Abstract
Organophosphate pesticides elicit developmental neurotoxicity through mechanisms over and above their shared property as cholinesterase inhibitors. We compared the consequences of neonatal exposure (postnatal days PN1-4) to diazinon or parathion on development of norepinephrine systems in rat brain, using treatments designed to produce equivalent effects on cholinesterase, straddling the threshold for barely-detectable inhibition. Norepinephrine levels were measured throughout development from the immediate posttreatment period (PN5), to early adolescence (PN30), young adulthood (PN60) and full adulthood (PN100); we assessed multiple brain regions containing all the major noradrenergic synaptic projections. Diazinon elicited a significant overall deficit of norepinephrine, whereas parathion produced a net increase. The effects were not immediately apparent (PN5) but rather emerged over the course of development, indicating that the organophosphate effects represent alteration of the trajectory of development, not just continuance of an initial injury. There were no comparable effects on β-adrenergic receptors, indicating that the presynaptic changes were not an adaptation to an underlying, primary effect on postsynaptic receptor signaling. Because we used the cholinesterase inhibition benchmark, the absolute dose of diazinon was much higher than that of parathion, since the latter is a more potent cholinesterase inhibitor. Our results are consistent with the growing evidence that the various organophosphates can differ in their impact on brain development and that consequently, the cholinesterase benchmark is an inadequate predictor of adverse neurodevelopmental effects.
Keywords: β-Adrenergic receptor, Brain development, Diazinon, Norepinephrine, Organophosphate pesticides, Parathion
INTRODUCTION
It is increasingly clear that the developmental neurotoxicity caused by organophosphate pesticides results from mechanisms other than their shared property as cholinesterase inhibitors (Slotkin, 2005). Consequently, the U.S. Environmental Protection Agency, after earlier restrictions on the use of chlorpyrifos, recently proposed to discontinue all use because of the inadequacy of the cholinesterase benchmark to establish safety standards (U.S. Environmental Protection Agency, 2015). Nevertheless, assigning a specific mechanism or mode of action for the adverse effects of organophosphates on brain development has proven elusive (U.S. Environmental Protection Agency, 2015), as it is likely that multiple pathways contribute to the ability of these compounds to elicit developmental neurotoxicity, with a spectrum of effects that shifts with progressive stages of brain maturation (Slotkin, 2005).
If the developmental neurotoxicity of organophosphates resides in cholinesterase-independent mechanisms, then it is likely that the various members of this pesticide class, which differ in their structural moieties other than the shared phosphothionate residue, could have divergent effects on specific neural circuits. We found that diazinon and parathion differed in their impact on development of serotonin and acetylcholine systems (Slotkin et al., 2006, 2008a, b, 2009b), contributing to disparities in some of their cognitive and emotional anomalies (Levin et al., 2010; Roegge et al., 2008; Timofeeva et al., 2008a, b). In the current study, we extend this comparison to development of norepinephrine systems, which in general have been much less studied for organophosphate effects. Norepinephrine plays critical roles in disorders of mood, attention, learning, memory and autonomic function (Ordway et al., 2007), some of the very same behavioral modalities for which there are divergent effects among the various organophosphates (Levin et al., 2010; Roegge et al., 2008; Timofeeva et al., 2008a, b). In earlier work, we explored the initial effects of chlorpyrifos on development of brain norepinephrine systems and persistence through the end of adolescence (Dam et al., 1999; Slotkin et al., 2002) but no studies of long-term, persistent effects, or comparative effects of other organophosphates have been published. Here, we exposed neonatal rats to diazinon or parathion using toxicodynamic dose-matching: each agent was given at two doses spanning the threshold for barely-detectable cholinesterase inhibition. If the effects on norepinephrine systems reside in their shared property as cholinesterase inhibitors, then we would expect to see similar outcomes from the two agents as the dose passes the threshold for inhibition. Norepinephrine measurements were then carried out longitudinally from early adolescence to young adulthood and then up to full adulthood (five months of age), encompassing multiple brain regions that comprise the major noradrenergic projections. In addition, we assessed the impact on β-adrenergic receptors (βARs) so as to determine whether changes in norepinephrine represent a primary presynaptic target of the organophosphate exposures, as opposed to compensation for a postsynaptic receptor defect. Adverse effects on cell signaling cascades, notably the pathway that generates cyclic AMP, are known to contribute to the developmental neurotoxicity of organophosphates (Slotkin, 2005), and noradrenergic control of cyclic AMP is exerted in large measure through βARs.
MATERIALS AND METHODS
Animal treatments
Although the determinations presented here are entirely new, the tissue samples were archived from several earlier studies (Adigun et al., 2010; Slotkin et al., 2008a, b, 2009a), so that no additional animals were actually used. All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague-Dawley rats were housed in breeding cages, with a 12-hr light/dark cycle and free access to food and water. On the day of birth, all pups were randomized and redistributed to the dams with a litter size of 10 to maintain a standard nutritional status. Randomization within their respective treatment groups was repeated at intervals of several days. In addition, dams were rotated among litters to distribute any maternal caretaking differences randomly across litters and treatment groups. Offspring were weaned on PN21. Because of their poor water solubility, diazinon and parathion were dissolved in dimethylsulfoxide to provide consistent absorption (Whitney et al., 1995) and were injected subcutaneously in a volume of 1 ml/kg once daily on PN1–4. Control animals received equivalent injections of dimethylsulfoxide vehicle, which does not itself produce developmental toxicity (Whitney et al., 1995). At the specified ages, one male and one female from each final litter assignment were decapitated. The cerebellum (including flocculi) was dissected from the rest of the brain, after which the midbrain/brainstem was separated from the forebrain by a cut rostral to the thalamus. The striatum and hippocampus were then dissected from these larger divisions and the midbrain and brainstem were divided from each other. The cerebral cortex was divided down the midline and then further sectioned into anterior and posterior regions (frontal/parietal cortex and temporal/occipital cortex, respectively). Tissues were blotted, frozen in liquid nitrogen and maintained at −45° C.
The dosing paradigms were chosen to achieve a toxicodynamic match between diazinon and parathion (Slotkin et al., 2006), straddling the threshold for barely-detectable cholinesterase inhibition (5–20% inhibition), well below the 70% inhibition required for signs of cholinergic hyperstimulation (Clegg and van Gemert, 1999): 0.5 and 2 mg/kg/day for diazinon; 0.1 and 0.2 mg/kg/day for parathion. Assessments were carried out in adolescence (PN30), early adulthood (PN60), and full adulthood (PN100).
Assays
For norepinephrine determinations, tissues were thawed on ice and deproteinized by homogenization (Polytron; Brinkmann Instruments, Westbury, NY) in 0.1 N perchloric acid containing 3,4-dihydroxybenzylamine as an internal standard. Homogenates were sedimented at 26,000 × g for 20 minutes, the supernatant solutions were decanted, and norepinephrine was then trace-enriched by alumina adsorption, separated by reverse-phase high performance liquid chromatography and quantitated by electrochemical detection (Seidler and Slotkin, 1981); values were corrected for recovery of the internal standard.
For βAR binding determinations, tissues were thawed and homogenized in buffer containing 145 mM sodium chloride, 2 mM magnesium chloride, and 20 mM Tris (pH 7.5), and were then sedimented at 40,000 × g for 15 min. The pellets were washed twice and then resuspended in the homogenization buffer. Aliquots of the suspension were incubated with 67 pM [125I]iodopindolol in 145 mM NaCl, 2 mM MgCl2, 1 mM sodium ascorbate, 20 mM Tris (pH 7.5), for 20 min at room temperature; samples were evaluated with and without 100 μM isoproterenol to displace specific binding. Incubations were stopped by addition of 3 ml ice-cold buffer, and the labeled membranes were trapped by rapid vacuum filtration onto glass fiber filters, which were washed with additional buffer and counted by liquid scintillation spectrometry. Binding was then assessed relative to membrane protein (Smith et al., 1985).
Data analysis
To avoid the increase in type 1 errors that could occur from multiple statistical tests on the same data, each set of determinations was first evaluated using multivariate ANOVA considering all relevant variables (treatment, sex, age, brain region) in a single test. Because we did not have samples for every region at each age, the initial ANOVA considered a given age-region combination as a single grouping, so that the factors were treatment, sex and [age, region]. Data were log-transformed because of heterogeneous variance among the different ages and regions. As permitted by the interaction terms, we conducted lower order tests on subdivisions of data (i.e. separated by sex or by region-age). Evaluations of individual differences between treatment groups were conducted post-hoc with Fisher’s Protected Least Significant Difference Test. Significance was assumed at the level of p < 0.05 (two-tailed).
Data were compiled as means and standard errors. For ready visualization of treatment effects across the different regions and ages, the results are given as the percent change from control; Table 1 provides the corresponding control values, normalized across all animal cohorts. Statistical procedures were always conducted on the original data, with log transforms because of heterogeneous variance as noted above, and with treatment comparisons made only against the contemporaneous control group.
TABLE 1.
CONTROL VALUES
| Age (days) | Region | Norepinephrine (ng/g) | |
|---|---|---|---|
| Male | Female | ||
| 5 | forebrain | 34 ± 1 | 34 ± 1 |
| midbrain + brainstem | 162 ± 8 | 163 ± 4 | |
| 30 | frontal/parietal cortex | 203 ± 5 | 214 ± 4 |
| temporal/occipital cortex | 163 ± 6 | 169 ± 7 | |
| hippocampus | 215 ± 11 | 221 ± 10 | |
| midbrain | 586 ± 15 | 550 ± 8 | |
| brainstem | 480 ± 11 | 467 ± 14 | |
| cerebellum | 181 ± 9 | 187 ± 11 | |
| 60 | frontal/parietal cortex | 348 ± 9 | 335 ± 6 |
| temporal/occipital cortex | 180 ± 10 | 198 ± 14 | |
| hippocampus | 293 ± 6 | 297 ± 20 | |
| midbrain | 710 ± 13 | 697 ± 13 | |
| brainstem | 459 ± 11 | 453 ± 10 | |
| cerebellum | 188 ± 6 | 198 ± 11 | |
| 100 | frontal/parietal cortex | 381 ± 14 | 374 ± 8 |
| hippocampus | 348 ± 12 | 303 ± 17 | |
| striatum | 133 ± 1 | 159 ± 13 | |
| midbrain | 604 ± 26 | 572 ± 13 | |
| brainstem | 449 ± 19 | 439 ± 9 | |
| cerebellum | 182 ± 5 | 200 ± 4 | |
| 100 | βAR Binding (fmol/mg protein) | ||
| frontal/parietal cortex | 38.4 ± 0.9 | 39.7 ± 0.8 | |
| temporal/occipital cortex | 34.0 ± 0.8 | 36.9 ± 0.9 | |
Data represent mean ± SE obtained from 12–24 animals of each sex at each age point for norepinephrine values and 12 animals for βAR binding.
Materials
Animals were purchased from Charles River Laboratories (Raleigh, NC). PerkinElmer Life Sciences (Boston, MA) was the source for [125I]iodopindolol (specific activity, 2200 Ci/mmol). Diazinon and parathion were obtained from Chem Service (West Chester, PA) and Sigma Chemical Co. (St. Louis, MO) was the source for all other reagents.
RESULTS
Early-life exposure to diazinon at either dose elicited a significant overall decrement in norepinephrine levels, without distinction between males and females (Figure 1). Although there were some disparities among regions at different age points, the regional differences were within the limits of random variation and thus did not achieve statistical significance.
Figure 1.
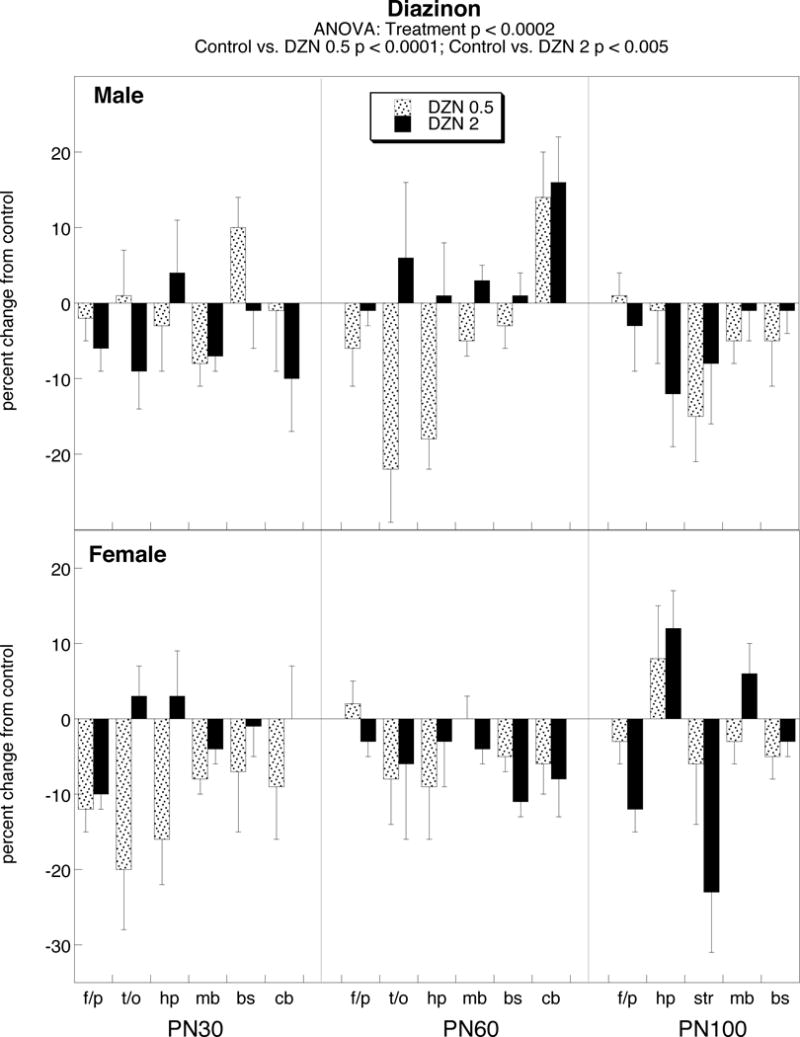
Effects of PN1-4 exposure to diazinon (DZN, 0.5 or 2 mg/kg) on norepinephrine levels. Data represent mean ± SE obtained from 6–12 animals of each sex at each age for each treatment group. Values are shown as percent change from control (see Table 1). Results of multivariate ANOVA appear at the top of the panel; lower-order tests for males and females or for individual ages or regions were not carried out because of the absence of a treatment interaction with these factors. Abbreviations: f/p frontal/parietal cortex, t/o temporal/occipital cortex, hp hippocampus, mb midbrain, bs brainstem, cb cerebellum, str striatum.
In contrast, parathion evoked increases in norepinephrine rather than decreases (Figure 2). Superimposed on this overall pattern, the treatment effects were sex-selective (treatment × sex interaction), with a more consistent increase seen in males than females. After separation of values by sex, significance was maintained at either parathion dose in males. In females, the effects were just at the margin of significance, largely because of a decrease in one region at one age (temporal/occipital cortex on PN30). Although the global ANOVA also identified an interaction of treatment × [age, region], this interaction was not maintained after separation of the values by sex.
Figure 2.
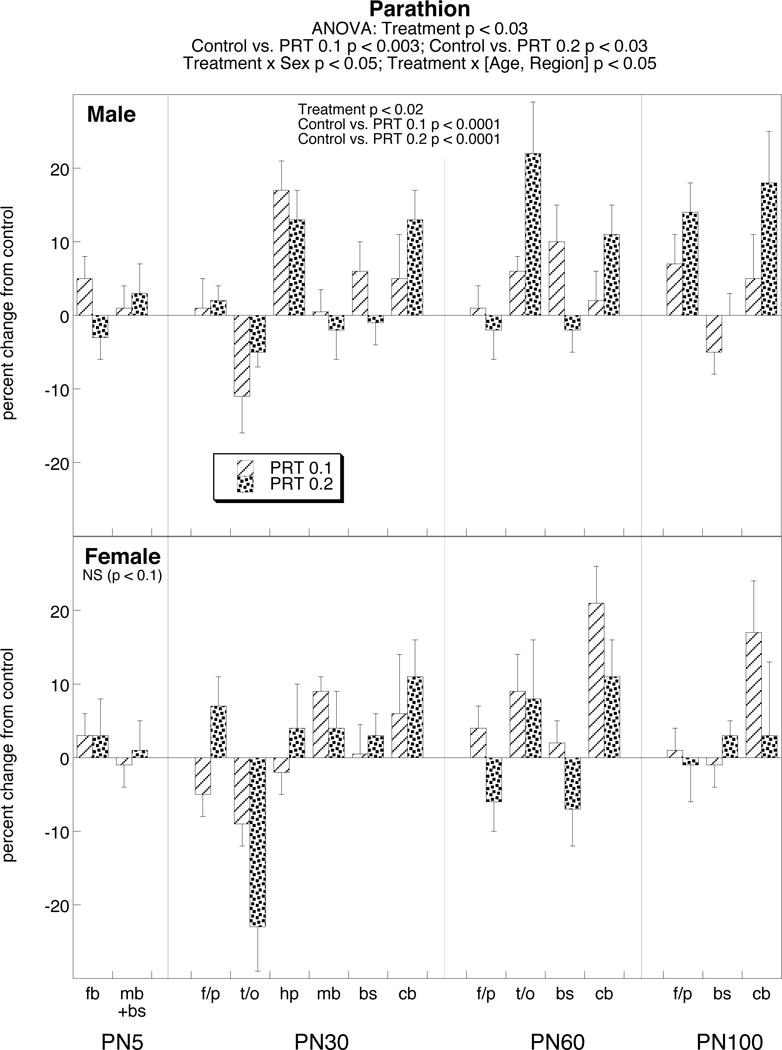
Effects of PN1-4 exposure to parathion (PRT, 0.1 or 0.2 mg/kg) on norepinephrine levels. Data represent mean ± SE obtained from 6–12 animals of each sex at each age for each treatment group. Values are shown as percent change from control (see Table 1). Results of multivariate ANOVA appear at the top of the panel; lower-order tests for males and females are shown within the panels as justified by the treatment × sex interaction. Treatment × [Age, Region] interactions were no longer detectable after separation by sex. Abbreviations: fb forebrain, mb+bs midbrain+brainstem, f/p frontal/parietal cortex, t/o temporal/occipital cortex, hp hippocampus, mb midbrain, bs brainstem, cb cerebellum.
We wanted to distinguish whether persistent effects on norepinephrine reflected continuance of an initial injury, or whether they resulted from an altered trajectory of noradrenergic development. Accordingly, we made assessments on PN5, twenty-four hours after the last dose of parathion. Because of tissue limitations, we assayed two larger regional groupings: the forebrain, comprising the frontal/parietal cortex, temporal/occipital cortex, hippocampus and striatum; and the combined midbrain + brainstem. There were no significant differences evoked by parathion at this early age point (Figure 2).
To illustrate the differences in the main treatment effects between diazinon and parathion, we calculated the mean values for effects on norepinephrine, collapsed across sex, region and dose (Figure 3). This simplified picture dilutes the effects seen by averaging regions or sexes for which there are relatively robust effects, with data points for which there was no effect or an opposite effect, so that the absolute magnitude becomes smaller. Thus, whereas affected regions or sexes showed effect sizes of as muchas 20–30%, averaged effect sizes were <10% when combined with the negative or opposite data points. Despite these limitations, there was an obvious overall pattern: diazinon decreased norepinephrine whereas parathion evoked an overall increase. The combined data also reinforce the progression of parathion’s effect, with an increase in intensity after adolescence.
Figure 3.
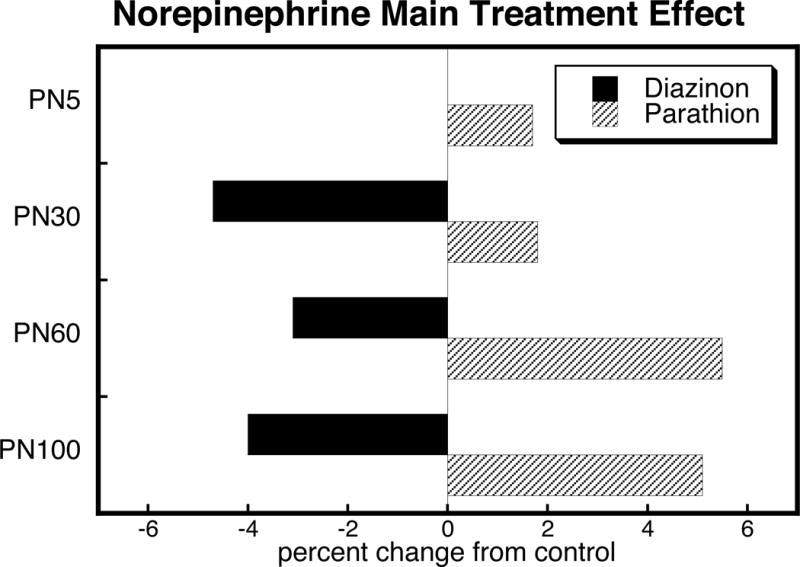
Main effects of diazinon and parathion, collapsed across sex, region and dose. Data are the mean values of the changes across all bars for each age point in Figures 1 and 2.
Unlike the profound effects of diazinon or parathion on norepinephrine levels, we did not find any significant changes in βAR binding (Figure 4).
Figure 4.
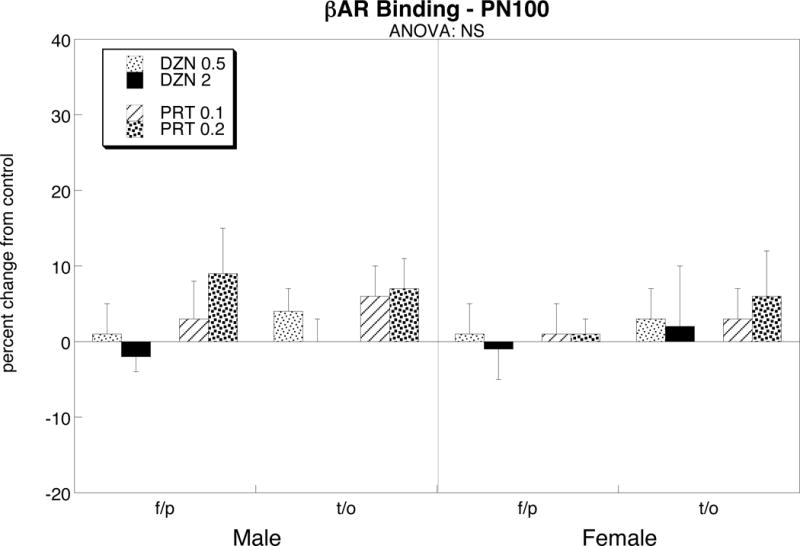
Effects of PN1-4 exposure to diazinon (DZN, 0.5 or 2 mg/kg) or parathion (PRT, 0.1 or 0.2 mg/kg) on βAR binding. Data represent mean ± SE obtained from 6 animals of each sex for each treatment group. Values are shown as percent change from control (see Table 1). Results of multivariate ANOVA appear at the top of the panel; lower-order tests were not carried out because there was no significant treatment effect or treatment interaction with sex or region. Abbreviations: f/p frontal/parietal cortex, t/o temporal/occipital cortex, NS not significant.
DISCUSSION
Our results show that diazinon and parathion elicit opposite effects on norepinephrine systems in the developing brain. Since the doses were matched to produce comparable effects on cholinesterase, the disparate outcomes are clearly unrelated to their shared property as cholinesterase inhibitors. Indeed, since the doses for each agent span below and above the threshold for inhibition, the fact that the outcome does not change when the threshold is passed, reinforces this conclusion. It is also important that the effects on norepinephrine were not seen in the immediate the posttreatment period (PN5), indicating that there is a change in the trajectory of development, leading to progressive changes in adolescence and adulthood.
At first glance, though, it seems puzzling that we would find opposite effects on norepinephrine, with diazinon producing a decrease and parathion an increase. Organophosphates in general promote the initial neuronal commitment to the norepinephrine/catecholamine phenotype, an effect known to be unrelated to cholinesterase inhibition (Slotkin et al., 2007; Slotkin and Seidler, 2007). So, the effects seen here for parathion are consistent with that cellular mechanism, whereas those for diazinon are not. Although these effects may be produced by different mechanisms, this paradox is readily resolved if one considers the fact that the doses of the two agents were matched for cholinesterase effects but differ in absolute doses because of the greater potency of parathion toward cholinesterase. In fact, if the parathion dose is raised further, to the point of overt intoxication, then there is a nonspecific decline in all monoamines in the developing brain, including norepinephrine (Mahaboob Basha et al., 2001). For endpoints that influence more general aspects of neural development, such as cell acquisition, apoptosis, neurodifferentiation and synaptogenesis, the organophosphates are approximately equipotent, a very different situation from their effects on cholinesterase (Slotkin et al., 2007). The reduction in norepinephrine caused by diazinon thus likely represents these mechanisms, which operate at higher exposures than those necessary to elicit more selective changes in neuronal phenotype. Indeed, the same result was seen earlier for the impact of these organophosphates on cell signaling mediated by adenylyl cyclase (Adigun et al., 2010): parathion elicited an increase in signaling, whereas diazinon, given at a higher dose, elicited a decrease, despite their similar effects on cholinesterase.
An alternative explanation is that the impact on presynaptic norepinephrine could represent a compensation resulting from a primary effect on development of βAR signaling. We effectively ruled out that possibility, since there were no significant changes in βARs, confirming and extending a prior report on βAR binding in one specific region (cerebellum) (Adigun et al., 2010). Nevertheless, our findings do not provide a complete picture of the potential impact on noradrenergic synaptic function. In our earlier work with neonatal chlorpyrifos exposure, we found that neural activity was affected to a greater extent than predicted solely from norepinephrine levels (Slotkin et al., 2002). Thus, the current results, while explaining some of the differences in behavioral outcomes after neonatal diazinon or parathion exposure (Levin et al., 2010; Roegge et al., 2008; Timofeeva et al., 2008a, b), may be just the tip of the iceberg.
In conclusion, our results show that diazinon and parathion have opposite effects on the developmental trajectory of norepinephrine systems throughout the brain, at doses producing comparable but minimal effects on cholinesterase. Using the cholinesterase benchmark can be misleading because the requisite dose of diazinon is then much higher than that of parathion, whereas effects on neurodevelopment are likely to include major contributions from mechanisms unrelated to cholinesterase inhibition. The promotional effects of parathion are representative of the ability of organophosphates to promote emergence of the norepinephrine/catecholamine phenotype, whereas diazinon, given at a higher absolute dose, elicits more injurious responses such as suppression of neurogenesis and axonogenesis, and promotion of apoptosis. Our findings support the recent U.S. Environmental Protection Agency’s proposal to cancel all use of chlorpyrifos, the most widely-used organophosphate, based on the inability to identify a safe exposure limit, given that developmental neurotoxicity occurs even in the absence of effects on the benchmark of cholinesterase inhibition (U.S. Environmental Protection Agency, 2015).
Highlights.
We compared neonatal diazinon vs. parathion for noradrenergic system effects
Diazinon produced a net decrease whereas parathion elicited a decrease
Effects emerged between juvenile stages and adolescence, persisting to 5 months
Effects were not due to an underlying defect in β-adrenergic receptor signaling
Organophosphates differ in their effects, unrelated to cholinesterase inhibition
Acknowledgments
Research was supported by NIH ES010356. The authors thank Ashley Stadler for technical assistance.
Abbreviations
- ANOVA
analysis of variance
- βAR
β-adrenergic receptor
- PN
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: TAS has received consultant income in the past three years from the following firms: Acorda Therapeutics (Ardsley NY), Pardieck Law (Seymour, IN), and Walgreen Co. (Deerfield, IL).
References
- Adigun AA, Wrench N, Seidler FJ, Slotkin TA. Neonatal organophosphorus pesticide exposure alters the developmental trajectory of cell signaling cascades controlling metabolism: differential effects of diazinon and parathion. Environ Health Perspect. 2010;118:210–215. doi: 10.1289/ehp.0901237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, van Gemert M. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J Toxicol Environ Health. 1999;2:211–255. doi: 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Timofeeva OA, Yang L, Ryde IT, Wrench N, Seidler FJ, Slotkin TA. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav Brain Res. 2010;208:319–327. doi: 10.1016/j.bbr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaboob Basha P, Begum S, Nayeemunnisa Methyl parathion induced alterations in monoaminergic system of developing rat pups. Indian J Exp Biol. 2001;39:276–279. [PubMed] [Google Scholar]
- Ordway G, Schwartz MA, Frazer A. Brain Norepinephrine: Neurobiology and Therapeutics. New York: Cambridge University Press; 2007. [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Development of central control of norepinephrine turnover and release in the rat heart: responses to tyramine, 2-deoxyglucose and hydralazine. Neuroscience. 1981;6:2081–2086. doi: 10.1016/0306-4522(81)90047-6. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. In: Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. Toxicity of Organophosphate and Carbamate Pesticides, editor. San Diego: Elsevier Academic Press; 2005. pp. 293–314. [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008a;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Levin ED, Seidler FJ. Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environ Health Perspect. 2008b;116:1308–1314. doi: 10.1289/ehp.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Lassiter TL, Ryde IT, Wrench N, Levin ED, Seidler FJ. Consumption of a high-fat diet in adulthood ameliorates the effects of neonatal parathion exposure on acetylcholine systems in rat brain regions. Environ Health Perspect. 2009a;117:916–922. doi: 10.1289/ehp.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Developmental neurotoxicity of parathion: progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol Teratol. 2009b;31:11–17. doi: 10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ Health Perspect. 2007;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Dev Brain Res. 2002;133:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol. 2008a;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, et al. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res Bull. 2008b;77:404–411. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Chlorpyrifos; tolerance revocations; Proposed Rule. Federal Register. 2015;80:69080–69110. [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]


