%) | 30 | (21.3%) | 23 | (15.3%) | |
| White | 58 | (61.7%) | 103 | (73.0%) | 111 | (74.0%) | |
| Others | 0 | (0.0%) | 2 | (1.4%) | 3 | (2.0%) | |
| Marital status, n (%) | 0.049 | ||||||
| Divorced or legally separated | 12 | (12.8%) | 15 | (10.6%) | 12 | (8.0%) | |
| Married, significant other or domestic partner | 30 | (31.9%) | 69 | (48.9%) | 81 | (54.0%) | |
| Single | 29 | (30.9%) | 32 | (22.7%) | 28 | (18.7%) | |
| Widowed | 23 | (24.5%) | 25 | (17.7%) | 29 | (19.3%) | |
| Primary insurance, n (%) | 0.86 | ||||||
| HMO | 9 | (9.6%) | 17 | (12.1%) | 13 | (8.7%) | |
| Medi-Cal | 1 | (1.1%) | 2 | (1.4%) | 3 | (2.0%) | |
| Medicare | 73 | (77.7%) | 104 | (73.7%) | 109 | (72.7%) | |
| PPO | 10 | (10.6%) | 15 | (10.6%) | 23 | (15.3%) | |
| Self-pay/others | 1 | (1.1%) | 3 | (2.1%) | 2 | (1.3%) | |
| Source of index admission, n (%) | 0.34 | ||||||
| Home | 91 | (96.8%) | 130 | (92.2%) | 145 | (96.7%) | |
| MD office | 2 | (2.1%) | 5 | (3.5%) | 3 | (2.0%) | |
| Transfer from another facility | 1 | (1.1%) | 6 | (4.3%) | 2 | (1.3%) | |
| Type of index admission, n (%) | 0.76 | ||||||
| Elective | 2 | (2.1%) | 2 | (1.4%) | 4 | (2.7%) | |
| Emergency | 92 | (97.9%) | 139 | (98.6%) | 146 | (97.3%) | |
| Discharge from an oncology service, n (%) | 10 | (10.6%) | 27 | (19.1%) | 21 | (14.0%) | 0.18 |
| Length of stay of the index admission, median (Q1, Q3) | 5.0 | (3.0, 7.0) | 5.0 | (3.0, 8.0) | 5.0 | (3.0, 8.0) | 0.59 |
| Number of hospital admissions in the past year, median (Q1, Q3) | 1.0 | (0.0, 3.0) | 1.0 | (0.0, 2.0) | 1.0 | (0.0, 2.0) | 0.07 |
| Number of PTA medications, median (Q1, Q3) | 20.0 | (16, 27) | 20.0 | (15, 25) | 20.0 | (16, 24) | 0.74 |
| Number of procedures during index admission, median (Q1, Q3) | 2.0 | (1.0, 4.0) | 2.0 | (1.0, 4.0) | 2.0 | (1.0, 4.0) | 0.91 |
| Hemoglobin level at discharge, median (Q1, Q3) | 10.7 | (9.3, 12.1) | 10.6 | (9.3, 11.8) | 10.7 | (9.6, 12.0) | 0.38 |
| Serum sodium level at discharge, median (Q1, Q3) | 138.0 | (135, 140) | 138.0 | (135, 141) | 138.0 | (136, 141) | 0.52 |
| Serum creatinine level at discharge, median (Q1, Q3) | 1.0 | (0.8, 2.0) | 1.1 | (0.8, 1.8) | 1.0 | (0.8, 1.6) | 0.18 |
| Primary diagnosis on admission, n (%) | 0.46 | ||||||
| Arrhythmia | 3 | (3.2%) | 2 | (1.4%) | 6 | (4.0%) | |
| Asthma/COPD | 6 | (6.4%) | 3 | (2.1%) | 5 | (3.3%) | |
| Bleeding | 3 | (3.2%) | 4 | (2.8%) | 8 | (5.4%) | |
| Bronchitis/pneumonia | 4 | (4.3%) | 4 | (2.8%) | 9 | (6.0%) | |
| Diabetes mellitus | 5 | (5.3%) | 4 | (2.8%) | 3 | (2.0%) | |
| Heart failure | 11 | (11.7%) | 17 | (12.1%) | 12 | (8.0%) | |
| Hypertension | 6 | (6.4%) | 2 | (1.4%) | 1 | (0.7%) | |
| Infection | 13 | (13.8%) | 31 | (22.0%) | 27 | (18.0%) | |
| Ischemic heart disease | 3 | (3.2%) | 10 | (7.1%) | 12 | (8.0%) | |
| Malignant neoplasm | 2 | (2.1%) | 6 | (4.3%) | 6 | (4.0%) | |
| Pancreatitis | 3 | (3.2%) | 4 | (2.8%) | 7 | (4.7%) | |
| Renal failure | 4 | (4.3%) | 8 | (5.7%) | 9 | (6.0%) | |
| Venous thromboembolism | 1 | (1.1%) | 2 | (1.4%) | 5 | (3.3%) | |
| Othersc | 30 | (31.9%) | 44 | (31.2%) | 40 | (26.7%) | |
| Primary diagnosis at 30-day readmission, n (%) | 0.42 | ||||||
| Arrhythmia | 1 | (5.3%) | 0 | (0.0%) | 0 | (0.0%) | |
| Asthma/COPD | 2 | (10.5%) | 0 | (0.0%) | 0 | (0.0%) | |
| Bleeding | 0 | (0.0%) | 1 | (3.6%) | 0 | (0.0%) | |
| Bronchitis/pneumonia | 0 | (0.0%) | 1 | (3.6%) | 1 | (7.1%) | |
| Diabetes mellitus | 0 | (0.0%) | 2 | (7.1%) | 1 | (7.1%) | |
| Heart failure | 1 | (5.3%) | 3 | (10.7%) | 1 | (7.1%) | |
| Hypertension | 2 | (10.5%) | 0 | (0.0%) | 0 | (0.0%) | |
| Infection | 4 | (21.1%) | 8 | (28.6%) | 8 | (57.1%) | |
| Ischemic heart diseases | 1 | (5.3%) | 1 | (3.6%) | 0 | (0.0%) | |
| Malignancy | 0 | (0.0%) | 1 | (3.6%) | 0 | (0.0%) | |
| Pancreatitis | 2 | (10.5%) | 2 | (7.1%) | 0 | (0.0%) | |
| Venous thromboembolism | 0 | (0.0%) | 2 | (7.1%) | 0 | (0.0%) | |
| Othersd | 6 | (31.6%) | 7 | (25.0%) | 3 | (21.4%) | |
| Comorbidity, n (%) | |||||||
| Atrial fibrillation | 34 | (36.2%) | 53 | (37.6%) | 62 | (41.3%) | 0.68 |
| COPD | 17 | (18.1%) | 20 | (14.2%) | 17 | (11.3%) | 0.33 |
| Diabetes mellitus | 43 | (45.7%) | 58 | (41.1%) | 68 | (45.3%) | 0.71 |
| Ischemic heart diseases | 43 | (45.7%) | 52 | (36.9%) | 55 | (36.7%) | 0.30 |
| Heart failure | 39 | (41.5%) | 53 | (37.6%) | 52 | (34.7%) | 0.56 |
| Malignant neoplasm | 13 | (13.8%) | 20 | (14.2%) | 18 | (12.0%) | 0.84 |
Notes:
For the assessment of medication adherence, we used MMAS-4. Use of the ©MMAS is protected by the US and International copyright laws. Permission for use is required. A license agreement is available from Donald E Morisky, MMAS Research (MORISKY), 294 Lindura Court, Las Vegas, NV 89135-1415; dmorisky@gmail.com © 2007 Donald E Morisky. All rights reserved. MMAS, Morisky Medication Adherence Scale, and Morisky are the trademarks of Donald E Morisky and may be used only with permission.
For categorical variables, we used chi-square tests. For nonnormally distributed variables, we used the two-sided Kruskal–Wallis rank sum test.
Other diagnoses at admission included anemia, bile duct obstruction, arthritis, calculus, cervical spondylosis, cholangitis, fluid overload, fracture, complications of transplanted organ, complications of implanted device, constipation, degeneration of lumber or lumbosacral intervertebral disk, dehydration, dementia, dislocation of prosthetic joint, disorder of autonomic nervous system, displacement of cervical intervertebral disk, electrolyte imbalance, encephalopathy, epilepsy, fecal impaction, gastritis, gastromy complications, gout, hallucinations, headache, hemiplegia, inguinal hernia with obstruction, intestinal obstruction, migraine, orthostatic hypotension, osteoarthrosis, pain, poisoning, pressure ulcer, prosthetic joint repair, sickle-cell crisis, spasm of muscle, sprain of neck, systemic lupus erythematosus, systemic sclerosis, psychosis, ulcerative colitis, and vascular insufficiency of intestine.
Other diagnoses at 30-day readmission included acute vascular insufficiency of intestine, altered mental status, cerebral infarction, electrolyte imbalance, fracture, hemiplegia, hepatic encephalopathy, complications due to renal dialysis device, hypersensitivity angitis, fall, pain, and unspecified disorder of stomach and duodenum.
Abbreviations: COPD, chronic obstructive pulmonary disease; HMO, Health Maintenance Organization; MD, Doctor of Medicine; MMAS-4, 4-item Morisky Medication Adherence Scale; PPO, Preferred Provider Organization; PTA, prior to admission.
Assessing patient medication adherence
Pharmacist consultations focused on obtaining the best possible prior to admission medication history, assessing medication adherence to all chronic medications, and providing education.
The consulting pharmacist assessed medication adherence via the MMAS-414–16 using the following questions:
Do you ever forget to take any of your chronic medicines?
Do you ever have problems remembering to take any of your chronic medicines?
When you feel better, do you sometimes stop taking any of your chronic medicines?
Sometimes, if you feel worse when you take any of your chronic medicines, do you stop taking them?
Since this was a retrospective analysis and we sought to control for confounding variables, the independent contribution of medication adherence to predict readmission was adjusted for seven HOSPITAL factors that are found to be significant independent predictors of 30-day hospital readmission.
Patient education
As part of the QI project, patients with medication knowledge deficits were educated at the time of the initial pharmacist consultation. Telephonic pharmacist counseling was also attempted within 72 hours after discharge for patients with low levels of adherence (and for some with medium levels, based on pharmacist judgment). During these phone calls, pharmacists reviewed the discharge medication list with the patient and identified any drug-related problems that warranted follow-up.
Statistical analysis
All covariates, including adherence, were tested individually to assess their association with 30-day readmission using bivariate nonparametric Wilcoxon rank-sum test and chi-square test for continuous and categorical covariates, respectively (Table 2). The previously published multiple logistic regression HOSPITAL model7 was then fitted with and without adherence to assess the contribution of adherence to the model. The odds ratios, Wald 95% confidence intervals (CIs), and P-values for the association of the full model results adding adherence are reported (Table 3). As a sensitivity analysis, we added the number of prior-to-admission medications into the model as both continuous and categorical variables (three levels: 2–9, 10–19, and 20–48).
Table 2.
Patient readmitted within 30 days vs not readmitted
| Patient characteristics | Not readmitted (N=324) | Readmitted (N=61) | P-valuea | ||
|---|---|---|---|---|---|
| Age, median (Q1, Q3) | 68.0 | (56.0, 80.0) | 66.0 | (59.0, 81.0) | 0.98 |
| Female, n (%) | 155 | (47.8%) | 35 | (57.4%) | 0.17 |
| Ethnicity, n (%) | 0.43 | ||||
| Hispanic | 27 | (8.3%) | 7 | (11.5%) | |
| Non-Hispanic | 297 | (91.7%) | 54 | (88.5%) | |
| Race, n (%) | 0.26 | ||||
| Asian | 18 | (5.6%) | 7 | (11.5%) | |
| Black or African American | 69 | (21.3%) | 14 | (23.0%) | |
| White | 232 | (71.6%) | 40 | (65.6%) | |
| Others | 5 | (1.5%) | 0 | (0.0%) | |
| Marital status, n (%) | 0.93 | ||||
| Divorced or legally separated | 33 | (10.2%) | 6 | (9.8%) | |
| Married, significant other or domestic partner | 152 | (46.9%) | 28 | (45.9%) | |
| Single | 73 | (22.5%) | 16 | (26.2%) | |
| Widowed | 66 | (20.4%) | 11 | (18.0%) | |
| Primary insurance, n (%) | 0.28 | ||||
| HMO | 36 | (11.1%) | 3 | (4.9%) | |
| Medi-Cal | 4 | (1.2%) | 2 | (3.3%) | |
| Medicare | 240 | (74.1%) | 46 | (75.4%) | |
| PPO | 40 | (12.3%) | 8 | (13.1%) | |
| Self-pay/others | 4 | (1.2%) | 2 | (3.3%) | |
| Source of index admission, n (%) | 0.35 | ||||
| Home | 306 | (94.4%) | 60 | (98.4%) | |
| MD office | 10 | (3.1%) | 0 | (0.0%) | |
| Transfer from another facility | 8 | (2.5%) | 1 | (1.6%) | |
| Type of index admission, n (%) | 0.79 | ||||
| Elective | 7 | (2.2%) | 1 | (1.6%) | |
| Emergency | 317 | (97.8%) | 60 | (98.4%) | |
| Discharge from an oncology service, n (%) | 43 | (13.3%) | 15 | (24.6%) | 0.023 |
| Length of stay of the index admission, median (Q1, Q3) | 5.0 | (3.0, 7.0) | 6.0 | (4.0, 10.0) | 0.003 |
| Number of hospital admissions in the past year, median (Q1, Q3) | 1.0 | (0.0, 2.0) | 1.0 | (0.0, 3.0) | 0.14 |
| Number of PTA medications, median (Q1, Q3) | 20.0 | (16.0, 25.0) | 20.0 | (16.0, 25.0) | 0.92 |
| Number of procedures during index admission, median (Q1, Q3) | 2.0 | (1.0, 4.0) | 2.0 | (1.0, 5.0) | 0.97 |
| Hemoglobin level at discharge, median (Q1, Q3) | 10.7 | (9.5, 12.1) | 10.3 | (9.3, 11.3) | 0.09 |
| Serum sodium level at discharge, median (Q1, Q3) | 138.0 | (136.0, 141.0) | 137.0 | (135.0, 140.0) | 0.044 |
| Serum creatinine level at discharge, median (Q1, Q3) | 1.1 | (0.8, 1.7) | 1.1 | (0.9, 2.4) | 0.33 |
| Medication adherence, n (%) | 0.020 | ||||
| Low | 75 | (23.1%) | 19 | (31.1%) | |
| Intermediate | 113 | (34.9%) | 28 | (45.9%) | |
| High | 136 | (42.0%) | 14 | (23.0%) | |
| Medication adherence collapsed, n (%) | 0.005 | ||||
| Low/intermediate | 188 | (58.0%) | 47 | (77.0%) | |
| High | 136 | (42.0%) | 14 | (23.0%) | |
| Primary diagnosis on admission, n (%) | 0.63 | ||||
| Arrhythmia | 10 | (3.1%) | 1 | (1.6%) | |
| Asthma/COPD | 11 | (3.4%) | 3 | (4.9%) | |
| Bleeding | 14 | (4.6%) | 0 | (0.0%) | |
| Bronchitis/pneumonia | 15 | (4.6%) | 2 | (3.3%) | |
| Diabetes mellitus | 9 | (2.8%) | 3 | (4.9%) | |
| Heart failure | 36 | (11.1%) | 4 | (6.6%) | |
| Hypertension | 8 | (2.5%) | 1 | (1.6%) | |
| Infection | 56 | (17.3%) | 15 | (24.6%) | |
| Ischemic heart diseases | 21 | (6.5%) | 4 | (6.6%) | |
| Malignant neoplasm | 11 | (3.4%) | 3 | (4.9%) | |
| Pancreatitis | 9 | (2.8%) | 5 | (8.2%) | |
| Renal failure | 18 | (5.6%) | 3 | (4.9%) | |
| Venous thromboembolism | 7 | (2.2%) | 1 | (1.6%) | |
| Othersb | 98 | (30.2%) | 16 | (26.2%) | |
| Primary diagnosis at 30-day readmission, n (%) | |||||
| Arrhythmia | 1 | (1.6%) | |||
| Asthma/COPD | 2 | (3.3%) | |||
| Bleeding | 1 | (1.6%) | |||
| Bronchitis/pneumonia | 2 | (3.3%) | |||
| Diabetes mellitus | 3 | (4.9%) | |||
| Heart failure | 5 | (8.2%) | |||
| Hypertension | 2 | (3.3%) | |||
| Infection | 20 | (32.8%) | |||
| Ischemic heart diseases | 2 | (3.3%) | |||
| Malignant neoplasm | 1 | (1.6%) | |||
| Pancreatitis | 4 | (6.6%) | |||
| Venous thromboembolism | 2 | (3.3%) | |||
| Othersc | 16 | (26.2%) | |||
| Comorbidity, n (%) | |||||
| Atrial fibrillation | 132 | (40.7%) | 17 | (27.9%) | 0.06 |
| COPD | 40 | (12.3%) | 14 | (23.0%) | 0.029 |
| Diabetes mellitus | 144 | (44.4%) | 25 | (41.0%) | 0.62 |
| Ischemic heart diseases | 134 | (41.4%) | 16 | (26.2%) | 0.026 |
| Heart failure | 125 | (38.6%) | 19 | (31.1%) | 0.27 |
| Atrial fibrillation | 132 | (40.7%) | 17 | (27.9%) | 0.06 |
Notes:
For categorical variables, we used Fisher’s exact and chi-square tests. For nonnormally distributed variables, we used the two-sided Kruskal–Wallis rank sum test.
Other diagnoses at admission included anemia, bile duct obstruction, arthritis, calculus, cervical spondylosis, cholangitis, fluid overload, fracture, complications of transplanted organ, complications of implanted device, constipation, degeneration of lumber or lumbosacral intervertebral disk, dehydration, dementia, dislocation of prosthetic joint, disorder of autonomic nervous system, displacement of cervical intervertebral disk, electrolyte imbalance, encephalopathy, epilepsy, fecal impaction, gastritis, gastromy complications, gout, hallucinations, headache, hemiplegia, inguinal hernia with obstruction, intestinal obstruction, migraine, orthostatic hypotension, osteoarthrosis, pain, poisoning, pressure ulcer, prosthetic joint repair, sickle-cell crisis, spasm of muscle, sprain of neck, systemic lupus erythematosus, systemic sclerosis, psychosis, ulcerative colitis, and vascular insufficiency of intestine.
Other diagnoses at 30-day readmission included acute vascular insufficiency of intestine, altered mental status, cerebral infarction, electrolyte imbalance, fracture, hemiplegia, hepatic encephalopathy, complications due to renal dialysis device, hypersensitivity angitis, fall, pain, and unspecified disorder of stomach and duodenum.
Abbreviations: COPD, chronic obstructive pulmonary disease; HMO, Health Maintenance Organization; MD, Doctor of Medicine; PPO, Preferred Provider Organization; PTA, prior to admission.
Table 3.
Multiple logistic regression model results for 30-day readmission, including adherence
| Association | HOSPITAL model with adherence
|
HOSPITAL model
|
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% confidence limits
|
P-valuea | Estimate | 95% confidence limits
|
P-valuea | |||
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Hemoglobin level at discharge (<12 g/dL) | 1.336 | 0.635 | 2.810 | 0.445 | 1.343 | 0.643 | 2.804 | 0.432 |
| Discharge from an oncology service | 2.219 | 1.078 | 4.566 | 0.031 | 2.210 | 1.086 | 4.494 | 0.029 |
| Sodium level at discharge (<135 mEq/L) | 1.646 | 0.807 | 3.359 | 0.171 | 1.764 | 0.872 | 3.567 | 0.114 |
| Procedure during hospital stay (any ICD-9-CM-coded procedure) | 1.098 | 0.579 | 2.082 | 0.773 | 1.130 | 0.601 | 2.127 | 0.704 |
| Index admission type: nonelective | 3.383 | 0.946 | 12.107 | 0.061 | 3.294 | 0.925 | 11.732 | 0.066 |
| Hospital admissions during the previous year | 0.676 | 0.295 | 1.551 | 0.356 | 0.721 | 0.317 | 1.636 | 0.433 |
| Length of stay ≥5 days | 1.974 | 1.062 | 3.669 | 0.032 | 1.838 | 0.996 | 3.393 | 0.052 |
| Medication adherence (low/intermediate vs high) | 2.545 | 1.323 | 4.902 | 0.005 | ||||
Notes: c-statistic =0.70 and 0.65 for the models with and without medication adherence, respectively.
Wald chi-square test.
The c-statistic was used to evaluate prediction accuracy, where c =1 for a perfect model and c =0.5 for a model with no better than random classification.20 Because we did not expect the predictive capacity of the HOSPITAL model to remain constant when we adapted this model to our data structures and our patient population, the most relevant c-statistic comparison was the c-statistic achieved by our model using our data with and without the inclusion of medication adherence data. All reported tests were two sided at a significance level of α =0.05. P-values were not adjusted for multiple testing. Data were analyzed using the SAS statistical software (SAS Institute, Cary, NC, USA).
Results
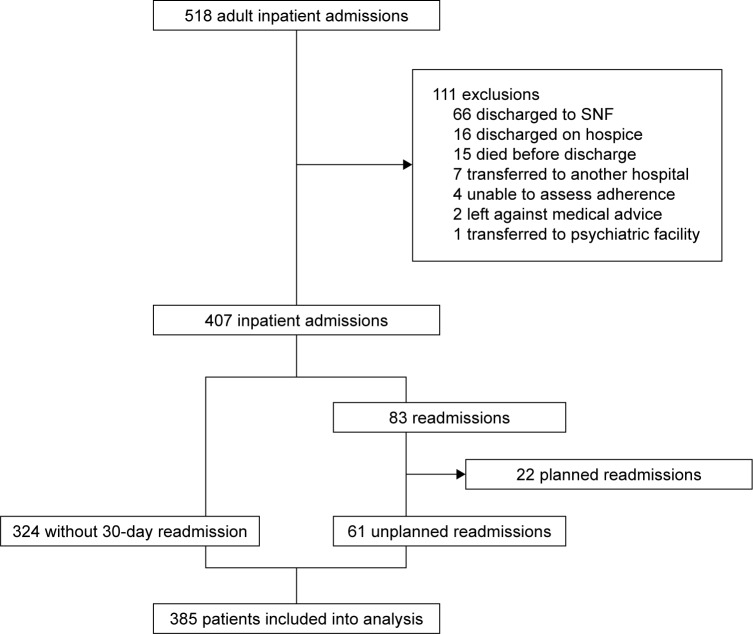
Five hundred and eighteen adult patients admitted under inpatient status received a pharmacist consultation during the study period (Figure 1). Of these patients, 111 patients were excluded due to having been transferred to another acute care facility or psychiatric facility, inability to assess adherence, being discharged to hospice or a skilled nursing facility, dying before discharge, or because the patient left against medical advice. Twenty-two additional patients were excluded due to their readmissions being planned. Ultimately, 385 patients were included in this analysis, of whom 61 patients experienced an unplanned 30-day readmission.
Figure 1.
Selection process for study inclusion.
Abbreviation: SNF, skilled nursing facility.
Baseline characteristics for patients with low, intermediate, and high adherence are reported in Table 1. Patients included in the analysis had diverse medical conditions including general medicine, cardiology, endocrinology, hematology, gastroenterology, nephrology, oncology, pulmonology, transplant, rheumatology, and infectious diseases. The patients’ average age at inclusion was 67 years (range from 21 to 99 years), and the average number of medications on the prior-to-admission medication list was 21 (range from 2 to 48). The variables with significant differences between groups were patients’ race (P=0.047) and marital status (P=0.049). Patients in the low-adherence group had the highest percentage of Black or African American patients compared to the intermediate- and high-adherence groups (31.9%, 21.3%, and 15.3%, respectively). Patients in the low-adherence group also had the lowest percentage being married/having domestic partner or significant other compared to the intermediate- and high-adherence groups (31.9%, 48.9%, and 54.0% respectively). There were no other clinical or demographic statistically significant differences observed between group characteristics.
Table 2 presents patients’ demographic and clinical characteristics by 30-day readmission status. Of the seven binary variables included in the previously published HOSPITAL model, bivariate analysis showed that three variables were significantly associated with 30-day readmission in our patient population: The involvement of an oncologist (relative risk [RR] =1.84, 95% CI: 1.10–3.07), sodium level at discharge (RR =1.65, 95% CI: 1.01–2.79), and length of stay (RR =1.09, 95% CI: 1.09–2.98). Patients with either low adherence or intermediate adherence had an unadjusted readmission rate of 20.0%, as compared to 9.3% in patients with high adherence (P=0.005).
When medication adherence was added to the model as low, intermediate, and high, the low and intermediate levels had similar associations with readmission (P=0.855); therefore, these levels were collapsed into one category (Table 3). Multiple regression modeling showed that patients with low or intermediate adherence (combined) had an adjusted 2.54-fold (95% CI: 1.32–4.90) higher odds of readmission (P=0.005) as compared to patients with high adherence. When we tested the HOSPITAL variables in our regression model without medication adherence, the c-statistic (Wald 95% CI) was 0.65 (0.58–0.73). After adding adherence to the model, the c-statistic (Wald 95% CI) improved to 0.70 (0.63–0.76). As a sensitivity analysis, we ran our final model with adherence adding the number of medications as an additional covariate. The number of medications, whether included as continuous or categorical variables, was highly insignificant (P=0.88 and P=0.57, respectively), while adherence stayed significant.
Discussion
Our study found that patients with either a low medication adherence or an intermediate medication adherence had >2.5 times greater odds of being rehospitalized within 30 days. Furthermore, when medication adherence was added to our model, its predictive capability was improved. In a systematic review of models that predict hospital readmission, the five models requiring information from hospital discharge had c-statistic ranging from 0.68 to 0.83.6 c-Statistic 0.70 is often used as the minimum cutoff for acceptable discrimination, so improving the baseline c-statistic from 0.65 to 0.70 represents a meaningful improvement.
The MMAS-4 provides clinicians with a reliable and quick assessment of medication adherence since it consists of four simple yes/no questions that takes less than a minute to administer. Based on 2015 US Bureau of Labor Statistics, the hourly wage data for pharmacists are $57.34.21 Having a pharmacist assess medication adherence using the MMAS-4 would cost less than a dollar. Like any self-reported measure, MMAS-4 may have several limitations that may compromise its accuracy (such as recall bias and/or overestimation).22 However, the benefit of allowing clinicians to focus on helping patients overcome barriers leading to nonadherence may offset this limitation.
Interestingly, we were only able to detect a difference in readmissions between high-adherence and nonhigh-adherence patients. It remains unclear whether intermediate adherence is better than low adherence as it relates to the primary outcome. It is possible that this study was not sufficiently powered to detect this difference or that the education provided equalized these groups but could not bring them up to the level of patients with high baseline adherence. Alternatively, it is conceivable that there is no significant difference in 30-day readmissions between patients who have low vs intermediate adherence and that the key determinant is simply whether or not they are highly adherent. More research will be required to definitively answer this question.
Another interesting finding was that race and marital status correlated with the level of adherence. This finding is consistent with the results of a sociodemographic analysis by Jankowska-Polańska et al23 who postulated that poor understanding of hypertension treatment was linked to patients having low adherence and living alone. This observation may warrant additional study to determine whether adherence is a modifiable predictor of hospital readmission or whether it is merely a measure of low socioeconomic status and poor social support that are the true root causes of readmission.
The initial variables in the HOSPITAL study were selected based on a priori hypotheses, and seven of them have been shown to be predictive of 30-day hospital readmission. We included all seven HOSPITAL variables in our regression model; however, only three of them (discharge from oncology service, sodium level at discharged, and length of stay) achieved statistical significance in our patient population. This could be due to statistical power, as we could include only 385 patients in our analysis that required an MMAS-4 for each patient vs 9,212 patients in HOSPITAL study, which used exclusively administrative data.
This study has several limitations. 1) This was a single-site study. Further study outside the CSMC would be necessary to confirm generalizability, although we have no reason to suspect that these results would not generalize. 2) As with almost any observational study, we cannot exclude the possibility that differences in readmission rates were due to unmeasured variables. 3) We were only able to identify readmissions occurring at CSMC. Prior investigators have found no reason to conclude that readmissions to other hospitals would have different predictors.7 4) Given our inclusion criteria, patients who were included in our study were more medically complicated than the patients who were not included. These criteria did identify patients who are systematically different, but not in any way that it should raise concern. In fact, one could consider this to be a strength of the study since we selected a potentially higher risk group of patients who may benefit most from intervention. Nonetheless, future research should consider expanding the inclusion criteria to include less sick patients. Finally, to the extent that patient education to improve adherence occurred in real time as part of the QI project, our results may actually underestimate the predictive power of medication adherence.
Conclusion
Good medication adherence assessed at the time of hospitalization appears to be independently associated with 30-day hospital readmission rates. Given that medication adherence could identify patients at higher risk of readmission, the information is easily obtainable in real time, and the fact that medication nonadherence has been shown to be a modifiable risk factor, a patient’s adherence level to medications is a uniquely valuable information. An unanswered research question is whether targeted interventions can be effectively deployed to reduce 30-day readmission rates in patients who are prospectively identified as not having good adherence.
Acknowledgments
The authors acknowledge the contributions of Donald Morisky, professor of the Department of Community Health Sciences at the UCLA Fielding School of Public Health. Authors obtained written permission from copyright owners for any excerpts from copyrighted works that are included and have credited the sources in the article. This research was supported by NIH/National Center for Advancing Translational Science UCLA CTSI Grant Number KL2TR000122 and National Institute on Aging Grant Number K23 AG049181-01 (JMP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The investigators retained full independence in the conduct of this research. OZR and BTR are married.
Footnotes
Author contributions
OZR, JMP, and RF contributed to study design. OZR, JMP, RF, BTR, and RS contributed to drafting the article. OZR and RF contributed to data acquisition. RF contributed to statistical analysis. OZR, RF, JMP, BTR, and RS contributed to data analysis and interpretation. OZR, JMP, RF, BTR, and RS contributed to article revisions.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 3.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 4.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 5.Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173(8):695–698. doi: 10.1001/jamainternmed.2013.171. [DOI] [PubMed] [Google Scholar]
- 6.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638. doi: 10.1001/jamainternmed.2013.3023. [DOI] [PubMed] [Google Scholar]
- 8.Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL Score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496–502. doi: 10.1001/jamainternmed.2015.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MJ, Shaykevich S, Cawthon C, Kripalani S, Paasche-Orlow MK, Schnipper JL. Predictors of medication adherence postdischarge: the impact of patient age, insurance status, and prior adherence. J Hosp Med. 2012;7(6):470–475. doi: 10.1002/jhm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. doi: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TM, La Caze A, Cottrell N. What are validated self-report adherence scales really measuring? A systematic review. Br J Clin Pharmacol. 2014;77(3):427–445. doi: 10.1111/bcp.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavsa SM, Holzworth A, Ansani NT. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc. 2003;51(1):90–94. doi: 10.1331/JAPhA.2011.09154. [DOI] [PubMed] [Google Scholar]
- 14.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence and long-term predictive validity of blood pressure control. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Morisky DE, Malotte CK, Choi P, et al. A patient education program to improve adherence rate with antituberculosis drug regimens. Health Educ Q. 1990;17(3):253–268. doi: 10.1177/109019819001700303. [DOI] [PubMed] [Google Scholar]
- 16.Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64(3):255–257. doi: 10.1016/j.jclinepi.2010.09.002. Discussion 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee WY, Ahn J, Kim JH, et al. Reliability and validity of a self-reported measure of medication adherence in patients with type 2 diabetes mellitus in Korea. J Int Med Res. 2013;41(4):1098–1110. doi: 10.1177/0300060513484433. [DOI] [PubMed] [Google Scholar]
- 18.Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12(1):118–123. doi: 10.1111/j.1524-4733.2008.00400.x. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz LI, Partovian C, Lin Z, et al. Development and use of an administrative claims measure for profiling hospital-wide performance on 30-day unplanned readmission. Ann Intern Med. 2014;161(10 suppl):S66–S75. doi: 10.7326/M13-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosmer DW, Lemeshow SL. Applied Logistic Regression. New York, NY: John Wiley & Sons Publications; 1989. [Google Scholar]
- 21.Bureau of Labor Statistics U.S. Department of Labor [webpage on the Internet] Occupational Employment Statistics, May 2015. Pharmacists and Pharmacist Technicians; [Accessed July 15, 2016]. Available from: http://www.bls.gov/oes/ [Google Scholar]
- 22.Gonzalez JS, Schneider HE. Methodological issues in the assessment of diabetes treatment adherence. Curr Diab Rep. 2011;11(6):472–479. doi: 10.1007/s11892-011-0229-4. [DOI] [PubMed] [Google Scholar]
- 23.Jankowska-Polańska B, Uchmanowicz I, Dudek K, Mazur G. Relationship between patients’ knowledge and medication adherence among patients with hypertension. Patient Prefer Adherence. 2016;10:2437–2447. doi: 10.2147/PPA.S117269. [DOI] [PMC free article] [PubMed] [Google Scholar]