ABSTRACT
Interstitial cystitis (IC) is a chronic bladder dysfunction characterized as urinary frequency, urgency, nocturia, and pelvic pain. The changes in urethra may wind up with the bladder changes in structure and functions, however, the functions of the urethra in IC remains elusive. The aim of this study was to understand the perturbed gene expression in urethra, compared with urinary bladder, associated with the defected urodynamics. Using female IC mimic rats, a comprehensive RNA-sequencing combined with a bioinformatics analysis was performed and revealed that IC-specific genes in bladder or urethra. Gene ontology analysis suggested that the cell adhesion or extracellular matrix regulation, intracellular signaling cascade, cardiac muscle tissue development, and second messenger-mediated signaling might be the most enriched cellular processes in IC context. Further study of the effects of these bladder- or urethra-specific genes may suggest underlying mechanism of lower urinary tract function and novel therapeutic strategies against IC.
KEYWORDS: bladder, gene expression, interstitial cystitis, rat model, RNA-Sequencing, urethra
Introduction
Interstitial cystitis (IC) is a chronic noninfectious inflammatory disease of the bladder wall, characterized by recurring discomfort or pain possibly related to the bladder symptoms such as urinary frequency, urgency, nocturia.8 Increasingly clinical important issues include significant negative impacts on the quality of life and social function as well as high prevalence in the general population1,6 Given that IC affects approximately 3.3 million women in the United States, IC is a major health issue largely affecting the quality of everyday life of patients—approximately 50% of those diagnosed with IC have difficulties working full-time, while approximately 70% of patients have trouble sleeping. Additionally, 75% of patients report dyspareunia.15 Multimodality therapy has become a standard treatment of IC, because of no notable responses to therapy as a single agent.7 This suggests that there are multiple underlying factors involved in the etiology of IC, and all of which have a direct impact on the symptom characteristics, disease course and variable responses to therapy. Many causative factors of IC have been documented, however, our current understanding of the causation still clings to the assumption of a leaky urothelium to toxic substances, allergens or bacteria from the urine. This may be the primary step on the inflammatory process of the deeper bladder wall layer.10 Although it is plausible, additional understanding of mechanisms involved in this theoretical mechanism may be required to clarify the mechanisms behind this devastating disease.
Various rodent models for IC study have been introduced to phenotype (a) nociception to bladder distention, (b) pelvic nociception, and/or (c) urinary frequency. IC animal models include the bladder injury rat model that mimics IC in which protamine sulfate (PS) and the endotoxin lipopolysaccharide (LPS) are administered intravesically to Sprague-Dawley rats. PS treatment destroys the bladder glycosaminoglycan (GAGs) layer, leading to enhancement of the LPS action. Animal models including ours bear 3 similarities to currently known pathophysiologic steps of human IC4,28 At first, PS damaged the bladder mucus, and then urothelium was injured by bacterial toxins such as LPS.4 Following these steps, the injured mucus and urothelium are exposed to rat's urine for an additional one month to give enough time for inducing chronic inflammation into the injured bladder wall by the toxins from urine. Our previous findings in this rat model demonstrated the involvement of degranulated mast cells, which was consistent with observation in the human bladder affected with IC. Over 50% of patients have mast cells in lamina propria4,26 Mast cells have been considered to play key pathophysiological roles in the initiation and propagation of inflammation, by the production proinflammatory mediators, neurotrophic factors and immunoregulatory cytokines.25 Mast cells also secrete nerve growth factor (NGF) leading to the neuropathic pain, one of IC characteristics.26
Another hypothesis for the etiology of IC is that an initial insult to the bladder such as urinary tract infection triggers neurogenic inflammation. Several studies have shown that there is an increase in the number and sensitivity of nerve fibers in the bladders of patients with IC19,22 A central role of this inflammation has been suggested in the pathogenesis of interstitial cystitis.9 The etiology of the IC is obscure, and some studies have been attributed to an anatomic problem such as bladder outlet obstruction (BOO) in LUT dysfunction, regardless of sex difference.5 Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a condition that is similar to IC in men.27 Many men who are categorized as having CP/CPPS have urodynamically proven BOO.20 Some evidence shows that the female paraurethral glands in the distal urethra are homologous to the prostate, and the inflammation in these glands may be related to the pathophysiology of IC.28 There may be a wind-up of urethral changes in structure and function with these bladder changes without any clear mechanistic explanation.
The urinary bladder and urethra are the 2 most relevant organs to understand the regulatory mechanism of IC. The normal function of the lower urinary tract is to store and periodically evacuate the urine, which is delicately regulated by the opposing actions of the bladder and the urethra during each phase of storage and voiding. The urethra, a tube through which urine is excreted from the urinary bladder inside the body for elimination to outside the body in urination, allows voluntary control over urination. In males, the urethra carries urine as well as semen. In females, it is much shorter than those in males, and is used only for urination. As the urinary bladder and urethra work together as a functional unit, the changes in bladder function are closely related to those of the urethral function.
In this study, we hypothesized that urethra and urinary bladder have different molecular signatures and regulatory mechanisms in IC patients. In this study using the IC rat model and comparable sham control model, we sought to understand the urethra- or bladder-specific gene signatures and the differences of biologic functions.
Results
The goal of this study was to identify the differentially expressed genes specifically in the bladder or urethra within the IC model, compared with the control. A workflow describing our analytic procedures for the construction of our IC rat model, measurement of cystometric parameters, harvests and extractions of bladder and urethra from animal model, and RNA-Sequencing down in this study were shown in Fig. 1.
Figure 1.
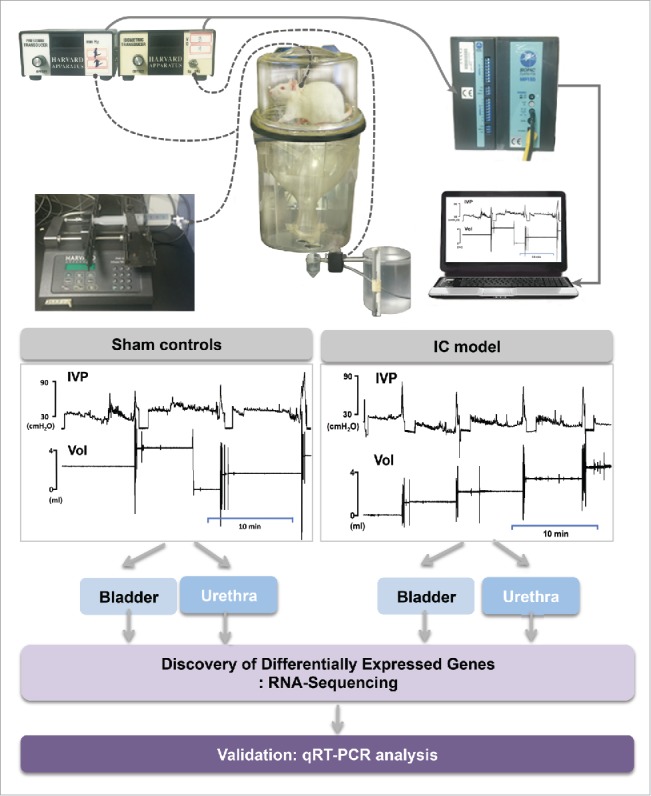
A workflow of this study.
Body weight, bladder weight and ratio were not changed in rat model
First, we wondered if there were any general health issues in IC rats compared with sham control rats. There was no significant difference in the body weight between the sham group (477.6 ± 10.5 g) and the IC group (495.6 ± 13.0 g), one month after intravesical instillations of PS and LPS or saline. The bladder weight did not differ significantly between the sham (0.22 ± 0.01 mg) and IC group (0.24 ± 0.02 mg). Although the bladder weight was normalized to body weight, there was no significant difference between the 2 groups (Sham; 0.47 ± 0.03, IC; 0.49 ± 0.04).
The cystometric parameters between the sham controls and IC rats were assessed
Rats of the IC group did not show any significant difference to sham rats in any pressure parameters - including BP, TP, and MP. However, all volume parameters except the RV (BC, MV and MI) decreased in IC rats comparing to sham, showing the features of IC. Although the value of RV did not show any significance between sham and IC, 57.1% (4 among 7 rats) of rats in IC group showed RV. However, no rats in the sham group showed RV (Table 1 and Fig. 1).
Table 1.
Cystometric parameters in conscious, unrestrained Sprague-Dawley rats in the sham and IC groups. Table 1. Cystometric parameters (including pressure and volume parameters) in awake rats subjected to sham-operation or intravesical PS/LPS-treated rats (IC rats).
| BP (cmH2O) | TP (cmH2O) | MP (cmH2O) | BC (mL) | MV (mL) | RV (mL) | MI (min) | |
|---|---|---|---|---|---|---|---|
| Sham | 16.14 ± 1.16 | 32.16 ± 2.04 | 74.49 ± 5.71 | 1.86 ± 0.18 | 1.86 ± 0.18 | 0 | 9.99 ± 0.91 |
| IC | 18.87 ± 2.89 | 38.10 ± 3.76 | 60.90 ± 4.68 | 1.31 ± 0.14* | 1.17 ± 0.10* | 0.15 ± 0.09 | 6.22 ± 0.83* |
BP, basal pressure; TP, threshold pressure; MP, micturition pressure; BC, bladder capacity; MV, micturition volume; RV, residual volume; MI, micturition interval; IC, interstitial cystitis; Results are expressed as the mean ± standard error. Pressure parameters were expressed by intravesical pressure.
p < 0.05,
RNA-Sequencing analysis identified the differentially expressed genes, which are specific to bladder or urethra
To understand the molecular responses associated with IC, we attempted to perform the next generation RNA sequencing analysis and to get the expression profile of the bladder and urethra in response to PS and LPS stimulation, and those of sham controls. Comparison of RNA-Sequencing status between raw and filtered reads was performed as follows.
Based on raw sequence data, the filtering processes were performed based on the following criteria; (i) position 1 to 15 base were removed because of hexamer-primed 2nd strand synthesis, (ii) reads with mean base quality ≤ 20 and total base quality (≥ 20) ≤ 80% were removed, and (iii) redundant reads (identical sequences) were collapsed into one read. Further calculation of expression level from the finally filtered reads was done as follows: filtered reads were aligned with the UCSC rn5 build of the Rattus-norvegicus genome using the Subread aligner.16 Gene-wise counts were obtained using the featureCounts.17 We compared normalized read count values across samples based on the annotation from UCSC rn5 build.
The differentially expressed genes in bladder and/or urethra in IC model vs. sham control
We identified differentially expressed genes (DEGs) with a false discovery rate (FDR)<0.05. A heatmap shown in Fig. 2A revealed that approximately 3-fold more DEGs were perturbed in urethra in comparison of the bladder. Forty-four DEGs were significantly perturbed in bladder tissues obtained from IC rat model, compared with those from control group (Fig. 2B and Supplementary Table 1A).
Figure 2.
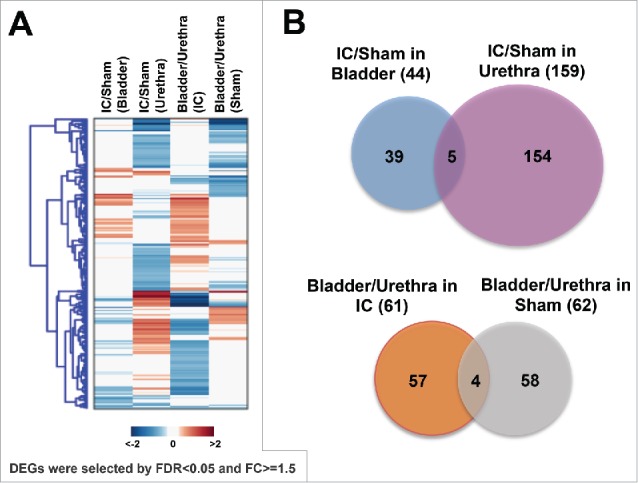
Identification of differentially expressed genes (DEGs) in bladder or urethra obtained from IC rats compared with sham controls. (A) A heatmap showing DEGs (B) Diagrams indicating IC-specific DEGs in bladder or urethra (upper), and bladder- or urethra-specific DEGs associated with IC (bottom).
The gene expression of 159 DEGs was significantly altered in urethras in the IC rat model (Fig. 2B and Supplementary Table 1B). Only 5 genes were commonly altered both in the bladder and urethra, suggesting that the gene expression of bladder and urethra were distinct (Fig. 2B). These 5 genes include collagen type VII α 1 (Col7a1), integrin α 7 (Itga7), Serpin Family A Member 3 (Serpina3n), Solute Carrier Family 25 Member 24 (Slc25a24) and Slit Guidance Ligand 3 (Slit3) (Table 2A). We also found 61 IC-specific (Fig. 2C and Supplementary Table 2A) or 62 Sham-specific genes (Fig. 2C and Supplementary Table 2B). Four commonly perturbed genes in bladder - compared with urethra - included Flavin Containing Monooxygenase 5 (Fmo5), Integrin Subunit α 7 (Itga7), Lymphocyte Cytosolic Protein 1 (Lcp1), and Methyltransferase Like 7B (Mettl7b) (Fig. 2C and Table 2B).
Table 2.
(A) List of 5 common genes in the comparison of IC vs. Sham controls, (B) List of 4 common genes in the comparison of bladder vs. urethra.
| A | |||
|
Symbol |
Description |
IC/Sham (Bladder) |
IC/Sham (Urethra) |
| Col7a1 | collagen, type VII, α 1 | −0.65 | −0.97 |
| Itga7 | integrin, α 7 | 0.81 | −0.6 |
| Serpina3n | serine (or cysteine) peptidase inhibitor, clade A, member 3N | −0.88 | −0.74 |
| Slc25a24 | solute carrier family 25 (mitochondrial carrier, phosphate carrier), member 24 | 0.7 | 1.14 |
|
Slit3 |
slit homolog 3 (Drosophila) |
−0.86 |
0.99 |
| B | |||
|
Symbol |
Description |
Bladder/Urethra (Sham) |
Bladder/Urethra (IC) |
| Fmo5 | flavin containing monooxygenase 5 | 0.77 | 0.99 |
| Itga7 | integrin, α 7 | −0.75 | 0.67 |
| Lcp1 | lymphocyte cytosolic protein 1 | 0.73 | 0.84 |
| Mettl7b | methyltransferase like 7B | −0.84 | 0.76 |
Enriched cellular processes perturbed in IC model and the differentially enriched cellular processes in bladder and urethra
We next attempted to understand the biologic and mechanistic meaning of these DEGs by examining the biologic pathways over-represented by the genes. The comparison between bladder and urethra revealed that responses of bladder or urethra induced IC model by PS and LPS treatment were different. GOBP and KEGG pathway enrichment analysis demonstrated that the altered genes in bladder tissues of IC model were mainly involved in response to disaccharide stimulus, response to sucrose stimulus, and regulation of blood pressure (Blue bars, Fig. 3A). The most enriched cellular processes in urethra in IC model included the response to abiotic stimulus, epithelial cell differentiation, extracellular matrix organization and response to wounding et al. (Orange bars, Fig. 3A). We also found that the cellular processes (e.g., intracellular signaling cascade, cardiac muscle tissue development, and second-messenger-mediated signaling) were enriched in IC-specific DEGs (Green bars, Fig. 3B).
Figure 3.
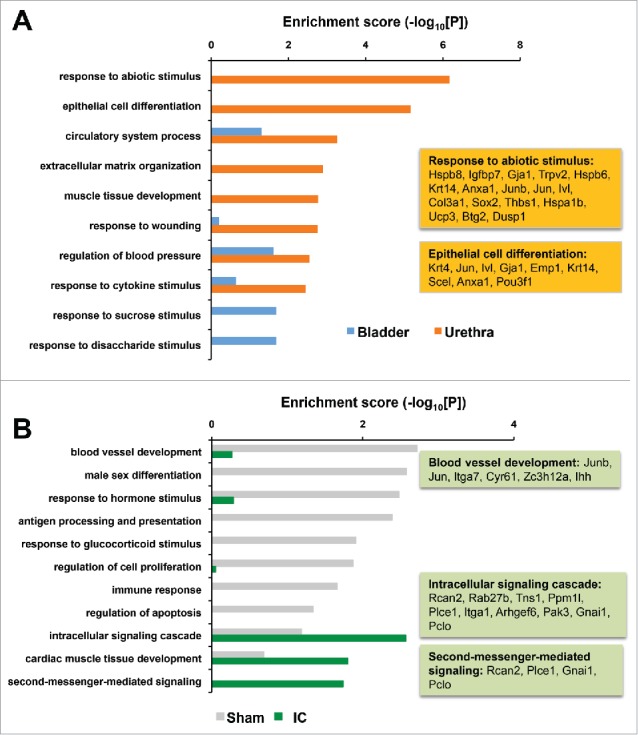
(A) Enriched cellular processes perturbed in IC rats by PS and LPS treatment. Representative DEGs of “response to abiotic stimulus” and “epithelial cell differentiation” were indicated in orange boxes. Blue, bladder specific; Orange, urethra-specific DEGs. (B) Differentially enriched cellular processes changed sham or IC specifically. Gray, sham control specific; Green, IC specific DEGs.
Differentially expressed genes in IC model suggest the bladder specific or urethra specific DEGs
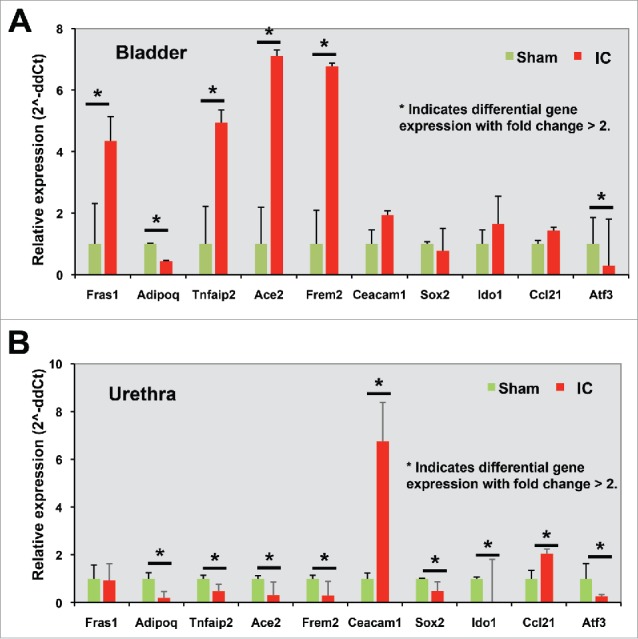
This pathway analysis could allow us to focus on the gene list of the greatest interest. We chose 5 genes whose expression levels were significantly increased in bladder, but not in urethra, of IC model for further validation. They were Fras1 (Fraser syndrome 1), Adipoq (adiponectin, C1Q and collagen domain containing), Tnfaip2 (tumor necrosis factor, α-induced protein 2), Ace2 (angiotensin I converting enzyme 2), and Frem2 (Fras1 related extracellular matrix protein 2). The fold changes of gene expression in IC model, compared with sham control, were presented in Fig. 4A.
Figure 4.
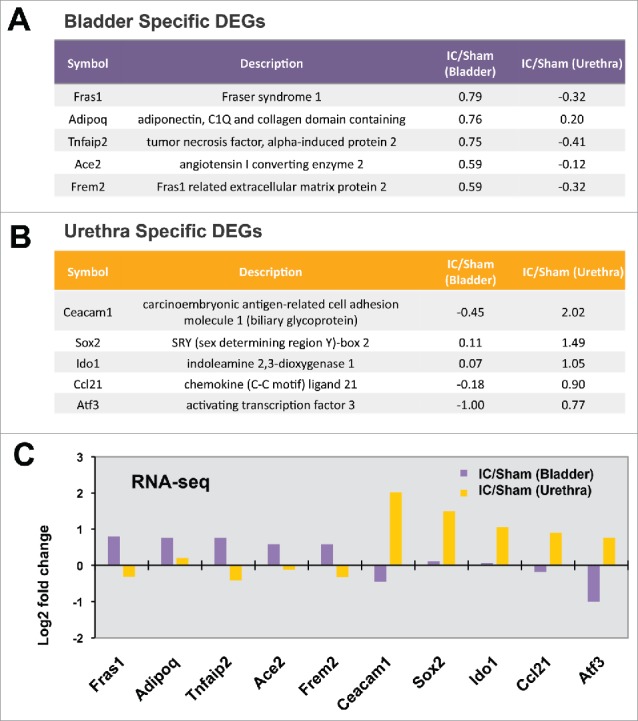
(A) Bladder specific DEGs, (B) Urethra specific DEGs, (C) Expression patterns obtained from RNA-Sequencing data of 10 genes.
Additionally we selected 5 more genes whose expression was significantly increased only in the urethra of IC model. They were Ceacam1 (carcinoembryonic antigen-related cell adhesion molecule 1, biliary glycoprotein), Sox2 (SRY, sex determining region Y)-box 2), Ido1 (indoleamine 2,3-dioxygenase 1), Ccl21 (chemokine (C-C motif) ligand 21), and Atf3 (activating transcription factor 3) (Fig. 4B). Fig. 4C shows the fold changes of 10 genes' gene expression levels in bladder or urethra in IC condition (fold change).
To validate the expression levels and to challenge the importance of these candidates as major modulators of important cellular processes, we performed qRT-PCR analysis using independent bladder (n = 3) or urethra (n = 3) tissues obtained from IC model (n = 3) or sham controls (n = 3). In total the top 10 genes, which were already selected based on our interest and representativeness of organ that they were belonged to, were analyzed (Fig. 5). Consistent with the expression levels quantified in the RNA-sequencing analysis, we found gene expression of Fras1, Tnfaip2, Ace2, and Frem2 were increased in IC model, and expression of Adipoq and activating transcription factor 3 (Atf3) was decreased in IC model, compared with sham controls (Fig. 6A). In the urethra, expression of genes such as Ceacam1, Ido1, and Ccl21 was increased in IC group, while gene expression of Adipoq, Tnfaip2, Ace2, Frem2, SRY, and Atf3 was significantly decreased (Fig. 6B).
Figure 5.
Further validation using qRT-PCR analysis.
Discussion
In this study, we sought to understand the functional mechanism associated with the altered gene expression in our IC rat model. Our global RNA profile using a comprehensive RNA-sequencing analysis identified genes whose expression levels were significantly altered in Sprague-Dawley rats by intravesical instillations of LPS following PS. Animal models are useful tools, in particular to study the symptom-driven human disorders of unknown etiology such as IC.2 Although IC is not an infectious disease, LPS - an endotoxin from E. coli - is commonly used as an acute precipitating factor in animal models of IC.29 Our previous series of animal experiments in Sprague-Dawley rats by intravesical instillations of LPS following PS demonstrated that this chronic rat model exhibited the uninhibited detrusor contractions proved by simultaneous intra-abdominal pressure (detrusor overactivity; DO) during the filling phase and the characteristic degranulatedmast cells in their histologic appearance.4
Our study took a systems approach to overview the enriched biologic pathways of these DEGs, and to identify an IC-regulatory bladder or urethra specific gene set. This approach provides a testable hypothesis that the bladder of IC patients may be different from the urethra of these same patients. We also performed independent biochemical validation experiments, which revealed the 10 most altered genes in bladder vs. urethra. These findings suggest the molecular mechanism in bladder or urethra tissues of IC patients may distinct, leading to the different responses to the therapeutic approaches of IC patients, by regulating downstream gene sets, and the understanding of the difference would benefit the patient treatment.
A central observation of the present study was that chronic IC with similar characteristics to the human disease was induced in rat model by intravesical instillations of LPS following PS.28 All rats in this chronic model except one exhibited degranulated mast cells, which was crucial evidence for the successful establishment of a model resembling the human disease. All rats demonstrated DO during the filling phase. These findings are similar to those of a previous rat model of IC induced by intravesical administration of HCl.13 Previous population-based studies on IC reported a prevalence of urodynamically verified DO ranging from 14–30% in IC patients.
Urine is a liquid-by-product of the body, which is made up of 95 percent water and 5 percent dissolved urinary toxic solutes including urea, chloride, sodium and potassium ions. These poisonous or toxic materials are always subject to invadinginto the bladder wall, which is protected in normal conditions by the tight junction of the umbrella cells and bladder mucus composed of GAGs and proteoglycans. This layer is known as a critical regulator of the bladder's permeability to water and urinary solutes, and is deficient in many patients with IC.23 The lower urinary tract (LUT) function results from simultaneous opposing interactions between the bladder and urethra, such as the bladder relaxation and urethral contraction during storage, and bladder contraction and active urethral relaxation during micturition. These continuously changing intravesical environments make the bladder wall more susceptible to these toxins. If there were an initial insult such like the LPS, the toxic urine results in urothelial hyperplasia and alterations of the LUT functions, forming a vicious cycle. According to the urodynamic study, obstruction is common in male or female patients with IC, which was postulated to be primarily due to pelvic floor dysfunction3,24 This may be caused by the bladder wall inflammation. However, these alterations that affect these interactions and result in voiding dysfunction in IC patients have not yet been fully elucidated.
Among 10 identified IC-specific DEGs from our female IC rats, we found that at least 3 of the DEGs (Fras1, Frem2, and Ceacam1) were related to the cell adhesion or extracellular matrix regulation. Further GO analysis suggested that the intracellular signaling cascade, cardiac muscle tissue development, and second messenger-mediated signaling were the most enriched cellular processes in bladder and urethra of IC group. Experimental data from quantitative RT-PCR analysis for further validation showed the patterns of gene expression in bladder and urethral cells were quite different. Expression of Tnfaip2, Ace2, and Frem2 was significantly changed but in the opposite way (Fig. 5). These findings strongly suggest that perturbed genes in IC context are significantly different in the bladder and urethra. In contrast, expression of Atf3 and Adipoq was both significantly downregulated both in bladder and urethra of IC rats, compared with sham controls. Although the biologic function of Atf3 in bladder or urethra remain unknown, a previous study suggested that Atf3 as a player involving in the growth of the detrusor muscle and its motor innervation following infravesical outlet obstruction,30 suggesting the possibility that Atf3 downregulation may cause bladder dysfunction observed in IC. Adiponectin, an adipose tissue-secreted adipocytokine, was reported to associate with bladder contraction, through PKCα signaling pathway and calcium sensitivity. Downregulation of Adipoq found in our study suggests a potential functional link to bladder contraction allowing proper voiding pattern - although this speculation should be tested in future work. We also found Ccl21 was significantly increased only in urethra, not in bladder tissues of IC rats. A previous study using 15 women with IC and 15 control subjects with stress urinary incontinence without bladder pain suggested that Ccl21 may be correlated significantly with clinical outcomes through an increased nociceptive signaling by Ccl21.21
In summary, we have identified the perturbed gene expression associated with the defected urodynamics in IC mimic animal model, thereby exhibiting the dysfunction of the urinary bladder. A comprehensive analysis based on the next generation RNA-sequencing method combined with a bioinformatics analysis was performed. Our experimental results revealed that the perturbed alterations in gene expression in bladder and urethra are distinct, along with different structure and anatomy, are functionally associated with extracellular matrix organization, wound healing, intracellular signaling cascade and second-messenger-mediated signaling. Further study of the effects on lower urinary tract function may suggest novel therapeutic strategies.
Materials and methods
Animals and study design
A total of 13 female Sprague-Dawley rats (Orient Bio Inc., Gyeonggi-do, South Korea), weighing 200–250 g, were used in the present study. In 7 rats, LPS was instilled intravesically, following the intravesical administration of PS. In 6 rats, the saline was instilled into the bladder and served as the sham group. To avoid potential leak of drugs during instillation of PS and LPS to the bladder, which might cause epithelial damage and subsequent inflammation, we were careful not to overflow the capacity of the individual bladders while the rats were anesthetized. In addition, the drugs were injected to ensure that the fluid does not flow to the urethra and were injected only into the bladder dome. The normal urethra is closed during the storage phase and anesthesia.
Continuous cystometry was performed in all of the rats under awake conditions, one month following intravesical instillation of LPS or saline. After cystometry, rats were killed by cervical dislocation. Following laparotomy, the bladder and urethra were obtained en bloc from all rats, separated at the level of the bladder neck, and the bladder was weighed. All experimental animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA) and were approved by the INHA Institutional Animal Care and Use Committee at the Inha University Medical School (Incheon, South Korea; approval ID: INHA 140731–321–1). The rats were maintained under a 12-h light:dark photoperiod and normal laboratory conditions, with free access to food pellets and tap water except during the experiments.
Surgical procedures
The rats were anesthetized with ketamine (Ketamine; Yuhan Corp., Seoul, Korea; 75 mg kg−1 intraperitoneally) and xylazine (Rompun; Bayer Korea Corp, Seoul, Korea; 15 mg kg−1 intraperitoneally) mixture, during the surgical procedures. Through a lower abdominal midline incision, the bladder and the proximal urethra were approached.
Induction of cystitis
Cystitis was induced by the intravesical instillation of LPS following PS, as described previously.28 Briefly, a 31 gauge needle attached to a syringe (Insulin syringe, SUNGSHIM MEDICAL CO., LTD, Gyeonggi-do, Korea) was inserted into the bladder dome, after the bladder was exposed. The bladder was then emptied by aspiration of urine, and then an appropriate volume of PS (10 mg ml−1) was instilled into the bladder. Twenty minutes later, the bladder was emptied, washed with phosphate-buffered saline (PBS) and then filled with the same volume of LPS (750 μg/ml) for another 20 min. The sham group was instilled with normal saline of the same volume.
Procedures for intra-vesicalcatheter implantation
Three days before cystometry, the catheterization for intravesical pressure (IVP) recordings was done, as described previously12,14 Briefly, after the bladder exposed, a polyethylene catheter (PE-50; Becton-Dickinson, Parsippany, NJ, USA) with a cuff was inserted into the dome of the bladder and held in place with a purse-string suture to record IVP. The catheter was tunneled through the subcutaneous space, exited through the back of the animals and anchored to the skin of the back with a silk ligature. The free end of the catheter was sealed. After surgery, the animals were caged individually and maintained in the same manner.
Functional evaluation
Cystometrograms were performed under unanesthetized, unrestrained conditions in metabolic cages. The external portion of the catheter, implanted into the bladder of the rat, was connected to a 2-way valve that was connected via a T-tube to a pressure transducer (Research Grade Blood Pressure Transducer; Harvard Apparatus, Holliston, MA, USA) and a microinjection pump (PHD22/2000 pump; Harvard Apparatus). This was used to record the IVP on the condition of continuous injection. Room-temperature saline was infused into the bladder by microinjection pump at a rate of 10 ml h−1. The micturition volume (MV) was recorded by means of a fluid collector connected to a force displacement transducer (Research Grade Isometric Transducer; Harvard Apparatus). IVP and MV were continuously recorded using Acq Knowledge 3.8.1 software and an MP150 data acquisition system (Biopac Systems, Goleta, CA, USA) at a sampling rate of 50 Hz. The mean values from 3 reproducible micturition cycles were used for evaluation of cystometric parameters.
Investigation of cystometric parameters
Cystometric parameters consisted of pressure and volume parameters of the model, including the lowest bladder pressure during filling phase (BP), bladder pressure immediately before micturition (TP), maximum bladder pressure during the micturition phase (MP), MV, remaining urine after micturition (RV), MV+RV (BC) and intervals between maximum micturition contractions (MI).
Next generation RNA-Sequencing analysis
RNA-Sequencing analysis identified the differentially expressed genes, which are specific to the bladder or urethra. To understand the molecular responses associated with IC, we attempted to perform the next generation RNA sequencing analysis and to get the expression profile of bladder and urethra in response to PS and LPS stimulation, and also those of sham controls. Comparison of RNA-Sequencing status between raw and filtered reads was performed as follows.
Bladder (n = 3) and urethra tissues (n = 3) were harvested from Sham controls (n = 6 in total) or IC rat model (n = 6 in total), and total RNA from the urine sediments were purified using the miRNeasy mini kit according to the manufacture's instruction (Qiagen). Concentration and yield of RNA samples was determined using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies). RNA integrity was determined by analysis on an Agilent 2100 Bioanalyzer (Agilent Technologies) following the manufacturer's recommendations. Only samples with a RIN score greater than 7.0 were used for the subsequent molecular analysis. Nugen Ovation RNA-Seq System V2 kit was used to generate the double-stranded cDNA using a mixture of random and poly (T) priming. cDNA library was prepared using commercial kits and following manufacturer's protocols. Kapa LTP library kit was used to make the sequencing library. The workflow consists of fragmentation of double stranded cDNA, end repair to generate blunt ends, A-tailing, adaptor ligation and PCR amplification Different adaptors were used for multiplexing samples in one lane. Sequencing was performed on Illumina NextSeq 500 for a single read 75 run. Data quality check was done on Illumina SAV. Demultiplexing was performed with Illumina CASAVA 1.8.2.
The quality of sequence reads was assessed using the FastQC tool (Babraham Bioinformatics, Cambridge, UK). The few low-quality bases were trimmed from read extremities using ShortRead (version 1.30.0) package from R bioconductor (version 3.3). More than 30 × 106 reads were generated for each replicate and were aligned with the UCSC rn5 build of the Rattusnorvegicus genome, through the use of the Subread aligner.16 Gene-wise counts were obtained with the featureCounts program.17 Genes were filtered out and excluded from downstream analysis if they failed to achieve raw read counts of at least 2 across all the libraries. DESeq218 (version 1.6.1) was used for calculating normalized data count data by regularized log transformation and conducting differential gene expression analyses. Differentially expressed genes (DEGs) were determined with false discovery rate (FDR) < 0.05 and fold change > = 1.5. If certain conditions didn't have replicate to estimate dispersion of the IC model versus Sham control in Urethra tissue and IC Bladder vs. IC Urethra, DEGs were determined by only using fold change of more than 1.5 folds due to no replicate in the IC model from Urethra tissue. Finally, to identify cellular processes represented by the DEGs, the enrichment analysis was performed using the DAVID software.11 Specifically enriched cellular processes between up- and downregulation was selected with enrichment P < 0.05. Bar graphs were used to represent the level of significance of each cellular process with enrichment score (−log10 [P]).
Reverse transcription and PCR analysis
Total RNA was separately purified from bladder (n = 3) or urethra tissues (n = 3) from the Sham controls (n = 6 in total) and IC model rates (n = 6 in total) using a QiagenRNEasy tissue extraction kit (Qiagen Inc., Valencia, California). RNA concentration was measured using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Willmington, DE). Primers for RT-PCR were designed based on sequence information suggested from RNA-sequencing data.
Statistical analysis
All results were analyzed using SigmaStat 2.0 (SPSS Inc., Chicago, USA). The results are presented as the mean values ± standard error of the mean. The Shapiro-Wilk W-test confirmed normal distributions. Statistical significance was determined by paired or unpaired t-tests. Unpaired t-tests were used to determine the statistical significance to detect differences in urodynamic parameters and histological data between the sham and IC groups. For multiple comparisons, one-way analysis of variance with Tukey's test was used to detect differences. Statistical significance was considered at p < 0.05. All calculations were made on the basis of n, which denoted the number of animals.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B03932278 (to T.L)) and National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2014R1A5A2009392 (to C-S.P., B-H.C.)). The authors acknowledge support from National Institutes of Health grants (1U01DK103260, 1R01DK100974, U24 DK097154, NIH NCATS UCLA CTSI UL1TR000124 (to J.K.)), Department of Defense grants ((W81XWH-15–1–0415) (to J.K.)), Centers for Disease Controls and Prevention (1U01DP006079 (to J.K.)), IMAGINE NO IC Research Grant, the Steven Spielberg Discovery Fund in Prostate Cancer Research Career Development Award, and US-Egypt Science and Technology Development Fund by the National Academies of Sciences, Engineering, and Medicine. J.K. is former recipient of Interstitial Cystitis Association Pilot Grant, a Fishbein Family IC Research Grant, New York Academy of Medicine, and Boston Children's Hospital Faculty Development. The funders had no role in the design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
JK, TL and SK designed the study, led obtaining funding, and overviewed the literature analysis and drafting the manuscript. BHC and CSP performed animal experiments. YJK, EC, and TDP performed data analysis and organized sample collection. SY performed the analysis of gene expression data. SK, YJK, and TDP assisted writing the manuscript. All authors read and approved the final manuscript.
References
- [1].Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011; 186:540-44; PMID:21683389; http://dx.doi.org/ 10.1016/j.juro.2011.03.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol Urodyn 2011; 30:673-82; PMID:21661012; http://dx.doi.org/ 10.1002/nau.21078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cameron AP, Gajewski JB. Bladder outlet obstruction in painful bladder syndrome/interstitial cystitis. Neurourol Urodyn 2009; 28:944-48; PMID:19301413; http://dx.doi.org/ 10.1002/nau.20729 [DOI] [PubMed] [Google Scholar]
- [4].Choi BH, Jin LH, Kim KH, Han JY, Kang JH, Yoon SM, Park CS, Lee T. Mast cell activation and response to tolterodine in the rat urinary bladder in a chronic model of intravesical protamine sulfate and bacterial endotoxin-induced cystitis. Mol Med Rep 2014; 10:670-76; PMID:24859757; http://dx.doi.org/ 10.3892/mmr.2014.2262 [DOI] [PubMed] [Google Scholar]
- [5].Dmochowski RR. Bladder outlet obstruction: Etiology and evaluation. Rev Urol 2005; 7(Suppl 6):S3-S13; PMID:16986027 [PMC free article] [PubMed] [Google Scholar]
- [6].El Khoudary SR, Talbott EO, Bromberger JT, Chang CC, Songer TJ, Davis EL. Severity of interstitial cystitis symptoms and quality of life in female patients. Journal Women's Health 2009; 18:1361-68; PMID:19743907; http://dx.doi.org/ 10.1089/jwh.2008.1270 [DOI] [PubMed] [Google Scholar]
- [7].Evans RJ. Treatment approaches for interstitial cystitis: Multimodality therapy. Rev Urol 2002; 4(Suppl 1):S16-20; PMID:16986029 [PMC free article] [PubMed] [Google Scholar]
- [8].Gillenwater JY, Wein AJ. Summary of the national institute of arthritis, diabetes, digestive and kidney diseases workshop on interstitial cystitis, national institutes of health, Bethesda, Maryland, August 28–29, 1987. J Urol 1988; 140:203-6; PMID:3379688 [DOI] [PubMed] [Google Scholar]
- [9].Grover S, Srivastava A, Lee R, Tewari AK, Te AE. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 2011; 3:19-33; PMID:21789096; http://dx.doi.org/ 10.1177/1756287211398255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hohlbrugger G. Leaky urothelium and/or vesical ischemia enable urinary potassium to cause idiopathic urgency/frequency syndrome and urge incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1996; 7:242-55; PMID:9127181; http://dx.doi.org/ 10.1007/BF01901246 [DOI] [PubMed] [Google Scholar]
- [11].Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44-57; PMID:19131956; http://dx.doi.org/ 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- [12].Jin LH, Park CS, Kim D, Choi BH, Park SH, Yoon SM, Lee T. Flow starting point and voiding mechanisms measured by simultaneous registrations of intravesical, intra-abdominal, and intraurethral pressures in awake rats. Int Neurourol J 2014; 18:68-76; PMID:24987559; http://dx.doi.org/ 10.5213/inj.2014.18.2.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jin LH, Shin HY, Kwon YH, Park CS, Yoon SM, Lee T. Urodynamic findings in an awake chemical cystitis rat model observed by simultaneous registrations of intravesical and intraabdominal pressures. Int Neurourol J 2010; 14:54-60; PMID:21120177; http://dx.doi.org/ 10.5213/inj.2010.14.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim KH, Jung HB, Choi DK, Park GH, Cho ST. Does methylphenidate affect cystometric parameters in spontaneously hypertensive rats? Int Neurourol J 2015; 19:67-73; PMID:26126435; http://dx.doi.org/ 10.5213/inj.2015.19.2.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koziol JA. Epidemiology of interstitial cystitis. Urol Clin North Am 1994; 21:7-20; PMID:8284848 [PubMed] [Google Scholar]
- [16].Liao Y, Smyth GK, Shi W. The subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res 2013; 41:e108; PMID:23558742; http://dx.doi.org/ 10.1093/nar/gkt214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liao Y, Smyth GK, Shi W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014; 30:923-30; PMID:24227677; http://dx.doi.org/ 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- [18].Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15:550; PMID:25516281; http://dx.doi.org/ 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marchand JE, Sant GR, Kream RM. Increased expression of substance P receptor-encoding mRNA in bladder biopsies from patients with interstitial cystitis. Br J Urol 1998; 81:224-28; PMID:9488063; http://dx.doi.org/ 10.1046/j.1464-410X.1998.00507.x [DOI] [PubMed] [Google Scholar]
- [20].Mayo ME, Ross SO, Krieger JN. Few patients with “chronic prostatitis” have significant bladder outlet obstruction. Urology 1998; 52:417-21; PMID:9730453; http://dx.doi.org/ 10.1016/S0090-4295(98)00202-7 [DOI] [PubMed] [Google Scholar]
- [21].Offiah I, Didangelos A, Dawes J, Cartwright R, Khullar V, Bradbury EJ, O'Sullivan S, Williams D, Chessell IP, Pallas K, et al.. The expression of inflammatory mediators in bladder pain syndrome. Eur Urol 2016; 70:283-90; PMID:26965559; http://dx.doi.org/ 10.1016/j.eururo.2016.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol 1995; 75:744-50; PMID:7542136; http://dx.doi.org/ 10.1111/j.1464-410X.1995.tb07384.x [DOI] [PubMed] [Google Scholar]
- [23].Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 2007; 69:9-16; PMID:17462486; http://dx.doi.org/ 10.1016/j.urology.2006.03.084 [DOI] [PubMed] [Google Scholar]
- [24].Payne C. Urodynamics for the evaluation of painful bladder syndrome/interstitial cystitis. J Urol 2010; 184:15-16; PMID:20478598; http://dx.doi.org/ 10.1016/j.juro.2010.04.026 [DOI] [PubMed] [Google Scholar]
- [25].Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: Role in pathophysiology and pathogenesis. Urology 2007; 69:34-40; PMID:17462477; http://dx.doi.org/ 10.1016/j.urology.2006.08.1109 [DOI] [PubMed] [Google Scholar]
- [26].Sant GR, Theoharides TC. The role of the mast cell in interstitial cystitis. Urol Clin North Am 1994; 21:41-53; PMID:8284844 [PubMed] [Google Scholar]
- [27].Schaeffer AJ, Datta NS, Fowler JE Jr., Krieger JN, Litwin MS, Nadler RB, Nickel JC, Pontari MA, Shoskes DA, Zeitlin SI, et al.. Overview summary statement. Diagnosis and management of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Urology 2002; 60:1-4; PMID:12521576 [DOI] [PubMed] [Google Scholar]
- [28].Stein PC, Pham H, Ito T, Parsons CL. Bladder injury model induced in rats by exposure to protamine sulfate followed by bacterial endotoxin. J Urol 1996; 155:1133-38; PMID:8583579; http://dx.doi.org/ 10.1016/S0022-5347(01)66406-1 [DOI] [PubMed] [Google Scholar]
- [29].Uchida K, Samma S, Rinsho K, Warren JR, Oyasu R. Stimulation of epithelial hyperplasia in rat urinary bladder by escherichia coli cystitis. J Urol 1989; 142:1122-26; PMID:2677413 [DOI] [PubMed] [Google Scholar]
- [30].Xu A, Frederiksen H, Kanje M, Uvelius B. Partial urethral obstruction: ATF3 and p-c-Jun are involved in the growth of the detrusor muscle and its motor innervation. Scand J Urol Nephrol 2011; 45:30-38; PMID:20969496; http://dx.doi.org/ 10.3109/00365599.2010.521188 [DOI] [PubMed] [Google Scholar]


