Abstract
Introduction
Sepsis and the ensuing immune dysfunction, continues to be a major contributor to neonatal morbidity and mortality. Neonatal sepsis also is associated with profound immune dysfunction. We have recently identified a role for a family of co-inhibitory molecules that are altered in murine sepsis and in critically ill adult patients, which may be a target for development of novel therapies. There is, however, a paucity of data pertaining to the role of co-inhibitory check point proteins in the control and modulation of neonatal sepsis.
Methods
The cecal slurry (CS) model consists of harvesting the cecal contents of an adult wild type (Wt) male mouse and combining it with 5% dextrose to create a cecal slurry (CS) with a concentration of 80mg/ml (LD70 at 7 days). Neonatal mice (5–7 days of age) underwent intraperitoneal injection (IP) of the CS or IP of 0.9% saline for sham procedure (Sh). Wild Type (C57BL/6) or PD-1−/− mice were used. 7 day survival study was undertaken. Cytometric bead array was used for cytokine expression. Blood and peritoneal fluid was cultured for bacterial burden. Flow cytometry was used to assess the peritoneal cavity cell populations.
Results
There was no mortality following Sh in either WT or PD-1−/− pups. PD-1 markedly affected sepsis survival with significantly improved survival in the PD-1−/− pups (40% versus 80%; p<0.01). This survival improvement was not associated with any difference in bacterial clearance. The bacterial burden was equivalent between WT and PD-1−/− pups at 24 hours following CS. However, PD-1−/− pups did display an increased circulating cytokine response to the CS compared with WT, with increased expression of IL-6, IL-10 and TNF-α levels. Within the peritoneal cavity, sepsis induced an influx of neutrophils, a finding that was increased in PD-1−/− pups. Although the T-cell response was unaffected by PD-1, it was noted that CS induced a loss of peritoneal B-cells in WT, while the peritoneal B-cell population was preserved in PD-1−/− pups.
Conclusion
Our data suggests that the checkpoint protein, PD-1, plays an important role in controlling the immune response to sepsis in the neonate, ultimately affecting sepsis related mortality in this neonatal murine model of sepsis. Akin to adult studies, this data further emphasizes the potential therapeutic target for PD-1 across a spectrum of septic patients.
Background
Sepsis continues to be a major contributor to morbidity and mortality in neonates worldwide. Indeed improving survival in children under 5 is one of the targets of the United Nations Millennium Development Goals(1). Nearly three quarters of all neonatal deaths are related to sepsis, with an estimated worldwide 4 million deaths annually in the first 7 days of life. Despite advances in the surgical and ICU care of neonates with sepsis, there remains a dearth of knowledge regarding the underlying mechanistic interactions between the bacterial burden and the neonatal immune system.
Healthy neonates, even without an infectious insult, are noted to display a degree of under-development of the immune system as well as immune dysfunction. These findings include an underdeveloped lymphocytic response, a relative inability of normal neutrophils and monocytes to migrate to a site of injury or infection, as well as a reduced ability of neutrophils to develop neutrophil extracellular traps (NETs)(2). Following an injury or an infection, akin to their adult counterparts, it is also evident that immune exhaustion and dysfunction occurs in neonates(3). Specifically, among both septic patients and adult murine models, sepsis induced immune dysfunction involves alterations in co-stimulatory/co-inhibitory receptors and the check point protein Programmed cell death receptor-1 (PD-1)(4–8).
PD-1 has emerged as a key regulator of immune function in a broad spectrum of illnesses from cancer(9) to sepsis(5). PD-1, a member of the B7-CD28 superfamily, functions as a co-inhibitory, co-stimulatory receptor. We have shown a role for PD-1 in adult sepsis in both murine models and critically ill patients(6, 10). Furthermore, the PD-1:PD-L1 pathway regulates the balance between a strong immune response with effective microbial clearance versus an over-exuberant response and end organ damage(4), a major contributor to sepsis related morbidity and mortality.
Information that is available regarding the control of neonatal sepsis appears to denote that mechanisms similar to those seen in adult sepsis may be affecting control and outcomes of neonatal sepsis(11). Given the clear and evident role of PD-1 in adult sepsis, we undertook an investigation of the role of PD-1 in modulating the neonatal response to peritoneal sepsis.
Materials and Methods
Mice
Wild type mouse pups were bred from C57BL/6J parents. C57BL/6 mice deficient in PD-1 (PD1−/−) were used to breed the knock-out strains (kindly provide by Tasuku Honjo, Kyoto University, Kyoto, Japan, via Megan Sykes at the Massachusetts General Hospital, Charlestown, MA). Both WT and PD-1−/− were developmentally normal with normal growth rates and weaning patterns. All mice were bred at Rhode Island Hospital and maintained at our institution’s rodent facility receiving standard care and dams received standard chow. All pups used were aged 5–7 days old at the start of any experimental procedure. Day of life was assessed either by known date of birth or by physical exam compared with “JAX Mice Pup Appearance by Age” (Jackson Laboratory). Research objectives and all animal protocols were approved by the Institutional Animal Care and Use Committee of Rhode Island Hospital (AWC# 0228-13) and conducted in accordance with the Animal Welfare Act and National Institutes of Health guidelines for animal care and use.
Cecal Slurry Model
The cecal slurry (CS) model at our institution is modified on the model previously described by Wynn et al(12). In brief, a naive wild type adult donor mouse (C57BL/6J) was euthanized and cecal contents are harvested. These cecal contents were mixed with 5% dextrose solution to create a CS with a concentration of 80 mg/mL. Pups from each litter used were randomly assigned to Naïve (N), Sham (Sh), or Cecal Slurry (CS) groups. These were repeated over across 4–5 litters. For the CS group, pups aged 5–7 days old underwent intra-peritoneal (IP) injection of cecal slurry at an LD70 (1.3mg/g BW) as a septic challenge(12). Matched pups from the same litter underwent IP injection of 0.9% saline served as the Sham (Sh) control. Selected pups from the same litter who were also brought down to the procedure room, but were not separated from the mother, and underwent no intervention served as naïve (N) controls. During all experiments, animals were closely watched and were euthanized if signs of distress were observed, such as scattering or absence of milk in the neonate, or were noted to be moribund,
Survival Study
Survival Studies were undertaken comparing survival of the pups up to 7 days from the N, Sh or CS. Survival checks were undertaken every 6 hours for the first three days, and then twice daily up to 7 days.
Peritoneal Culture analysis
Peritoneal fluid was lavaged and collected using a sterile technique. Blood was collected following decapitation and collected with sterile technique. Blood and fluid were then aliquoted and plated onto blood agar plates and incubated at 40°C for 48 hours. Colony forming units (CFUs) were counted. Data is expressed as Log10 CFU/100μL peritoneal fluid(4).
Circulating cytokines
Circulating cytokine analysis was undertaken using Cytokine Bead Array (BD Mouse Inflammation Kit Catalogue #552364) was prepared per manufacturer’s protocol and analyzed with FACS Aria(4). Given the very small quantity of circulating blood volume of the murine pups, we chose selected cytokines, specifically IL-6 as a pro-inflammatory and IL-10 as an anti-inflammatory marker, as well as TNF-α.
Flow Cytometry
Peritoneal cells were collected by lavage, centrifuged and analyzed fresh by flow cytometry as previously described by our laboratory(4). Cell counts were also undertaken to calculate absolute numbers of cells within each population. Cell populations were identified by forward and side scatter and subsequently gated using monoclonal antibodies along with appropriate isotype controls according to both manufacturer’s recommendation and our prior publications.
The following mAb conjugated to fluorochromes were used:, PE-labeled anti-CD3e (clone 145-2C11) (T-cells), APC-labeled anti-Gr1 (clone RB6-8C5) (Neutrophils) and FITC-labeled anti-CD45R (B220) (clone RA3-6B2) (B-cells) from eBioscience. BD FACS Aria III was used to assess fluorescence. Data was analyzed with FlowJo version 9.3.2.
Statistical Analysis
SigmaPlot 12.5 (Systat Software, San Jose, CA) was used for all analysis. Data are expressed as mean and standard error of the mean. Categorical data was assessed using Chi-squared or Fisher’s exact test. One way analysis of variance (ANOVA) with Holm-Sidak or Dunn’s post-hoc analysis was used for continuous data across multiple groups. Survival curves were created using Kaplan Meier curves. Alpha was set to 0.05.
Results
Survival study
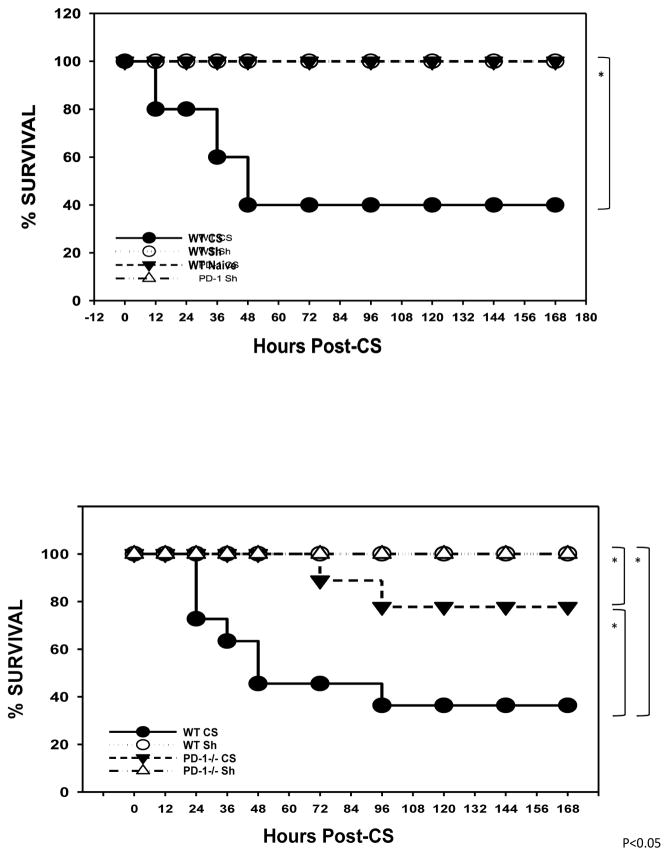
Akin to previous observations(12), in the wild type animals, no mortality was noted in either the naive pups nor pups undergoing sham peritoneal injection. IP CS injection induced an early mortality of 60% at 48 hours (Figure 1a). Similar to wild-type pups, PD-1−/− pups undergoing sham sustained no mortality. However, following cecal slurry PD-1−/− pups compared to wild type demonstrated markedly improved survival with only 20% versus 60% mortality (p<0.05). Furthermore, when PD-1−/− pups did die, the mortality occurred later in the time course compared with wild type pups. (Figure 1a and 1b).
Figure 1a).
Survival study following cecal slurry in wild type wherein CS induced approximately 60% mortality. 1b) Mortality was significantly lessened in PD1−/− mice. (N=20–25 mice per group). *=p<0.05
Bacterial burden
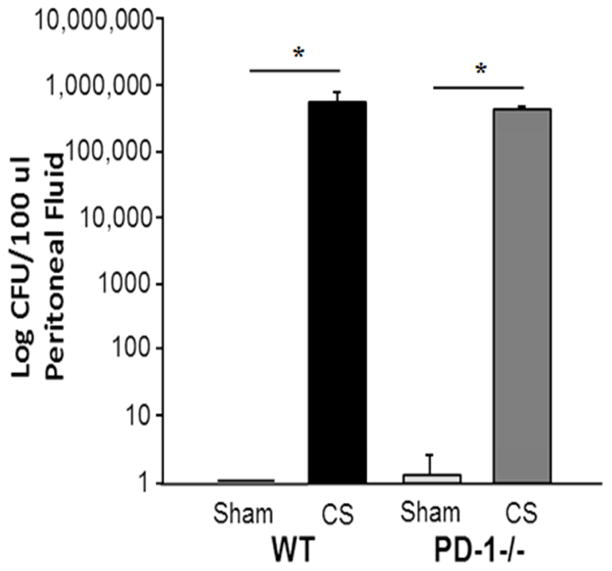
The improved survival noted in the PD1−/− pups was not associated with any change in the peritoneal bacterial burden at 24 hours. In the WT pups, CS when compared to sham injection, was noted to create a large peritoneal bacterial burden at 24 hours (0 versus 5.9×106 CFU/microliter; p<0.001). This was not associated with the development of a bacteremia response. Likewise, in PD1−/− pups, the sham procedure did not induce any significant bacterial burden, whereas CS did induce a marked peritoneal bacterial burden at 24 hours. This bacterial burden in the survivors at 24 hours following CS was not different between WT or PD1−/− pups (5.9×106 versus 3.8×106 CFU/microliter; (p=NS)) (Figure 2).
Figure 2.
There was no difference in the bacterial burden 24 hours following CS between WT versus PD-1−/− mice. N=6–9 per group. *=p<0.05; ANOVA.
Circulating cytokines
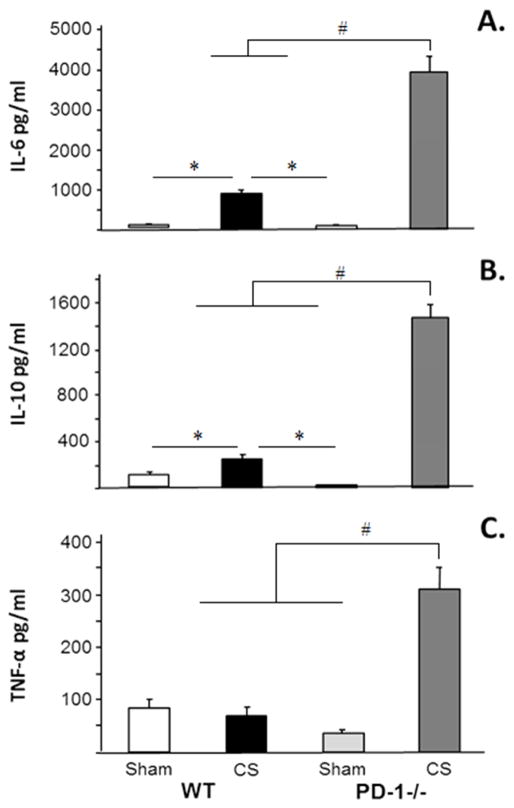
As expected, 24 hours following CS compared to sham, an increased concentration of both IL-6 and IL-10 was noted in circulation in WT pups. However, in the PD1−/− pups this finding was even more marked. Following CS, the elevation of IL-6 and IL-10 was markedly elevated in PD1−/− pups when compared to CS in WT pups. In the WT pups, there was no difference in circulating TNF-α expression at 24 hours following CS. However, CS in PD1−/− pups induced a significant elevation of circulating TNF-α (Figure 3a, 3b and 3c).
Figure 3a, 3b and 3c.
The circulating cytokine response to peritoneal sepsis was markedly more pronounced in PD-1−/− pups compared with WT. N=6–9 per group. #p<0.05 comparing CS in PD-1−/− pups to all other groups. *=p<0.05; ANOVA.
Peritoneal cavity cell populations
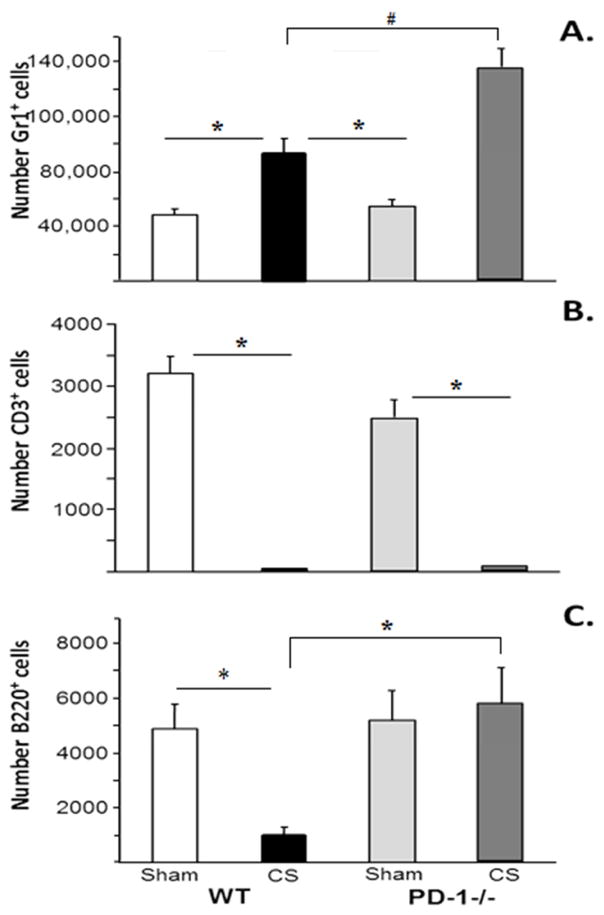
There was no difference in the neutrophil populations following sham between WT and PD1−/− pups. Whilst sepsis, compared with sham, induced an influx of neutrophils into the peritoneal cavity in WT pups, it was noted that in PD1−/− pups CS was associated with a significantly greater neutrophil influx when compared to that seen in WT pups. As has been observed in adult mice, sepsis, (CS) in WT pups induced a marked loss of T-lymphocytes in the peritoneal cavity. This finding was also observed in PD1−/− pups following CS with no difference in T-cell populations following CS between WT and PD-1−/− pups. Conversely, although there was a marked decline in the peritoneal B-cell population following sepsis in the WT pups, it was noted that these B-cell populations were preserved in PD1−/− pups (Figure 4a, 4b and 4c).
Figure 4a, 4b &4c.
Peritoneal cell influx in response to CS across WT and PD1−/− pups. 4a: Peritoneal neutrophil influx increased in WT, but was the neutrophil influx was more marked in PD-1−/− pups. 4b: Peritoneal lymphocyte loss was noted in both WT and PD-1−/− following CS. 4c: Peritoneal B-Cell population was decreased following CS in WT, but was preserved in PD-1−/− pups. N=6–9 per group. #p<0.05 comparing CS in PD-1−/− pups to all other groups. *=p<0.05; ANOVA.
Discussion
The immune dysfunction induced by sepsis is a major contributor to mortality and, among survivors, long term morbidity. This is especially true at the extremes of age. Our lab has a longstanding interest in understanding the perturbations in the immune system within both a murine model as well with critically ill septic patients(7, 13). We have previously noted a key role for co-inhibitory, check point molecules and the alterations in expression of these receptors and ligands, specifically Programmed Death receptor-1 (PD-1)(5, 6, 10, 14–16).
PD-1 is a check point protein that exists on a wide variety of immune cells. Among its recognized roles, PD-1:PD-L1 is a major negative co-stimulatory pathway that regulates and maintains peripheral tolerance(17). It has been postulated that the PD-1:PD-L1 pathway developed to blunt immune responses in conditions such as infections, allergies, ischemia/injury and autoimmunity to prevent the tissue destruction and end organ damage associated with an over-exuberant immune response(18, 19). Within adults, PD-1 is also noted to negatively modulate the immune response to sepsis(4, 18).
With respect to the neonate, the role of PD-1 in neonatal sepsis may be left over from the immunosuppressive functions of the PD-1:PD-L1 pathway in the developing fetus. PD-1 is involved in the earliest stages of immune development contributing towards the tolerance of the allogenic fetus at the feto-maternal interface(20). PD-L1 is expressed by fetal tissue and is known to interact with PD-1 expressing maternal cells. Without the immunosuppressive effect of PD-1, fetal loss occurs through a robust maternal immune response(20, 21). Thus the neonate is born with a negative inhibitory mechanism aimed at preventing an over-exuberant response to the myriad of environmental allergens, including bacterial exposures.
We identified a survival benefit for neonatal pups lacking PD-1 following an abdominal septic challenge. This current finding is in keeping with prior observations in adult mice following CLP, wherein adult PD-1−/− mice displayed improved survival(10). However, within the neonate, this survival advantage appears to be independent of the ability to clear the peritoneal bacterial burden by 24 hours. Following CS, there was no difference in the peritoneal bacterial burden between WT and PD-1−/− neonatal pups. This differs from adult murine data demonstrating that PD-1−/− mice display greater bactericidal activity(4). A single time point does not fully address the dynamics of how the under-developed neonatal immune system handles a bacterial burden. However, given the difference seen in mortality by 24 hours, the lack of difference in bacterial clearance by this time point would imply mechanisms other than merely bacterial clearance are involved in the survival benefit seen in PD-1−/− pups.
In keeping with the concept that PD-1 acts in a suppressive fashion, we noted that PD-1−/− pups displayed a markedly elevated cytokine response. The neonate has emerged from an intrauterine environment aimed specifically against developing pro-inflammatory responses(22–24). The cytokine milieu of the neonate is considerably different from adults(25), and is largely under the control of negative signaling to prevent an over-exuberant response to commensal or environmental organisms(22, 24). Roger et al(22) noted that releasing the immune system from these immunosuppressive pathways improved both cytokine responses and cellular responses to bacteria. We contend that our cytokine findings are in keeping with these observations. Removing the immunosuppressive effect of PD-1 (PD-1−/− pups), thereby allowing for a more robust immune response, may be critical for neonatal survival from sepsis. A more comprehensive assessment of the dynamics of cytokine fluxes, with associated downstream pathway alterations will be critical to understanding timing of any intervention aimed at altering the cytokine response in the immunosuppressed neonate.
We have previously demonstrated a key role for PD-1 in modulating the influx of immune cells into the peritoneal cavity in response to sepsis(14). We, therefore, reviewed the effect of PD-1 upon neonatal peritoneal immune cellular response to sepsis. Neutrophils from neonates display decreased ability to traffic to a source of infection, decreased NET formation and decreased ability to clear bacteria following sepsis(2). We noted that neutrophils did enter the peritoneal cavity in response to sepsis in WT pups. However when the negative regulation of PD-1 was lacking (PD-1−/− pups), the neutrophil influx was noted to be more pronounced. This response is in keeping with other observations in non-infectious acute models(26), noting that PD-1−/− mice have a more pronounced neutrophil influx into areas of injury. This suppressive role for PD-1 is believed to limit further tissue destruction. Although our findings support the concept that PD-1 appears to alter neutrophil trafficking, once arrived at the source of infection, namely the peritoneal cavity, PD-1 may not play a role in other neutrophil functioning such as bacterial clearance.
We noted a divergent effect of PD-1 upon the T-cell versus B-cell peritoneal populations. We believe that this may be a reflection of the differentially developed lymphocytic populations in the neonate(27), as well as potential differences in the role of PD-1 in affecting neonatal T- versus B-cell responses. Within adult populations, PD-1 is noted to be expressed at low levels on naïve T-cells, but following activation of these T-Lymphocytes, PD-1 expression is rapidly up-regulated on T-lymphocytes(28). Over-expression of PD-1 has been denoted as a marker of cellular exhaustion(29). Akin to our adult observations, sepsis induced a marked loss of CD3+T-lymphocytes in the WT pups. However, unlike adult observations(30, 31), PD-1 in the neonatal pups did not appear to play a role in the peritoneal T-lymphocyte response to sepsis. PD-1−/− pups still displayed a profound loss of peritoneal CD3+lymphocytes similar to their WT counterparts.
However, the peritoneal B-cell response was affected by PD-1. In WT pups, sepsis induced a loss of B-cells, but this finding was reversed in PD-1−/− pups. Sepsis did not induce a loss of peritoneal B-cells in PD-1−/− pups. Although both neonatal T- and B-cells display a degree of developmental immaturity, our findings may reflect differences in the mechanistic pathways by which neonatal T- versus B-cells signal. Neonatal B-cell responses to antigen are affected by the relatively high expression of negative regulators of B-cell receptor signaling or cross-linking of antigen. This has been strongly linked to CD40 and CD22 expression(32), regulatory molecules that prevent over-activation of the immune system. However, neonatal T-cell signaling dysfunction appears more to be related to alterations in downstream signaling in response to strong antigenic responses(33) rather than the contribution from surface co-stimulatory signaling.
Conclusion
Advances in the care of the septic neonatal patient will only come about through a better understanding of the pathways of sepsis. Although differences exist between neonatal and adult responses to sepsis, the role of PD-1 appears to be consistent across the age spectrum. Further work will be required to focus on the mechanistic steps involved in the PD-1 mediated immune alterations. However, we have demonstrated a key role for the checkpoint protein PD-1 in regulating both the immune response to and ultimately mortality from neonatal sepsis.
Acknowledgments
This project was supported by NIH grant R35-GM118097 (to A.A.), a NIH grant K08-GM110495 (D.S.H.) and “Armand D. Versaci” Research Scholar in Surgical Sciences Fellowship awards (to W.A.Y. & E.A.F.). We appreciate the contributions, insights and reviews of Lyle Moldawer PhD Department of Surgery, Gainesville, Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu L, Johnson H, Cousens S, Perin J, Scott S, Lawn J, et al. Global, regional and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Yost C, Cody M, Harris E, Thornton N, McInturff A, Artinez MM, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113(25):6419–27. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haeney M. Infection determinants at extremes of age. Journal of Antimicrobial Chemotherapy. 1994;(Suppl A):1–9. doi: 10.1093/jac/34.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Venet F, Wang Y, Lepape A, Yuan Z, Chen Y, et al. PD-1 expression by macrophages plays a pathologic role in alterting microbial clearance and the innate inflammatory response to sepsis. Proceedings of the National Academy of Sciences. 2009;106(15):6303–8. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchins N, Unsinger J, Hotchkiss R, Ayala A. The new normal: immunomodulatory agents against sepsis immune suppression. Trends in Molecular Medicine. 2014;20(4):224–33. doi: 10.1016/j.molmed.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monaghan S, Thakkar R, Tran M, Huang X, Cioffi W, Ayala A, et al. Programmed death 1 expression as a marker for immune and physiological dysfunction in the critically ill surgical patient. Shock. 2012;38(2):117–22. doi: 10.1097/SHK.0b013e31825de6a3. [DOI] [PubMed] [Google Scholar]
- 7.Thakkar R, Huang X, Lomas-Neira J, Heffernan D, Ayala A. Sepsis and the immune response. In: Eremin O, Sewell H, editors. Essential immunology for surgeons. Oxford University Press; 2011. pp. 303–42. [Google Scholar]
- 8.Lewis A, Billiar T, Rosengart M. Biology and metabolism of sepsis: Innate immunity, bioenergetics and autophagy. Surgical Infections. 2016;17(3) doi: 10.1089/sur.2015.262. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present and future. Journal of Clinical Investigation. 2015;125(9):3384–91. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monaghan S, Thakkar R, Heffernan D, Tran M, Huang X, Chung C, et al. Mechanisms of indirect acute lung injury: a novel role for the coinhibitory receptor programmed death-1. Annals of Surgery. 2012;255(1):158–64. doi: 10.1097/SLA.0b013e31823433ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberger B, Laskin D, Mariano T, Sunil V, DeCoste C, Heck D, et al. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. Journal of Leukocyte Biology. 2001;70(6):969–76. [PMC free article] [PubMed] [Google Scholar]
- 12.Wynn J, Scumpia P, Delano M, O’Malley K, Ungaro R, Abouhamze A, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28(6):675–83. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 13.Heffernan D, Monaghan S, Chung C, Cioffi W, Gravenstein S, Ayala A. A divergent response of innate regulatory T-cells to sepsis in humans: circulating invariant natural killer T-cells are preserved. Human Immunology. 2014;75(3):277–82. doi: 10.1016/j.humimm.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Young J, Heffernan D, Chung C, Kettenmann M, Young W, Guillen V, et al. Effect of PD-1:PD-L1 in invariant Natural Killer T-cell emigration and chemotaxis following sepsis. Shock. 2015 doi: 10.1097/SHK.0000000000000553. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayala A, Elphick G, Kim Y, Huang X, Carreira-Rosario A, Santos S, et al. Sepsis-induced potentiation of peritoneal macrophage migration is mitigated by programmed cell death receptor-1 gene deficiency. Journal of Innate Immunity. 2014;6(3):325–38. doi: 10.1159/000355888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shubin N, Chung C, Heffernan D, Irwin L, Monaghan S, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. Journal of Leukocyte Biology. 2012;92(3):593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okazaki T, Honjo T. The PD1:PDL pathway in immunological tolerance. Trends in Immunology. 2006;27(4):195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Tang L, Bai J, Chung C, Lomas-Neira J, Chen Y, Huang X, et al. Active players in resolution of shock/sepsis induced indirect lung injury: immunomodulatory effects of Tregs and PD-1. Journal of Leukocyte Biology. 2014;96(5):809–20. doi: 10.1189/jlb.4MA1213-647RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tumeh P, Harview C, Yearley J, Shintaku I, Taylor E, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guleria I, Khosroshahi A, Ansari M, Habicht A, Azuma M, Yagita H, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. Journal of Experimental Medicine. 2005;202(2):231–7. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habicht A, Dada S, Jurewicz M, Fife B, Yagita H, Azuma M, et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. Journal of Immunology. 2007;179(8):5211–9. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 22.Roger T, Schneider A, Weier M, Sweep F, Le Roy D, Bernhagen J, et al. High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates. Proceedings of the National Academy of Sciences. 2016;113(8):E997–1005. doi: 10.1073/pnas.1514018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belderbos M, Levy O, Meyaard L, Bont L. Plasma-mediated immune suppression: a neonatal perspective. Pediatric allegy and immunology. 2013;24(2):102–13. doi: 10.1111/pai.12023. [DOI] [PubMed] [Google Scholar]
- 24.Chiesa C, Pacifico L, Natale F, Hofer N, Osborn J, Resch B. Fetal and early neonatal interleukin-6 response. Cytokine. 2015;76(1):1–12. doi: 10.1016/j.cyto.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Lusyati S, Hulzebos C, Zandvoort J, Sauer P. Levles of 25 cytokines in the first seven days of life in newborn infants. BMC Research Notes. 2013;6:547. doi: 10.1186/1756-0500-6-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarrio M, Grabie N, Bu D, Sharpe A, Lichtman A. PD-1 protects against inflammation and myocyte damage in T cell mediated myocarditis. Journal of Immunology. 2012;188(10):4876–84. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert review of clinical immunology. 2014;10(9):1171–84. doi: 10.1586/1744666X.2014.942288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman G, Long A, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. Journal of Experimental Medicine. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang W, Kim J, Kim Y, Kim Y, Lee J, Azuma M, et al. Cutting edge: Programmed death-1/programmed death ligand-1 interaction regulates the induciton and maintainance of invariant NKT cell anergy. Journal of Immunology. 2008;181(10):6707–10. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 30.Licata L, Nguyen C, Burga R, Falanga V, Espat N, Ayala A, et al. Biliary obstruction results in PD1 dependent liver T-cell dysfunction and acute inflammation mediated by Th17 cells and neutrophils. Journal of Leukocyte Biology. 2013;94(4):813–23. doi: 10.1189/jlb.0313137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. International Immunology. 2007;19(7):813–24. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 32.Tian C, Kron G, Dischert K, Higginbotham J, Crowe J. Low expression of the interleukin (IL)-4 receptor alpha chain and reduced signaling via the IL-4 receptor complex in human neonatal B cells. Immunology. 2006;119(1):54–62. doi: 10.1111/j.1365-2567.2006.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miscia S, Di Baldassarre A, Sabatino G, Bonvini E, Rana R, Vitale M, et al. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T-lymphocytes. Journal of Immunology. 1999;163(5):2416–24. [PubMed] [Google Scholar]