Abstract
Atopic dermatitis (AD), the most common chronic inflammatory skin disease, is driven by both terminal keratinocyte differentiation defects and strong type 2 immune responses. In contrast to chronic plaque-type psoriasis, AD is now understood to be a much more heterogeneous disease, with additional activation of Th22, Th17/IL-23 and Th1 cytokine pathways, depending on the subtype of the disease. In this review, we discuss our current understanding of the AD immune map in both early-onset as well as chronic disease. Clinical studies using broad and targeted therapeutics have helped to elucidate the contribution of various immune axes to the disease phenotype. Importantly, immune activation extends well beyond lesional AD, as non-lesional skin and the blood component harbor AD-specific inflammatory changes. For this reason, future therapeutics will need to focus on a systemic treatment approach, especially in patients suffering from moderate-to-severe disease.
Keywords: Atopic dermatitis, eczema, keratinocyte, immune, T helper cell, skin immune map, targeted therapy
Introduction
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease, with a prevalence of up to 7% in adults and up to 25% among children.1–5 Characteristically, symptoms start within the first 5 years of life, and in adult patients, the disease has generally been present for decades. Similar to psoriasis,6,7 AD is now considered a primarily T-cell driven disease,8,9 as proven by the clinical efficacy of broad T-cell targeting therapeutics such as cyclosporine, efalizumab, and alefacept.10,11,12 While the latter two are no longer available due to safety concerns, cyclosporine, oral glucocorticosteroids (GCS) and phototherapy (NB-UVB) are often used to treat moderate-to-severe disease.13–15 However, cyclosporine and even more so GCS are not suitable for long term use due to multiple side effects. Phototherapy is very time consuming and not feasible for most patients.16 Therefore, AD presents a large unmet need for both effective and safe therapeutics.2 While animal models have been instrumental in deciphering general components of cutaneous biology in health and disease, the complex interplay between immune mechanisms, skin barrier and potential intrinsic and extrinsic triggers of disease are not well represented in a single animal model, and thus need to be addressed and characterized in humans.17,18
One strategy that was instrumental in psoriasis to educate on disease pathogenesis and activated cytokines is through clinical trials with broad and specific immune antagonists coupled with tissue biomarkers.19 Such an approach is also being successfully implemented in AD.20 Broad therapeutics such as GCS, cyclosporine, topical calcineurin inhibitors and NB-UVB have suggested the immune nature of AD, and indicated possible involvement of more than one cytokine pathway.13,14,21,22 These studies not only provided the final proof of the immune nature of AD, but also of the pathogenic role of the Th2 axis in this disease. Although increased IL-4 and IL-13 in lesional and non-lesional AD was first described in 1994, it was not until recent that studies demonstrated the clinical efficacy of dupilumab, an IL4R antagonist, and that conclusive clinical proof became available supporting the importance of the type 2 immune pathway in AD.23–26
The emerging immune map of AD
Similar to psoriasis, that is centered around a Th17/IL-23 axis, AD has been associated with activation of T-cell subsets.27 Although AD seems to be unanimously characterized by a strong activation of Th2 immune responses in lesions and even in non-lesional skin,20 Th22, Th17/IL-23 and Th1 cytokine pathways likely play a role in the disease, particularly in some AD subtypes.8
In acute lesions, AD onset is characterized by profound increases of Th2 (IL-4, IL-5, IL-13, IL-31, CCL18) and Th22 (IL-22, S100A proteins) responses.28,29 These mediators have been demonstrated to down-regulate terminal differentiation genes and tight junction products such as claudins, contributing to the barrier defect in AD.30–40 Recently, it has been demonstrated that group 2 innate lymphoid cells (ILC) can also produce Th2 cytokines. While present at much lower frequencies than T cells, type 2 ILC have been found at increased levels in AD lesions compared to healthy control skin,41–43 thereby possibly promoting Th2 responses.41,44
Among Th2 immune mediators, IL-4 and IL-13 have been demonstrated to play a key role in AD pathogenesis. Genetically, AD has been shown to be associated with IL-4 and IL-13 polymorphisms,45–48 and eczema-like features can be induced in transgenic mice overexpressing these cytokines.49–52 In humans, mRNA in situ hybridization studies by Hamid et al. demonstrated increased levels of IL-4 and IL-13 in both acute and chronic AD, to a higher degree than IFN-γ.26,53 IL-4 decreases the expression of multiple genes in the epidermal differentiation complex (EDC) that regulate epidermal barrier function.54 Keratinocytes differentiated in the presence of IL-4 and IL-13 exhibited significantly reduced filaggrin gene expression, even in patients without filaggrin mutations.38 Aside from filaggrin, loricrin and involucrin are also downregulated in lesional and nonl-lesional AD skin by IL-4 and IL-13, contributing to a defective skin barrier in AD.31 A compromised barrier allows penetration of bacteria and allergens in to the skin, leading to infections and allergen sensitization, both being highly characteristic of AD.31
Th2 polarization facilitates Staphylococcus aureus binding and colonization,55,56 and IL-4 and IL-13 inhibit skin production of antimicrobial peptides (AMP),56 predisposing AD skin to S. aureus infections,57 which, in turn, further exacerbates skin inflammation and barrier defects.58–62 Also, eczema vaccinatum, a disseminated viral skin infection that occurs in AD following inoculation with vaccinia virus, has been demonstrated to depend on IL-4/IL-13 expression via AMP downregulation.63 Mechanistically, it has been shown that IL-4 and IL-13 inhibit TNF-α and IFN-γ-induced human beta-defensin(HBD)-3 via activation of STAT-6 production in keratinocytes,64,65 as well as TNF-α-induced cathelicidin production.57 Despite the fact that IL-17 can be found in AD lesions, its antimicrobial effects (via the up-regulation of antimicrobial peptides such as HBD-2 in keratinocytes) are inhibited when IL-4 and/or IL-13 are present.62 The fact that IL-4/IL-13-driven inflammation can truncate these key Th1 (IFN-γ) and Th17 (IL-17) dependent skin defense mechanisms in AD, as well as the successful treatment of AD with dupilumab, which blocks receptor binding of both IL-4 and IL-13,23–25 proves their central role in disease pathogenesis.
Th17-associated molecules (IL-17A, PI3/elafin, CCL20) are consistently up-regulated in both acute and chronic AD, but at lower levels than in psoriasis (as compared to normal skin).66,67 IL-17A could possibly contribute to the immune dysregulation in AD by synergistically upregulating S100A7/8/9 together with IL-22.68 The S100A proteins, which are highly upregulated in AD, can act as both antimicrobials and inflammatory molecules.69 There is also evidence that IL-17 can contribute to barrier abnormalities by down-regulating filaggrin, and by affecting keratinocyte expression of genes associated with cellular adhesion.34
Th2 and Th22 responses are intensified in chronic AD lesions, with parallel activation of the Th1 axis (IFN-γ, CXCL9, CXCL10), rather than a “switch” to a Th1-only signature.66,70 IL-22 has also been identified as a key mediator of epidermal hyperplasia.68 IL-31, a cytokine associated with itch,71,72 shows large increases in acute lesions, correlating with disease severity in some studies.29,66,73,74
AD shows phenotypic variations
Several AD subtypes have been described, with considerable variations (Figure 1).8,75 These are based on IgE levels (intrinsic versus extrinsic AD),76 filaggrin mutations status, race, and age.3,29,77–79
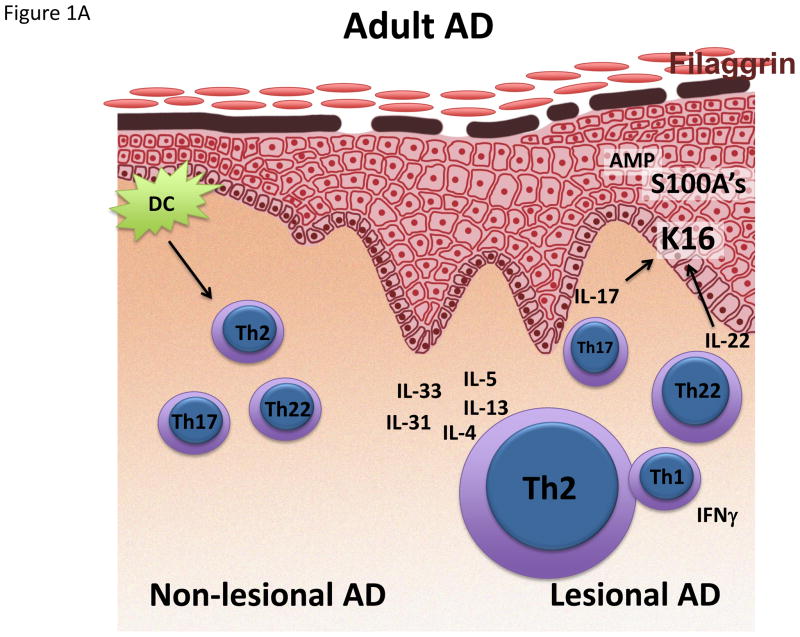
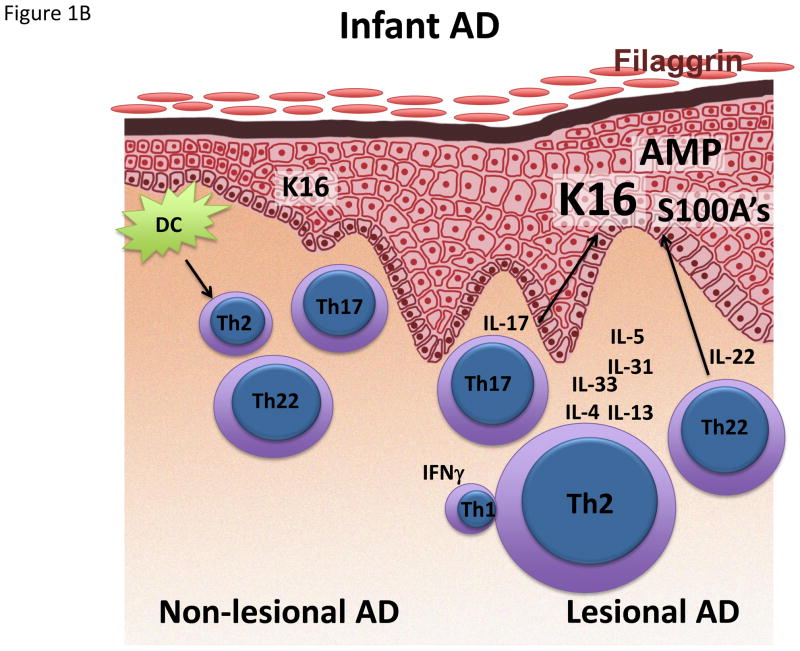
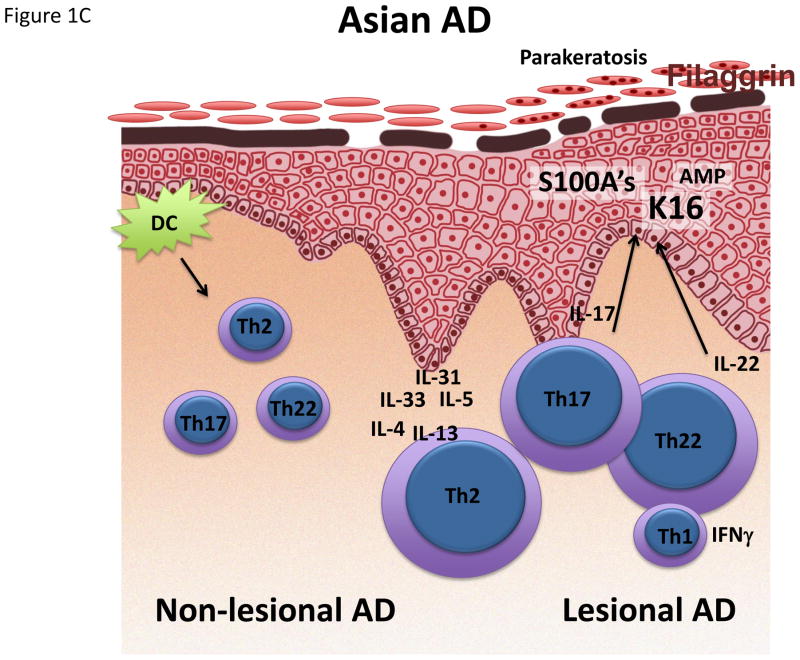
Figure 1.
Schematic representation/activation levels of selected immune pathways and epidermal responses in lesional and non-lesional skin in (A) infant, (B) early-onset AD and (C) Asian AD, (D) compared to psoriasis. AMP Antimicrobial peptide. K16 Keratin 16.
Mutations in the FLG gene, leading to a deficiency in filaggrin, have been associated with AD that is more severe and persistent than its wild type counterpart. This includes a higher degree of immune dysregulation with type 1 interferon-mediated stress responses and higher IL-1 cytokine levels, and higher rates of skin infections and allergies.28,29,34,35,80–84 However, FLG mutations are only detected in up to 30% of individuals (and rarely occurs in African-American populations with AD),84 and patients with FLG mutations have been shown to outgrow their disease.35 Consistently, dupilumab treatment was demonstrated to work equally well independent of filaggrin status.24
Extrinsic AD is characterized by an increase in total and allergen-specific IgE levels, higher rates of eosinophils, and a family history of atopic diseases. In contrast, intrinsic AD shows normal IgE levels, and patients usually lack a personal or familial history of atopy.85 Both intrinsic and extrinsic subtypes show strong Th2 activation,79 consistent with similar treatment efficacy of dupilumab in both conditions.25 However, intrinsic AD shows a stronger activation of Th17 and Th22 responses, with levels of some Th17-related mediators (i.e. CCL20) correlating with AD disease severity.79
Ethnic differences have also been demonstrated to contribute to AD disease heterogeneity.86–90 In Asian AD patients, the Th17 axis was significantly increased compared to European American patients, and its overall cytokine profile, together with features atypical for AD such as parakeratosis, suggest that Asian AD is likely a blend between AD and psoriasis.78 Future studies will show whether this effect is genetic or environmental, and whether Asian AD can be successfully targeted with the IL-17-targeting drugs originally developed for psoriasis.91–93
Pediatric versus adult AD – Different immune phenotypes on a common Th2 background
Despite the fact that AD usually starts early in childhood, most AD studies have only investigated adult patients. However, there are some clinical clues for differences between early pediatric and adult AD, such as lesions on extensor surfaces in infants, whereas adults typically show flexor involvement.3 Furthermore, the skin microbiome differs in pediatric vs. adult AD.94 Most studies in AD children are limited to studies of peripheral blood,80,95–107 demonstrating that disease activity correlates with several serum biomarkers (i.e. IL-31, CCL17, CCL22, CCL27, eosinophils, IgE), and a limited array of Th2/Th1 markers using mRNA expression.108–112 Recently, the peripheral blood phenotype of early pediatric AD has been characterized only by Th2 expansion, without other polar T-cell subsets in blood.113 In contrast, adult AD blood also shows increases in Th22 polarization, possibly reflecting continuous immune stimulation over time.113 Remarkable differences also have been detected in a recent study between the skin profiles of infants and adults.114 While both early-onset pediatric as well as adult AD show a strong Th2 activation, there is increased innate and IL-17-related inflammation in early AD lesions of infants. This dual upregulation of both Th2 and Th17 responses might be explained by profoundly increased levels of IL-19, a cytokine that can be induced by both IL-17 and IL-4/IL-13, and which has been shown to amplify the effects of IL-17 on keratinocytes.115 Besides Th17 responses, early-onset pediatric AD showed increased levels of antimicrobial peptides (AMP),114 comparable to levels in adult psoriasis. This increase in AMP might serve as a danger signal triggering disease, as demonstrated in psoriasis, where complexes of AMP with either self-DNA or RNA can stimulate dendritic cell activation.116,117 However, control skin from healthy infants also showed elevated levels of Th17 and Th22 associated mediators, including AMPs,114,118,119 possibly rooted in the necessity of newborn skin to combat infections when the skin immune system is not yet fully developed. Thus, the pathogenic role of these immune axes in children need to ultimately be evaluated through clinical trials.
Strikingly, the filaggrin deficiency of adult AD was missing in early AD,114 perhaps challenging the notion of defective filaggrin as primary factor for disease elicitation and an instigator of the atopic march. Future studies will need to further characterize epidermal barrier features in early-onset AD.
Surprisingly, the non-lesional skin of infants and young children also showed significant hyperplasia, and activated cytokines to levels as high or even higher than in adult non-lesional skin.114 Thus, the non-lesional skin of children with early AD can be viewed as a true state of disease initiation. Interestingly, at 2 months of age prior to onset of AD, infant non-lesional skin contain increased TSLP, a cytokine that drives differentiation of Th2 cells.120 In sum, the Th2 axis seems to be pathogenic across all AD phenotypes. But, other cytokine axes may have a pathogenic role is some AD subtypes. Clinical trials with specific Th2, Th17/IL-23 and Th22 antagonists are needed in different parts of the world and in different phenotypes to be able to dissect the pathogenic contribution of each axis to the disease.
AD as a systemic disease
Often AD begins during early infancy or childhood, and adult patients usually have longstanding disease for decades.121,122 Circulating skin homing T-cells, marked by cutaneous lymphocyte antigen/CLA, in severe AD patients show significant increases in activation markers, and polar cytokines, even compared to those seen in psoriasis, as compared to healthy individuals.123 Significant increases in B-cells in blood are also seen in AD, but not in psoriasis, perhaps reflecting the atopic or allergic associations characterizing the disease,124 and the atopic march.4,125 The systemic nature of AD is also reflected in the wide abnormalities seen in the non-lesional skin of adult patients with severe, chronic disease, since even non-lesional skin harbors considerable immune activation and terminal differentiation defects.114,126 Non-lesional AD shows increased expressions levels of Th2 (CCL22, CCL18, and IL-13), Th22 (L-22), and Th1 (MX-1) cytokines, significantly correlating with disease severity.126 In addition, it is characterized by profound decreases in terminal differentiation genes, and their expression is inversely correlation to disease activity as defined by SCORAD.126 These non-lesional abnormalities have therapeutic implications, suggesting the need for systemic treatments for patients with severe AD.
AD is increasingly recognized to also be associated with other, non-allergic conditions.127,128 Similar to psoriasis, adult AD patients harbor an increased risk of cardiovascular disease.129 So far, people suffering from AD were shown to have higher odds of heavy smoking, increased alcohol intake, and decreased rates of vigorous physical activity compared to non-AD individuals.130 In line, adult AD patients were identified to have increased cardiovascular risk factors such as a higher BMI, higher odds of arterial hypertension and lifetime pre-diabetes, and a sedentary lifestyle.131–134 Recently, an increased prevalence of coronary artery disease has been reported in severe AD patients without known cardiovascular disease, showing the presence of coronary plaques in 48.1% of AD patients, being significantly increased compared to healthy controls which showed a rate of 21.2%, as assessed by coronary computed tomography angiography.135
It is now well established that chronic inflammation accelerates atherosclerosis due to repetitive vascular injury.136 Mechanistically, elevated levels of TNF-α, IL-17 and IL-22 are currently thought to contribute to the increased cardiovascular risk in chronic plaque-type psoriasis, another chronic inflammatory skin disease.137,138 These cytokines are also activated in skin of AD patients, and circulating T cells skewed towards the production of several of these markers are also increased in AD,79,139–142 possibly mediating endothelial damage in this patient population. In vitro data suggest that IL-17 can indeed contribute to pro-inflammatory changes in endothelial cells, and the inhibition of IL-17 in a mouse model of atherosclerosis significantly decreased disease.143,144
It will be important to characterize serum markers of cardiovascular risk (and associated inflammatory markers) to better estimate disease risk, and to monitor therapeutics on their effect of cardiovascular risk factors.
Targeted therapies as milestones in understanding pathogenesis
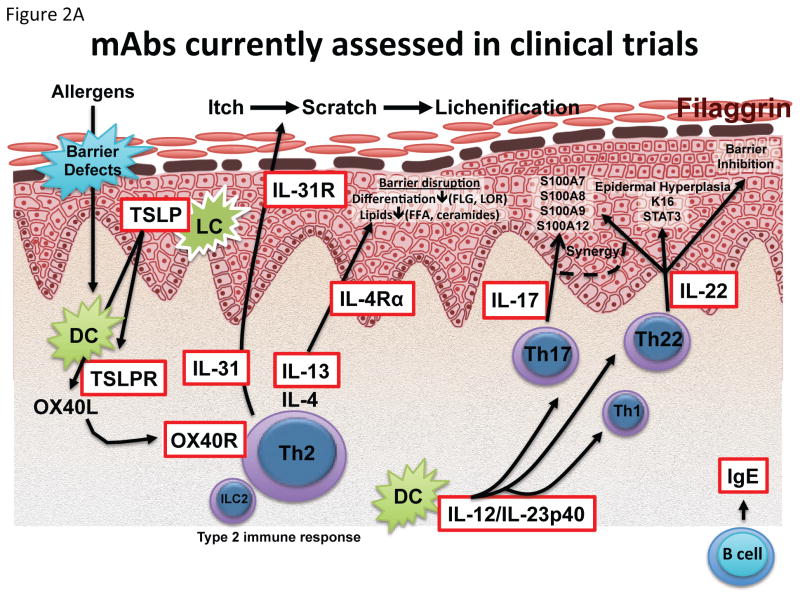
Due to the advent of new, targeted therapeutics (Figure 2A), our knowledge in key disease pathways is rapidly expanding. Ongoing or recently published controlled trials are summarized in Table 1.
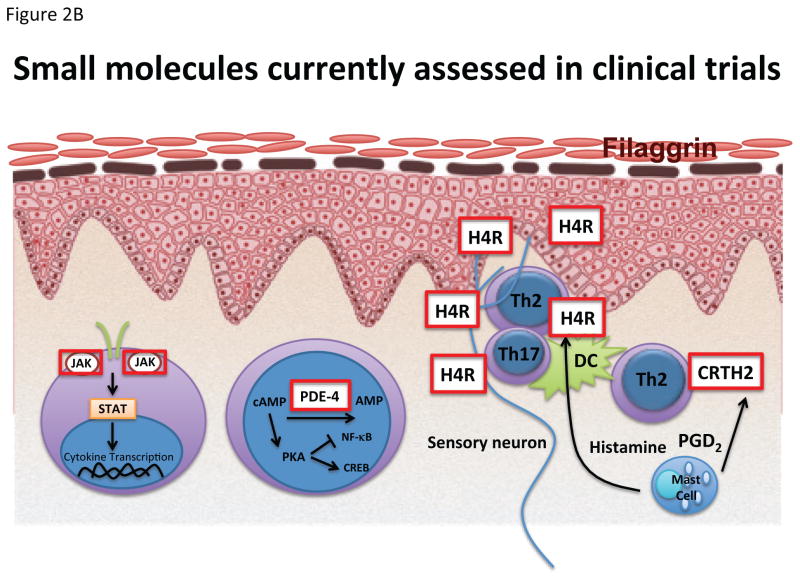
Figure 2.
Targets of (A) biologics and (B) small molecules recently published or currently being assessed in clinical trials. AMP adenosine monophosphate; cAMP cyclic adenosine monophosphate; CREB cAMP response element-binding protein; CRTH2 Prostaglandin DP2 receptor; FFA Free fatty acids; H4R Histamine H4 receptor; JAK Janus kinase; NF-kB Nuclear factor kappa-light-chain-enhancer of activated B cells; PGD2 Prostaglandin D2; PKA Protein kinase A; STAT signal transducer and activator of transcription.
Table 1. Recent controlled trials in AD.
CRTH2 Prostaglandin D2 receptor 2; H4R Histamine H4 receptor; JAK Janus kinase; PDE4 Phosphodiesterase 4; TSLP thymic stromal lymphopoietin; TSLPR thymic stromal lymphopoietin receptor;
| Agent | Trade name | Target | Drug | Phase | Manufacturer | ClinicalTrials.gov |
|---|---|---|---|---|---|---|
| Dupilumab | IL-4Rα | Anti-IL-4Rα mAb | Phase III published | Regeneron | NCT01949311 | |
| Crisaborole | PDE4 | Topical PDE4 Inhibitor | Phase III published | Pfizer |
NCT02118766 NCT02118792 |
|
| Ustekinumab | Stelara | IL-12/23p40 | Anti-p40 mAb | Phase II published | Janssen | NCT01806662 |
| Tralokinumab | IL-13 | Anti-IL-13 mAb | Phase II completed | MedImmune | NCT02347176 | |
| Tofacitinib | JAK1/3 | Topical JAK1/3 Inhibitor | Phase II published | Innovaderm | NCT02001181 | |
| Lebrikizumab | IL-13 | Anti-IL-13 mAb | Phase II completed | Hoffmann-La Roche | NCT02340234 | |
| CIM331/Nemolizumab | IL-31R | Anti-IL-31R mAb | Phase II completed | Chugai | NCT01986933 | |
| QGE031 | IgE | Anti-IgE mAb | Phase II completed | Novartis | NCT01552629 | |
| Apremilast | Otezla | PDE4 | PDE4 Inhibitor - Oral small molecule | Phase II completed | Celgene | NCT02087943 |
| QAW039/Fevipiprant | CRTH2 | CRTH2 Inhibitor - Oral small molecule | Phase II completed | Novartis | NCT01785602 | |
| ILV-094 | IL-22 | Anti-IL-22 mAb | In Phase II | Pfizer | NCT01941537 | |
| GBR830 | OX40 | Anti-OX40 mAb | In Phase II | Glenmark | NCT02683928 | |
| Secukinumab | Cosentyx | IL-17 | Anti-IL-17 mAb | In Phase II | Novartis | NCT02594098 |
| OC000459 | CRTH2 | CRTH2 Inhibitor - Oral small molecule | In phase II | Atopix | NCT02002208 | |
| Baricitinib | JAK1/2 | Jak1/2 inhibitor – Oral small molecule | In Phase II | Eli Lilly | NCT02576938 | |
| PF-04965842 | JAK1/2 | Jak1/2 inhibitor – Oral small molecule | In Phase II | Pfizer | NCT02780167 | |
| ZPL389 | H4R | Histamine H4 receptor inhibitor – Oral small molecule | Phase II completed | Ziarco Pharma | NCT02424253 | |
| BMS-981164 | IL-31 | Anti-IL-31 mAb | Phase I completed | BMS | NCT01614756 | |
| AMG157/Tezepelumab | TSLP | Anti-TSLP mAb | Phase I completed | Amgen | NCT00757042 | |
| MK-8226 | TSLPR | Anti-TSLPR mAb | In Phase I | Merck | NCT01732510 |
IgE, which is profoundly increased in 80% of patients suffering from extrinsic disease, has long been regarded as key in the development of eczema.145 So far, two randomized-controlled studies failed to show clinical effects of the IgE-blocker omalizumab,146,147 suggesting that increased IgE levels are an epiphenomenon of AD, mediating comorbidities such as food allergy, asthma and rhinoconjunctivitis, but not AD itself. However, results from a current trial with a higher affinity anti-IgE antibody (QGE031) are currently pending. Eosinophils, which can be found at increased levels in AD patients both in blood and skin, are also likely not central to disease development. In this regard, IL-5 which specifically acts on eosinophils resulting in accelerated eosinophilopoiesis, chemotaxis, cell activation, and delayed apoptosis,148 may not play a key role in AD as mepolizumab, a monoclonal IL-5 antagonist did not show efficacy in early trials.149 However, more definitive longer trials are needed to define the role of IL-5 in AD, since the initial studies were of only two-week duration, which may be potentially too short a time frame to judge treatment effect in this disease.149,150
On the other hand, dupilumab, a monoclonal antibody that specifically targets IL-4Rα, thereby blocking the two key mediators of the Th2 pathway, IL-4 and IL-13 is highly efficacious for controlling skin disease in moderate-to-severe AD patients (Figure 3).23–25 Dupilumab has shown excellent safety and efficacy in phase II trials, with Eczema Area and Severity Index (EASI)50, EASI75 and EASI90 responses of 82.5%, 60.3% and 36.5%, respectively, after 16 weeks of treatment (300mg once a week), compared to 29.5%, 11.5%, and 3.3% of respective responses in the placebo group.23 These results have also been confirmed in two large phase III studies with dupilumab (SOLO1 and SOLO2) in 671 and 708 moderate-to-severe AD patients, respectively (Table 2). Weekly doses of 300mg dupilumab (without concomitant topical glucocorticosteroids or calcineurin inhibitors) elicited a 72% and 69% improvement of baseline EASI, and 37% and 36% of patients achieved clearing or near-clearing of skin lesions, compared to only 10% and 8% in the placebo group (p<0.001).151
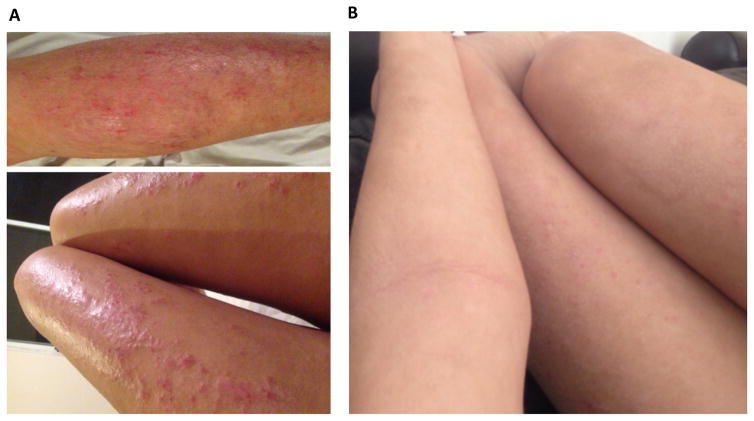
Figure 3.
Clinical responses in an AD patient before (A) and after (B) treatment with dupilumab 300mg eow. Hallmarks of AD such as widely distributed erythema and excoriations are largely relieved after 16 weeks of treatment.
Table 2. Study results from two independent, randomized, placebo-controlled, phase 3 trials of identical design (SOLO1 and SOLO 2).
16 weeks treatment, randomized 1:1:1 to subcutaneous dupilumab 300mg weekly (QW), every other week (Q2W), or placebo. In addition, each patient randomized to dupilumab received a single loading dose of 600mg on day 1. EASI Eczema Area and Severity Index; EASI-75 Proportion of patients with an EASI improvement from baseline at week 16 of at least 75%; IGA Investigator’s Global Assessment; LS least-squares; NRS numerical rating scale; wks weeks;
| SOLO 1 | SOLO 2 | All comparisons | ||
|---|---|---|---|---|
| Patients enrolled | 671 | 708 | ||
| Primary end point (16wks): IGA of 0/1 – clear/almost clear | Dupilumab Q2W | 85 (38%) | 84 (36%) | P<0.001 |
| Dupilumab QW | 83 (37%) | 87 (36%) | ||
| Placebo | 23 (10%) | 20 (8%) | ||
| Key secondary/coprimary end point (16wks): EASI-75 | Dupilumab Q2W | 115 (51%) | 103 (44%) | P<0.001 |
| Dupilumab QW | 117 (52%) | 115 (48%) | ||
| Placebo | 33 (15%) | 28 (12%) | ||
| LS mean % change (±SE) in EASI from baseline (16wks) | Dupilumab Q2W | −72.3±2.6 | −67.1±2.5 | P<0.001 |
| Dupilumab QW | −72.0±2.6 | −69.1±2.5 | ||
| Placebo | −37.6±3.3 | −30.9±3.0 | ||
| LS mean % change from baseline in peak score on NRS for pruritus (16wks) | Dupilumab Q2W | −51.0±2.5 | −44.3±2.3 | P<0.001 |
| Dupilumab QW | −48.9±2.6 | −48.3±2.4 | ||
| Placebo | −26.1±3.0 | −15.4±3.0 |
Current trials with monoclonal antibodies that exclusively target IL-13 (tralokinumab - NCT02347176, lebrikizumab - NCT02340234) will shed further light on the question whether IL-4 and IL-13 are redundant, or complementary, in the pathogenesis of AD. Blockade of IL-31 (BMS-981164), the Th2-associated itch cytokine,71 is also currently being investigated (NCT01614756).8 A single subcutaneous dose of CIM331 (nemolizumab), a monoclonal antibody blocking IL-31 receptor A, was well tolerated in a phase I study in healthy volunteers and patients with AD, decreasing pruritus, sleep disturbance and topical use of glucocorticosteroids in the latter.152 Future studies should clarify the role of anti IL-31 treatment for AD disease activity, versus control of the itch associated with the disease.
The thymic stromal lymphopoietin (TSLP)-OX40 ligand (OX40L) pathway has recently been suggested to be an initiation factor for exacerbated Th2 immune activation.153,154 Keratinocytes and Langerhans cells in lesional skin of AD patients were shown to highly express TSLP,155 triggering the expression of OX40L on dendritic cells. TSLP blockade is currently assessed in a phase I clinical trial (AMG-157, NCT00757042; MK-8226 NCT01732510). OX40L and OX40 (a co-stimulatory receptor expressed on activated T cells) are important in generating and maintaining Th2 responses as well as in the development of adaptive and innate allergic inflammation.156 OX40-OX40L interaction has also been demonstrated in a variety of inflammatory conditions associated with allergy, including allergic asthma, rhinitis, and conjunctivitis.153,154 Blocking this Th2 biased costimulation might be a therapeutic target in the future, and is currently assessed in a clinical trial (NCT02683928).
The prostaglandin DP2 receptor CRTH2 (CD294), a G protein-coupled receptor expressed by CLA+ Th2 cells,157 has been shown to be important for allergic skin inflammation after epicutaneous antigen challenge.158,159 Polymorphisms in CRTH2 have been associated with allergic sensitization.160 CRTH2 blockade (Figure 2B) via the small molecules fevipiprant (QAW039, NCT01785602) and OC000459 (NCT02002208) are currently being assessed in clinical trials.
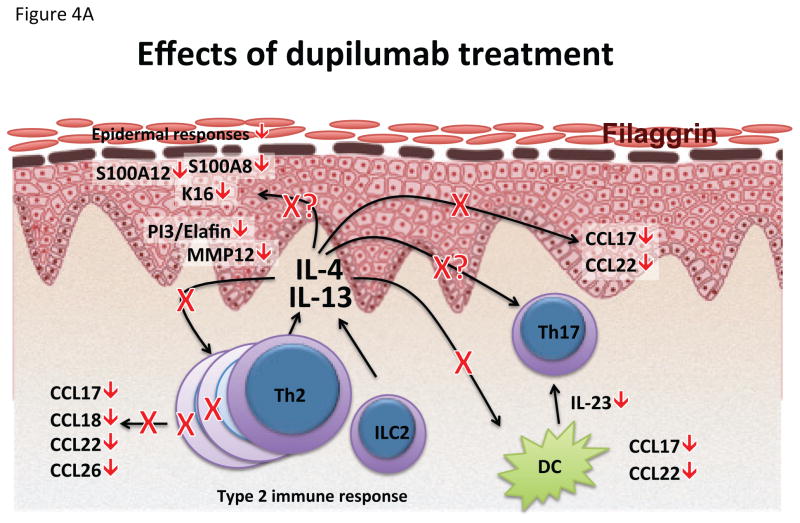
While the efficacy of dupilumab proves the pathogenic role of type 2 immune responses in AD, the role of other cytokine pathways remains to be elucidated, as dupilumab not only reduces Th2 associated molecules such as CCL17, CCL18 and CCL26, but also strongly decreased mediators associated with Th17 and Th22 responses, such as S100A proteins, PI3/elafin and IL-23p19 (Figure 4).24 Th17/IL-23 axis is up-regulated in AD patients and might have a role in AD development, in line with recent findings in a flaky tail mouse model showing that IL-4 signaling can be regulated by the IL-17 pathway,161 and the up-regulated Th17 responses in early-onset AD in children.114
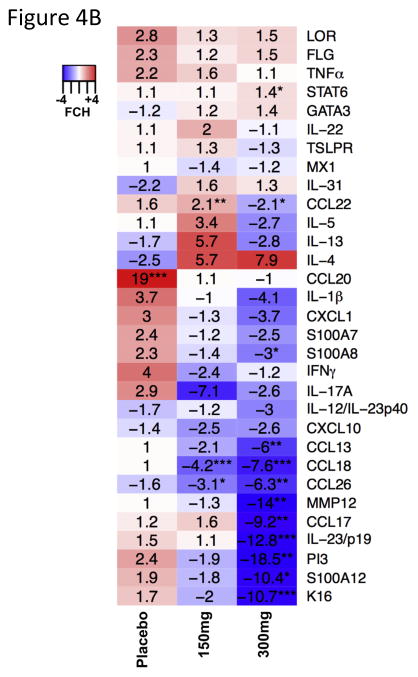
Figure 4.
Effects of dupilumab on lesional AD skin. (A) Schematic representation of pathways influenced by dupilumab treatment. (B) Summary heat map of quantitative RT-PCR mRNA expression changes in placebo, 150mg and 300mg dupilumab after 4 weeks of treatment. Values represent mean fold change (FCH) +/− SEM. *p<0.1, **p<0.05, ***p<0.01. Figure reproduced with permission of publisher from Hamilton et al.24 FLG Filaggrin; K16 Keratin 16; LOR Loricrin; MMP12 Matrix metalloproteinase-12; PI3 Peptidase inhibitor 3; TSLPR: Thymic stromal lymphopoietin receptor.
Ustekinumab is an IL-12/IL-23p40 blocker inhibiting Th1 and Th17/Th22 responses, successfully used for the treatment of moderate-to-severe psoriasis.162 In a small phase II study163 using the FDA-approved psoriasis dosing, ustekinumab had clear and sustained clinical and molecular effects,163 but outcomes (as compared to the “placebo” arm) were likely obscured by the allowed background topical glucocorticosteroid use,21 and waning treatment effects after 8–10 weeks from each ustekinumab administration, suggesting under-dosing of the drug. Interestingly, ustekinumab treatment in AD163 and alopecia areata patients164 induced significant reductions in Th2 axis, in addition to the expected reductions in Th1, Th17 and Th22 axes.
Since Th22 and Tc22 T-cells have been correlated with AD disease severity,32 and the Th22 cytokine, IL-22, is involved in epidermal hyperplasia and barrier defects in AD,32,68 an anti IL-22 treatment might prove to be effective in chronic AD patients. This approach is currently being investigated using the IL22 blocking antibody ILV-094 (NCT01941537). Anti-IL-17 (secukinumab - NCT02594098) treatment is also being explored for AD in both intrinsic and extrinsic AD patients.68
Broader treatment approaches (Figure 2B) that show first, promising results, but need to be verified in larger, controlled studies, include apremilast (anti-phosphodiesterase (PDE)-4),165,166 JAK inhibition,167,168 and H4R antagonists.169
Apremilast, which showed treatment effects in psoriasis170 and is currently being evaluated in a controlled trial in AD (NCT02087943), inhibits PDE-4, thereby increasing the intracellular cAMP levels, which in turn results in a reduction in inflammatory mediators (e.g. IFN-γ, TNF-α, IL-12, IL-17, IL-23), and an increase in anti-inflammatory effects.15 Crisaborole, a topical PDE-4 inhibitor, demonstrated a favorable safety profile and improvement in clinical disease severity in phase III studies, both in children and adults with AD.171
In AD, the JAK-STAT signaling pathway is thought to have multiple effects, including the induction of Th2 polarization and skin barrier disruption,172 the activation of eosinophils and B cell maturation, the upregulation of epidermal chemokines, and the downregulation of AMPs.173 Topical tofacitinib, a JAK1/3-inhibitor, showed promising results in a placebo controlled phase II trial.167 Several oral JAK 1 and 2 inhibitors are now in phase II trials in moderate-to-severe AD patients (baricitinib - NCT02576938; PF-04965842 - NCT02780167).
The histamine H4 receptor has recently been identified to be involved in keratinocyte proliferation174 in patients with AD. ZPL389, a small molecule blocking this receptor, is currently evaluated in clinical trials for psoriasis (NCT02618616) and AD (NCT02424253). In AD, significant improvement of EASI and SCORAD over placebo have been announced in a congress report.8
Outlook
Currently, clinical trials with targeted therapeutics have become key in the advancement of understanding the pathophysiology of this debilitating skin disease. Both successful treatment approaches, as well as failing therapies, have profoundly increased our understanding of AD, and will help to shape future therapies, hopefully at a similar successful pace as seen for psoriasis in the last 15 years.
Acknowledgments
Funding: PMB was supported in part by grant # UL1TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
Abbreviations
- AD
Atopic dermatitis
- AMP
Antimicrobial peptide
- CLA
Cutaneous lymphocyte antigen
- CRTH2
Prostaglandin DP2 receptor
- EASI
Eczema Area and Severity Index
- EDC
Epidermal differentiation complex
- FLG
Filaggrin
- GCS
Glucocorticosteroid
- HBD
Human beta-defensin
- H4R
Histamine H4 receptor
- ILC
Innate lymphoid cells
- IL4R
Interleukin 4 receptor
- JAK
Janus kinase
- NB-UVB
Narrow-band ultraviolet B
- OX40L
OX40 ligand
- PDE
Phosphodiesterase
- PGD2
Prostaglandin D2
- PI3
Peptidase inhibitor 3
- SCORAD
SCORing Atopic Dermatitis
- STAT
Signal Transducer and Activator of Transcription
- TSLP
Thymic stromal lymphopoietin
- TSLPR
Thymic stromal lymphopoietin receptor
Footnotes
Disclosures: EGY is a board member for Sanofi Aventis, Regeneron, Stiefel/GlaxoSmithKline, MedImmune, Celgene, Anacor, AnaptysBio, Celsus, Dermira, Galderma, Glenmark, Novartis, Pfizer, Vitae and Leo Pharma; has received consultancy fees from Regeneron, Sanofi, MedImmune, Celgene, Stiefel/GlaxoSmithKline, Celsus, BMS, Amgen, Drais, AbbVie, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, LEO Pharma, Novartis, Pfizer, Vitae, Mitsubishi Tanabe and Eli Lilly; and has received research support from Janssen, Regeneron, Celgene, BMS, Novartis, Merck, LEO Pharma and Dermira. DYML has received research support from Pfizer, AstraZeneca and MedImmune, is on the advisory board for Celgene and Anacor; and has received consultancy fees from Aimmune Therapeutics and Novartis. PMB declares not to have a relevant conflict of interest.
References
- 1.Hanifin JM, Reed ML, Eczema P Impact Working G. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18:82–91. doi: 10.2310/6620.2007.06034. [DOI] [PubMed] [Google Scholar]
- 2.Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014;69:3–16. doi: 10.1111/all.12270. [DOI] [PubMed] [Google Scholar]
- 3.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–22. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 4.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–37. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107–14. doi: 10.1097/DER.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5:5621. doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- 7.Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203–12. doi: 10.1084/jem.20151093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werfel T, Allam JP, Biedermann T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336–49. doi: 10.1016/j.jaci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Biedermann T, Skabytska Y, Kaesler S, Volz T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front Immunol. 2015;6:353. doi: 10.3389/fimmu.2015.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon D, Wittwer J, Kostylina G, Buettiker U, Simon HU, Yawalkar N. Alefacept (lymphocyte function-associated molecule 3/IgG fusion protein) treatment for atopic eczema. J Allergy Clin Immunol. 2008;122:423–4. doi: 10.1016/j.jaci.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Harper EG, Simpson EL, Takiguchi RH, et al. Efalizumab therapy for atopic dermatitis causes marked increases in circulating effector memory CD4+ T cells that express cutaneous lymphocyte antigen. J Invest Dermatol. 2008;128:1173–81. doi: 10.1038/sj.jid.5701169. [DOI] [PubMed] [Google Scholar]
- 12.Hijnen DJ, ten Berge O, Timmer-de Mik L, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Efficacy and safety of long-term treatment with cyclosporin A for atopic dermatitis. J Eur Acad Dermatol Venereol. 2007;21:85–9. doi: 10.1111/j.1468-3083.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- 13.Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626–34. doi: 10.1016/j.jaci.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tintle S, Shemer A, Suarez-Farinas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583–93. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooderham M, Lynde CW, Papp K, et al. Review of Systemic Treatment Options for Adult Atopic Dermatitis. J Cutan Med Surg. 2016 doi: 10.1177/1203475416670364. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Simon D, Bieber T. Systemic therapy for atopic dermatitis. Allergy. 2014;69:46–55. doi: 10.1111/all.12339. [DOI] [PubMed] [Google Scholar]
- 17.Graham MT, Nadeau KC. Lessons learned from mice and man: mimicking human allergy through mouse models. Clin Immunol. 2014;155:1–16. doi: 10.1016/j.clim.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka A, Amagai Y, Oida K, Matsuda H. Recent findings in mouse models for human atopic dermatitis. Exp Anim. 2012;61:77–84. doi: 10.1538/expanim.61.77. [DOI] [PubMed] [Google Scholar]
- 19.Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324–36. doi: 10.1016/j.jaci.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134:769–79. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner PM, Khattri S, Garcet S, et al. A mild topical steroid leads to progressive anti-inflammatory effects in the skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2016;138:169–78. doi: 10.1016/j.jaci.2015.12.1323. [DOI] [PubMed] [Google Scholar]
- 22.Suarez-Farinas M, Gittler JK, Shemer A, Cardinale I, Krueger JG, Guttman-Yassky E. Residual genomic signature of atopic dermatitis despite clinical resolution with narrow-band UVB. J Allergy Clin Immunol. 2013;131:577–9. doi: 10.1016/j.jaci.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40–52. doi: 10.1016/S0140-6736(15)00388-8. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton JD, Suarez-Farinas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293–300. doi: 10.1016/j.jaci.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–9. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 26.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harden JL, Krueger JG, Bowcock AM. The immunogenetics of Psoriasis: A comprehensive review. J Autoimmun. 2015;64:66–73. doi: 10.1016/j.jaut.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371–9. doi: 10.1016/j.jaip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Oliva M, Renert-Yuval Y, Guttman-Yassky E. The ‘omics’ revolution: redefining the understanding and treatment of allergic skin diseases. Curr Opin Allergy Clin Immunol. 2016;16:469–76. doi: 10.1097/ACI.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 30.Danso MO, van Drongelen V, Mulder A, et al. TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014;134:1941–50. doi: 10.1038/jid.2014.83. [DOI] [PubMed] [Google Scholar]
- 31.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–7. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nograles KE, Zaba LC, Shemer A, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–52. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sa SM, Valdez PA, Wu J, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–40. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 34.Gutowska-Owsiak D, Schaupp AL, Salimi M, et al. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp Dermatol. 2012;21:104–10. doi: 10.1111/j.1600-0625.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 35.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 36.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 37.Hijnen D, De Bruin-Weller M, Oosting B, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell- attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113:334–40. doi: 10.1016/j.jaci.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egawa G, Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J Allergy Clin Immunol. 2016;138:350–8. doi: 10.1016/j.jaci.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Nomura T, Kabashima K. Advances in atopic dermatitis in 2015. J Allergy Clin Immunol. 2016;138:1548–55. doi: 10.1016/j.jaci.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Bruggen MC, Bauer WM, Reininger B, et al. In Situ Mapping of Innate Lymphoid Cells in Human Skin: Evidence for Remarkable Differences between Normal and Inflamed Skin. J Invest Dermatol. 2016;136:2396–405. doi: 10.1016/j.jid.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Bonefeld CM, Geisler C. The role of innate lymphoid cells in healthy and inflamed skin. Immunol Lett. 2016;179:25–8. doi: 10.1016/j.imlet.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novak N, Kruse S, Kraft S, et al. Dichotomic nature of atopic dermatitis reflected by combined analysis of monocyte immunophenotyping and single nucleotide polymorphisms of the interleukin-4/interleukin-13 receptor gene: the dichotomy of extrinsic and intrinsic atopic dermatitis. J Invest Dermatol. 2002;119:870–5. doi: 10.1046/j.1523-1747.2002.00191.x. [DOI] [PubMed] [Google Scholar]
- 46.He JQ, Chan-Yeung M, Becker AB, et al. Genetic variants of the IL13 and IL4 genes and atopic diseases in at-risk children. Genes Immun. 2003;4:385–9. doi: 10.1038/sj.gene.6363985. [DOI] [PubMed] [Google Scholar]
- 47.Lesiak A, Kuna P, Zakrzewski M, et al. Combined occurrence of filaggrin mutations and IL-10 or IL-13 polymorphisms predisposes to atopic dermatitis. Exp Dermatol. 2011;20:491–5. doi: 10.1111/j.1600-0625.2010.01243.x. [DOI] [PubMed] [Google Scholar]
- 48.Namkung JH, Lee JE, Kim E, et al. Association of polymorphisms in genes encoding IL-4, IL-13 and their receptors with atopic dermatitis in a Korean population. Exp Dermatol. 2011;20:915–9. doi: 10.1111/j.1600-0625.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 49.Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001;117:977–83. doi: 10.1046/j.0022-202x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen L, Martinez O, Overbergh L, Mathieu C, Prabhakar BS, Chan LS. Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin Exp Immunol. 2004;138:375–87. doi: 10.1111/j.1365-2249.2004.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129:742–51. doi: 10.1038/jid.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L, Overbergh L, Mathieu C, Chan LS. The development of atopic dermatitis is independent of Immunoglobulin E up-regulation in the K14-IL-4 SKH1 transgenic mouse model. Clin Exp Allergy. 2008;38:1367–80. doi: 10.1111/j.1365-2222.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 53.Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–31. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- 54.Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186–90. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brauweiler AM, Goleva E, Leung DY. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6) J Invest Dermatol. 2014;134:2114–21. doi: 10.1038/jid.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kisich KO, Carspecken CW, Fieve S, Boguniewicz M, Leung DY. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J Allergy Clin Immunol. 2008;122:62–8. doi: 10.1016/j.jaci.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 57.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 58.Niebuhr M, Scharonow H, Gathmann M, Mamerow D, Werfel T. Staphylococcal exotoxins are strong inducers of IL-22: A potential role in atopic dermatitis. J Allergy Clin Immunol. 2010;126:1176–83. e4. doi: 10.1016/j.jaci.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura Y, Oscherwitz J, Cease KB, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasraie S, Niebuhr M, Werfel T. Interleukin (IL)-31 induces pro-inflammatory cytokines in human monocytes and macrophages following stimulation with staphylococcal exotoxins. Allergy. 2010;65:712–21. doi: 10.1111/j.1398-9995.2009.02255.x. [DOI] [PubMed] [Google Scholar]
- 61.Lehmann HS, Heaton T, Mallon D, Holt PG. Staphylococcal enterotoxin-B-mediated stimulation of interleukin-13 production as a potential aetiologic factor in eczema in infants. Int Arch Allergy Immunol. 2004;135:306–12. doi: 10.1159/000082324. [DOI] [PubMed] [Google Scholar]
- 62.Eyerich K, Pennino D, Scarponi C, et al. IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol. 2009;123:59–66. doi: 10.1016/j.jaci.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 63.Howell MD, Gallo RL, Boguniewicz M, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–9. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 65.Albanesi C, Fairchild HR, Madonna S, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–92. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 66.Gittler JK, Shemer A, Suarez-Farinas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhingra N, Guttman-Yassky E. A possible role for IL-17A in establishing Th2 inflammation in murine models of atopic dermatitis. J Invest Dermatol. 2014;134:2071–4. doi: 10.1038/jid.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mansouri Y, Guttman-Yassky E. Immune Pathways in Atopic Dermatitis, and Definition of Biomarkers through Broad and Targeted Therapeutics. J Clin Med. 2015;4:858–73. doi: 10.3390/jcm4050858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thepen T, Langeveld-Wildschut EG, Bihari IC, et al. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol. 1996;97:828–37. doi: 10.1016/s0091-6749(96)80161-8. [DOI] [PubMed] [Google Scholar]
- 71.Rabenhorst A, Hartmann K. Interleukin-31: a novel diagnostic marker of allergic diseases. Curr Allergy Asthma Rep. 2014;14:423. doi: 10.1007/s11882-014-0423-y. [DOI] [PubMed] [Google Scholar]
- 72.Lee CH, Yu HS. Biomarkers for itch and disease severity in atopic dermatitis. Curr Probl Dermatol. 2011;41:136–48. doi: 10.1159/000323307. [DOI] [PubMed] [Google Scholar]
- 73.Guttman-Yassky E, Dhingra N, Leung DY. New era of biologic therapeutics in atopic dermatitis. Expert Opin Biol Ther. 2013;13:549–61. doi: 10.1517/14712598.2013.758708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szegedi K, Kremer AE, Kezic S, et al. Increased frequencies of IL-31-producing T cells are found in chronic atopic dermatitis skin. Exp Dermatol. 2012;21:431–6. doi: 10.1111/j.1600-0625.2012.01487.x. [DOI] [PubMed] [Google Scholar]
- 75.Bieber T. Atopic dermatitis 2. 0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy. 2012;67:1475–82. doi: 10.1111/all.12049. [DOI] [PubMed] [Google Scholar]
- 76.Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58:1–7. doi: 10.1016/j.jdermsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 77.McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol. 2013;131:280–91. doi: 10.1016/j.jaci.2012.12.668. [DOI] [PubMed] [Google Scholar]
- 78.Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254–64. doi: 10.1016/j.jaci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Suarez-Farinas M, Dhingra N, Gittler J, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013;132:361–70. doi: 10.1016/j.jaci.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cole C, Kroboth K, Schurch NJ, et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol. 2014;134:82–91. doi: 10.1016/j.jaci.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malajian D, Guttman-Yassky E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine. 2015;73:311–8. doi: 10.1016/j.cyto.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 82.Kezic S, O’Regan GM, Lutter R, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129:1031–9. doi: 10.1016/j.jaci.2011.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kono M, Nomura T, Ohguchi Y, et al. Comprehensive screening for a complete set of Japanese-population-specific filaggrin gene mutations. Allergy. 2014;69:537–40. doi: 10.1111/all.12369. [DOI] [PubMed] [Google Scholar]
- 84.Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912–7. doi: 10.1016/j.jaci.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akdis CA, Akdis M. Immunological differences between intrinsic and extrinsic types of atopic dermatitis. Clin Exp Allergy. 2003;33:1618–21. doi: 10.1111/j.1365-2222.2003.01803.x. [DOI] [PubMed] [Google Scholar]
- 86.Vachiramon V, Tey HL, Thompson AE, Yosipovitch G. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395–402. doi: 10.1111/j.1525-1470.2012.01740.x. [DOI] [PubMed] [Google Scholar]
- 87.Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48:S139–42. doi: 10.1067/mjd.2003.273. [DOI] [PubMed] [Google Scholar]
- 88.Berardesca E, Pirot F, Singh M, Maibach H. Differences in stratum corneum pH gradient when comparing white Caucasian and black African-American skin. Br J Dermatol. 1998;139:855–7. doi: 10.1046/j.1365-2133.1998.02513.x. [DOI] [PubMed] [Google Scholar]
- 89.Bhattacharya T, Silverberg JI. Efficacy of systemic treatments for atopic dermatitis in racial and ethnic minorities in the United States. JAMA Dermatol. 2014;150:1232–4. doi: 10.1001/jamadermatol.2014.1674. [DOI] [PubMed] [Google Scholar]
- 90.Margolis DJ, Gupta J, Apter AJ, et al. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J Allergy Clin Immunol. 2014;133:784–9. doi: 10.1016/j.jaci.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–38. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 92.Lebwohl M, Strober B, Menter A, et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015;373:1318–28. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 93.Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N Engl J Med. 2016;375:345–56. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 94.Shi B, Bangayan NJ, Curd E, et al. The skin microbiome is different in pediatric versus adult atopic dermatitis. J Allergy Clin Immunol. 2016;138:1233–6. doi: 10.1016/j.jaci.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Velden VH, Laan MP, Baert MR, de Waal Malefyt R, Neijens HJ, Savelkoul HF. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: risk factors and regulatory role of IFN-gamma, IL-4 and IL-10. Clin Exp Allergy. 2001;31:997–1006. doi: 10.1046/j.1365-2222.2001.01176.x. [DOI] [PubMed] [Google Scholar]
- 96.Herberth G, Heinrich J, Roder S, et al. Reduced IFN-gamma- and enhanced IL-4-producing CD4+ cord blood T cells are associated with a higher risk for atopic dermatitis during the first 2 yr of life. Pediatr Allergy Immunol. 2010;21:5–13. doi: 10.1111/j.1399-3038.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 97.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:983–5. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 98.Kaminishi K, Soma Y, Kawa Y, Mizoguchi M. Flow cytometric analysis of IL-4, IL-13 and IFN-gamma expression in peripheral blood mononuclear cells and detection of circulating IL-13 in patients with atopic dermatitis provide evidence for the involvement of type 2 cytokines in the disease. J Dermatol Sci. 2002;29:19–25. doi: 10.1016/s0923-1811(01)00174-8. [DOI] [PubMed] [Google Scholar]
- 99.Kawamoto N, Kaneko H, Takemura M, et al. Age-related changes in intracellular cytokine profiles and Th2 dominance in allergic children. Pediatr Allergy Immunol. 2006;17:125–33. doi: 10.1111/j.1399-3038.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 100.La Grutta S, Richiusa P, Pizzolanti G, et al. CD4(+)IL-13(+) cells in peripheral blood well correlates with the severity of atopic dermatitis in children. Allergy. 2005;60:391–5. doi: 10.1111/j.1398-9995.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 101.Campbell DE, Fryga AS, Bol S, Kemp AS. Intracellular interferon-gamma (IFN-gamma) production in normal children and children with atopic dermatitis. Clin Exp Immunol. 1999;115:377–82. doi: 10.1046/j.1365-2249.1999.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katsunuma T, Kawahara H, Yuki K, Akasawa A, Saito H. Impaired interferon-gamma production in a subset population of severe atopic dermatitis. Int Arch Allergy Immunol. 2004;134:240–7. doi: 10.1159/000078772. [DOI] [PubMed] [Google Scholar]
- 103.Machura E, Mazur B, Kwiecien J, Karczewska K. Intracellular production of IL-2, IL-4, IFN-gamma, and TNF-alpha by peripheral blood CD3+ and CD4+ T cells in children with atopic dermatitis. Eur J Pediatr. 2007;166:789–95. doi: 10.1007/s00431-006-0319-5. [DOI] [PubMed] [Google Scholar]
- 104.Antunez C, Torres MJ, Corzo JL, et al. Different lymphocyte markers and cytokine expression in peripheral blood mononuclear cells in children with acute atopic dermatitis. Allergol Immunopathol (Madr) 2004;32:252–8. doi: 10.1016/s0301-0546(04)79251-4. [DOI] [PubMed] [Google Scholar]
- 105.Leonardi S, Rotolo N, Vitaliti G, Spicuzza L, La Rosa M. IgE values and T-lymphocyte subsets in children with atopic eczema/dermatitis syndrome. Allergy Asthma Proc. 2007;28:529–34. doi: 10.2500/aap2007.28.3038. [DOI] [PubMed] [Google Scholar]
- 106.Antunez C, Torres MJ, Mayorga C, et al. Cytokine production, activation marker, and skin homing receptor in children with atopic dermatitis and bronchial asthma. Pediatr Allergy Immunol. 2006;17:166–74. doi: 10.1111/j.1399-3038.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 107.Chernyshov PV. Expression of activation inducer molecule (CD69) on CD3+CD8+ T lymphocytes in children with atopic dermatitis correlates with SCORAD but not with the age of patients. J Eur Acad Dermatol Venereol. 2009;23:462–3. doi: 10.1111/j.1468-3083.2008.02909.x. [DOI] [PubMed] [Google Scholar]
- 108.Wu KG, Li TH, Chen CJ, Cheng HI, Wang TY. Correlations of serum Interleukin-16, total IgE, eosinophil cationic protein and total eosinophil counts with disease activity in children with atopic dermatitis. Int J Immunopathol Pharmacol. 2011;24:15–23. doi: 10.1177/039463201102400103. [DOI] [PubMed] [Google Scholar]
- 109.Ezzat MH, Hasan ZE, Shaheen KY. Serum measurement of interleukin-31 (IL-31) in paediatric atopic dermatitis: elevated levels correlate with severity scoring. J Eur Acad Dermatol Venereol. 2011;25:334–9. doi: 10.1111/j.1468-3083.2010.03794.x. [DOI] [PubMed] [Google Scholar]
- 110.Nakazato J, Kishida M, Kuroiwa R, Fujiwara J, Shimoda M, Shinomiya N. Serum levels of Th2 chemokines, CCL17, CCL22, and CCL27, were the important markers of severity in infantile atopic dermatitis. Pediatr Allergy Immunol. 2008;19:605–13. doi: 10.1111/j.1399-3038.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 111.Hon KL, Leung TF, Ma KC, Li AM, Wong Y, Fok TF. Serum levels of cutaneous T-cell attracting chemokine (CTACK) as a laboratory marker of the severity of atopic dermatitis in children. Clin Exp Dermatol. 2004;29:293–6. doi: 10.1111/j.1365-2230.2004.01501.x. [DOI] [PubMed] [Google Scholar]
- 112.Leung TF, Ma KC, Hon KL, et al. Serum concentration of macrophage-derived chemokine may be a useful inflammatory marker for assessing severity of atopic dermatitis in infants and young children. Pediatr Allergy Immunol. 2003;14:296–301. doi: 10.1034/j.1399-3038.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 113.Czarnowicki T, Esaki H, Gonzalez J, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol. 2015;136:941–51. doi: 10.1016/j.jaci.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Esaki H, Brunner PM, Renert-Yuval Y, et al. Early onset pediatric atopic dermatitis is Th2, but also Th17 polarized in skin. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.07.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 115.Witte E, Kokolakis G, Witte K, et al. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol. 2014;134:2757–67. doi: 10.1038/jid.2014.308. [DOI] [PubMed] [Google Scholar]
- 116.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 117.Ganguly D, Chamilos G, Lande R, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–94. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dorschner RA, Lin KH, Murakami M, Gallo RL. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res. 2003;53:566–72. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- 119.Iram N, Mildner M, Prior M, et al. Age-related changes in expression and function of Toll-like receptors in human skin. Development. 2012;139:4210–9. doi: 10.1242/dev.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim J, Kim BE, Lee J, et al. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.12.1306. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 121.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA dermatology. 2014;150:593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Silverberg JI. Persistence of childhood eczema into adulthood. JAMA dermatology. 2014;150:591–2. doi: 10.1001/jamadermatol.2013.10267. [DOI] [PubMed] [Google Scholar]
- 123.Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136:208–11. doi: 10.1016/j.jaci.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 124.Czarnowicki T, Gonzalez J, Bonifacio KM, et al. Diverse activation and differentiation of multiple B-cell subsets in patients with atopic dermatitis but not in patients with psoriasis. J Allergy Clin Immunol. 2016;137:118–29. doi: 10.1016/j.jaci.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 125.Alduraywish SA, Lodge CJ, Campbell B, et al. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71:77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 126.Suarez-Farinas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954–64. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deckert S, Kopkow C, Schmitt J. Nonallergic comorbidities of atopic eczema: an overview of systematic reviews. Allergy. 2014;69:37–45. doi: 10.1111/all.12246. [DOI] [PubMed] [Google Scholar]
- 128.Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing Comorbidities Suggest that Atopic Dermatitis Is a Systemic Disorder. J Invest Dermatol. 2017;137:18–25. doi: 10.1016/j.jid.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 129.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy. 2015;70:1300–8. doi: 10.1111/all.12685. [DOI] [PubMed] [Google Scholar]
- 130.Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol. 2015;135:721–8. doi: 10.1016/j.jaci.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 131.Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. 2015;72:606–16. doi: 10.1016/j.jaad.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 132.Silverberg JI, Simpson EL. Association between obesity and eczema prevalence, severity and poorer health in US adolescents. Dermatitis. 2014;25:172–81. doi: 10.1097/DER.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 133.Silverberg JI, Becker L, Kwasny M, Menter A, Cordoro KM, Paller AS. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144–52. doi: 10.1001/jamadermatol.2014.3059. [DOI] [PubMed] [Google Scholar]
- 134.Strom M, Silverberg JI. Associations of Physical Activity and Sedentary Behavior with Atopic Disease in US Children. J Pediatrics. 2016;174:247–253. doi: 10.1016/j.jpeds.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 135.Hjuler KF, Bottcher M, Vestergaard C, et al. Increased Prevalence of Coronary Artery Disease in Severe Psoriasis and Severe Atopic Dermatitis. Am J Med. 2015;128:1325–34. doi: 10.1016/j.amjmed.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 136.Steyers CM, 3rd, Miller FJ., Jr Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–49. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vena GA, Vestita M, Cassano N. Psoriasis and cardiovascular disease. Dermatol Ther. 2010;23:144–51. doi: 10.1111/j.1529-8019.2010.01308.x. [DOI] [PubMed] [Google Scholar]
- 138.Kupetsky EA, Mathers AR, Ferris LK. Anti-cytokine therapy in the treatment of psoriasis. Cytokine. 2013;61:704–12. doi: 10.1016/j.cyto.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 139.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–30. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 140.Kanda N, Watanabe S. Increased serum human beta-defensin-2 levels in atopic dermatitis: relationship to IL-22 and oncostatin M. Immunobiology. 2012;217:436–45. doi: 10.1016/j.imbio.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 141.Leonardi S, Cuppari C, Manti S, et al. Serum interleukin 17, interleukin 23, and interleukin 10 values in children with atopic eczema/dermatitis syndrome (AEDS): association with clinical severity and phenotype. Allergy Asthma Proc. 2015;36:74–81. doi: 10.2500/aap.2015.36.3808. [DOI] [PubMed] [Google Scholar]
- 142.Hayashida S, Uchi H, Takeuchi S, Esaki H, Moroi Y, Furue M. Significant correlation of serum IL-22 levels with CCL17 levels in atopic dermatitis. J Dermatol Sci. 2011;61:78–9. doi: 10.1016/j.jdermsci.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 143.Erbel C, Chen L, Bea F, et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–75. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 144.Griffin GK, Newton G, Tarrio ML, et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–99. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000;105:860–76. doi: 10.1067/mai.2000.106484. [DOI] [PubMed] [Google Scholar]
- 146.Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89–93. doi: 10.1159/000350486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990–8. doi: 10.1111/j.1610-0387.2010.07497.x. [DOI] [PubMed] [Google Scholar]
- 148.Simon D, Braathen LR, Simon HU. Eosinophils and atopic dermatitis. Allergy. 2004;59:561–70. doi: 10.1111/j.1398-9995.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 149.Oldhoff JM, Darsow U, Werfel T, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693–6. doi: 10.1111/j.1398-9995.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 150.Phipps S, Flood-Page P, Menzies-Gow A, Ong YE, Kay AB. Intravenous anti-IL-5 monoclonal antibody reduces eosinophils and tenascin deposition in allergen-challenged human atopic skin. J Invest Dermatol. 2004;122:1406–12. doi: 10.1111/j.0022-202X.2004.22619.x. [DOI] [PubMed] [Google Scholar]
- 151.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016 doi: 10.1056/NEJMoa1610020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 152.Nemoto O, Furue M, Nakagawa H, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2016;174:296–304. doi: 10.1111/bjd.14207. [DOI] [PubMed] [Google Scholar]
- 153.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–44. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 155.Nakajima S, Igyarto BZ, Honda T, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol. 2012;129:1048–55. doi: 10.1016/j.jaci.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–14. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 157.Iwasaki M, Nagata K, Takano S, Takahashi K, Ishii N, Ikezawa Z. Association of a new-type prostaglandin D2 receptor CRTH2 with circulating T helper 2 cells in patients with atopic dermatitis. J Invest Dermatol. 2002;119:609–16. doi: 10.1046/j.1523-1747.2002.01862.x. [DOI] [PubMed] [Google Scholar]
- 158.He R, Oyoshi MK, Wang JY, Hodge MR, Jin H, Geha RS. The prostaglandin D(2) receptor CRTH2 is important for allergic skin inflammation after epicutaneous antigen challenge. J Allergy Clin Immunol. 2010;126:784–90. doi: 10.1016/j.jaci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ulven T, Kostenis E. Novel CRTH2 antagonists: a review of patents from 2006 to 2009. Expert Opin Ther Pat. 2010;20:1505–30. doi: 10.1517/13543776.2010.525506. [DOI] [PubMed] [Google Scholar]
- 160.Cameron L, Depner M, Kormann M, et al. Genetic variation in CRTh2 influences development of allergic phenotypes. Allergy. 2009;64:1478–85. doi: 10.1111/j.1398-9995.2009.02053.x. [DOI] [PubMed] [Google Scholar]
- 161.Nakajima S, Kitoh A, Egawa G, et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J Invest Dermatol. 2014;134:2122–30. doi: 10.1038/jid.2014.51. [DOI] [PubMed] [Google Scholar]
- 162.Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and Safety of Systemic Long-Term Treatments for Moderate-to-Severe Psoriasis: A Systematic Review and Meta-Analysis. J Invest Dermatol. 2015;135:2641–8. doi: 10.1038/jid.2015.206. [DOI] [PubMed] [Google Scholar]
- 163.Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. 2016 doi: 10.1111/exd.13112. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301–4. doi: 10.1016/j.jaci.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 165.Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890–7. doi: 10.1001/archdermatol.2012.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Volf EM, Au SC, Dumont N, Scheinman P, Gottlieb AB. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341–6. [PubMed] [Google Scholar]
- 167.Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: A Phase 2a randomised trial. Br J Dermatol. 2016 doi: 10.1111/bjd.14871. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 168.Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395–9. doi: 10.1016/j.jaad.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 169.De Benedetto A, Yoshida T, Fridy S, Park JE, Kuo IH, Beck LA. Histamine and Skin Barrier: Are Histamine Antagonists Useful for the Prevention or Treatment of Atopic Dermatitis? J Clin Med. 2015;4:741–55. doi: 10.3390/jcm4040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gisondi P, Girolomoni G. Apremilast in the therapy of moderate-to-severe chronic plaque psoriasis. Drug Des Devel Ther. 2016;10:1763–70. doi: 10.2147/DDDT.S108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494–503. e4. doi: 10.1016/j.jaad.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 172.Amano W, Nakajima S, Kunugi H, et al. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J Allergy Clin Immunol. 2015;136:667–77. doi: 10.1016/j.jaci.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 173.Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137. doi: 10.4161/jkst.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Glatzer F, Gschwandtner M, Ehling S, et al. Histamine induces proliferation in keratinocytes from patients with atopic dermatitis through the histamine 4 receptor. J Allergy Clin Immunol. 2013;132:1358–67. doi: 10.1016/j.jaci.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]