Abstract
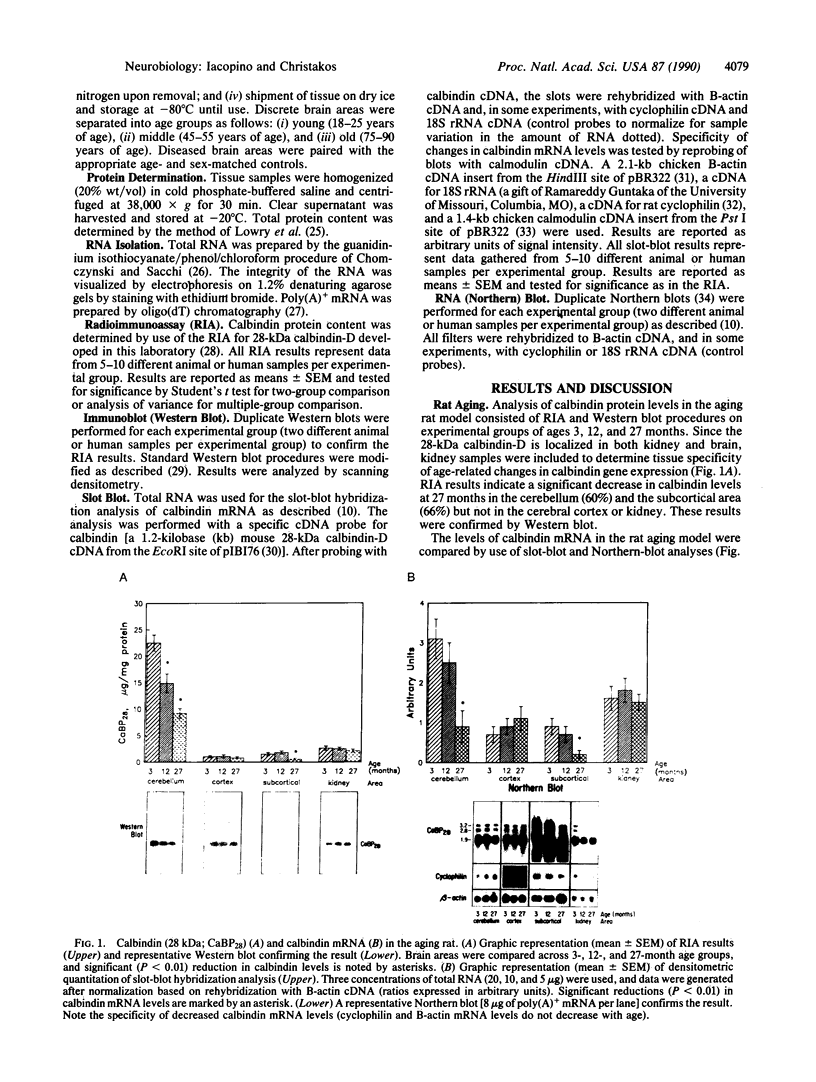
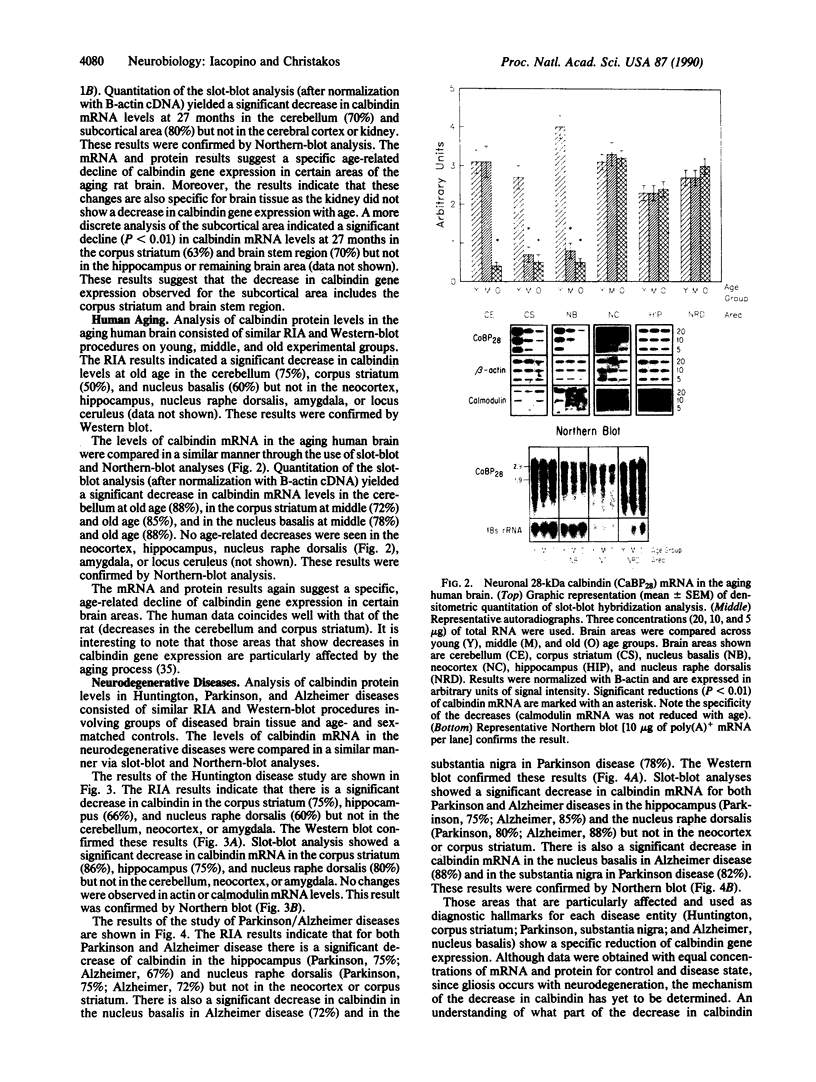
The present studies establish that there are specific, significant decreases in the neuronal calcium-binding protein (28-kDa calbindin-D) gene expression in aging and in neurodegenerative diseases. The specificity of the changes observed in calbindin mRNA levels was tested by reprobing blots with calmodulin, cyclophilin, and B-actin cDNAs. Gross brain regions of the aging rat exhibited specific, significant decreases (60-80%) in calbindin mRNA and protein levels in the cerebellum, corpus striatum, and brain-stem region but not in the cerebral cortex or hippocampus. Discrete areas of the aging human brain exhibited significant decreases (50-88%) in calbindin protein and mRNA in the cerebellum, corpus striatum, and nucleus basalis but not in the neocortex, hippocampus, amygdala, locus ceruleus, or nucleus raphe dorsalis. Comparison of diseased human brain tissue with age- and sex-matched controls yielded significant decreases (60-88%) in calbindin protein and mRNA in the substantia nigra (Parkinson disease), in the corpus striatum (Huntington disease), in the nucleus basalis (Alzheimer disease), and in the hippocampus and nucleus raphe dorsalis (Parkinson, Huntington, and Alzheimer diseases) but not in the cerebellum, neocortex, amygdala, or locus ceruleus. Since calbindin gene expression decreased specifically in brain areas known to be particularly affected in aging and in each of the neurodegenerative diseases, these findings suggest that decreased calbindin gene expression may lead to a failure of calcium buffering or intraneuronal calcium homeostasis, which contributes to calcium-mediated cytotoxic events during aging and in the pathogenesis of neurodegenerative diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altavista M. C., Bentivoglio A. R., Crociani P., Rossi P., Albanese A. Age-dependent loss of cholinergic neurones in basal ganglia of rats. Brain Res. 1988 Jul 5;455(1):177–181. doi: 10.1016/0006-8993(88)90130-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge K. G., Miller J. J., Parkes C. O. Calcium-binding protein distribution in the rat brain. Brain Res. 1982 May 13;239(2):519–525. doi: 10.1016/0006-8993(82)90526-1. [DOI] [PubMed] [Google Scholar]
- Baudier J., Cole R. D. Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987 Dec 25;262(36):17577–17583. [PubMed] [Google Scholar]
- Celio M. R., Norman A. W. Nucleus basalis Meynert neurons contain the vitamin D-induced calcium-binding protein (Calbindin-D 28k). Anat Embryol (Berl) 1985;173(2):143–148. doi: 10.1007/BF00316296. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christakos S., Gabrielides C., Rhoten W. B. Vitamin D-dependent calcium binding proteins: chemistry, distribution, functional considerations, and molecular biology. Endocr Rev. 1989 Feb;10(1):3–26. doi: 10.1210/edrv-10-1-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- De Boni U., McLachlan D. R. Senile dementia and Alzheimer's disease: a current view. Life Sci. 1980 Jul 7;27(1):1–14. doi: 10.1016/0024-3205(80)90013-2. [DOI] [PubMed] [Google Scholar]
- Deary I. J., Hendrickson A. E. Calcium and Alzheimer's disease. Lancet. 1986 May 24;1(8491):1219–1219. doi: 10.1016/s0140-6736(86)91205-5. [DOI] [PubMed] [Google Scholar]
- DiFiglia M., Christakos S., Aronin N. Ultrastructural localization of immunoreactive calbindin-D28k in the rat and monkey basal ganglia, including subcellular distribution with colloidal gold labeling. J Comp Neurol. 1989 Jan 22;279(4):653–665. doi: 10.1002/cne.902790411. [DOI] [PubMed] [Google Scholar]
- Enderlin S., Norman A. W., Celio M. R. Ontogeny of the calcium binding protein calbindin D-28k in the rat nervous system. Anat Embryol (Berl) 1987;177(1):15–28. doi: 10.1007/BF00325286. [DOI] [PubMed] [Google Scholar]
- Farber J. L. The role of calcium in cell death. Life Sci. 1981 Sep 28;29(13):1289–1295. doi: 10.1016/0024-3205(81)90670-6. [DOI] [PubMed] [Google Scholar]
- Feldman S. C., Christakos S. Vitamin D-dependent calcium-binding protein in rat brain: biochemical and immunocytochemical characterization. Endocrinology. 1983 Jan;112(1):290–302. doi: 10.1210/endo-112-1-290. [DOI] [PubMed] [Google Scholar]
- Gilbert D. S., Newby B. J. Neurofilament disguise, destruction and discipline. Nature. 1975 Aug 14;256(5518):586–589. doi: 10.1038/256586a0. [DOI] [PubMed] [Google Scholar]
- Gona A. G., Pendurthi T. K., Al-Rabiai S., Gona O., Christakos S. Immunocytochemical localization and immunological characterization of vitamin D-dependent calcium-binding protein in the bullfrog cerebellum. Brain Behav Evol. 1986;29(3-4):176–183. doi: 10.1159/000118679. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A. Calcium channels and calcium channel antagonists. Ann Neurol. 1987 Apr;21(4):317–330. doi: 10.1002/ana.410210402. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., McDonald J. W., Parnisari R. M., Camel J. E. Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Res. 1986 Aug 13;380(1):136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- Ichimiya Y., Emson P. C., Mountjoy C. Q., Lawson D. E., Heizmann C. W. Loss of calbindin-28K immunoreactive neurones from the cortex in Alzheimer-type dementia. Brain Res. 1988 Dec 13;475(1):156–159. doi: 10.1016/0006-8993(88)90210-7. [DOI] [PubMed] [Google Scholar]
- Ingersoll R. J., Wasserman R. H. Vitamin D3-induced calcium-binding protein. Binding characteristics, conformational effects, and other properties. J Biol Chem. 1971 May 10;246(9):2808–2814. [PubMed] [Google Scholar]
- Iqbal K., Grundke-Iqbal I., Wisniewski H. M. Alzheimer's disease, microtubule and neurofilament proteins, and axoplasmic flow. Lancet. 1987 Jan 10;1(8524):102–102. doi: 10.1016/s0140-6736(87)91937-4. [DOI] [PubMed] [Google Scholar]
- Jande S. S., Maler L., Lawson D. E. Immunohistochemical mapping of vitamin D-dependent calcium-binding protein in brain. Nature. 1981 Dec 24;294(5843):765–767. doi: 10.1038/294765a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Martinez A., Vitórica J., Satrústegui J. Cytosolic free calcium levels increase with age in rat brain synaptosomes. Neurosci Lett. 1988 Jun 7;88(3):336–342. doi: 10.1016/0304-3940(88)90234-0. [DOI] [PubMed] [Google Scholar]
- McLachlan D. R., Wong L., Bergeron C., Baimbridge K. G. Calmodulin and calbindin D28K in Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1(3):171–179. doi: 10.1097/00002093-198701030-00009. [DOI] [PubMed] [Google Scholar]
- Metuzals J., Pant H., Gainer H., Eagles P. A., White N. S., Houghton S. In vitro polymorphism and phase transitions of the neurofilamentous network isolated from the giant axon of the squid (Loligo pealei L.). Cell Tissue Res. 1988 May;252(2):249–262. doi: 10.1007/BF00214367. [DOI] [PubMed] [Google Scholar]
- Morgan D. W., Welton A. F., Heick A. E., Christakos S. Specific in vitro activation of Ca,Mg-ATPase by vitamin D-dependent rat renal calcium binding protein (calbindin D28K). Biochem Biophys Res Commun. 1986 Jul 31;138(2):547–553. doi: 10.1016/s0006-291x(86)80531-9. [DOI] [PubMed] [Google Scholar]
- Pansini A. R., Christakos S. Vitamin D-dependent calcium-binding protein in rat kidney. Purification and physiocochemical and immunological characterization. J Biol Chem. 1984 Aug 10;259(15):9735–9741. [PubMed] [Google Scholar]
- Perl D. P., Gajdusek D. C., Garruto R. M., Yanagihara R. T., Gibbs C. J. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and Parkinsonism-dementia of Guam. Science. 1982 Sep 10;217(4564):1053–1055. doi: 10.1126/science.7112111. [DOI] [PubMed] [Google Scholar]
- Peterson C., Goldman J. E. Alterations in calcium content and biochemical processes in cultured skin fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2758–2762. doi: 10.1073/pnas.83.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C., Ratan R. R., Shelanski M. L., Goldman J. E. Altered response of fibroblasts from aged and Alzheimer donors to drugs that elevate cytosolic free calcium. Neurobiol Aging. 1988 May-Jun;9(3):261–266. doi: 10.1016/s0197-4580(88)80063-0. [DOI] [PubMed] [Google Scholar]
- Putkey J. A., Ts'ui K. F., Tanaka T., Lagacé L., Stein J. P., Lai E. C., Means A. R. Chicken calmodulin genes. A species comparison of cDNA sequences and isolation of a genomic clone. J Biol Chem. 1983 Oct 10;258(19):11864–11870. [PubMed] [Google Scholar]
- Scharfman H. E., Schwartzkroin P. A. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989 Oct 13;246(4927):257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C., Ihara Y. Brain transglutaminase: in vitro crosslinking of human neurofilament proteins into insoluble polymers. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6070–6074. doi: 10.1073/pnas.79.19.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto-Ohshima A., Emson P. C., Lawson E., Mountjoy C. Q., Carrasco L. H. Loss of matrix calcium-binding protein-containing neurons in Huntington's disease. Lancet. 1988 Jun 4;1(8597):1252–1255. doi: 10.1016/s0140-6736(88)92073-9. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J., Pansini A. R., Christakos S. Vitamin D-dependent rat renal calcium-binding protein: development of a radioimmunoassay, tissue distribution, and immunologic identification. Endocrinology. 1984 Aug;115(2):640–648. doi: 10.1210/endo-115-2-640. [DOI] [PubMed] [Google Scholar]
- Taylor A. N., Wasserman R. H. Correlations between the vitamin D-induced calcium binding protein and intestinal absorption of calcium. Fed Proc. 1969 Nov-Dec;28(6):1834–1838. [PubMed] [Google Scholar]
- Terry R. D. Some biological aspects of the aging brain. Mech Ageing Dev. 1980 Sep-Oct;14(1-2):191–201. doi: 10.1016/0047-6374(80)90119-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese S., Lee S., Huang Y. C., Christakos S. Analysis of rat vitamin D-dependent calbindin-D28k gene expression. J Biol Chem. 1988 Jul 15;263(20):9776–9784. [PubMed] [Google Scholar]
- Wasserman R. H., Corradino R. A., Taylor A. N. Vitamin D-dependent calcium-binding protein. Purification and some properties. J Biol Chem. 1968 Jul 25;243(14):3978–3986. [PubMed] [Google Scholar]
- Whitehouse P. J. Clinical and neurochemical consequences of neuronal loss in the nucleus basalis of Meynert in Parkinson's disease and Alzheimer's disease. Adv Neurol. 1987;45:393–397. [PubMed] [Google Scholar]
- Wood T. L., Kobayashi Y., Frantz G., Varghese S., Christakos S., Tobin A. J. Molecular cloning of mammalian 28,000 Mr vitamin D-dependent calcium binding protein (calbindin-D28K): expression of calbindin-D28K RNAs in rodent brain and kidney. DNA. 1988 Nov;7(9):585–593. doi: 10.1089/dna.1988.7.585. [DOI] [PubMed] [Google Scholar]









