Highlights
► Candidate TB vaccine MVA85A is well tolerated intramuscularly or intradermally. ► Both routes are highly immunogenic. ► MVA85A-induced CD4+ T cell cytokine production was similar between the two routes.
Keywords: Tuberculosis, Vaccine, MVA85A, Intradermal, Intramuscular
Abstract
Background
New vaccines to prevent tuberculosis are urgently needed. MVA85A is a novel viral vector TB vaccine candidate designed to boost BCG-induced immunity when delivered intradermally. To date, intramuscular delivery has not been evaluated. Skin and muscle have distinct anatomical and immunological properties which could impact upon vaccine-mediated cellular immunity.
Methods
We conducted a randomised phase I trial comparing the safety and immunogenicity of 1 × 108 pfu MVA85A delivered intramuscularly or intradermally to 24 healthy BCG-vaccinated adults.
Results
Intramuscular and intradermal MVA85A were well tolerated. Intradermally-vaccinated subjects experienced significantly more local adverse events than intramuscularly-vaccinated subjects, with no difference in systemic adverse events. Both routes generated strong and sustained Ag85A-specific IFNγ T cell responses and induced multifunctional CD4+ T cells. The frequencies of CD4+ T cells expressing chemokine receptors CCR4, CCR6, CCR7 and CXCR3 induced by vaccination was similar between routes.
Conclusions
In this phase I trial the intramuscular delivery of MVA85A was well tolerated and induced strong, durable cellular immune responses in healthy BCG vaccinated adults, comparable to intradermal delivery. These findings are important for TB vaccine development and are of relevance to HIV, malaria, influenza and other intracellular pathogens for which T cell-inducing MVA-based vaccine platforms are being evaluated.
1. Introduction
In 2010, 1.45 million people died from tuberculosis (TB) and there were 8.8 million incident cases [1]. TB control is confounded by diagnostic difficulties, drug resistance, lack of new therapeutic agents and coexisting HIV infection. Preventative vaccination is widely regarded as the most cost-effective approach to reduce TB incidence. The Bacille–Calmette–Guérin (BCG) vaccine, in use for 90 years, is protective against meningeal and disseminated TB in children but its efficacy against adult pulmonary disease is highly variable [2], [3], [4]. Moreover, it is not recommended for HIV-infected infants [5]. There is an urgent need for a more universally effective TB vaccine platform.
The most clinically advanced new vaccine candidate is MVA85A, a recombinant replication-deficient Modified Vaccinia virus Ankara (MVA) expressing the immunodominant Mycobacterium tuberculosis (M. tb) Antigen 85A (Ag85A). MVA85A is well tolerated and highly immunogenic when administered as a boost to BCG-primed individuals [6]. The protective efficacy of MVA85A is currently being evaluated in two phase IIb efficacy trials: one in BCG-vaccinated infants in South Africa (NCT00953927) and the second in HIV-infected adults in South Africa and Senegal (NCT01151189).
In all clinical trials to date, MVA85A has been administered by the intradermal route like BCG. The skin is a large, highly vascular organ with superior lymphatic drainage. It plays a specialised role in innate and adaptive immunity [7]. Its numerous antigen presenting cells (APCs) including epidermal (Langerhans) and dermal dendritic cells (DC) are capable of antigen cross-presentation and induction of cell-mediated immunity [8], [9], [10]. It could therefore be considered an optimal site for vaccination against TB.
Nevertheless, the intramuscular route is a well-established route for immunisation, favoured in worldwide immunisation programmes. Advantages include reduced injection site pain, minimal injection site reactions, technical ease and capacity for larger injectate volume. Injecting into muscle may increase antigen persistence and therefore exposure to APCs by virtue of a “depot effect” [8], [11]. Other recombinant MVA constructs have been safely given intramuscularly [12], [13].
We postulated that the safety and immunogenicity of intramuscular MVA85A would be non-inferior to intradermal MVA85A. To test our hypothesis we designed a randomised phase I trial to compare intramuscular and intradermal delivery of MVA85A in BCG-vaccinated healthy adults.
2. Methods
2.1. Study design
This phase I trial (ClinicalTrial.gov registry number NCT01181856) was approved by the Medicines and Healthcare products Regulatory Agency (MHRA, EudraCT 2009-015973-11) and Oxfordshire Research Ethics Committee A (reference 09/H0604/128). Twenty four previously BGC-vaccinated healthy subjects aged between 18 and 55 years were enrolled at the Centre for Clinical Vaccinology & Tropical Medicine, Churchill Hospital, Oxford, UK. All subjects gave written informed consent and the trial was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice (GCP).
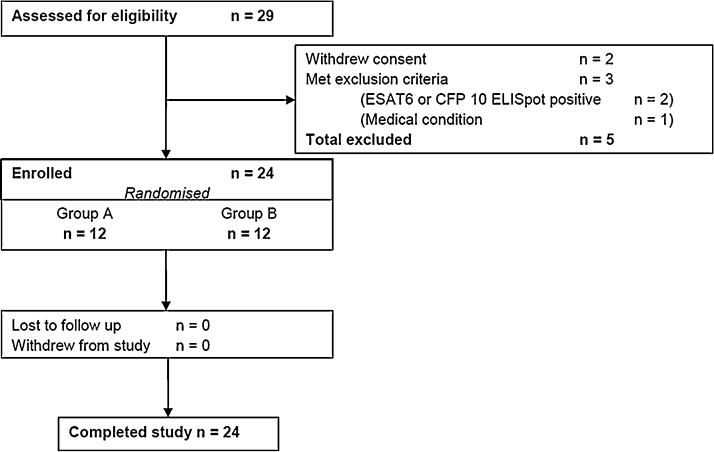
Enrolled subjects were in good health, had normal baseline haematology and biochemistry, and negative serological testing for hepatitis B, hepatitis C and HIV. Latent infection with M. tb was excluded by means of a negative ex vivo ELISpot response to ESAT-6 and CFP-10 peptides. The reasons for exclusion of five subjects are shown in Fig. 1. All subjects were followed for six months post-vaccination.
Fig. 1.
Consort diagram showing subject recruitment and follow-up.
2.2. Treatment groups, randomisation and blinding
The 24 subjects were randomised in a 1:1 ratio to group A (intramuscular MVA85A, deltoid muscle, 23G diameter 25 mm length needle, dose 1 × 108 pfu) or group B (intradermal MVA85A, upper arm, 29G diameter 12.7 mm length needle, dose 1 × 108 pfu) using sequentially-numbered, opaque, sealed envelopes opened on the day of vaccination after confirming eligibility. Variable block randomisation was used to ensure allocation concealment. The Investigator performing vaccination was not blinded however anonymisation of blood samples ensured the laboratory staff were blinded and vaccination route was not divulged to subjects.
2.3. Endpoints
The primary endpoint in the trial was safety, as assessed by the frequency and severity of vaccine-related local and systemic adverse events (AEs). Expected local AEs (including pain, erythema, and swelling) and systemic AEs (fever, feverishness, fatigue, malaise, headache, myalgia, arthralgia, and nausea) were solicited from subjects using a diary card for seven days following vaccination and at each follow-up visit. Routine laboratory biochemical and haematological parameters were measured at 7 and 84 days post-vaccination.
The secondary endpoint was the immunogenicity of MVA85A measured by the ex vivo interferon-gamma (IFNγ) ELISpot assay performed on fresh peripheral blood mononuclear cells (PBMC) stimulated with pools of mycobacterial peptides. Exploratory pre-defined immunological analyses included multi-parameter flow cytometry as detailed below.
2.4. Vaccine
Clinical grade MVA85A (lot number 010507) was constructed as previously described [14] and produced under Good Manufacturing Practice conditions by IDT Biologika GmbH (Dessau-Rosslau, Germany).
2.4.1. Ex vivo IFNγ ELISpot assay
PBMC were isolated from whole blood using Leucosep tubes (Greiner Bio-One), with LymphoPrep™ (Axis-Shield) providing a density gradient for separation. IFNγ ELISpot assays were performed on freshly isolated PBMC from all 24 subjects at screening and at 1, 2, 4, 12 and 24 weeks post-vaccination, using the Human IFNγ ELISpot (ALP) kit (Mabtech). 3 × 105 PBMC, suspended in 80 μl media, were added to each ELISpot well. Twenty microlitres of antigen was added to duplicate wells to give a total volume of 100 μl per well. The antigens used were a single pool of Ag85A peptides, consisting of 66 15mers, overlapping by 10 amino acids, (2 μg/ml each peptide). The 66 peptides were also split into 7 pools (A–G) of 9 or 10 peptides (10 μg/ml each peptide). Responses to purified protein derivative (PPD) from M. tb (SSI) were also assessed at each time-point (20 μg/ml). Staphylococcus enterotoxin B (SEB) and phytohemagglutinin (PHA) (Sigma) were used as positive controls (10 μg/ml). Unstimulated PBMC were used as a measure of background IFNγ production. ELISpot plates were incubated for 18–20 h at 37 °C, 5% CO2, before being developed. Spots were counted on an ELISpot reader (AID Germany, software version 5.0). Results are reported as spot-forming cells (SFC) per million PBMC, calculated by subtracting the mean count of the unstimulated PBMC from the mean count of duplicate antigen wells and correcting for number of PBMC in the well. Responses were considered positive if the count was twice (but at least 5 spots greater) than that of the unstimulated wells.
2.4.2. Surface and intracellular cytokine staining
Frozen PBMC were thawed and rested overnight in R10 with 10 U/ml Benzonaze (Merck Chemicals). The following morning, PBMC were washed and 1 × 106 cells were stimulated with 20 μg/ml of PPD, 2 μg/ml of each 85A peptide in a pool of 66 peptides, 5 μg/ml staphylococcal enterotoxin (Sigma) or left unstimulated as negative control. αCD28 and αCD49d (both from BD Bioscience) were added to all conditions at 1 μg/ml. Stimulated cells were incubated for 2 h at 37 °C, 5% CO2 after which Brefeldin A (Sigma) was added at 3 μg/ml followed by an overnight incubation under the same conditions. PBMC were washed the following morning with PBS and then stained with amine-reactive Live/Dead fixable red (Molecular Probes, Invitrogen) for 10 min at 4 °C followed by surface staining for 30 min at 4 °C with a mix of mouse anti-human CD4/PB (Biolegend), CD14/ECD and CD19/ECD (Beckman Coulter) (clones RPA-T4, RMO52 and J3-119 respectively). Cells were then washed, permeabilised using Cytofix/Cytoperm kit (BD Pharmingen) following the manufacturer's recommendations and stained intracellularly for 30 min in the cold with a mix of: CD3/AF700, IFNγ/PECy7 (eBioscience), CD8/APC-AF750, IL2/PE (Beckman Coulter) and TNFα/AF647 (clones UCHT1, 4S.B3, B9.11, N7.48A, MAB11 and BL168 respectively). Cells were then washed and resuspended in PBS containing 1% Bovine Serum Albumin (Sigma) and 0.1% Sodium Azide (Sigma). Stained cells were immediately acquired using an LSR-II (Becton Dickinson). Data were analysed using FlowJo 8.7 (Treestar, Inc., USA). Lymphocytes were gated on a forward scatter height (FSC-H) versus side scatter area plot, followed by exclusion of duplets on a FSC-H versus forward scatter area plot. Only live CD3+ cells were included in the analysis of CD4+ and CD8+ cell cytokine responses.
To assess the effect of vaccination route on cell trafficking we measured the expression of chemokine receptors by surface staining PBMC for 30 min at 37 °C with CCR4/PERCPCY5.5, CCR6/AF647 (Biolegend), CCR7/PECY7 and CXCR3/PECY5 (BD Bioscience) (clones TG6/CCR4, TG7/CCR6, 3D12 and 1C6/CXCR3 respectively).
2.4.3. IgG enzyme linked immunosorbent assay (ELISA)
Anti-r85A IgG was measured in serum samples collected at week 0, 2, 4 and 24. ELISA plates (NUNC ImmunoPlates, Fisher) were coated overnight with 2 μg/ml r85A (Lionex, Germany) in PBS. Samples were diluted 1:10 in 1% Casein/PBS (Fisher Scientific) and were tested in duplicates. A pool of IgG-positive sera was included in each plate. Blank wells were left without serum incubation to assess background. Serum IgG was captured by goat anti-human IgG alkaline phosphatase antibody (Sigma). Plates were developed using Diethanolamine/4-nitrophenylphosphate kit (Sigma) according to the manufacturer's recommendations and were read at 405 nm. Background-subtracted optical density (OD) values are presented.
2.5. Statistical analysis
Safety data were summarised by frequency and median number of AEs per subject. The IFNγ ELISpot data at each time-point were summarised using medians and interquartile ranges. An area-under-the-curve (AUC) analysis was performed to summate each subject's response over time. The Mann Whitney U-test was used to compare AEs and immune responses between groups. Differences between time-points within the groups were compared using Wilcoxon matched pairs test. Data were analysed using Microsoft Excel 2010 and Prism 5 for Windows (GraphPad Software, Inc.).
3. Results
The two groups were well matched with regard to age, gender, continent of birth and timing of prior BCG vaccination (Table 1). All subjects were followed up to trial completion.
Table 1.
Demographics of enrolled subjects.
| Characteristic | Group A intramuscular (n = 12) | Group B intradermal (n = 12) | p value |
|---|---|---|---|
| Female, n (%) | 7 (58%) | 8 (66%) | 1.0 |
| Median age in years (range) | 25.5 (18–52) | 30.5 (19–55) | 0.73 |
| Median time interval since BCG in years | 13.0 | 22.0 | 0.71 |
| Current tobacco smoker, n (%) | 2 (17%) | 1 (8%) | 1.0 |
| Continent of birth | |||
| Europe | 10 | 10 | 1.0 |
| Africa | 1 | 1 | 1.0 |
| Asia | 1 | 1 | 1.0 |
3.1. MVA85A was well tolerated by both the intramuscular and intradermal routes
All 24 vaccinations were well tolerated with 96% of the 215 AEs reported during the trial being mild in severity, no vaccine-related severe AEs, and only one incidence of fever (mild, 37.5 °C, occurring 29 h post vaccination). There were no vaccine-related serious adverse events (SAE) and no vaccine-related laboratory abnormalities. One unrelated SAE occurred (traumatic fracture of left elbow sustained during a fall 5 months after vaccination necessitating admission to hospital for manipulation under anaesthetic).
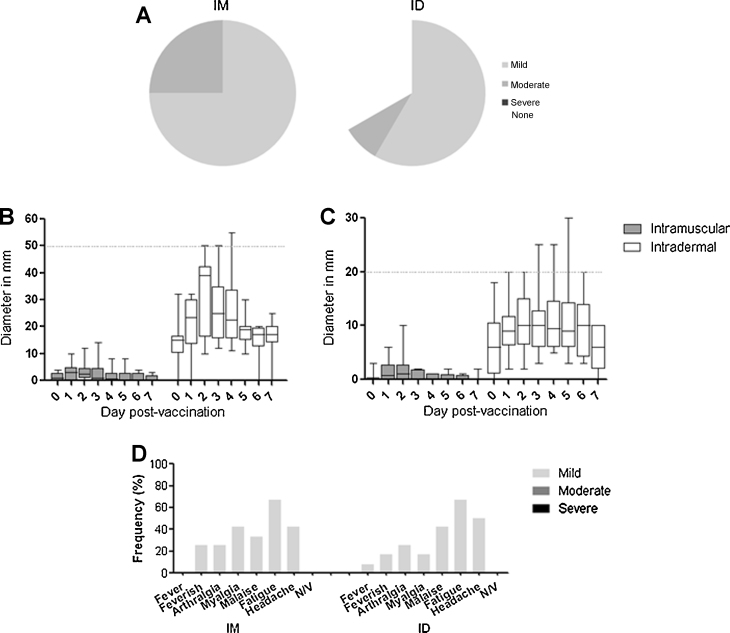
Subjects receiving intradermal MVA85A experienced more expected local AEs than those receiving intramuscular MVA85A (median 3 vs. 6 AEs per subject, p = 0.0002, Mann Whitney U test) but there was no difference for expected systemic AEs (median 2 vs. 2.5 AEs per subject, p = 0.48). In the intramuscular group, local pain at the vaccination site was more frequently reported (100% vs. 66% subjects) and more likely to be rated as moderate rather than mild (25% vs. 17% of subjects) though this was not statistically significant (Fig. 2A). Erythema and swelling were mild for all but one subject and differed markedly between groups (Fig. 2(B) and (C)).
Fig. 2.
Local and systemic adverse events. (A) shows the proportion of subjects reporting mild, moderate, and severe local pain after intramuscular and intradermal vaccination. No subjects experienced severe pain. Subsequent figures show the diameter of erythema (B) and swelling (C) in mm at the injection site of subjects during the first 7 days after vaccination, and the frequency of systemic adverse events (D) reported by subjects during the first 7 days after vaccination. All data are shown by group. Box and whisker plots show median, interquartile range, and minimum and maximum values. The horizontal dashed line on (B) and (C) indicate the threshold between “mild” severity and “moderate” severity which is 50 mm for erythema and 20 mm for swelling. Only one subject experienced moderate erythema and swelling. N/V = nausea or vomiting.
All systemic adverse events were mild. The most common systemic symptom was fatigue (67% of subjects) followed by headache (46%) and malaise (38%). Only 20% reported feverishness and there was only one recorded fever (37.5 °C on a single occasion 24 h after vaccination). There was no clinically significant difference in the occurrence of systemic symptoms between intramuscularly and intradermally-vaccinated subjects (Fig. 2D). There were no vaccine-related laboratory AEs. The safety data in this trial are in keeping with the Investigators’ clinical experience using MVA85A to date [6], [15], [16], [17], [18], [19].
3.2. Intradermal and Intramuscular MVA85A vaccination induced strong and sustained antigen-specific responses
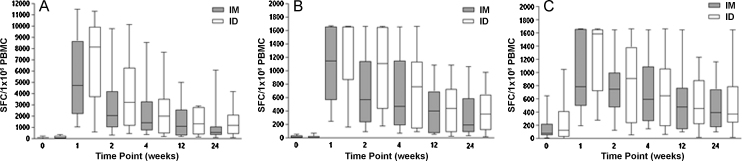
Both intradermal and intramuscular MVA85A induced high levels of Ag85A-specific IFNγ at the peak (day 7) time-point. The responses were durable, staying significantly above baseline six months post-vaccination (Wilcoxon matched pairs test). The median antigen-specific IFNγ response in the intradermal group exceeded that of the intramuscular group (Fig. 3) but this difference did not reach statistical significance at any time-point. An AUC analysis of the response summated over six months showed no difference between the two groups (p = 0.62, Mann Whitney U test). IFNγ ELISpot responses were substantially higher for the summed 85A pools than the single pool at the peak time-point for all subjects. However, there was no difference in the breath of response across the peptide pools between the two groups.
Fig. 3.
IFNγ ELISpot responses post-vaccination for intramuscular and intradermal routes. (A) Shows responses to 85A summed pooled peptides calculated by summing across all peptide pools A–G at each time-point. This could potentially result in duplicate counting of cells that responded to any of the 10mer overlap regions, since these could occur in two pools with adjacent peptides, but allows direct comparison with immunogenicity data from previous trials with MVA85A. (B) Shows responses to the 85A single pool and (C) to PPD. Box and whisker plots show median, interquartile range, and minimum and maximum values. In the 85A single pool and PPD graphs, a value of 1667 SFC/1 × 106 PBMC represents a blackout in the ELISpot well.
3.3. Intradermal and intramuscular MVA85A vaccination induced IFNγ, tumour necrosis factor-alpha (TNFα) and interleukin-2 (IL2) producing CD4+ T cells
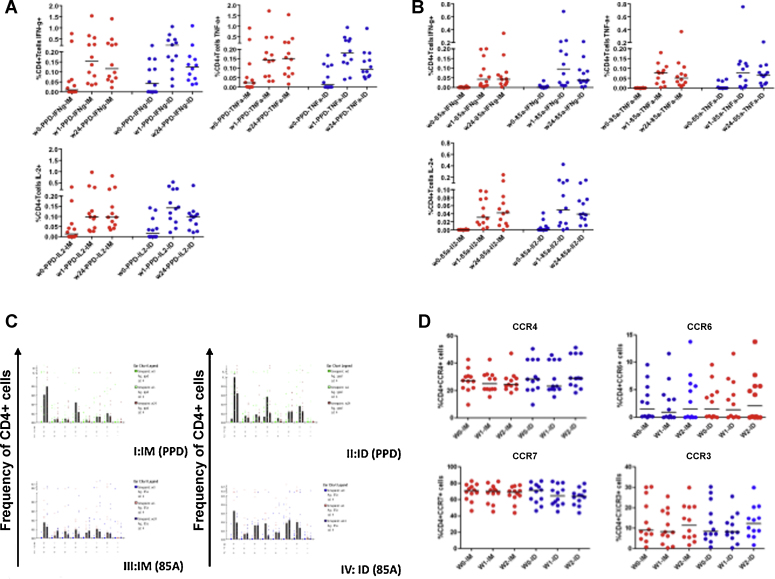
Mycobacteria-specific cytokines (IFNγ, TNFα and IL2) were measured in subjects’ cryopreserved PBMC after an overnight stimulation with PPD and a single pool of 85A peptides (Fig. 4). Both routes of vaccination induced significant IFNγ, TNFα and IL2 at weeks 1 and 24 post vaccination. Levels of produced cytokines between the two groups did not significantly differ at any time-point.
Fig. 4.
Mycobacteria-specific cytokine responses in cryopreserved PBMC stimulated overnight with PPD or 85A peptide pools. Comparable levels of PPD (A) and 85A-specific (B) IFNγ, TNFα and IL2 were detected with no significant differences between the two vaccination groups at any time-point (Mann Whitney U test). Significant cytokine production was detected at all time-points compared to baseline (p < 0.05, Wilcoxon matched pairs test). Polyfunctional PPD and 85A-specific CD4+ T cells (C) producing multiple cytokines were detected following vaccination. The patterns of cytokine production are comparable between both groups. Median values are shown. (D) Shows the expression of CCR4, CCR6, CCR7 and CXCR3 on CD4+ cells in unstimulated cryopreserved PBMC. Individual values for subjects are shown with horizontal bars representing median values.
3.4. Intradermal and intramuscular MVA85A induced similar profiles of CD4+ T cell chemokine receptor expression
The effect of vaccination route on cell trafficking was evaluated by measurement of CD4+ T cell surface expression of chemokine receptors CCR4, CCR6, CCR7 and CXCR3. This was initially performed in both mycobacteria-stimulated and unstimulated cells for 4 subjects. Expression of these markers was not affected by stimulation (data not shown). Fig. 4D shows median expression of these chemokine markers in unstimulated cells. Substantial levels of expression were detected in both groups, which were not significantly different.
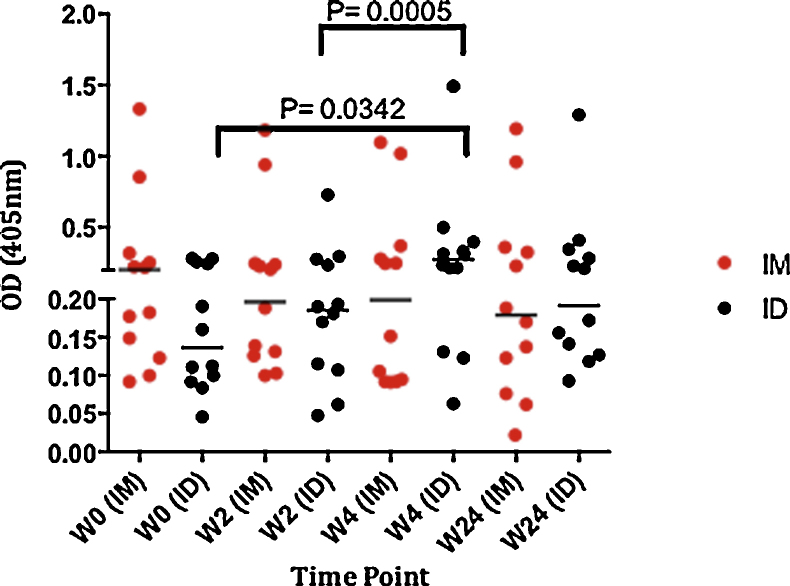
3.5. Serum IgG
We looked at the humoral (IgG) immune response to 85A in both groups in serum samples at week 0, 2, 4 and 24 following vaccination (Fig. 5). Volunteers who were vaccinated intradermally with MVA85A had significantly higher serum IgG 4 weeks post-vaccination when compared to baseline (p = 0.0342) and week-2 (p = 0.0005). There was no significant increase during follow-up in IgG levels in the intramuscularly immunised group, similarly no significant differences were observed between the two groups at any of the time points. At baseline the intramuscular group had IgG levels higher than the intradermal group.
Fig. 5.
Serum IgG. Antigen 85A-specific IgG was measured in samples from subjects in the ID and IM groups (12 subjects in each group) at weeks 0, 2, 4 and 24. Median OD values are shown with individual values for each volunteer.
4. Discussion
This randomised phase I trial is the first clinical evaluation of intramuscular delivery of MVA85A. In keeping with our experience of the intradermal route, MVA85A was well tolerated. As expected, we observed a marked reduction in the extent of local erythema and swelling in the intramuscular group. However systemic symptoms between the two groups were comparable. This is in line with previous route comparison studies of recombinant MVA vaccines which report more frequent local AEs in the intradermal group than the intramuscular group but similar levels of systemic reactogenicity [12], [13], [20], [21].
We observed a trend towards more frequent and more appreciable injection-site pain in intramuscular-vaccinated than intradermal-vaccinated subjects. This could reflect a difference in pain quality as much as severity, which we did not specifically assess. Intramuscular-vaccinated subjects may experience a deeper muscular ache whereas intradermal-vaccinated subjects may experience more superficial tenderness. The routes may also differ in their propensity to generate needle insertion (“prick”) pain and dose delivery (“administration”) pain [22], [23]. These qualitative assessments of pain are important determinants of vaccine acceptability which could be investigated in future route-comparison studies.
The magnitudes of the antigen-specific immune responses in this trial were comparable with previous trials of this dose of MVA85A in BCG-vaccinated healthy adults. The overall median peak response was 7437 SFC/million PBMC and occurred at seven days post-vaccination for both routes, in keeping with previous data. The ex vivo IFNγ responses contracted over time but remained significantly above the pre-vaccination baseline at the week 24 time-point indicating that a durable vaccine-induced immune response can be induced by either route.
Both intradermal and intramuscular routes of MVA85A vaccination induced mycobacteria-specific polyfunctional CD4+ T cell populations producing IFNγ, TNFα and IL2. There were no significant differences in the magnitude of these responses between the two routes. These three detected cytokines have been shown to be important in controlling mycobacterial infection [24], [25], [26], [27], [28]. Both routes of vaccination studied here induced polyfunctional CD4+ T cells; in a previous Leishmania major vaccine study, polyfunctional cells producing these three cytokines – rather than single producing cells – were essential for protection against leishmaniasis [29].
We investigated whether intradermal and intramuscular vaccination would direct cells to different compartments within the immune system. To do this we studied the expression of the chemokine receptors, CCR4, CCR6, CCR7 and CXCR3. Through their ability to control leucocyte migratory and trafficking characteristics, chemokines and their receptors play important roles in modulating the host immune response. We looked at: CCR4, a chemokine receptor associated with Th2 cells shown to impair IFNγ production in a mycobacteria mouse model [30]; CCR6, found to be upregulated in TB patients [31]; CCR7, which is involved in the control of immune cell trafficking to lymph nodes; and CXCR3 shown to be involved in directing Th1 trafficking [32]. The route of MVA85A vaccination did not impact on the expression of these markers at the studied time-points (weeks 0, 1 and 2).
We measured serum total IgG as an indication of the humoral immune response in the two groups. Subjects vaccinated intradermally had higher IgG levels post-vaccination, and this was not seen in subjects vaccinated intramuscularly. Further work to evaluate levels of Th2 immunity would be important in subsequent studies.
The relatively small numbers of subjects limit the statistical power of this study. The data suggest a non-significant trend of higher antigen-specific IFNγ responses in the intradermal group than the intramuscular group, more marked at early time-points. However we are unable to comment on whether a larger study would show a statistically significant difference between the groups and, if so, whether this difference would persist at late time-points. The lack of any differences detected in intracellular cytokine staining analysis suggests that there is no real immunological difference between the two routes.
The lack of blinding of subjects and placebo control could have introduced bias. However these design features would be unusual in a phase I study with safety as a primary outcome. Our design incorporated variable block randomisation in order to limit operator bias and facilitate matching of groups. We blinded laboratory staff to the intervention. We chose an equivalent dose (1 × 108 pfu) and volume (135 μL) for both groups in this study in order to compare ‘like with like’ and maximise the relevance of our findings to our past and ongoing studies. We have previously performed dose finding studies of intradermal MVA85A and found that 1 × 108 pfu was well tolerated and highly immunogenic [17].
Our study demonstrated no difference between cellular immunogenicity induced by intramuscular or intradermal MVA85A vaccination. Interestingly Abadie et al. performed IFNγ ELISpot and intracellular cytokine staining assays on the skin and muscle-draining lymph node T cells of MVA vaccinated mice and found that intradermal delivery resulted in markedly higher magnitude and quality of peak T cell response than intramuscular delivery [10]. Thus preclinical observations may not be echoed in humans and clinical evaluation of delivery routes for novel vaccines is of crucial importance.
Vaccine dosage and volume are important considerations when comparing vaccination routes because muscle and skin have fundamental anatomical differences. There is accumulating evidence in other fields, particularly influenza vaccinology, that the intradermal route offers the opportunity to dose-spare [13], [22], [33], [34], [35]. As little as one-fifth of the intramuscular dose may be required to achieve the same efficacy by intradermal injection. The data presented here do not suggest dose sparing can be achieved with intradermal vaccination with MVA85A.
The intradermal and intramuscular routes have been extensively compared for licensed vaccines against hepatitis A, hepatitis B, influenza and rabies [36], [37], [38], [39]. Both routes are safe and immunogenic in clinical trials however the generalisability of these findings to other vaccines is limited. These vaccines target predominantly humoral immunity rather than the cell mediated mechanisms considered crucial for protection against TB, and being targeted by viral vector vaccine candidates such as MVA85A.
Data from this trial will inform TB vaccine research in a promising era for the field as the two leading viral-vector TB vaccine candidates undergo phase IIb efficacy trials. The findings are relevant to global vaccine-preventable diseases caused by other intracellular pathogens including HIV, malaria, influenza and hepatitis C, as recombinant MVA-based vaccine constructs designed to induce cellular immunity are currently being developed for all of these diseases.
Acknowledgement
The authors wish to thank Nicola Williams, Mary Smith, Cynthia Bateman, Laura Dinsmore and all the trial participants.
Conflicts of interest statement: HMcS is a named inventor in a patent filing related to MVA85A and is a shareholder in a joint venture, OETC, formed for the future development of this vaccine. HMcS is named as a co-inventor on patents related to heterologous prime-boost immunisation. All other authors: no conflict. Funding: This work was supported by the Wellcome Trust (Senior Clinical Research Fellowship held by HMcS) and the NIHR Biomedical Research Centre, Oxford.
Footnotes
Clinical trial registration: www.clinicaltrials.gov, NCT01181856.
References
- 1.WHO. Global Tuberculosis Control; 2011. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf [accessed 27.02.11].
- 2.Colditz G.A., Brewer T.F., Berkey C.S., Wilson M.E., Burdick E., Fineberg H.V. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 3.Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 4.Bhat G.J., Diwan V.K., Chintu C., Kabika M., Masona J. HIV, BCG and TB in children: a case control study in Lusaka, Zambia. J Trop Pediatr. 1993;39:219–223. doi: 10.1093/tropej/39.4.219. [DOI] [PubMed] [Google Scholar]
- 5.Hesseling A.C., Cotton M.F., Fordham von Reyn C., Graham S.M., Gie R.P., Hussey G.D. Consensus statement on the revised World Health Organization recommendations for BCG vaccination in HIV-infected infants. Int J Tuberc Lung Dis. 2008;12:1376–1379. [PubMed] [Google Scholar]
- 6.McShane H., Pathan A.A., Sander C.R., Keating S.M., Gilbert S.C., Huygen K. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 7.Koblin B.A., Casapia M., Morgan C., Qin L., Wang Z.M., Defawe O.D. Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: a randomized clinical trial. PLoS ONE. 2011;6:e24517. doi: 10.1371/journal.pone.0024517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent P.E., Bonnet S., Alchas P., Regolini P., Mikszta J.A., Pettis R. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Romani N., Thurnher M., Idoyaga J., Steinman R.M., Flacher V. Targeting of antigens to skin dendritic cells: possibilities to enhance vaccine efficacy. Immunol Cell Biol. 2010;88:424–430. doi: 10.1038/icb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abadie V., Bonduelle O., Duffy D., Parizot C., Verrier B., Combadiere B. Original encounter with antigen determines antigen-presenting cell imprinting of the quality of the immune response in mice. PLoS ONE. 2009;4:e8159. doi: 10.1371/journal.pone.0008159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriksen-Lacey M., Bramwell V.W., Christensen D., Agger E.M., Andersen P., Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010;142:180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud T.K., Hamill M., Lillie P.J., Hwenda L., Collins K.A., Ewer K.J. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP + M1. Clin Infect Dis. 2011;52:1–7. doi: 10.1093/cid/ciq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilck M.B., Seaman M.S., Baden L.R., Walsh S.R., Grandpre L.E., Devoy C. Safety and immunogenicity of modified vaccinia Ankara (ACAM3000): effect of dose and route of administration. J Infect Dis. 2010;201:1361–1370. doi: 10.1086/651561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McShane H., Brookes R., Gilbert S.C., Hill A.V. Enhanced immunogenicity of CD4(+) t-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect Immun. 2001;69:681–686. doi: 10.1128/IAI.69.2.681-686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beveridge N.E., Price D.A., Casazza J.P., Pathan A.A., Sander C.R., Asher T.E. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minassian A.M., Rowland R., Beveridge N.E., Poulton I.D., Satti I., Harris S. A Phase I study evaluating the safety and immunogenicity of MVA85A, a candidate TB vaccine, in HIV-infected adults. BMJ Open. 2011;1:e000223. doi: 10.1136/bmjopen-2011-000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathan A.A., Minassian A.M., Sander C.R., Rowland R., Porter D.W., Poulton I.D. Effect of vaccine dose on the safety and immunogenicity of a candidate TB vaccine, MVA85A, in BCG vaccinated UK adults. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander C.R., Pathan A.A., Beveridge N.E., Poulton I., Minassian A., Alder N. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med. 2009;179:724–733. doi: 10.1164/rccm.200809-1486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelan K.T., Pathan A.A., Sander C.R., Fletcher H.A., Poulton I., Alder N.C. Safety and immunogenicity of boosting BCG vaccinated subjects with BCG: comparison with boosting with a new TB vaccine, MVA85A. PLoS ONE. 2009;4:e5934. doi: 10.1371/journal.pone.0005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters B.S., Jaoko W., Vardas E., Panayotakopoulos G., Fast P., Schmidt C. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine. 2007;25:2120–2127. doi: 10.1016/j.vaccine.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Currier J.R., Ngauy V., de Souza M.S., Ratto-Kim S., Cox J.H., Polonis V.R. Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS ONE. 2010;5:e13983. doi: 10.1371/journal.pone.0013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belshe R.B., Newman F.K., Cannon J., Duane C., Treanor J., Van Hoecke C. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 23.Frenck R.W., Jr., Belshe R., Brady R.C., Winokur P.L., Campbell J.D., Treanor J. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone(R)) administered by intradermal and intramuscular route in healthy adults. Vaccine. 2011;29:5666–5674. doi: 10.1016/j.vaccine.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn J.L., Chan J., Triebold K.J., Dalton D.K., Stewart T.A., Bloom B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lammas D.A., De Heer E., Edgar J.D., Novelli V., Ben-Smith A., Baretto R. Heterogeneity in the granulomatous response to mycobacterial infection in patients with defined genetic mutations in the interleukin 12-dependent interferon-gamma production pathway. Int J Exp Pathol. 2002;83:1–20. doi: 10.1046/j.1365-2613.2002.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roach D.R., Bean A.G., Demangel C., France M.P., Briscoe H., Britton W.J. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 27.Millington K.A., Gooding S., Hinks T.S., Reynolds D.J., Lalvani A. Mycobacterium tuberculosis-specific cellular immune profiles suggest bacillary persistence decades after spontaneous cure in untreated tuberculosis. J Infect Dis. 2010;202:1685–1689. doi: 10.1086/656772. [DOI] [PubMed] [Google Scholar]
- 28.Millington K.A., Innes J.A., Hackforth S., Hinks T.S., Deeks J.J., Dosanjh D.P. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darrah P.A., Patel D.T., De Luca P.M., Lindsay R.W., Davey D.F., Flynn B.J. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 30.Imai T., Nagira M., Takagi S., Kakizaki M., Nishimura M., Wang J. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 31.Stolberg V.R., Chiu B.C., Martin B.E., Shah S.A., Sandor M., Chensue S.W. Cysteine–cysteinyl chemokine receptor 6 mediates invariant natural killer T cell airway recruitment and innate stage resistance during mycobacterial infection. J Innate Immun. 2011;3:99–108. doi: 10.1159/000321156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin S., Rottman J.B., Myers P., Kassam N., Weinblatt M., Loetscher M. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Investig. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez M.L., Usandizaga M., Alomar P., Salva F., Martin F., Erroz M.J. Intradermal and intramuscular route for vaccination against hepatitis B. Vaccine. 1990;8:402–405. doi: 10.1016/0264-410x(90)90102-r. [DOI] [PubMed] [Google Scholar]
- 34.Kenney R.T., Frech S.A., Muenz L.R., Villar C.P., Glenn G.M. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 35.Playford E.G., Hogan P.G., Bansal A.S., Harrison K., Drummond D., Looke D.F. Intradermal recombinant hepatitis B vaccine for healthcare workers who fail to respond to intramuscular vaccine. Infect Control Hosp Epidemiol. 2002;23:87–90. doi: 10.1086/502012. [DOI] [PubMed] [Google Scholar]
- 36.Pancharoen C., Mekmullica J., Thisyakorn U., Kasempimolporn S., Wilde H., Herzog C. Reduced-dose intradermal vaccination against hepatitis A with an aluminum-free vaccine is immunogenic and can lower costs. Clin Infect Dis. 2005;41:1537–1540. doi: 10.1086/497266. [DOI] [PubMed] [Google Scholar]
- 37.Wistrom J. Intramuscular vs. intradermal hepatitis B vaccination: a 6-year follow-up. JAMA. 1995;273:1835–1836. doi: 10.1001/jama.1995.03520470043027. [DOI] [PubMed] [Google Scholar]
- 38.Belshe R.B., Newman F.K., Wilkins K., Graham I.L., Babusis E., Ewell M. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25:6755–6763. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabchareon A., Chantavanich P., Pasuralertsakul S., Pojjaroen-Anant C., Prarinyanupharb V., Attanath P. Persistence of antibodies in children after intradermal or intramuscular administration of preexposure primary and booster immunizations with purified Vero cell rabies vaccine. Pediatr Infect Dis J. 1998;17:1001–1007. doi: 10.1097/00006454-199811000-00007. [DOI] [PubMed] [Google Scholar]