Abstract
Objective:
To evaluate in vivo the dynamics of endogenous dopamine (DA) neurotransmission during migraine ictus with allodynia.
Methods:
We examined 8 episodic migraineurs and 8 healthy controls (HC) using PET with [11C]raclopride. The uptake measure of [11C]raclopride, nondisplaceable binding potential (BPND), would increase when there was a reduction in endogenous DA release. The opposite is true for a decrease in [11C]raclopride BPND. Patients were scanned twice: one PET session was during a spontaneous migraine ictus at rest, followed by a sustained thermal pain threshold (STPT) challenge on the trigeminal region, eliciting an allodynia experience; another was during interictal phase.
Results:
Striatal BPND of [11C]raclopride in migraineurs did not differ from HC. We found a significant increase in [11C]raclopride BPND in the striatum region of migraineurs during both headache attack and allodynia relative to interictal phase. However, when compared to the migraine attack at rest, migraineurs during the STPT challenge had a significant sudden reduction in [11C]raclopride BPND in the insula. Such directional change was also observed in the caudate of HC relative to the interictal phase during challenge. Furthermore, ictal changes in [11C]raclopride BPND in migraineurs at rest were positively correlated with the chronicity of migraine attacks, and negatively correlated with the frequency during challenge.
Conclusions:
Our findings demonstrate that there is an imbalanced uptake of [11C]raclopride during the headache attack and ictal allodynia, which indicates reduction and fluctuation in ictal endogenous DA release in migraineurs. Moreover, the longer the history and recurrence of migraine attacks, the lower the ictal endogenous DA release.
Globally, migraine has an estimated prevalence of 14.7%.1 Considering the headache attack (ictal) phase alone, migraine is universally ranked seventh among specific causes of disability due to the severe pain and increased sensitivity to innocuous stimuli from the environment, including allodynia.2 Ultimately, these symptoms force patients' isolation until the attack resolves. Recently, MRI-based studies have revealed, in migraineurs, functional and structural changes in brain regions that are not commonly associated with the sensory-discriminative processing of pain,3 including sensory cortex and insula.4 One of these regions is the basal ganglia (BG), especially the striatum,5 where dopamine (DA) plays a crucial role in multiple tasks, for instance pain modulation. Interestingly, migraine has been associated with a higher prevalence of DA-deficient disorders including Parkinson disease and restless legs syndrome.6,7 Yet, in emergency care departments, DA receptor antagonists are commonly prescribed to provide relief to children and adults during attacks.8,9 DA function in acute migraine and treatment in vivo are unknown. In addition, MRI-based10 and animal studies11,12 have not revealed the central dopaminergic mechanisms of the migraine attack experience in humans.
Here, we studied episodic migraineurs during a non-drug-induced spontaneous migraine attack (ictal phase) and during a nonheadache phase (interictal) using PET with [11C]raclopride, a selective radiotracer for DA D2/D3 receptors, mostly in the striatal regions.13,14 We hypothesized that patients with episodic migraine would have an imbalance in DA release, based on the uptake of [11C]raclopride levels, during the headache attack and allodynia in vivo.
METHODS
Standard protocol approvals, registrations, and patient consents.
University of Michigan institutional review board approval (HUM00027383) was obtained. All patients provided written informed consent before enrollment.
Participants and protocol.
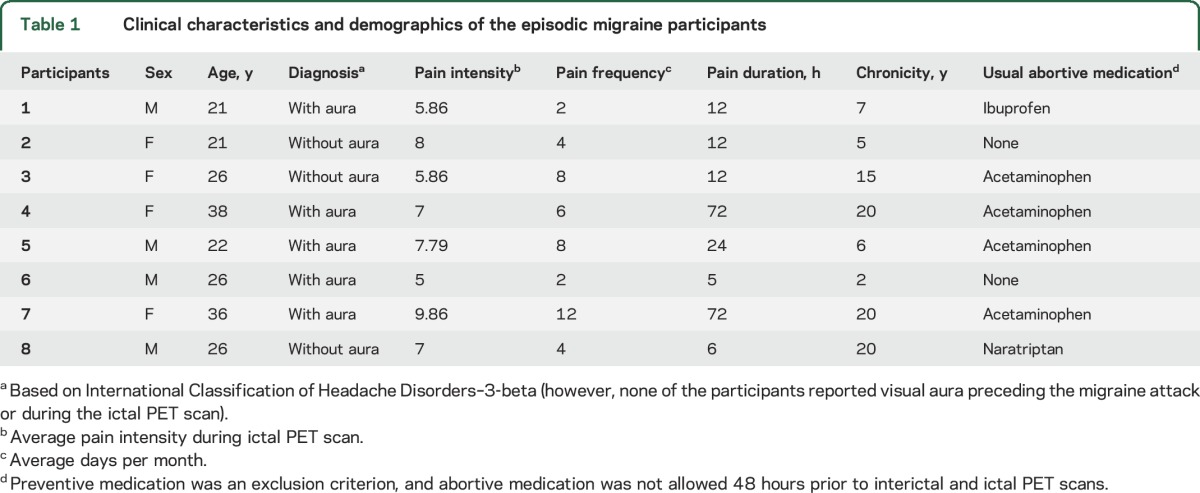
Eight patients with episodic migraine, diagnosed according to the International Classification of Headache Disorders (ICHD-3-beta),15 were enrolled in the study with 8 age- and sex-matched healthy controls (HC). After an initial phone prescreening, and screening appointment, written informed consent was obtained, followed by full evaluation by a pain specialist to confirm the diagnosis. The clinical characteristics of migraineurs and allodynia are summarized in table 1. Patients were excluded in case of pregnancy, current use of preventive migraine medications, opiate or hormonal contraceptive use in the 6 months prior to enrollment, or presence of comorbid chronic pain or other neurologic disorders. The protocol consisted of 1 screening appointment, 1 MRI session, and 2 PET sessions. One PET scan occurred during the interictal phase, and another during the ictal phase, in which participants were instructed to tolerate all headache symptoms without abortive medication until the scan was completed.
Table 1.
Clinical characteristics and demographics of the episodic migraine participants
PET sessions required participants to be headache-free for at least 48 hours prior to the scan time, in addition to having abstained from abortive medications. All PET appointments were scheduled in advance; however, for the ictal PET scan, patients were contacted by phone the morning of the session by the pain specialist to confirm the presence of a spontaneous migraine attack (ICHD-3-beta15). If a migraine attack was absent, the PET session was rescheduled. For female participants, PET sessions were scheduled during 2 separate mid-late follicular phases to assure similar hormonal status. During each step of the protocol, participants recorded their headache pain intensity and area of pain (described elsewhere16) on a mobile application developed in-house (GeoPain; MoxyTech LLC).
Neuroimaging acquisition.
Both 90-minute PET scans with [11C]raclopride were acquired with a Siemens (Knoxville, TN) HR+ scanner in 3D mode (reconstructed full-width at half maximum resolution of ∼5.5 mm in-plane and 5.0 mm axially) with septa retracted and scatter correction. Radiotracer synthesis was done using a cyclotron in the vicinity. Each [11C]raclopride dose of 15 ± 1 mCi (555 ± 37 MBq), ≤50 μg, was administered 50% as a bolus with the remainder continuously infused throughout of the scan using a computer-controlled pump to achieve steady-state tracer levels approximately 30 minutes after tracer administration. A light forehead restraint was used to eliminate head movement. Twenty-one sets of scans were acquired over 90 minutes with an increasing duration (30 seconds up to 10 minutes). During the PET early phase (30–40 minutes), participants were resting without challenge, which was followed by a sustained thermal pain threshold (STPT) challenge during the late phase (60–90 minutes).
Axial T1-weighted anatomic MRI scans were acquired on a 3T scanner (General Electric, Milwaukee, WI) using fast spoiled gradient recalled echo, echo time 3.4, repetition time 10.5, inversion time 200, flip angle 25°, field of view 24 cm, 1.5-mm-thick slices, number of excitations 1, and acquisition matrix 256 × 256, 60 slices.
STPT challenge: PET.
This challenge, developed in-house,17 was applied on the trigeminal ophthalmic region to test thermal sensitivity and cutaneous allodynia during the late phase of the PET session. A 16 mm2 thermal probe (Pathway model; MEDOC, Ramat Yishai, Israel) was applied to the forehead area (V1) ipsilateral to the headache and, starting from a baseline of 32°C, multiple ascending heat cycles occurred at a constant rate (increasing 1°C/s). The participant controlled the heat intensity, as participants were instructed to click a mouse at the first perception of pain to instantly return temperature to baseline level. The heat cycles recurred every 10 seconds for 20 minutes (40–60 minutes post [11C]raclopride injection) during PET, and multiple measurements were recorded to provide the average pain threshold of each PET.
Neuroimaging preprocessing and analysis.
All image data were analyzed from 30 to 40 minutes post tracer administration for the binding potential changes related to the resting state, and 60–90 minutes for the STPT analysis. For each participant, the MRI scans were linearly coregistered to the PET images using a mutual information algorithm,18 and then warped to Montreal Neurological Institute (MNI) International Consortium of Brain Mapping space using DARTEL. The PET images were normalized into MNI space with the resulting deformation parameters, resampled to 2-mm voxels, and smoothed with a 3-3-2 mm Gaussian kernel. Image orientation for participant 1 was flipped after preprocessing with the purpose of matching the migraine location of the remaining participants. Therefore, all participants had right-sided headache for the neuroimaging analyses.
Only regions with specific [11C]raclopride nondisplaceable binding potential (BPND) were included in the analyses (voxels with a distribution volume ratio [DVR] value >1.1 times the mean global image). [11C]Raclopride BPND is an objective measurement in vivo of endogenous D2/D3R availability. No global normalization was applied to the data; therefore, the calculations presented are based on absolute f2Bmax/Kd estimates (or DVR − 1), where f2 is the free fraction of tracer in the nondisplacement tissue compartment, Bmax is the density of available binding sites, and Kd is the affinity constant. DVR was calculated using the Logan method19 and the reference region used was cerebellar gray matter. The resulting parametric maps were produced with voxelwise thresholds at p < 0.01 and with an extent threshold of 288 voxels.
A paired t test between ictal and interictal [11C]raclopride BPND was performed on a voxel-by-voxel basis using the statistical parametric mapping (SPM8) package in MATLAB (MathWorks, Natick, MA). Associations of crude differences of interictal and ictal [11C]raclopride BPND with age, average thermal sensitivity, frequency, and chronicity of migraine attacks were analyzed by linear regression.
In addition, to examine changes in [11C]raclopride BPND between the early (at rest) and late (STPT challenge) phases in the same PET session, paired t tests on voxel and sphere regions were performed. A linear mixed model (LMM) was used to analyze [11C]raclopride BPND change (late − early) across all 3 groups (HC, interictal, and ictal) and areas. The LMM included fixed effects for areas, groups, and their interactions.
RESULTS
The average age of migraineurs (4 F/4 M) was 27 ± 6.45 (mean ± SD) years, with an average migraine chronicity of 11.9 ± 7.66 years, and an average frequency of 5.75 ± 3.45 attacks per month. The average age of the HC was 26 ± 7.17 years. All participants denied the presence of additional migraine attacks 3 days preceding or following the PET scans. The average intensity of the migraine attacks during PET was moderate (7.05 ± 1.53; visual analog scale 1–10) and constant without relief across the early phase, with a total pain area of 40.5 cells (18.4%) out of 220 in the head and facial area, and the summation all rated together of 94.1 (42.78%) (pain area and intensity number summation [PAINS]16) measured by GeoPain. On average, the ictal PET scans for migraineurs occurred 9 hours and 34 minutes (SD 4 hours) after onset of the migraine.
There were no differences between migraineurs and HC in striatal [11C]raclopride BPND at rest or during challenge.
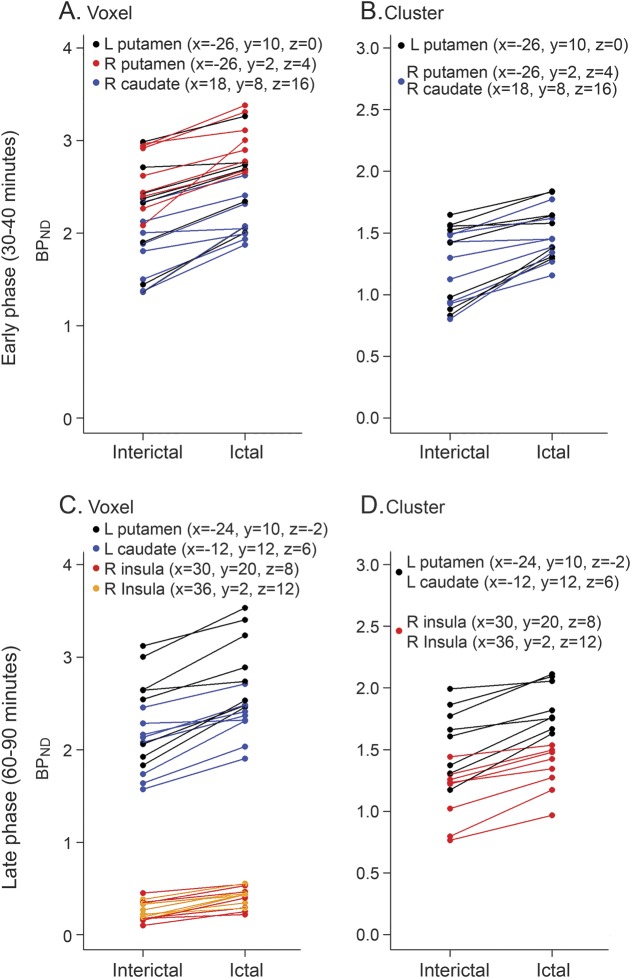
During early phase analyses (at rest), a paired t test between ictal and interictal migraine phases showed higher [11C]raclopride BPND during the ictal phase compared to the interictal phase at rest (figure 1 and table e-1 at Neurology.org). This was observed both on voxel (figure 1A) and cluster (figure 1B) levels (the anatomical locations of the findings are represented as ipsilateral or contralateral to the side of the headache). The contralateral putamen presented an increase of [11C]raclopride BPND (p < 0.001 and p = 0.01 for voxel and cluster level, respectively). The ipsilateral putamen and ipsilateral caudate also presented higher [11C]raclopride BPND during the ictal phase at rest (p = 0.001 and p = 0.003 for voxel and cluster level, respectively, for both areas). At cluster levels, [11C]raclopride BPND in the ipsilateral putamen during the ictal phase at rest was on average 24% higher in comparison to the interictal phase. The ictal [11C]raclopride BPND changes (ictal − interictal) observed at rest in the ipsilateral striatum region at the sphere level were positively associated with longer chronicity (number of years) of the migraine attacks (p = 0.047, figure e-1A).
Figure 1. Striatal [11C]raclopride nondisplaceable binding potential (BPND) scatterplots for the early (30–40 minutes) and late phase (60–90 minutes) analyses.
Upper images: During early phase analyses, a paired t test between ictal and interictal migraine phases showed higher [11C]raclopride BPND during the ictal phase compared to the interictal phase both on voxel (A) and cluster (B) levels. Lower images: During late phase analyses, a higher [11C]raclopride BPND was also observed during the ictal compared to the interictal phase with sustained thermal pain threshold (sustained thermal pain threshold challenge) and with ictal spontaneous migraine and allodynia, both on voxel (C) and cluster (D) levels.
During late phase analyses (STPT challenge), a higher [11C]raclopride BPND was also observed during the ictal compared to the interictal phase (figure 1, table e-2). The contralateral putamen and contralateral caudate presented significantly higher [11C]raclopride BPND during ictal compared to interictal phase (p < 0.001 and p = 0.004 for voxel and cluster level, respectively, for both areas). The ipsilateral insula also presented significantly higher [11C]raclopride BPND during the ictal compared to the interictal phase. At cluster levels, [11C]raclopride BPND during the challenge was 18% higher in the contralateral putamen or caudate and 20% higher in the ipsilateral insula when compared to the interictal phase. Changes in [11C]raclopride BPND observed during the challenge in the contralateral striatum region were negatively correlated with the monthly frequency of the migraine attacks at cluster levels (p = 0.016, figure e-1B).
In paired t tests of late − early phase analysis, 9 areas were significant in at least 1 group (table e-3). In LMM analysis of late − early phase, there was evidence of changes in [11C]raclopride BPND between groups (table e-4 and figure e-2) in the ipsilateral insula (area 1; p = 0.030) and bilateral caudate (area 7; p = 0.035, and area 8; p = 0.014). We observed significant decrease in [11C]raclopride BPND during challenge from baseline (at rest) in the ipsilateral insula (area 1) for patients in the ictal state (−27%) relative to interictal (50%; p = 0.009). Such directional change was also observed during challenge in the contralateral caudate (area 8) of HC (−11%) relative to interictal (5%; p = 0.004), but reversed in the ipsilateral caudate (area 7) of HC (1%) relative to interictal (−9%; p = 0.012).
Since the BPND values in insula are low compared to caudate or putamen, changes within individuals have a high degree of uncertainty. Hence, we report in table e-4 the percent change in insula only for the group mean BPND values.
DISCUSSION
Our study compared in vivo the activity of the endogenous DA system in migraineurs during headache-free and during a drug-free spontaneous migraine headache and cutaneous heat allodynia. The analysis of [11C]raclopride BPND revealed no changes in striatal [11C]raclopride BPND between migraineurs and HC, but rather an increase in striatal [11C]raclopride BPND of migraineurs during the ictal phase at rest and during an ictal thermal challenge, relative to their interictal phases, specifically in the bilateral putamen and ipsilateral caudate. This could be interpreted as a reduction of endogenous DA release during a migraine attack at rest and allodynia (figures 2–4). In contrast, intra-analysis of the ictal phase revealed a sudden decrease in [11C]raclopride BPND in the insula (table e-4) during the ictal challenge relative to at rest, which could also be interpreted as a sudden increase of endogenous DA release during migraine-associated heat allodynia.
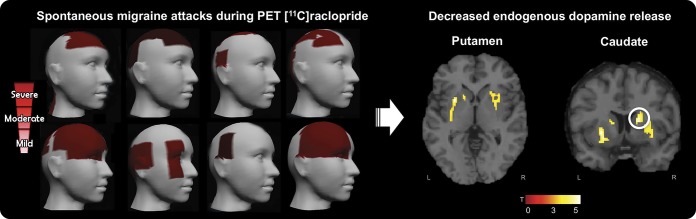
Figure 2. Migraine headache severity and decreased endogenous dopamine release during ictal PET with [11C]raclopride.
Left image: Migraine pain intensity was recorded from the 8 migraine participants during the ictal PET sessions (sequence of participants follows table 1, from top left to lower right). We used a free and interactive mobile application developed in-house (GeoPain; MoxyTech LLC). Headache color scale: mild (visual analog scale [VAS] [1–10] 1–3), moderate (VAS 4–7), and severe (VAS 8–10). Participants 2 and 7 had severe pain. Right image: Decreased endogenous dopamine release in the striatum region during spontaneous migraine headache attack. Montreal Neurological Institute coordinates: contralateral putamen (x = −26, y = 10, z = 0), ipsilateral putamen (x = 26, y = 2, z = 4), and ipsilateral caudate (x = 18, y = 8, z = 16).
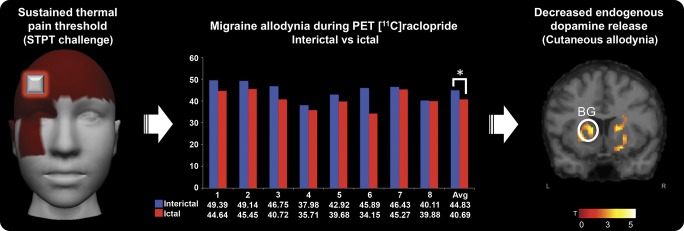
Figure 3. Migraine allodynia during PET with [11C]raclopride and decreased endogenous dopamine release.
Left image: Sustained thermal pain threshold (STPT challenge). Average migraine pain intensity for the 8 migraine participants, and the highlighted area of the application of the STPT challenge during the PET scans. Center: Thermal pain threshold levels (in Celsius) of cutaneous allodynia during PET scan. Average heat levels were statistically significant (*p = 0.01) with a lower threshold for pain during ictal compared to interictal PET scans. Right: Decreased endogenous dopamine release during cutaneous allodynia. Montreal Neurological Institute coordinates: contralateral putamen (x = −24, y = 10, z = −2) and contralateral caudate (x = −12, y = 12, z = 6). BG = basal ganglia.
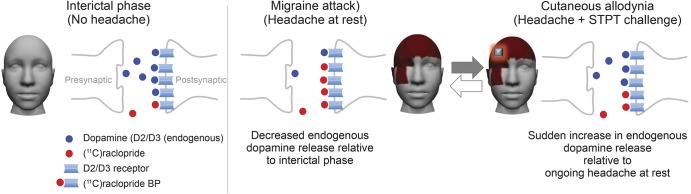
Figure 4. Imbalanced endogenous dopamine release during migraine attack and allodynia in vivo.
Left: Stable levels of endogenous dopamine during interictal phase (without headache). Center: Decreased endogenous dopamine release during spontaneous migraine headache attack (relative to interictal phase) on the striatum region (headache at rest). Right: Sudden increase in endogenous dopamine release during cutaneous allodynia relative to ongoing headache at rest (headache with sustained thermal pain threshold challenge [STPT]). BP = binding potential.
The BG is associated with important aspects of pain processing, including its sensory-discriminative, cognitive, and affective dimensions,20 and DA plays a crucial role in BG function. Migraine has been linked to reduced gray matter in the striatum part of the BG,21 and an elevated frequency of attacks is linked to increased volume and decreased activations in the region during attacks.5 Our findings in the dorsal striatum region, including putamen and caudate, clarify some of the endogenous DA mechanisms in the brains of migraineurs. Because we only observed changes in [11C]raclopride BPND in migraineurs during the ictal phase when compared to their interictal phase, but not when we compared the interictal phase of migraineurs to HC, the dysfunction is more indicative of provisional shift in receptor occupancy by endogenous DA rather than an abrupt change in the number of receptors available in migraineurs.
Given the substantial evidence that striatal DA is an endogenous attenuator of nociception in animals and of pain in humans,22–24 it is reasonable to suggest that the pain experienced during the ictal phase is due, at least in part, to the concurrently reduced striatal DA we detected when compared to the interictal phase. However, reduced DA release alone is not known to be painful, so it is necessary to consider that the reduced endogenous modulation could cause pain by enhancing sensory responses to otherwise innocuous ongoing somatosensory afferent input during the migraine ictus.25 This speculation is supported by the observation that iontophoretically applied DA strongly inhibits the response of rodent trigeminal system neurons to meningeal stimulation.26 There is also evidence that rodent trigeminal medullary dorsal horn neurons are tonically inhibited by a descending dopaminergic pathway acting via D2 type receptors.27
The evidence cited above13–21 shows that DA has hypalgesic effects in humans and in animal experiments, and that the endogenous release of DA is increased, not decreased, by painful stimulation. Although the changes in [11C]raclopride BPND that we observed during headache and allodynia were negatively correlated with the frequency of migraine attacks, the baseline level of interictal DA activity was not different from that of HC. Moreover, there is no clear correlation of the duration or intensity of chronic pain with DA activity among patients with a variety of chronically painful conditions. The weight of current evidence is that, overall, patients with chronic pain may have normal, decreased, or increased striatal DA activity at baseline and their DA activation responses to noxious stimulation are highly variable.28
Given the complex relationship of DA activity to migraine pathophysiology,29 additional studies are required to elucidate how endogenous striatal DA might otherwise affect the ictal pain and other clinical components of migraine. For example, DA participates in the control of motivation, reward/aversion, and salience, so reduced DA activity could lead to the patient's withdrawal and seclusion during pain.11,30,31 The lack of thermal challenge during the early phase of our protocol revealed the reduced DA release uniquely related to the migraine at rest. Although DA release in the striatum region could potentially be affected by a reward mechanism due to relief of the headache intensity while at rest,32 our analysis demonstrated that pain levels in our migraine patients did not change during the ictal PET session at the early phase.
The sudden increased DA release during ictal allodynia in the insula, relative to rest, is consistent with evidence that DA activity is known to modulate the threshold required for a particular stimulus, making it more salient and aversive.33,34 In fact, even patients with DA deficiency disorders can increase DA release as much as HC when confronted with relevant stimuli.35 This phenomenon was observed in our study (figure e-2), where migraineurs, despite their low baseline DA levels during headache attack, had similar sudden DA release response to allodynia experience as HC during challenge relative to at rest in most of the areas. It is noteworthy that migraineurs with cutaneous allodynia more commonly report general sensitivity to other stimuli such as photophobia, phonophobia, and nausea,36 and DA D2/D3 agonists elicit robust emesis in humans. It is also possible that DA receptor hypersensitivity plays a role. Studies indicate that migraineurs more frequently have DA receptor hypersensitivity when compared to HC.29,37 Increased discomfort in migraineurs could result from a hypersensitivity of DA postsynaptic receptor sites due to a chronic deficiency of the physiologic agonist.38 Indeed, receptor hypersensitivity can be induced in animals by persistent interruption of the dopaminergic activity.39 Our results indicate that although migraine patients during headache attacks have lower DA activity compared to the interictal phase, they can suddenly increase or recover DA endogenous neurotransmission when challenged with innocuous stimuli from the environment, worsening even further migraine-associated symptoms and allodynia experience. Interestingly, there is a report on the efficacy of a prophylactic DA agonist in reducing migraine severity after careful modulation of DA hypersensitivity.40 These results combined indicate that migraineurs are susceptible to DA deficiency disorders.6,7 Nonetheless, additional studies are needed to evaluate the implication of our results toward therapeutic recommendations.
This study investigated the endogenous DA D2/D3 system using [11C]raclopride during drug-free spontaneous migraine attacks and allodynia in vivo. Although more studies are needed to confirm our findings, this study demonstrates that there is a transient DA reduction and imbalance in the striatum region and insula during migraine attacks contributing to patients' pain and discomfort, and increasing their global sensory sensitivity and aversive reactions to environmental stimuli.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the PET Center Nuclear Medicine Technologists (Jill M. Rothley, Edward J. McKenna, Andrew R. Weeden, Paul Kison, and Caitlin Hendriks), the Functional MRI Laboratory personnel (Scott Peltier and Keith Newnham), and previous research fellow Dr. Ilkka Martikainen.
GLOSSARY
- BG
basal ganglia
- BPND
nondisplaceable binding potential
- DA
dopamine
- DVR
distribution volume ratio
- HC
healthy control
- ICHD
International Classification of Headache Disorders
- LMM
linear mixed model
- MNI
Montreal Neurological Institute
- STPT
sustained thermal pain threshold
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. DaSilva had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: Dr. DaSilva. Acquisition of data: Drs. DaSilva, Nascimento, DosSantos, and Smith, S. Lucas, and R.L. Toback. Neuroimaging analysis and interpretation of data: Drs. DaSilva and Nascimento, J. Heffernam, Dr. Jassar, D. Lucas, and Drs. Casey, Koeppe, and Zubieta. Biostatistical analysis: E.L. Bellile and Drs. Boonstra and Taylor.
STUDY FUNDING
This study was supported by the following grants (Dr. DaSilva): National Institute of Health–National Institute of Neurological Disorders and Stroke–K23 NS062946, R01 NS094413, Dana Foundation's Brain and Immuno-Imaging Award, and the Migraine Research Foundation Research Grant Award. This study is not industry-sponsored.
DISCLOSURE
A. DaSilva co-created GeoPain (previously named PainTrek) and also co-founded MoxyTech LLC, which licensed the technology from the University of Michigan. T. Nascimento, H. Jassar, J. Heffernan, R. Toback, S. Lucas, M. DosSantos, E. Bellile, P. Boonstra, J. Taylor, K. Casey, R. Koeppe, Y. Smith, and J. Zubieta report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. J Headache Pain 2013;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May A. New insights into headache: an update on functional and structural imaging findings. Nat Rev Neurol 2009;5:199–209. [DOI] [PubMed] [Google Scholar]
- 4.Borsook D, Veggeberg R, Erpelding N, et al. The insula: a “hub of activity” in migraine. Neuroscientist 2016;22:632–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maleki N, Becerra L, Nutile L, et al. Migraine attacks the basal ganglia. Mol Pain 2011;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher AI, Ross GW, Sigurdsson S, et al. Midlife migraine and late-life parkinsonism: AGES-Reykjavik study. Neurology 2014;83:1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervenka S, Palhagen SE, Comley RA, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain 2006;129:2017–2028. [DOI] [PubMed] [Google Scholar]
- 8.Bachur RG, Monuteaux MC, Neuman MI. A comparison of acute treatment regimens for migraine in the emergency department. Pediatrics 2015;135:232–238. [DOI] [PubMed] [Google Scholar]
- 9.Marmura MJ. Use of dopamine antagonists in treatment of migraine. Curr Treat Options Neurol 2012;14:27–35. [DOI] [PubMed] [Google Scholar]
- 10.Yuan K, Zhao L, Cheng P, et al. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain 2013;14:836–844. [DOI] [PubMed] [Google Scholar]
- 11.De Felice M, Eyde N, Dodick D, et al. Capturing the aversive state of cephalic pain preclinically. Ann Neurol 2013;74:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charbit AR, Akerman S, Holland PR, Goadsby PJ. Neurons of the dopaminergic/calcitonin gene-related peptide A11 cell group modulate neuronal firing in the trigeminocervical complex: an electrophysiological and immunohistochemical study. J Neurosci 2009;29:12532–12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GJ, Volkow ND, Fowler JS, et al. Age associated decrements in dopamine D2 receptors in thalamus and in temporal insula of human subjects. Life Sci 1996;59:PL31–PL35. [DOI] [PubMed] [Google Scholar]
- 14.Cervenka S, Varrone A, Fransen E, Halldin C, Farde L. PET studies of D2-receptor binding in striatal and extrastriatal brain regions: biochemical support in vivo for separate dopaminergic systems in humans. Synapse 2010;64:478–485. [DOI] [PubMed] [Google Scholar]
- 15.Headache Classification Committee of the International Headache Society (IHS). The International Classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 16.DaSilva AF, Nascimento TD, DosSantos MF, et al. μ-Opioid activation in the prefrontal cortex in migraine attacks: brief report I. Ann Clin Transl Neurol 2014;1:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nascimento TD, DosSantos MF, Lucas S, et al. μ-Opioid activation in the midbrain during migraine allodynia: brief report II. Ann Clin Transl Neurol 2014;1:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 2001;293:311–315. [DOI] [PubMed] [Google Scholar]
- 19.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16:834–840. [DOI] [PubMed] [Google Scholar]
- 20.Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain 1995;60:3–38. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz N, Admiraal-Behloul F, Arkink EB, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache 2008;48:1044–1055. [DOI] [PubMed] [Google Scholar]
- 22.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother 2008;8:781–797. [DOI] [PubMed] [Google Scholar]
- 23.Potvin S, Grignon S, Marchand S. Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse 2009;63:390–402. [DOI] [PubMed] [Google Scholar]
- 24.Hagelberg N, Jaaskelainen SK, Martikainen IK, et al. Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol 2004;500:187–192. [DOI] [PubMed] [Google Scholar]
- 25.Nathan PW, Noordenbos W, Wall PD. Ongoing activity in peripheral nerve: interactions between electrical stimulation and ongoing activity. Exp Neurol 1973;38:90–98. [DOI] [PubMed] [Google Scholar]
- 26.Bergerot A, Storer RJ, Goadsby PJ. Dopamine inhibits trigeminovascular transmission in the rat. Ann Neurol 2007;61:251–262. [DOI] [PubMed] [Google Scholar]
- 27.Lapirot O, Melin C, Modolo A, et al. Tonic and phasic descending dopaminergic controls of nociceptive transmission in the medullary dorsal horn. Pain 2011;152:1821–1831. [DOI] [PubMed] [Google Scholar]
- 28.Martikainen IK, Nuechterlein EB, Pecina M, et al. Chronic back pain is associated with alterations in dopamine neurotransmission in the ventral striatum. J Neurosci 2015;35:9957–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peroutka SJ. Dopamine and migraine. Neurology 1997;49:650–656. [DOI] [PubMed] [Google Scholar]
- 30.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann NY Acad Sci 2008;1129:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 1995;83:1197–1209. [DOI] [PubMed] [Google Scholar]
- 32.Navratilova E, Atcherley CW, Porreca F. Brain circuits encoding reward from pain relief. Trends Neurosci 2015;38:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakravarthy VS, Joseph D, Bapi RS. What do the basal ganglia do? A modeling perspective. Biol Cybern 2010;103:237–253. [DOI] [PubMed] [Google Scholar]
- 34.Boehme R, Deserno L, Gleich T, et al. Aberrant salience is related to reduced reinforcement learning signals and elevated dopamine synthesis capacity in healthy adults. J Neurosci 2015;35:10103–10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008;79:368–376. [DOI] [PubMed] [Google Scholar]
- 36.Ashkenazi A, Yang I, Mushtaq A, Oshinsky ML. Is phonophobia associated with cutaneous allodynia in migraine? J Neurol Neurosurg Psychiatry 2010;81:1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerbo R, Barbanti P, Buzzi MG, et al. Dopamine hypersensitivity in migraine: role of the apomorphine test. Clin Neuropharmacol 1997;20:36–41. [DOI] [PubMed] [Google Scholar]
- 38.Fanciullacci M, Alessandri M, Del Rosso A. Dopamine involvement in the migraine attack. Funct Neurol 2000;15(suppl 3):171–181. [PubMed] [Google Scholar]
- 39.Kim DS, Szczypka MS, Palmiter RD. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J Neurosci 2000;20:4405–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai M, Loi V, Pisano MR, Del Zompo M. Therapy of migraine by modulating dopamine hypersensitivity: its effect on mood and pain. Int J Clin Pharmacol Res 1997;17:101–103. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.