Abstract
Objective
Mesenteric lymphatic vessels pumping, important to propel lymph and immune cells from the intestinal interstitium to the mesenteric lymph nodes, is compromised during intestinal inflammation. The objective of this study was to test the hypothesis that the pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α), is a significant contributor to the inflammation-induced lymphatic contractile dysfunction, and to determine its mode of action.
Methods
Contractile parameters were obtained form isolated rat mesenteric lymphatic vessels mounted on a pressure myograph after 24-h incubation with or without TNF-α. Various inhibitors were administered and quantitative real-time PCR, western blotting and immunofluorescence confocal imaging were applied to characterize the mechanisms involved in TNF-α actions.
Results
Vessel contraction frequency was significantly decreased after TNF-α treatment and could be restored by selective inhibition of NF-кB, iNOS, guanylate cyclase and ATP-sensitive K+ channels. We further demonstrated that NF-кB inhibition also suppressed the significant increase in iNOS mRNA observed in TNF-α-treated lymphatic vessels and that TNF-α treatment favor the nuclear translocation of the p65 NF-κB subunit.
Conclusions
These findings suggest that TNF-α decreases mesenteric lymphatic contractility by activating the NF-κB - iNOS signaling pathway. This mechanism could contribute to the alteration of lymphatic pumping reported in intestinal inflammation.
Keywords: Lymphatic vessel contraction, inflammation, cytokine, nitric oxide synthase, nuclear factor-kappa B
Introduction
Lymphatic vessels serve as a route whereby lymph, interstitial fluid, containing immune cells, antigens, protein and other macromolecules, gains access to lymph nodes and is returned to the blood circulation. The flow of lymph is promoted by an active process called lymphatic pumping, characterized by the rhythmical contraction-relaxation cycle of the muscular wall of collecting lymphatic vessels. Lymphatic pumping is tightly regulated by the volume of lymph filling up the vessel chambers, but also by chemical mediators, such as nitric oxide (NO) and prostaglandins. Given their role in tissue fluid homeostasis and immune cell trafficking, lymphatic vessels are critically involved in edema clearance and initiation of an immune response that occur in most inflammatory insults. In this context, we previously investigated the effect of inflammation on mesenteric lymphatic vessel pumping in rodent models of TNBS-induced ileitis and demonstrated that it was significantly altered [49,77]. These findings suggest that if mesenteric lymphatic pumping, the main contributor to drainage of intestinal lymph [9], is impaired during intestinal inflammation, then lymph drainage from the gut mucosa is likely compromised. Such dysfunction causes lymph stasis, which may enhance the severity of the mucosal edema and contribute to the fibrotic and hypoxic environment characteristic of chronic intestinal inflammatory diseases [13,26]. Inhibition of lymph flow also likely affects the transportation of antigens and immune cells such as macrophages and dendritic cells to draining lymph nodes, and compromises the development of a proper immune response. In support of this proposal, pathological features, such as dilated or obstructed lymphatic vessels, formation of intestinal lymphoid aggregates and granulomas, suggesting compromised lymph flow and immune cell trafficking, have been reported in patients suffering from inflammatory bowel disease (IBD) [29,61,68]. Thus, impaired lymphatic pumping may play an essential role in the perpetuation of chronic gut inflammation and IBD. However, the mechanisms leading to impaired lymphatic pumping remain unknown.
Many pro-inflammatory cytokines are involved in the generation and progression of IBD [59]. Among them, tumor necrosis factor alpha (TNF-α) is abundantly present in the serum of IBD patients [38] and is highly expressed in the gut of animal models of TNBS-induced colitis [14,28,43]. Moreover, several studies demonstrated that TNF-α impairs the contractility of arterial vessels [7,8,78]. Based on these findings, we explored the possibility that TNF-α might also alter the contractile ability of lymphatic vessels and hypothesized that dysfunctional lymphatic pumping we demonstrated in model of TNBS-induced ileitis [49,77] is possibly due to the increased, inflammation-induced production of TNF-α and its detrimental action on mesenteric lymphatic vessels. Although TNF-α may not be the only cytokine contributing to the impaired lymphatic contractile function in the complex inflammatory milieu, investigating the effects of this cytokine may help to identify one potential mechanism whereby inflammation causes lymphatic contractile dysfunction.
Methods
Ethical approval
Male Sprague Dawley rats (200–300 g, Charles River, Montreal, QC, Canada) were used to perform all experiments. Animals were housed at a room temperature of 22°C with 12-hour day and light cycles and allowed full access to water and food at all times; both normal circadian rhythms and body temperatures were maintained. All animal protocols were reviewed and approved by the University of Calgary Animal Care and Ethics Committee and were conducted in accordance with the guidelines of the Canadian Council on Animal Care and the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Lymphatic vessel preparation
Rats were anaesthetized by isofluorane (Pharmaceutical Partners of Canada, Richmond Hill, ON, Canada) inhalation in a sealed box and euthanized by decapitation. The small intestine including jejunum and ileum was exteriorized after laparotomy and kept in cold (4°C) Dulbecco’s phosphate buffered saline (DPBS; Sigma-Aldrich, St-Louis, MO, USA). A piece of ileal mesentery containing a mesenteric arcade (artery, vein and two collecting lymphatic vessels on each side of the blood vessels) was isolated from the gut and pinned down on a Sylgard-coated Petri dish filled with cold DPBS and set on the stage of a dissection microscope. Collecting lymphatic vessels were then immediately and carefully dissected out of the mesentery and surrounding adipose tissue and cut into two halves. Each half was placed into one 3.5 mm sterile petri dish filled with DMEM/F12 solution (Thermo Fisher Scientific, Burlington, ON, Canada), the first one serving as a sham control vessel and the other half incubated with TNF-α alone or a combination of TNF-α plus relevant drug. Vessels were then placed in an incubator (5% CO2, 37°C) for a period of 3, 4, 6, 12 or 24 h.
Surgical induction of ileitis
Experimental ileitis was induced in rats as published previously [56]. Briefly, rats were fasted for 4–6 h and prepared for anaesthesia (4% isofluorane induction, maintained at 2% throughout the procedure). A surgical laparotomy was performed under sterile conditions to allow a segment of the ileum, 10 cm proximal to the ileocecal junction to be exteriorized. A solution of 40 mg/ml 2,4,6-trinitrobenzenesulfonic acid (TNBS, 50% ethanol in sterile 0.9% NaCl) was injected intraluminally into the ileum segment in a volume of 1 ml. An equivalent volume of sterile physiological saline (0.9% NaCl) was injected in the same manner to produce sham animals. Animals were euthanized 3 days after surgery by inhalation of an overdose of isofluorane followed by decapitation as described above. Severity of inflammation was assessed using three parameters: macroscopic damage score, weight loss and myeloperoxidase (MPO) activity as published previously [56], and mesenteric lymphatic vessels draining the inflamed site isolated.
Pressure myography
Following lymphatic vessel incubation, vessels were placed in a 2-ml pressure myograph organ bath and cannulated with two pulled and matched glass micropipettes (original size: OD 1.2 mm, ID 0.69 mm; approximate final ID 80–120 μm). Lymphatic vessels were tied at each end with a fine suture to the glass pipettes, which restricted fluid leakage as well as pressure drops inside the lymphatic vessel. The pipettes were positioned just above the glass bottom of the organ bath, which was transferred to the stage of a microscope. Each glass pipette was connected to independently adjustable pressure reservoirs filled with physiologic saline solution (PSS) of the following composition (mM): CaCl2, 2.5; KCl, 5; MgCl2, 2; NaCl, 120; NaHCO3, 25; NaH2PO4, 1; glucose, 11. The pH was maintained at 7.4 by constant bubbling with 95% O2/5% CO2 [73]. The reservoirs were set at the same height so that flow was not generated by pressure differences and pressure inside the vessel (transmural pressure) was constant and equal to that determined by the level of PSS in the reservoirs. The organ bath was superfused at a rate of 3 ml/min with PSS kept at 37°C. Vessel leak, integrity and twist were assessed first by applying a positive transmural pressure (3–5 cmH2O) and approximate in situ length established by briefly increasing transmural pressure to 10 cmH2O. The vessel was then set to a transmural pressure of 5 cmH2O for a 10–15 min equilibration period, or until phasic contractions were observed and kept at that pressure for an additional 30 min. Vessel contractions were observed via a microscope-CCD video camera connected to a television monitor and recorded using a video-dimension analyzer (Living Systems Instrumentation, Burlington, VT, USA), which tracks the optically denser lymphatic vessel wall, and measurements sent to a computer via an analog-to-digital converter (PowerLab/4SP, ADInstruments, Mountain View, CA, USA) and recorded with Chart v5 software (ADInstruments). Phasic contractile activity was assessed by measuring outer end-diastolic and outer end-systolic diameters, frequency and amplitude of contractions (decrease of more than 50% in diameter) during the last 2 min of recording in each experimental condition. Sham vessels were used as a control and were considered if they displayed regular rhythmical contractions of 4 or more/min. They were compared to vessels treated with different drugs. The contractile performance of sham lymphatic vessels incubated for 24 h in our experimental conditions was compared to that of freshly isolated mesenteric lymphatic vessels. No significant difference in contraction frequency could be observed (respective values 5.8 ± 0.51 and 4.7 ± 0.39 at 5 cmH2O, n=6, P=0.183) confirming that incubated sham vessels were suitable for investigating changes in contractile function following exposure to different treatments [23]. Tone index was measured to characterize the level of tonic contraction. It was determined as the difference between passive outer lymphatic diameter in Ca2+-free PSS and outer end-diastolic diameter in PSS at a given transmural pressure, expressed as a percentage of the passive outer lymphatic diameter [19].
Quantitative real-time polymerase chain reaction (qPCR)
Following dissection and incubation procedures similar to those described above, lymphatic vessels were placed in RNA-later (Thermo Fisher Sientific) for 24 h, homogenized using a QIAshredder (Qiagen, Mississauga, ON, Canada) and RNA extracted using the Qiagen Micro RNeasy Kit. The extracted RNA was used to synthesize cDNA with SuperscriptⅡReverse Transcriptase enzyme and oligo (dT) primers (both from Thermo Fisher Sientific) and the target genes (β-actin, eNOS, iNOS, COX-1, COX-2, TNF-α and TNFR1) were amplified by using QuantiTect Syber Green (Qiagen) in an iCycler iQ Real-Time PCR Instrument (Bio-Rad, Mississauga, ON, Canada). The sequences of all primers were determined according to the NCBI published rat sequences and forward and reverse primers were designed using Primer3 Software [39,66] (see Table 1). PCR results were quantified using the ΔΔCT method [81], and mRNA expression of the target genes was normalized to that of β-actin.
Table 1.
Quantitative real-time primer sequences (all 5′–3′)
| Gene | Forward | Reverse | Size (bp) |
|---|---|---|---|
| β-actin | CGTGGGCCGCCCTAGGCACCA | TTGGCCTTAGGGTTCAGGGGG | 150 |
| iNOS | CACCACCCTCCTTGTTCAAC | CAATCCACAACTCGCTCCAA | 120 |
| eNOS | TGCAACAAACCGAGGCAATC | CACCAGCTGGCTGTTCCAGA | 114 |
| COX-1 | ACTGGAAACCCAGCACATTC | AAGGAGACATAGGGGCAGGT | 108 |
| COX-2 | GGCCATGGAGTGGACTTAAA | CTCTCCACCGATGACCTGAT | 109 |
| TNF-α | GCCGATTTGCCACTTCATAC | AAGTAGACCTGCCCGGACTC | 113 |
| TNFR1 | CTCCCAGAAAGCAAGCAAAC | CCTCTGGCAGGTCAACATCT | 142 |
Western blot
Western blots were performed as described previously for small resistance arteries [33,50]. Briefly, isolated lymphatic vessels were lysed in extraction buffer, loaded on SDS-polyacrylamide gels and subjected to the electrophoresis (100 V, 120 min). Proteins in the separating gel were then transferred to 0.2 μm nitrocellulose or polyvinylidene difluoride (PVDF) membranes (Bio-Rad and Roche, Indianapolis, IN, USA, respectively) and fixed to the membranes with 0.5% glutaraldehyde in phosphate buffered saline (PBS) for 45 min. A blocking solution of 5% BSA/non-fat milk in 0.1% TBST for 90 min was employed to minimize non-specific binding. The membranes were then incubated with goat polyclonal anti-smooth muscle (SM)-22 antibody (1:2000 dilution; Novus Canada, Oakville, ON, Canada), anti-IκB/phospho-IκB antibodies (1:1000 dilution; Cell Signaling Technology, Danvers, MA, USA) in 0.1% TBST, a mixture of Tris-buffered saline (TBS, 150 mM NaCl, 25 mM Tris HCl with pH 7.5) and Tween 20 (0.1%) with 5% bovine serum albumin (BSA) or 5% non-fat milk overnight. After washing, the membranes were then incubated with appropriate secondary antibodies, anti-goat IgG-horseradish peroxidase-conjugated secondary antibody (1:5000 dilution) for SM-22, anti-mouse IgG biotin-SP conjugated antibody (1:10000 dilution) for phospho-IκB, anti-rabbit IgG biotin-SP conjugated antibody (1:10000 dilution; all from Millipore, Bellerica, MA, USA) for IκB and iNOS, in 0.1% TBST for 1 h. After extensive washing in TBST, membranes were incubated with horseradish peroxidase-conjugated streptavidin (1:200000, Pierce Biotechnology, Rockford, IL, USA) for 30 min and further washed with TBST and TBS prior to detection of the chemiluminescence signal with an Amersham ECL advanced western blotting detection kit (GE Healthcare, Mississauga, ON, Canada). Images were obtained using a Fuji CCD camera (Fujifilm Canada, Mississauga, ON, Canada) and chemiluminescence imaging software (LAS3000 Mini; Fujifilm Canada). Blots were quantified using MultiGauge v3.0 software (Fujifilm Canada) and the levels of IκB, phospho-IκB protein normalized to SM-22 expression.
Immunofluorescence imaging
Lymphatic vessels were dissected as described above and similarly incubated in the absence (sham) or presence of TNF-α (10 ng/ml) for 24 h. The vessels were then fixed in PBS containing 4% formalin (Sigma-Aldrich, Oakville, ON, Canada) for 30 min at 37°C, and permeabilized in chilled methanol at −20°C for 5 min.
Rat mesenteric lymphatic muscle and endothelial cells were kindly provided by Dr. W. E. Cromer and D. C. Zawieja (TAMUHSC, Temple, TX) and cultured according to their publication [17]. Briefly, both types of cells were grown on 12 mm round coverslips (Warner Instruments, Hamden, CT, USA) pre-coated with 2% porcine gelatin (LECs) in DMEM/F12 supplemented with Antibiotic-Antimycotic (Thermo Fisher Scientific) and FBS (Sigma-Aldrich) for LMCs and EGM-2 (Lonza, Walkersville, MD, USA) for LECs. LECs and LMCs were allowed to reach 80% confluence before being treated with TNF-α at 3 ng/ml for 6 h and 10 ng/ml for 3 h respectively, after which cover slips were removed from culture media and placed in DPBS. Cells were then fixed in 2% paraformaldehyde for 15 min at room temperature, washed in glycine-DPBS, rinsed in DPBS and permeabilized in chilled methanol on a bed of ice for 4 min.
All samples were blocked in DPBS supplemented with 5% goat serum and 1% BSA (both Sigma-Aldrich) and incubated overnight with primary antibody against the p65 subunit of NF-κB (Sigma-Aldrich) at dilutions of 1:100 (primary antibody to blocking solution). Vessels were counterstained with smooth muscle α-actin (1:500; Abcam, Toronto, ON, Canada). Negative controls were prepared with mouse IgG1 (BD Biosciences, Mississauga, ON, Canada). Both cells and vessels were then incubated in secondary goat anti-mouse IgG1 antibody Alexa Fluor 647 (Thermo Fisher Scientific) and goat anti-rabbit antibody Alexa Fluor 488 (Thermo Fisher Scientific; vessels only) at dilutions of 1:250 for 1 h at room temperature. For nuclear labelling, 4′,6-diamidino-2-phenylindole (DAPI, Thermo Fisher Scientific) was used at a dilution of 1:500 in DPBS to incubate the vessels 5 min at room temperature. Vessels and cells were then mounted on slides with FluorSave reagent (EMD Millipore, Etobicoke, ON, Canada) and imaged using a resonant scanning inverted confocal microscope (TCS SP8, Leica Microsystems, Concord, ON, Canada). The immunofluorescence was detected and images recorded in z stacks.
NF-kB translocation analysis
Acquired images were analyzed with the open source image processing package Fiji. Background was subtracted and threshold adjusted on stacks selected to contain a majority of smooth muscle cells. Regions of Interests (ROIs) were manually drawn in the DAPI channel before being overlayed in the p65 channel. The intensity of the signal for that channel in the ROIs was then averaged for each experiment and values compared for sham and TNF-α treated vessels respectively.
Chemicals
TNF-α (Sigma-Aldrich) was diluted in distilled water to make a stock solution of 0.1 mg/ml and was subsequently diluted in DMEM/F12 supplemented with Antibiotic-Antimycotic) to achieve final concentrations of 0.1, 1, 3 and 10 ng/ml. Stock solutions of pyrrolidine dithiocarbamate (PDTC), 1400W (both from Tocris Bioscience, Minneapolis, MI, USA), indomethacin and glibenclamide (both from Sigma-Aldrich), 1H-(1,2,4) oxadiazolo [4,3-a] quinoxalin-1-one (ODQ, Alexis Biochemicals, Farmingdale, NY, USA) were all prepared in dimethyl sulfoxide at a concentration of 10 mM. PDTC, 1400W, glibenclamide and ODQ were diluted in DMEM/F12 to achieve a final concentration of 10 μM. When used to treat vessels during myography experiments, inhibitors were diluted to achieve final concentrations of 10 μM in PSS. The working concentration of PDTC, 1400W, indomethacin, ODQ and glibenclamide were determined based on previous studies showing effective restoration of contraction in inflamed lymphatic vessels [15,47,49,77].
Data and statistical analysis
Contraction data were expressed relative to each vessel’s own sham control value. Statistical significance was then assessed on the raw data using two-tailed paired and unpaired Student’s t-test or ANOVA with an appropriate post-hoc test as indicated in the text was used. Experimental data were expressed as mean values ± one standard error of the mean (SEM) and P value less than 0.05 was considered significant.
Results
TNF-α decreases lymphatic contraction frequency in a concentration-dependent manner
Isolated lymphatic vessels incubated with increasing concentrations of TNF-α (0.1 – 10 ng/ml) for 24 h displayed a decreased contraction frequency compared to sham-treated vessels when mounted in the pressure myograph system at a transmural pressure of 5 cmH2O (Fig. 1). The TNF-α-induced decrease in contraction frequency was concentration-dependent (IC50 ~4 ng/ml) and significant differences were observed between sham and vessels treated with 1 ng/ml and 10 ng/ml TNF-α. However, the amplitude of contractions and tone index were not significantly different at any of the TNF-α concentrations. Vessels treated with 100 ng/ml TNF-α, did not respond with increased contraction frequency to sequential increase in pressure (n=4, data not shown), unlike sham-treated vessels [44].
Figure 1. Effects of TNF-α on the contractile activity of pressurized rat mesenteric lymphatic vessels.
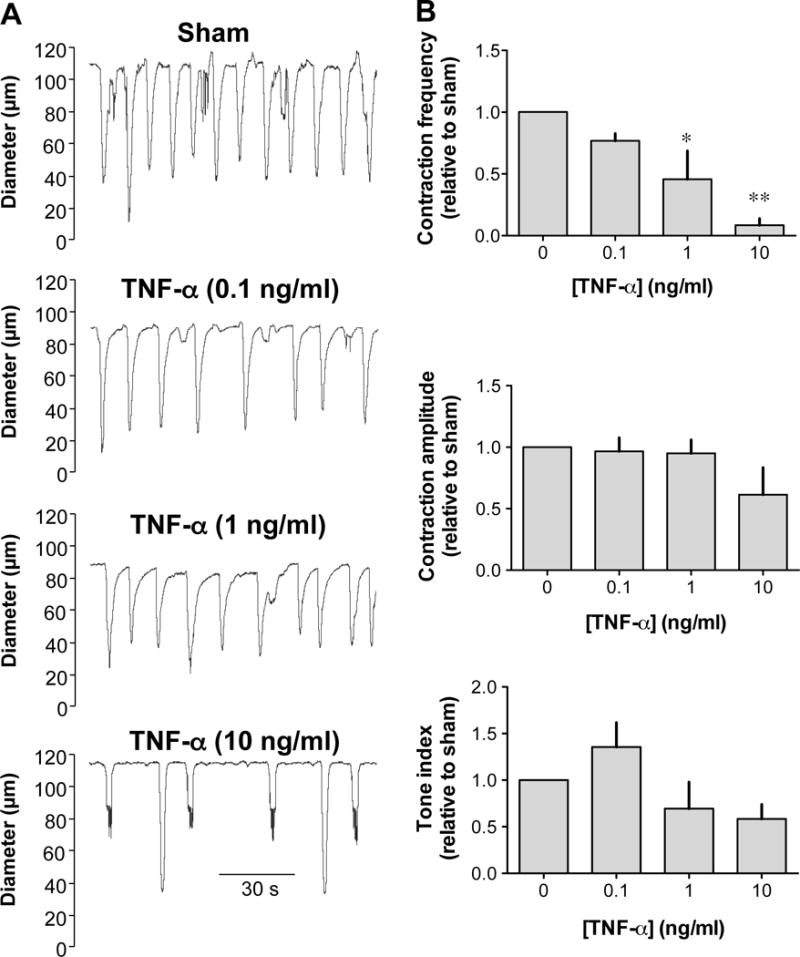
A. Original recordings of the contractile activity of lymphatic vessels pressurized at 5 cmH2O after a 24-h incubation without (sham) and with increasing concentrations (0.1, 1.0 and 10 ng/ml) of TNF-α. Time scale bar applies to all traces. B. TNF-α caused a concentration-dependent decrease in contraction frequency (top bar graph), but did not affect the amplitude of contractions or the tone index (n=4; *P<0.05, **P<0.01, repeated measures of ANOVA with Dunnett’s post-hoc test).
TNF-α-induced decrease in lymphatic contraction frequency depends on NF-κB activation
TNF-α-induced cellular effects are typically mediated through the activation of NF-κB. To assess the role of this transcription factor in the lymphatic contractile inhibition caused by TNF-α, vessels were incubated with the NF-κB inhibitor, PDTC (10 μM). Under these conditions, the contraction frequency of vessels treated with TNF-α was restored, reaching values not significantly different from sham vessels (Fig. 2). The involvement of NF-κB was further investigated by measuring the phosphorylated form of IκB following TNF-α treatment. Western blot of proteins extracted from rat mesenteric lymphatic vessels incubated for 4 h with 3ng/ml or 10ng/ml TNF-α, showed a concentration-dependent increase in phospho-IκB when compared to sham-treated vessels (Fig. 3A & B). Finally, nuclear translocation of the NF-κB subunit p65 was assessed 3 h after TNF-α treatment by confocal immunofluorescence. As illustrated in figure 3C, TNF-α treatment produced a significant increase in p65 staining in the nucleus of lymphatic cells, but more evidently in muscle cells, while no nuclear staining was seen in cells from sham-treated vessels. Nuclear translocation of p65 after TNF-α treatment was also evident in cultures of endothelial and muscle cells isolated from rat mesenteric vessels.
Figure 2. Effect of PDTC on the TNF-α-induced decrease in lymphatic contraction frequency.
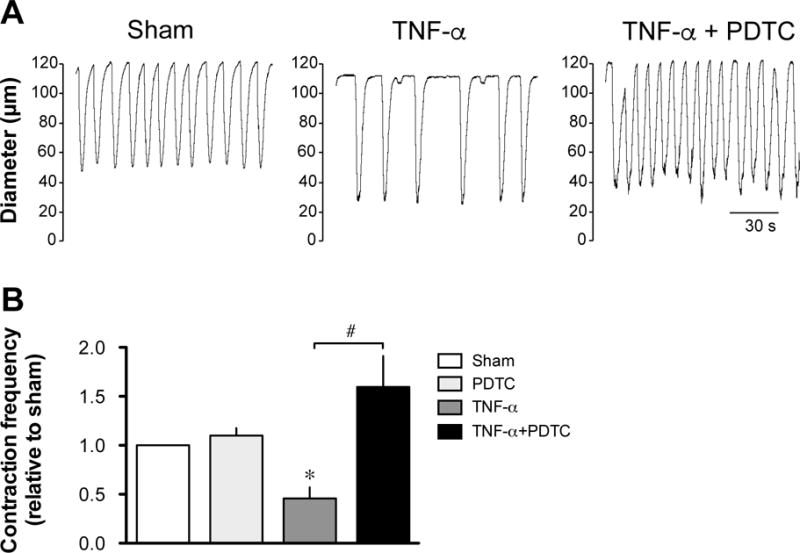
A. Original recordings comparing the contractile activity of lymphatic vessels pressurized at 5 cmH2O after a 24-h incubation in DMEM/F12 media (sham), in media plus 3 ng/ml TNF-α and in media/TNF-α plus 10 μM PDTC. Time scale bar applies to all traces. B. Summary bar graph of the effect of PDTC on TNF-α-induced decreased response relative to the contraction frequency measured in the respective sham vessels (n=4–5; *P<0.05 vs own sham, paired Student t-test and ##P<0.05, repeated measures of ANOVA with Bonferroni’s post-hoc test).
Figure 3. Effect of TNF-α on NF-κB activation in lymphatic vessels.
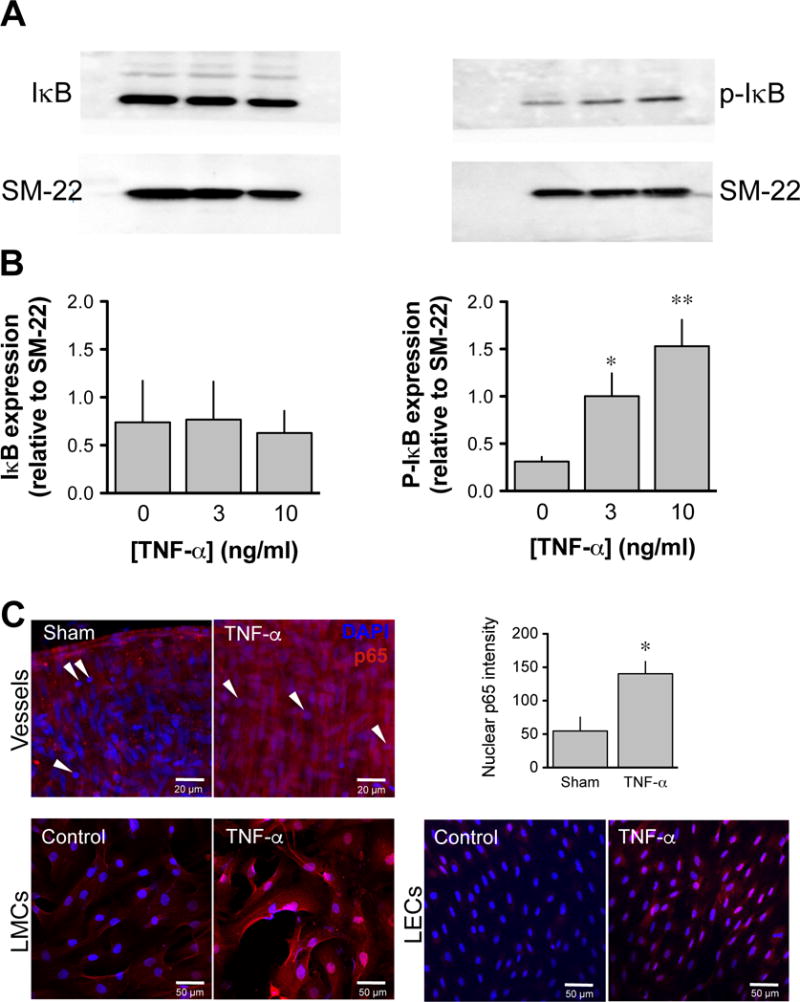
A. Representative western blots of unphosphorylated (left panel) and phosphorylated (right panel) IκB protein following 4-h incubation without (sham) and with 3ng/ml and 10 ng/ml TNF-α. B. Summary densitometry bar graph of the level of IκB and p-IκB in the three vessel groups normalized to the level of SM22 (n=4; *P<0.05, **P<0.01, repeated measures of ANOVA with Dunnett’s post-hoc test). C. Level of nuclear translocation of the NF-κB subunit, p65 in lymphatic cells, illustrated by confocal immunofluorescence. Representative images depicting nucleus (blue, DAPI) and p65 (red) in a sham (top left panel) and a TNF-α-treated lymphatic vessel (middle panel, 10 ng/ml, 3-h incubation). Maximal projection of a stack of 3 images taken at the level of the muscles, as denoted by the vertical (perpendicular to the vessel length) orientation of most of the nucleus (note immune cells identified by their round nucleus, arrowheads). Nuclear translocation (pink nuclear staining) is apparent in most cells of the TNF-α-treated vessel as confirmed by the summary bar graph representing the level of nuclear p65 fluorescence (right panel; n=4; *P<0.05 vs own sham, paired Student t-test). Nuclear translocation of the p65 subunit is also evident in cultures of lymphatic endothelial (bottom left panels) and muscle cells (bottom right panels) isolated from the same rat mesenteric vessels when treated with TNF-α. Images are representative of 3–5 similar experiments.
TNF-α-induced, NF-κB-mediated, decreased in lymphatic contraction frequency requires activation of iNOS but not COXs
In an inflammatory context, NF-κB is classically involved in upregulating the transcription of iNOS and COX-2. As these enzymes catalyze the synthesis of potent modulators of lymphatic pumping, NO and prostaglandins, respectively, changes in mRNA expression of these enzymes, as well as the constitutively expressed eNOS and COX-1, were assessed by qPCR. Figure 4A shows that the relative expression of iNOS mRNA in TNF-α-treated vessels varied over time and was significantly increased after 6-h incubation, when compared to expression levels in sham or control vessels (n=4, P<0.05). Expression gradually returned to control values after 12 and 24-h incubations. There was no significant difference in the expression of eNOS mRNA between control, sham and TNF-α-treated vessels after 6, 12 or 24 h (Fig. 4B). On the other hand, the mRNA expression of COX-1 and COX-2 in lymphatic vessels at the same time points, while showing some variability, was not significantly affected by the TNF-α -treatment (Fig. 4C & D).
Figure 4. Effect of TNF-α on the gene expression of NOSs and COXs in lymphatic vessels.
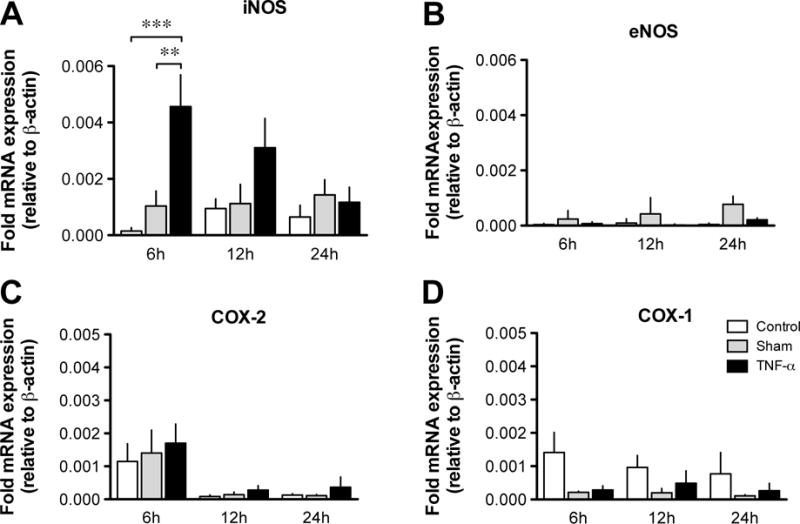
Bar graphs representing time-course mRNA expression as measured by qPCR of genes for iNOS (A), eNOS (B) COX-2 (C) and COX-1 (D) in fresh lymphatic vessels (control), and in vessels incubated for 6, 12 and 24 h in normal media (sham) or in the presence of TNF-α (3 ng/ml). Gene expressions were normalized to the expression of the housekeeping gene β-actin (n=3–7; *P<0.05, **P<0.01, two-way ANOVA with Bonferroni’s post-hoc test).
To examine whether the increased iNOS expression resulted in increased production of NO that would mediate the TNF-α-induced decrease in lymphatic contractions, isolated lymphatic vessels treated for 24 h with 3 ng/ml of TNF-α were pressurized at 5 cmH2O and treated with the iNOS inhibitor 1400W (10 μM). In these conditions, contraction frequency of TNF-α-treated vessels was significantly lower than sham vessels (3.3 ± 0.3 cpm, vs 6.0 ± 0.3 cpm, n=4, P<0.05) and was considerably increased to 4.7 ± 0.5 cpm after a 20-min incubation with 1400W (10 μM; n=4, P<0.05), while frequency of sham vessels treated in the same manner was not changed (6.4 ± 0.4 cpm, n=4). To further demonstrate the role of iNOS in the lymphatic dysfunction, 1400W was administered to the vessels together with the TNF-α at the beginning of the 24 h incubation. Under these conditions, the TNF-α-induced decreased lymphatic contraction frequency could be completely reversed (Fig. 5A & B). Consistent with the inability of TNF-α to increase the mRNA expression of COX-1 and COX-2, the TNF-α-induced decrease in lymphatic contraction frequency could not be restored in vessels co-incubated with the nonselective COX inhibitor, indomethacin (10 μM, Fig. 5C).
Figure 5. Effect of 1400W on the TNF-α-induced decrease in lymphatic contraction frequency.
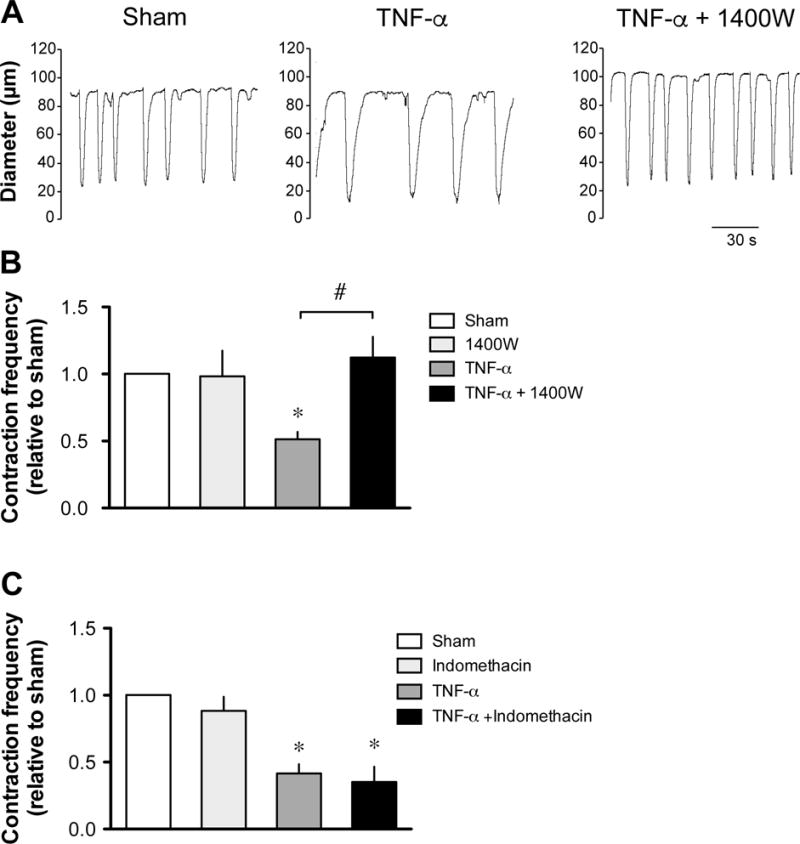
A. Original recordings comparing the contractile activity of lymphatic vessels pressurized at 5 cmH2O after a 24-h incubation in DMEM/F12 media (sham), in media plus 3 ng/ml TNF-α and in media/TNF-α plus 10 μM 1400W. Time scale bar applies to all traces. B. Summary bar graph of the effect of 1400W on TNF-α-induced decreased response relative to the contraction frequency measured in the respective sham vessels (n=5; *P<0.05 vs own sham, paired Student t-test and ##P<0.01, repeated measures of ANOVA with Bonferroni’s post-hoc test. C. Summary bar graph of the absence of effect of a 24-h incubation with 10 μM indomethacin on TNF-α-induced decreased response relative to the contraction frequency measured in the respective sham vessels (n=4).
Finally, the role of NF-κB activation in TNF-α-induced upregulation of iNOS mRNA was confirmed by qPCR in TNF-α-treated vessels during co-incubation with PDTC (10 μM). Under these conditions, the relative expression of iNOS mRNA was again significantly increased in TNF-α-treated vessels, compared to that in sham vessels, while iNOS mRNA expression decreased in TNF-α-treated vessels co-incubated with PDTC to a level not significantly different from that of sham and PDTC-treated vessels (Fig. 6).
Figure 6. Effect of PDTC on the TNF-α-induced upregulation of iNOS in lymphatic vessels.
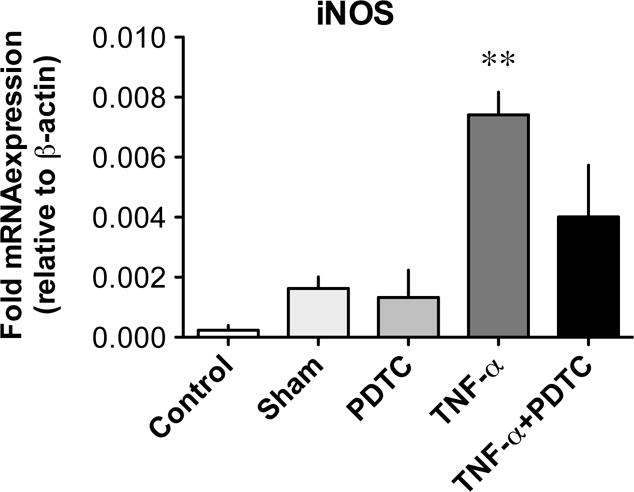
Bar graphs representing iNOS mRNA expression as measured by qPCR in lymphatic vessels without (control), and after 6 h of incubation in media alone (sham), 10 μM PDTC, TNF-α (3 ng/ml) and TNF-α plus PDTC. Gene expression was normalized to the expression of β-actin (n=4; **P<0.01 vs sham, one-way ANOVA with Bonferroni’s post-hoc test).
TNF-α-induced, NO-dependent, decreased in lymphatic contraction frequency is mediated by activation of sGC and KATP channels
Previous studies have demonstrated that the NO-induced inhibition of lymphatic pumping was mediated by activation of the soluble guanylyl cyclase (sGC) and opening of KATP channels leading to hyperpolarization of the muscle cell layer [49,77]. Involvement of this signaling pathway in the TNF-α-induced decrease in lymphatic contraction frequency was thus examined. First, the contractile activity was compared between lymphatic vessels incubated for 24 h with TNF-α and with TNF-α plus the sGC inhibitor ODQ (10 μM). As shown in Fig. 7A & B, addition of ODQ to the incubation media allowed lymphatic contraction frequency of TNF-α-treated vessels to reach values significantly greater than vessels treated with TNF-α alone and not different from sham-treated vessels. A role for KATP channels was then investigated and contractile activity in sham and TNF-α-treated vessels assessed in the presence of the blocker glibenclamide. After 20 min of glibenclamide (10 μM) incubation, the low contractile activity of TNF-α-treated vessels was significantly increased to values not significantly different from sham values (Fig. 8A & B).
Figure 7. Effect of ODQ on the TNF-α-induced decrease in lymphatic contraction frequency.
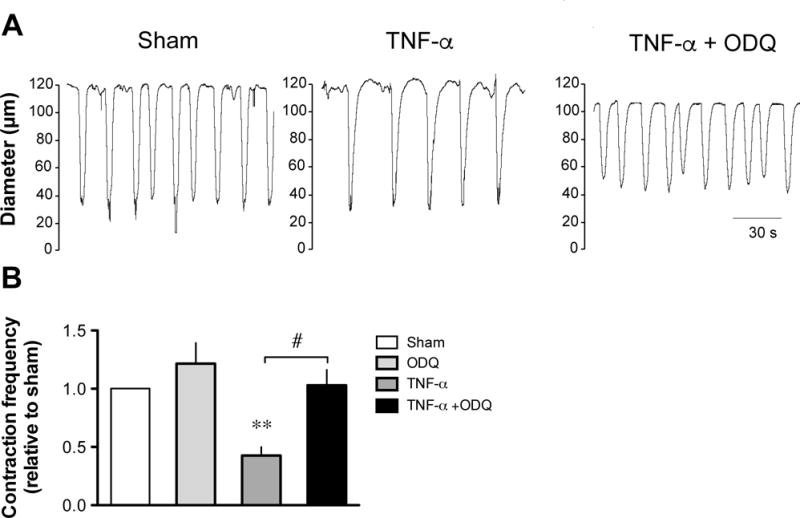
A. Original recordings comparing the contractile activity of lymphatic vessels pressurized at 5 cmH2O after a 24-h incubation in DMEM/F12 media (sham), in media plus 3 ng/ml TNF-α and in media/TNF-α plus 10 μM ODQ. Time scale bar applies to all traces. B. Summary bar graph of the effect of ODQ on TNF-α-induced decreased response relative to the contraction frequency measured in the respective sham vessels (n=4; **P<0.01 vs own sham, paired Student t-test; #P<0.05, repeated measures of ANOVA with Bonferroni’s post-hoc test).
Figure 8. Effect of glibenclamide on the TNF-α-induced decrease in lymphatic contraction frequency.
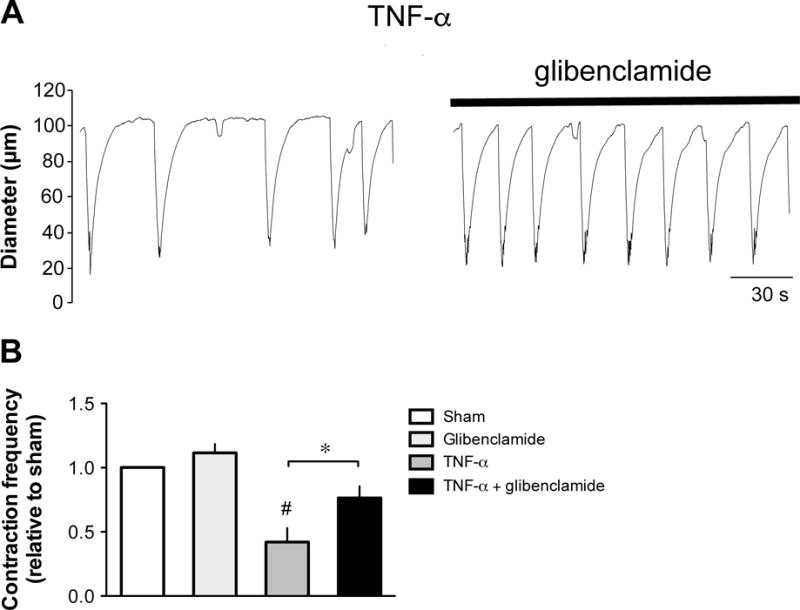
A. Original recordings of the contractile activity of lymphatic vessels pressurized at 5 cmH2O after a 24-h incubation in media plus 3 ng/ml TNF-α before (left trace) and during incubation with 10 μM glibenclamide (right trace). Time scale bar applies to both traces. B. Summary bar graph of the effect of glibenclamide administration on TNF-α-induced decreased response relative to the contraction frequency measured in sham vessels (n=4; *P<0.05, paired Student t-test; #P<0.05 vs sham, repeated measures of ANOVA with Dunnett’s post-hoc test).
TNF-α production and expression of its receptor TNFR1 are elevated in mesenteric lymphatic vessels isolated from a rat model of acute ileitis
We previously reported lymphatic contractile dysfunction in TNBS-induced ileitis and demonstrated that pumping inhibition was mediated in part by increase in iNOS-dependent NO production [49,77], suggesting involvement of signalling pathway similar to that activated by TNF-α, as demonstrated above. We thus examined whether TNF-α was upregulated in mesenteric lymphatic vessels of sham and TNBS-treated rats. We measured the mRNA expression levels of TNF-α by qPCR and showed that TNF-α expression was significantly increased in lymphatic vessels from inflamed rats compared to their sham counterparts, reaching values similar to those of the inflamed ileum (Fig. 9A). Examination of the mRNA expression of the TNF-α receptor TNFR1 also revealed and increased level in vessels from TNBS-treated rats (Fig. 9B).
Figure 9. Expression of TNF-α and its receptor in ileum and mesenteric lymphatic vessels of TNBS-treated rats.
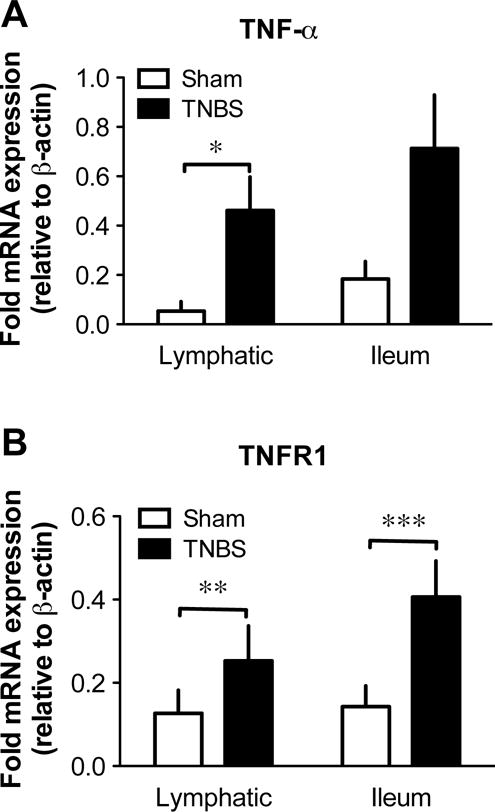
Bar graphs representing mRNA expression as measured by qPCR of genes for TNF-α (A) and TNFR1 (B) in lymphatic vessels and ileum collected 3 days after surgical injection of saline (sham) and TNBS in rat ileum. Gene expressions were normalized to the expression of the housekeeping gene β-actin (n=4; *P<0.05, **P<0.01, ***P<0.001, unpaired Student t-test).
Discussion
The ability of mesenteric lymphatic vessels to transport lymph and immune cells hence regulating tissue fluid volume and immunity is especially critical during intestinal inflammation. However in these conditions, as demonstrated in rodent models of TNBS-induced ileitis [49,77], the ability of the mesenteric lymphatics to pump and thus promote intestinal lymph drainage is compromised. The findings presented here suggest that part of this dysfunction might be due to TNF-α, one of the pro-inflammatory cytokines upregulated in the ileum during TNBS-induced inflammation [16,64]. Indeed, we found that mRNA expressions of TNF-α and the TNF-α receptor TNFR1 were significantly upregulated in lymphatic vessels following TNBS-induced ileitis, suggesting that the inflammation spread to the mesentery and that the lymphatic vessels were indeed inflamed. We demonstrated that isolated and pressurized rat mesenteric lymphatic vessels treated for 24 h with TNF-α display a compromised ability to rhythmically contract and to adequately respond to changes in transmural pressure. In our hands, TNF-α specifically decreased lymphatic contraction frequency in a concentration-dependent manner with an IC50 of ~4 ng/ml, compatible with TNF-α concentrations reported in the serum of inflamed rats [76,82], or IBD patients [38]. Our data corroborate findings showing that intradermal injection of TNF-α in the mouse paw caused a decrease in contractile function of the axillary lymphatic vessel [3].
We obtained convincing pharmacological, biochemical and functional evidences that the TNF-α-induced lymphatic contractile dysfunction was mediated by activation of NF-κB, a crucial contributor of TNF-α-induced inflammatory cell signals [30,36]. We first demonstrated a significant restoration of the contraction frequency in TNF-α-treated lymphatic vessels when co-incubated with PDTC, a direct inhibitor of IκB kinase (IKK) [48]. Secondly, we showed by western blotting that the phosphorylation level of IκB increased in lymphatic vessels within hours of the initiation of TNF-α treatment. Finally, we demonstrated nuclear translocation of the p65 NF-κB subunit in cells of TNF-α treated lymphatics. Although only muscular activation could be visually ascertained in these experiments, p65 nuclear translocation likely occurred in other lymphatic cell types and we indeed observed such translocation in cultured lymphatic endothelial and muscle cells isolated from the same vessels.
One of the main transcriptional targets of TNF-α and NF-κB is iNOS. The upregulation of this inducible enzyme in many cell types during inflammation leads to high levels of NO, an important relaxing factor modulating lymphatic pumping [55,70,80]. While in physiological states this action essentially relies on eNOS [10,11], several studies demonstrated that NO produced under the catalytic action of iNOS plays a major role in lymphatic dysfunction observed during inflammation [46,49,65,77]. By showing restoration of lymphatic contraction frequency in TNF-α-treated vessels upon selective inhibition of iNOS with 1400W, our findings provide a convincing indication of the pre-eminent role of iNOS in the TNF-α-induced decrease in pumping. These data are also consistent with studies demonstrating iNOS-dependent impairment of arterial contractility by TNF-α [2,27]. Involvement of iNOS was further strengthened by qPCR data showing that the significant up-regulation of iNOS mRNA expression in lymphatic vessels treated with TNF-α was reversed in the presence of PDTC. Similar to that observed in cultured cells incubated with cytokines [1,37], the increase was transient, peaking at 6 h and gradually decreasing at later time points. Endothelial and muscle cells are the main cellular components of lymphatic vessels and both cell-types play important roles in regulating lymphatic contraction [24,55,70]. Although the PCR experiments on intact lymphatics do not permit us to determine in which cell type the iNOS upregulation occurred, we observed that both lymphatic endothelial and muscle cells in culture exhibited upregulated iNOS mRNA expression following TNF-α treatment (Rehal, Roizes & von der Weid, unpublished data) and we can reasonably infer that both cell types are sensitive to TNF-α and contribute to lymphatic contractile dysfunction by upregulating iNOS expression and NO production. This proposal is further supported by other studies demonstrating iNOS upregulation in cultured lymphatic endothelial cells isolated from sheep, mouse and rat mesenteric lymphatic vessels upon stimulation with LPS, TNF-α, and other cytokines [17,42,45] and in mesenteric lymphatic vessels from old rats, which display an inflamed phenotype[51] Alternatively, NO could also be generated by immune cells reminiscent of macrophages that have recently been reported to populate the wall of mesenteric lymphatic vessels [12,16,40]. Indeed, some of these cells display NF-κB activation (see arrowheads in Fig. 3C).
Although we cannot exclude that, in addition to iNOS, other genes upregulated by NF-κB activation contribute to lymphatic dysfunction, our data strongly suggest that cyclooxygenases (COXs) and arachidonate metabolites, other important contributors of lymphatic pumping modulation [34,35] are not players in the TNF-α -induced lymphatic contractile dysfunction. Unlike the involvement of COXs in impaired lymphatic pumping during intestinal inflammation demonstrated by Wu et al. [77] and Rehal & von der Weid [56], we observed no restoration of lymphatic pumping by indomethacin and no significant changes in COX-1 and COX-2 mRNA expression in TNF-α -treated vessels at least up to 24 h after treatment. These results are surprising because COX-2 is usually upregulated by TNF-α via the NF-κB pathway [5,79], as well as other pathways [31], but may suggest difference in qualitative or temporal gene regulation in different tissues or cell types.
Previous studies have shown that sGC and KATP channels are involved in both physiological and inflammation-induced regulation of mesenteric lymphatic pumping by NO [49,69]. Using a pharmacological approach, we confirm here that sGC and KATP channel activity are also important elements in the chain of events leading to lymphatic pumping inhibition by TNF-α. In light of previous findings [69,72], we can suggest that the opening of KATP channels inhibits contractile activity by hyperpolarizing the lymphatic muscle. Our current finding that TNF-α treatment mainly affects lymphatic contraction frequency further corroborates an alteration of the pacemaking mechanism regulating this rhythmical activity [67,71,72]. A similar ability of NO and KATP channels to inhibit pacemaking was reported in interstitial cells of Cajal from mouse small intestine during LPS treatment via a NF-κB-dependent pathway [83].
Although lymphatic pumping has been suggested to be dysfunctional in IBD [68,74], whether restoration of pumping is beneficial to the disease resolution is not known. This question could be explored with glibenclamide, as it restores TNF-α-induced dysfunction. However, reports show that this blocker leads to increased severity of experimental IBD [21,75] and that the opening of KATP channels has anti-inflammatory effects by suppressing the production of pro-inflammatory cytokines such as TNF-α and IL-1β in the gut [18,32].
Anti-TNF-α antibodies such as infliximab have been used for the treatment of IBD for many years [60]. Our findings suggest that the efficacy of the anti-TNF-α treatment may be due, at least in part, to its ability to restore mesenteric lymphatic pumping. However, it will be difficult to experimentally test this idea, as, given the strong correlation between inflammation and lymphatic dysfunction, the expected amelioration of gut inflammation caused by infliximab will inevitably lead to restoration of pumping, hindering a direct effect of the drug on the lymphatic function. Furthermore, by demonstrating the inhibitory effect of TNF-α on lymphatic pumping, our study suggests that anti-TNF-α treatments may be of value to alleviate lymphedema, a chronic and disabling condition, characterized by an unmanageable edema due to the inability of the lymphatic vessels to drain fluid away from an affected limb or organ [58,62]. Interestingly, anti-TNF-α therapies used in the context of chronic inflammatory diseases have incidentally been shown to ameliorate the lymphedema sometimes associated with these diseases. For example, in a case study, infliximab administered to children with Crohn’s disease alleviated the extraintestinal dermatologic manifestations of the disease, which presented as lymphedema [41]. Other case studies also reported improvement of lymphedema associated with chronic inflammatory diseases, such as rheumatoid arthritis [20,53], ankylosing spondylitis [4] or psoriatic arthritis [63] following anti-TNF-α treatment. While the mechanisms of action are still unknown, we can speculate that amelioration of lymphedema by targeting TNF-α results from the restoration of the TNF-α-induced inhibition of lymphatic pumping we report here, which would improve lymph drainage. Intriguingly, Nakamura et al. [52] reported that inhibition of TNF-α action with pegsunercept, a soluble TNF-α receptor antagonist worsened lymphedema in an experimental postsurgical mouse model. However in the latter study, another important role of TNF-α, which is to promote lymphangiogenesis, was likely at play [6,57]. Inhibition of TNF-α would then impair pro-lymphangiogenic processes beneficial to the resolution of lymphedema in this experimental model. Lymphangiogenesis is also consistently observed in the gut of IBD patients [22,25,54], but the role of this inflammation-driven process in the disease progression still remains uncertain.
In conclusion, TNF-α decreases the frequency of lymphatic vessel contractions by activating NF-κB, the transcription of iNOS gene, and the NO-dependent activation of sGC and KATP channels to impair lymphatic pumping. These findings, together with the additional observation that TNF-α and TNFR1 are upregulated in mesenteric lymphatic vessels from rats with TNBS-induced acute ileitis, suggest that the TNF-α-NF-κB-iNOS pathway is potentially one of the mechanisms involved in impairment of mesenteric lymphatic pumping associated with intestinal inflammation.
Perspectives.
Lymphatic pumping, the main driving force for lymph drainage, is compromised during inflammation and evidences of lymphatic dysfunctions have been reported in patients suffering from inflammatory bowel disease. Our findings that TNF-α, a pro-inflammatory cytokine significantly upregulated in IBD, inhibits lymphatic pumping provides new information about potential mechanisms affecting lymphatic functions during intestinal inflammation and suggest a role for the mesenteric lymphatic system in the perpetuation of IBD.
Acknowledgments
The authors thank Dr. Pina Colarusso from the Snyder Institute Live Cell Imaging for her help with the nuclear co-localization data analysis.
Financial support: This study was supported by grants from the National Institutes of Health (NIH HL096552) and the Canadian Institutes of Health Research (CHIR MOP-89975).
Footnotes
Disclosures
The authors have no conflict of interest to disclose.
References
- 1.Adams V, Nehrhoff B, Spate U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Schuler G. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54:95–104. doi: 10.1016/s0008-6363(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 2.Aires RD, Capettini LS, Silva JF, Rodrigues-Machado Mda G, Pinho V, Teixeira MM, Cortes SF, Lemos VS. Paraquat poisoning induces TNF-alpha-dependent iNOS/NO mediated hyporesponsiveness of the aorta to vasoconstrictors in rats. PLoS One. 2013;8:e73562. doi: 10.1371/journal.pone.0073562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldrich MB, Sevick-Muraca EM. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine. 2013;64:362–369. doi: 10.1016/j.cyto.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almodovar R, Zarco P, Quiros FJ, Mazzucchelli R. Infliximab treatment efficacy in lymphoedema associated with ankylosing spondylitis. Rheumatology (Oxford) 2004;43:1456. doi: 10.1093/rheumatology/keh341. [DOI] [PubMed] [Google Scholar]
- 5.Arias-Negrete S, Keller K, Chadee K. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem Biophys Res Commun. 1995;208:582–589. doi: 10.1006/bbrc.1995.1378. [DOI] [PubMed] [Google Scholar]
- 6.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baudry N, Vicaut E. Role of nitric oxide in effects of tumor necrosis factor-alpha on microcirculation in rat. J Appl Physiol (1985) 1993;75:2392–2399. doi: 10.1152/jappl.1993.75.6.2392. [DOI] [PubMed] [Google Scholar]
- 8.Baxter G. Effects of tumor necrosis factor on in vitro digital arterial responses in horses. Am J Vet Res. 1994;55:551–555. [PubMed] [Google Scholar]
- 9.Benoit JN, Zawieja D. Gastrointestinal Lymphatics. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 1669–1692. [Google Scholar]
- 10.Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol. 2011;301:H1897–1906. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol. 2009;297:H1319–1328. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridenbaugh EA, Nizamutdinova IT, Jupiter D, Nagai T, Thangaswamy S, Chatterjee V, Gashev AA. Lymphatic muscle cells in rat mesenteric lymphatic vessels of various ages. Lymphat Res Biol. 2013;11:35–42. doi: 10.1089/lrb.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, O’Connell PR. Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007;102:439–448. doi: 10.1111/j.1572-0241.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty S, Zawieja SD, Wang W, Lee Y, Wang YJ, von der Weid PY, Zawieja DC, Muthuchamy M. Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. Am J Physiol Heart Circ Physiol. 2015;309:H2042–2057. doi: 10.1152/ajpheart.00467.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiba Y, Matsuo K, Sakai H, Abe K, Misawa M. Effects of 1400W, a potent selective inducible NOS inhibitor, on histamine- and leukotriene D4-induced relaxation of isolated guinea pig nasal mucosa. Nitric Oxide. 2006;15:142–147. doi: 10.1016/j.niox.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Cromer W, Wang W, Zawieja SD, von der Weid PY, Newell-Rogers MK, Zawieja DC. Colonic Insult Impairs Lymph Flow, Increases Cellular Content of the Lymph, Alters Local Lymphatic Microenvironment, and Leads to Sustained Inflammation in the Rat Ileum. Inflamm Bowel Dis. 2015;21:1553–1563. doi: 10.1097/MIB.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK, Zawieja DC. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis. 2014;17:395–406. doi: 10.1007/s10456-013-9393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daneshmand A, Mohammadi H, Rahimian R, Habibollahi P, Fakhfouri G, Talab SS, Mehr SE, Dehpour AR. Chronic lithium administration ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats; potential role for adenosine triphosphate sensitive potassium channels. J Gastroenterol Hepatol. 2011;26:1174–1181. doi: 10.1111/j.1440-1746.2011.06719.x. [DOI] [PubMed] [Google Scholar]
- 19.Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol. 2006;4:3–10. doi: 10.1089/lrb.2006.4.3. [DOI] [PubMed] [Google Scholar]
- 20.Eyigor S, Karapolat H, Kirazli Y. Efficacy of etanercept and complete decongestive physical therapy in bilateral lower-limb lymphoedema associated with rheumatoid arthritis: a case report. Adv Ther. 2008;25:23–28. doi: 10.1007/s12325-008-0006-1. [DOI] [PubMed] [Google Scholar]
- 21.Fakhfouri G, Rahimian R, Hashemi S, Rasouli MR, Bahremand A, Mehr SE, Khorramizadeh MR, Dehpour AR. Sildenafil attenuates TNBS-induced colitis in rats: possible involvement of cGMP and KATP channels. Fundam Clin Pharmacol. 2013;26:190–193. doi: 10.1111/j.1472-8206.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- 22.Fogt F, Pascha TL, Zhang PJ, Gausas RE, Rahemtulla A, Zimmerman RL. Proliferation of D2–40-expressing intestinal lymphatic vessels in the lamina propria in inflammatory bowel disease. Int J Mol Med. 2004;13:211–214. [PubMed] [Google Scholar]
- 23.Gashev AA, Davis MJ, Gasheva OY, Nepiushchikh ZV, Wang W, Dougherty P, Kelly KA, Cai S, von der Weid PY, Muthuchamy M, Meininger CJ, Zawieja DC. Methods for Lymphatic Vessel Culture and Gene Transfection. Microcirculation. 2009:1–14. doi: 10.1080/10739680903120778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geleff S, Schoppmann SF, Oberhuber G. Increase in podoplanin-expressing intestinal lymphatic vessels in inflammatory bowel disease. Virchows Arch. 2003;442:231–237. doi: 10.1007/s00428-002-0744-4. [DOI] [PubMed] [Google Scholar]
- 26.Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–213. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunnett CA, Heistad DD, Loihl A, Faraci FM. Tumor necrosis factor-alpha impairs contraction but not relaxation in carotid arteries from iNOS-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1558–1564. doi: 10.1152/ajpregu.2000.279.5.R1558. [DOI] [PubMed] [Google Scholar]
- 28.He Y, Lin LJ, Zheng CQ, Jin Y, Lin Y. Cytokine expression and the role of Th17 cells in a mouse model of colitis. Mol Med Rep. 2012;6:1438–1442. doi: 10.3892/mmr.2012.1111. [DOI] [PubMed] [Google Scholar]
- 29.Heatley RV, Bolton PM, Hughes LE, Owen EW. Mesenteric lymphatic obstruction in Crohn’s disease. Digestion. 1980;20:307–313. doi: 10.1159/000198452. [DOI] [PubMed] [Google Scholar]
- 30.Heyninck K, Wullaert A, Beyaert R. Nuclear factor-kappa B plays a central role in tumour necrosis factor-mediated liver disease. Biochem Pharmacol. 2003;66:1409–1415. doi: 10.1016/s0006-2952(03)00491-x. [DOI] [PubMed] [Google Scholar]
- 31.Hobbs SS, Goettel JA, Liang D, Yan F, Edelblum KL, Frey MR, Mullane MT, Polk DB. TNF transactivation of EGFR stimulates cytoprotective COX-2 expression in gastrointestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 301:G220–229. doi: 10.1152/ajpgi.00383.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseini-Tabatabaei A, Abdollahi M. Potassium channel openers and improvement of toxic stress: do they have role in the management of inflammatory bowel disease? Inflamm Allergy Drug Targets. 2008;7:129–135. doi: 10.2174/187152808785748164. [DOI] [PubMed] [Google Scholar]
- 33.Johnson RP, El-Yazbi AF, Takeya K, Walsh EJ, Walsh MP, Cole WC. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol. 2009;587:2537–2553. doi: 10.1113/jphysiol.2008.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston MG, Feuer C. Suppression of lymphatic vessel contractility with inhibitors of arachidonic acid metabolism. J Pharmacol Exp Ther. 1983;226:603–607. [PubMed] [Google Scholar]
- 35.Johnston MG, Gordon JL. Regulation of lymphatic contractility by arachidonate metabolites. Nature. 1981;293:294–297. doi: 10.1038/293294a0. [DOI] [PubMed] [Google Scholar]
- 36.Karin M. The NF-kappa B activation pathway: its regulation and role in inflammation and cell survival. Cancer J Sci Am. 1998;4(Suppl 1):S92–99. [PubMed] [Google Scholar]
- 37.Kolios G, Brown Z, Robson RL, Robertson DA, Westwick J. Inducible nitric oxide synthase activity and expression in a human colonic epithelial cell line, HT-29. Br J Pharmacol. 1995;116:2866–2872. doi: 10.1111/j.1476-5381.1995.tb15938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komatsu M, Kobayashi D, Saito K, Furuya D, Yagihashi A, Araake H, Tsuji N, Sakamaki S, Niitsu Y, Watanabe N. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem. 2001;47:1297–1301. [PubMed] [Google Scholar]
- 39.Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23:1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 40.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol. 2015;194:5200–5210. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kugathasan S, Miranda A, Nocton J, Drolet BA, Raasch C, Binion DG. Dermatologic manifestations of Crohn disease in children: response to infliximab. J Pediatr Gastroenterol Nutr. 2003;37:150–154. doi: 10.1097/00005176-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Leak LV, Cadet JL, Griffin CP, Richardson K. Nitric oxide production by lymphatic endothelial cells in vitro. Biochem Biophys Res Commun. 1995;217:96–105. doi: 10.1006/bbrc.1995.2750. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Kim MS, Kim EY, Park HJ, Chang CY, Jung DY, Kwon CH, Joh JW, Kim SJ. 15-deoxyspergualin prevents mucosal injury by inhibiting production of TNF-alpha and down-regulating expression of MD-1 in a murine model of TNBS-induced colitis. Int Immunopharmacol. 2007;7:1003–1012. doi: 10.1016/j.intimp.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Roizes S, von der Weid PY. Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol. 2014;592:5409–5427. doi: 10.1113/jphysiol.2014.280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang Q, Ju Y, Chen Y, Wang W, Li J, Zhang L, Xu H, Wood RW, Schwarz EM, Boyce BF, Wang Y, Xing L. Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice. Arthritis Res Ther. 2015;18:62. doi: 10.1186/s13075-016-0963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2011;108:18784–18789. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin CC, Shih CH, Yang YL, Bien MY, Lin CH, Yu MC, Sureshbabu M, Chen BC. Thrombin induces inducible nitric oxide synthase expression via the MAPK, MSK1, and NF-kappaB signaling pathways in alveolar macrophages. Eur J Pharmacol. 2011;672:180–187. doi: 10.1016/j.ejphar.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Liu SF, Ye X, Malik AB. Pyrrolidine dithiocarbamate prevents I-kappaB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol Pharmacol. 1999;55:658–667. [PubMed] [Google Scholar]
- 49.Mathias R, von der Weid PY. Involvement of the NO-cGMP-KATP channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G623–634. doi: 10.1152/ajpgi.00392.2012. [DOI] [PubMed] [Google Scholar]
- 50.Moreno-Dominguez A, Colinas O, El-Yazbi A, Walsh EJ, Hill MA, Walsh MP, Cole WC. Ca2+ sensitization due to myosin light chain phosphatase inhibition and cytoskeletal reorganization in the myogenic response of skeletal muscle resistance arteries. J Physiol. 2013;591:1235–1250. doi: 10.1113/jphysiol.2012.243576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation. 2011;18:463–473. doi: 10.1111/j.1549-8719.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura K, Radhakrishnan K, Wong YM, Rockson SG. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One. 2009;4:e8380. doi: 10.1371/journal.pone.0008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ostrov BE. Beneficial effect of etanercept on rheumatoid lymphedema. Arthritis Rheum. 2001;44:240–241. doi: 10.1002/1529-0131(200101)44:1<240::AID-ANR32>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 54.Rahier JF, De Beauce S, Dubuquoy L, Erdual E, Colombel JF, Jouret-Mourin A, Geboes K, Desreumaux P. Increased lymphatic vessel density and lymphangiogenesis in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:533–543. doi: 10.1111/j.1365-2036.2011.04759.x. [DOI] [PubMed] [Google Scholar]
- 55.Reeder LB, Yang LH, Ferguson MK. Modulation of lymphatic spontaneous contractions by EDRF. J Surg Res. 1994;56:620–625. doi: 10.1006/jsre.1994.1098. [DOI] [PubMed] [Google Scholar]
- 56.Rehal S, von der Weid PY. Experimental ileitis alters prostaglandin biosynthesis in mesenteric lymphatic and blood vessels. Prostaglandins Other Lipid Mediat. 2014;116–117:37–48. doi: 10.1016/j.prostaglandins.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem. 1998;273:8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- 58.Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- 59.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 60.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Sura R, Colombel JF, Van Kruiningen HJ. Lymphatics, tertiary lymphoid organs and the granulomas of Crohn’s disease: an immunohistochemical study. Aliment Pharmacol Ther. 2011;33:930–939. doi: 10.1111/j.1365-2036.2011.04605.x. [DOI] [PubMed] [Google Scholar]
- 62.Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc Med. 1998;3:145–156. doi: 10.1177/1358836X9800300209. [DOI] [PubMed] [Google Scholar]
- 63.Tong D, Eather S, Manolios N. Psoriatic arthritis and chronic lymphoedema: treatment efficacy by adalimumab. Clin Rheumatol. 2009;28:1349–1350. doi: 10.1007/s10067-009-1259-z. [DOI] [PubMed] [Google Scholar]
- 64.Tsujikawa T, Ohta N, Nakamura T, Satoh J, Uda K, Ihara T, Okamoto T, Araki Y, Andoh A, Sasaki M, Fujiyama Y, Bamba T. Medium-chain triglycerides modulate ileitis induced by trinitrobenzene sulfonic acid. J Gastroenterol Hepatol. 1999;14:1166–1172. doi: 10.1046/j.1440-1746.1999.02024.x. [DOI] [PubMed] [Google Scholar]
- 65.Umarova BA, Lelekova TV, Kopylova GN, Goncharova EL, Bakaeva ZV, Samonina GE. The role of protective effects of proline-containing peptides (PGP, PG, and GP) in contractile dysfunction of mesenteric lymphatic vessels in rats with experimental acute peritonitis. Bull Exp Biol Med. 2006;142:279–282. doi: 10.1007/s10517-006-0346-2. [DOI] [PubMed] [Google Scholar]
- 66.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3–new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol. 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Kruiningen HJ, Colombel JF. The forgotten role of lymphangitis in Crohn’s disease. Gut. 2008;57:1–4. doi: 10.1136/gut.2007.123166. [DOI] [PubMed] [Google Scholar]
- 69.von der Weid P-Y. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and beta-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol. 1998;125:17–22. doi: 10.1038/sj.bjp.0702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von der Weid P-Y, Crowe MJ, van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol. 1996;493:563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von der Weid P-Y, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol. 2008;295:H1989–2000. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- 72.von der Weid P-Y, Zhao J, van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol. 2001;280:H2707–2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- 73.von der Weid PY, Lee S, Imtiaz MS, Zawieja DC, Davis MJ. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphat Res Biol. 2014;12:66–75. doi: 10.1089/lrb.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von der Weid PY, Rehal S, Ferraz JG. Role of the lymphatic system in the pathogenesis of Crohn’s disease. Curr Opin Gastroenterol. 2011;27:335–341. doi: 10.1097/MOG.0b013e3283476e8f. [DOI] [PubMed] [Google Scholar]
- 75.Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. 578 e561. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 76.Wang K, Wang DC, Feng YQ, Leng XF. Lymph invasion route of bacteria and endotoxin and CD4+/CD8+ T cells ratio changes of draining lymph node of burn infection wound. Burns. 2008;34:234–240. doi: 10.1016/j.burns.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 77.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G566–574. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- 78.Xie J, Wang Y, Kolls J, Malinski T, Nelson S, Summer W, Greenberg SS. Tumor necrosis factor alpha inhibits contractions to sympathetic nerve stimulation by a nitric oxide-dependent mechanism. Proc Soc Exp Biol Med. 1993;203:446–453. doi: 10.3181/00379727-203-43621. [DOI] [PubMed] [Google Scholar]
- 79.Yamamoto S, Yamamoto K, Kurobe H, Yamashita R, Yamaguchi H, Ueda N. Transcriptional regulation of fatty acid cyclooxygenases-1 and -2. Int J Tissue React. 1998;20:17–22. [PubMed] [Google Scholar]
- 80.Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol. 1993;264:H1460–H1464. doi: 10.1152/ajpheart.1993.264.5.H1460. [DOI] [PubMed] [Google Scholar]
- 81.Yuan JS, Wang D, Stewart CN., Jr Statistical methods for efficiency adjusted real-time PCR quantification. Biotechnol J. 2008;3:112–123. doi: 10.1002/biot.200700169. [DOI] [PubMed] [Google Scholar]
- 82.Zou B, Chen Q, Tang S, Gao T, Zhang J, Xi F, Yu W. Timing of insulin therapy affects the inflammatory response in endotoxemic rats. Inflammation. 2012;35:723–729. doi: 10.1007/s10753-011-9367-8. [DOI] [PubMed] [Google Scholar]
- 83.Zuo DC, Choi S, Shahi PK, Kim MY, Park CG, Kim YD, Lee J, Chang IY, So I, Jun JY. Inhibition of pacemaker activity in interstitial cells of Cajal by LPS via NF-kappaB and MAP kinase. World J Gastroenterol. 2013;19:1210–1218. doi: 10.3748/wjg.v19.i8.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]


